Abstract
OBJECTIVE—The unfolding of type 1 diabetes involves a number of steps: defective immunological tolerance, priming of anti-islet autoimmunity, and destruction of insulin-producing β-cells. A number of genetic loci contribute to susceptibility to type 1 diabetes, but it is unclear which stages of the disease are influenced by the different loci. Here, we analyzed the frequency of type 1 diabetes–risk alleles among individuals from the Diabetes Prevention Trial–Type 1 (DPT-1) clinical trial, which tested a preventive effect of insulin in at-risk relatives of diabetic individuals, all of which presented with autoimmune manifestations but only one-third of which eventually progressed to diabetes.
RESEARCH DESIGN AND METHODS—In this study, 708 individuals randomized into DPT-1 were genotyped for 37 single nucleotide polymorphisms in diabetes susceptibility loci.
RESULTS—Susceptibility alleles at loci expected to influence immunoregulation (PTPN22, CTLA4, and IL2RA) did not differ between progressors and nonprogressors but were elevated in both groups relative to general population frequencies, as was the INS promoter variant. In contrast, HLA DQB1*0302 and DQB1*0301 differed significantly in progressors versus nonprogressors (DQB*0302, 42.6 vs. 34.7%, P = 0.0047; DQB*0301, 8.6 vs. 14.3%, P = 0.0026). Multivariate analysis of the factors contributing to progression demonstrated that initial titers of anti-insulin autoantibodies (IAAs) could account for some (P = 0.0016) but not all of this effect on progression (P = 0.00038 for the independent effect of the number of DQB*0302 alleles). The INS-23 genotype was most strongly associated with anti-IAAs (median IAA levels in TT individuals, 60 nU/ml; AT, 121; and AA, 192; P = 0.000037) and only suggestively to the outcome of oral insulin administration.
CONCLUSIONS—With the exception of HLA, most susceptibility loci tested condition the risk of autoimmunity rather than the risk of failed immunoregulation that results in islet destruction. Future clinical trials might consider genotyping INS-23 in addition to HLA alleles as disease/treatment response modifier.
Type 1 diabetes is an autoimmune disease characterized by destruction of the insulin-producing β-cells in the pancreatic islets. Although its etiology is not yet understood, strong genetic and environmental components appear to modulate individual disease susceptibility in patients and in animal models (1). The major histocompatibility complex (MHC) is the primary genetic determinant of susceptibility to type 1 diabetes in human patients and in the NOD mouse model (2). In addition, numerous genetic studies in humans and mice have led to the description of additional susceptibility loci (IDDM and Idd, respectively), for which the causative genes have yet to be fully defined in most cases. The best evidence exists for polymorphisms in the promoter region of the Insulin gene, which may impact ectopic expression of this locus in the thymus and thereby modulate immunological tolerance through clonal deletion of autoreactive thymocytes (3,4). More recently, polymorphisms in PTPN22 and CTLA4, two genes key in the fine-tuning of immune responses, have been associated with type 1 diabetes (5,6). Several other susceptibility loci have recently been described in genome-wide association studies performed in large cohorts (7–9). Although their identity, biological significance, and functional impact remain to be elucidated, some of these susceptibility loci appear to be shared across several autoimmune diseases, suggesting the existence of common regulatory steps whose dysfunction may lead to autoimmunity (e.g., PTPN22 is associated with Graves’ thyroiditis, type 1 diabetes, and Rheumatoid Arthritis, among others).
This complex genetic determinism matches the multiple steps and checkpoints involved in the pathogenesis of type 1 diabetes. A likely first step is the defective induction of tolerance to self-antigens in immature thymocytes, as demonstrated in the NOD mouse model (10–12) and suggested by the impact of INS promoter polymorphisms in human patients (3,4). A second phase involves activation of autoreactive cells in the periphery, followed by lymphocytic infiltration of pancreatic islets and the production of autoantibodies. Although clinically silent, metabolic studies can demonstrate impaired insulin secretion or altered first-phase insulin release after intravenous glucose challenge (IVGTT) and a flattening of the physiological increase in C-peptide with age (13,14).
This prodromic phase can persist for long periods of time in mice and in humans; many such pre-diabetic individuals may never progress to overt diabetes. In the final disease stage, the insulitic infiltration results in massive destruction/functional incapacitation of β-cells, culminating in loss of glycemic control. In animal models, several factors appear to impact on these checkpoints, in particular the timing of self-antigen availability for presentation in the pancreatic lymph nodes, the functionality of regulatory T-cells, and infectious or related environmental challenges (rev. in 15,16). In such models, loci that affect the breakdown of immunological tolerance could be distinguished from others that control later steps of immunoregulation or the aggressivity of the attack on the islets (10,11,17,18). Whether such checkpoints occur in humans is unknown, although the long prodromic phase that precedes onset in at-risk individuals suggests the existence of similar immunological and genetic steps.
The notion of checkpoints controlled by different mediators raised the hope that pre-diabetic individuals might be prevented from developing full-blown diabetes by reestablishing tolerance and halting the autoimmune process, allowing islet regeneration to take place naturally. The Diabetes Prevention Trial–Type 1 (DPT-1) was set-up with the specific goal of identifying anti-islet cytoplasmic antibody–positive (ICA+) first-degree relatives of type 1 diabetic patients at risk for developing type 1 diabetes themselves and treating them with daily low-dose subcutaneous and yearly 4-day intravenous insulin (parenteral insulin trial) or oral insulin to prevent loss of glycemic control (19). This study was based on results from NOD mice (although the protocol used was very different) and small pilot studies in human patients (20–23). Neither the parenteral nor the oral insulin treatments resulted in significant modification of diabetes incidence, although post hoc analysis suggested a slight treatment effect in the subgroup with the highest insulin autoantibody (IAA) titers at baseline (19,24). In the context of this trial, a large amount of high-quality longitudinal data was collected, representing a unique opportunity to study the genetic factors underlying progression to overt diabetes in antibody-positive individuals.
The primary goal of the present study was to gauge the contribution of type 1 diabetes susceptibility loci to the transition to full-blown diabetes and thus to elucidate which stage of the disease is impinged on by such loci. We genotyped single nucleotide polymorphisms (SNPs) in a number of known or putative type 1 diabetes susceptibility loci and also reconsidered the HLA data (19) from the particular angle of distinguishing progressor from nonprogressor individuals. Beyond the implications for our understanding of the pathogenic processes in type 1 diabetes, this distinction could have direct clinical consequences, by allowing a more accurate definition of the risk of progression in pre-diabetic individuals. Improved prediction would facilitate the design of prevention trials, and/or define individuals at particularly high risk for whom more aggressive therapeutic regimens might be warranted.
RESEARCH DESIGN AND METHODS
Identification and randomization of subjects.
This study is based on the data published by the DPT-1 Study Group (19) and Skyler et al. (24). Briefly, relatives of type 1 diabetic patients were screened for ICAs. In case of a positive result, IAAs, β-cell function, glucose tolerance status, and HLA genotype were determined. ICA+ and IAA+ individuals with preserved islet function without HLA DQB1*0602 alleles were deemed to be at intermediate risk and eligible for randomization to oral insulin or placebo, whereas similar individuals with an abnormal response to IVGTT were considered high risk and were randomized in the parenteral insulin trial (intravenous and subcutaneous).
In this study, all demographic, clinical, biochemical, and HLA data were obtained from the original study database after de-identification of the subjects (the 30 April 2003 release of the clinical and phenotypic data, with antibody data from the 1 November 2004 release). Allele frequencies of parents of type 1 diabetic patients were retrieved from the Type 1 Diabetes Genetic Consortium (T1DGC) study (25).
SNP typing.
Type 1 diabetes susceptibility SNPs were chosen based on the available literature (5,7–9,26–32; V.B., C.C., D.M., and C.B., unpublished data). Starting from amplified genomic DNA provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Central Repository, SNPs were genotyped with fluorogenic allele-discrimination chemistry, as described previously (11). Primers and probes are described in Supplementary Table 1 (available in an online appendix at http://dx.doi.org/10.2337/db07-1736).
Statistical analyses.
Categorical variables were analyzed by the χ2 test or Fisher's exact test if the counts were less than five in one of the cells. Survival differences among groups were assessed by the log-rank test. Cox proportional hazard modeling was applied to multivariate analysis of survival parameters. Kaplan-Meier survival probability function was used to plot risk of diabetes onset among subgroups. Impact of SNP genotype classes on IAA levels was assessed by linear regression on log-transformed initial IAA values. Data were analyzed with the survival package in the R statistical environment (http://cran.r-project.org). Because most showed no significance, P values reported here are not corrected for multiple sampling; those tests showing potential significance were reconsidered with a simple Bonferroni correction, based on the number of SNPs or alleles tested (for HLA alleles, we only corrected for frequent alleles or haplotypes, ignoring those present in <10 individuals). DPT-1 parental HLA allele frequencies were estimated based on published transmission disequilibrium of HLA alleles to type 1 diabetes probands (33–35), according to the following formula: ProAF = ParAF2 + [ParAF × (1 − ParAF) × (%transmission to probands/50%)], where ProAF and ParAF are the proband and parental allele frequencies, respectively. Hazard ratios for INS-23 genotype subsets were computed by Cox proportional modeling. Subgroups of individuals showing maximal treatment efficacy were identified by computing survival differences across individual subsets ranked according to the distribution of log-transformed initial IAA quantiles.
Haplotype reconstruction.
CTLA4 haplotypes were reconstructed using PHASE2.1 (36) and pooling data from progressors and nonprogressors. The algorithm was run according to the authors’ recommended procedure, and the output was checked for consistency with different seeds for random numbers generation.
RESULTS
The goal of this study was to test whether any of the loci so far associated with susceptibility to type 1 diabetes might condition progression from the pre-diabetic to the diabetic state, as opposed to influencing the autoimmune deviation that results in pre-diabetes. To that end, DNA samples and full clinical information were obtained from individuals randomized into the DPT-1 study. As described previously (19), >100,000 relatives of type 1 diabetic patients were screened for autoantibodies (ICA test), identifying 3,483 positive individuals (Supplementary Fig. 1). To be further considered in the study, ICA+ individuals had to lack protective HLA-DQA1*0102/DQB1*0602 alleles. Individuals with an abnormal IVGTT or insulin-release assay were eligible for randomization into the high-risk/parenteral insulin group, whereas subjects with high IAA titers and preserved IVGTT/oral glucose tolerance test were eligible for the medium-risk oral insulin trial. Four hundred eighteen high-risk and 388 medium-risk individuals were identified, who were randomized between the arms of the study and for whom excellent follow-up was performed during the years of the DPT-1 trial. Of these, 258 individuals progressed to clinical diabetes during the follow-up period. The age distribution (Supplementary Fig. 2) showed the existence of one group of individuals (n = 638) whose mean age at screening was 3,461 days (9.5 years, range 372–9,628 days) and a second group (n = 70) with a mean age at randomization of 13,597 days (37.3 years, range 10,075–16,340 days), leading to a bimodal distribution (goodness-of-fit to normality by Kolmogorov-Smirnov P = 10−70). More importantly, the incidence of progression for the individuals screened after 10,000 days of age was distinctly lower (14 vs. 39%). Thus, we did not include this subgroup to avoid diluting true genetic effects. The remaining group of 638 individuals included in our studies encompassed 485 children screened at age <4,500 days (12.3 years, 76%). In the 258 individuals who progressed to type 1 diabetes, the median time from screening to diagnosis of type 1 diabetes was 3.7 years (5–95%, 1.2–7 years).
Non-HLA diabetes susceptibility loci and progression.
We first addressed the impact of convincingly associated, non-HLA type 1 diabetes susceptibility polymorphisms on diabetes progressor/nonprogressor outcome. These included the INS-23 promoter polymorphism, the PTPN22 R620W coding-region change, and several 3′-untranslated and intronic polymorphisms in the CTLA4 costimulatory gene (the CT60 marker and other SNPs, because the causal polymorphism in the costimulatory region remains in question). We also genotyped a number of variants for which the evidence is more recent or less substantially replicated, including some discovered in the context of genome-wide association studies (acknowledging that the low effect of these recently described variants would likely make the present cohort underpowered) (5,7–9,26–32,37).
These SNPs were genotyped using fluorogenic PCR with a success rate of 98.3% (96% of individuals with 93% or more genotyping success and 100% concordance rate on repeated genotyping of a handful of markers in all individuals). Because some of these markers have shown strong population differentiation (e.g., the absence of PTPN22 R620W T in Asian populations), we restricted our analyses to Caucasian individuals screened at <10,000 days (n = 575), who constitute the majority of subjects recruited into DPT-1. As depicted in Table 1, no polymorphism showed a significant difference between DPT-1 progressors and nonprogressors, with the exception of a SNP in Lymphotoxin-α, likely reflecting HLA haplotypes (see below). For a number of SNPs, the allele frequencies in the progressors and in the nonprogressor groups matched well those usually reported in type 1 diabetic patients. For example, the PTPN22 R620W T variant is present in ∼8% of nonaffected U.S. Caucasian populations, 15–20% of type 1 diabetic patients, and 15 and 17% of nonprogressor and progressor DPT-1 individuals, respectively. A similar distribution was found for the INS-23 A variant (unaffected, 24–32%; type 1 diabetes, 12–18%; nonprogressor, 20%; and progressor, 17%) and for CTLA4 CT60 G (unaffected, 52%; type 1 diabetes, 57–63%; nonprogressor, 60%; and progressor, 61%).
TABLE 1.
Allele frequencies in DPT-1 participants and comparative cohorts
| Chromosome | Gene name | Polymorphism | Alleles |
rs number | Allele counts 0/1/2 |
MAF |
OR (95% CI) | P value | Control subjects MAF |
Case subjects MAF |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | Nonprogressor | Progressor | Nonprogressor | Progressor | Lit. | HapMap CEU | DCGS | Lit. | DCGS | ||||||
| 6 | Lymphotoxin-α | +10 | G | A | rs1800683 | 137/173/39 | 115/95/17 | 0.36 | 0.28 | 1.41 (1.1–1.83) | 9.4E–03 | 0.36 | 0.34 | 0.44 | ||
| 11 | Insulin | −23 Hphl | T | A | rs689 | 16/109/221 | 7/63/153 | 0.20 | 0.17 | 1.23 (0.9–1.67) | 0.22 | 0.32 | 0.24 | 0.28 | 0.12 | 0.18 |
| 1 | PTPN22 | R620W | C | T | rs2476601 | 253/86/9 | 155/66/6 | 0.15 | 0.17 | 1.18 (0.86–1.63) | 0.35 | 0.06–0.15 | 0.14 | 0.08 | 0.15–0.17 | 0.19 |
| 10 | IL-2 Ra (CD25) | Intron 1 | G | A | rs706778 | 106/175/64 | 57/124/45 | 0.44 | 0.47 | 1.15 (0.91–1.46) | 0.28 | 00.4–0.45 | 0.45 | 0.39* | 0.45 | 0.48* |
| 10 | IL-2 Ra (CD25) | Intron 1 | T | C | rs3118470 | 145/160/41 | 85/115/26 | 0.35 | 0.37 | 1.09 (0.8–1.39) | 0.54 | 0.32–0.34 | 0.29 | 0.25* | 0.36 | 0.38* |
| 10 | IL-2 Ra (CD25) | IL-2 Ra region | C | A | rs41295061 | 307/36/5 | 194/28/1 | 0.07 | 0.07 | 1.02 (0.63–1.64) | 0.96 | 0.11 | 0.07 | |||
| 10 | IL-2 Ra (CD25) | IL-2 Ra region | T | A | rs11594656 | 204/119/20 | 141/67/13 | 0.23 | 0.21 | 1.13 (0.85–1.51) | 0.44 | 0.25 | 0.21 | 0.22 | ||
| 2 | IFIH1 | A946T | C | T | rs1990760 | 43/139/143 | 25/104/93 | 0.35 | 0.35 | 1.00 (0.78–1.29) | 0.97 | 0.39 | 0.39 | 0.35 | ||
| 1 | Fc receptor-like 3 | Prom −169 | A | G | rs7528684 | 99/174/67 | 74/107/42 | 0.45 | 0.43 | 1.11 (0.87–1.41) | 0.45 | 0.45 | 0.44 | 0.49 | 0.44 | |
| 6 | C6orf118 | M256I | C | A | rs510579 | 144/158/42 | 88/111/25 | 0.35 | 0.36 | 1.03 (0.81–1.33) | 0.84 | 0.35 | 0.36 | 0.38 | ||
| 5 | CAPSL | R75Q | A | G | rs1445898 | 55/184/94 | 41/103/68 | 0.44 | 0.44 | 1.02 (0.8–1.31) | 0.92 | 0.44 | 0.39 | 0.41 | ||
| 2 | CTLA4 | Prom −1577 | G | A | rs11571316 | 129/176/42 | 90/102/32 | 0.37 | 0.37 | 1.02 (0.8–1.3) | 0.94 | 0.42 | 0.47 | 0.46 | 0.35 | 0.42 |
| 2 | CTLA4 | Prom −318 | T | C | rs5742909 | 0/60/278 | 2/34/188 | 0.09 | 0.08 | 1.05 (0.69–1.61) | 0.90 | 0.11 | 0.06 | 0.06 | 0.09 | 0.08 |
| 2 | CTLA4 | T49A | G | A | rs231775 | 57/175/111 | 43/115/66 | 0.42 | 0.45 | 1.12 (0.88–1.42) | 0.40 | 0.43 | 0.38 | 0.38 | 0.29 | 0.42 |
| 2 | CTLA4 | 3'UTR 6230 (CT60) | G | A | rs3087243 | 117/181/48 | 84/105/35 | 0.40 | 0.39 | 1.04 (0.82–1.33) | 0.79 | 0.48 | 0.46 | 0.47 | 0.37 | 0.43 |
| 2 | CTLA4 | JO31 | C | A | rs11571302 | 101/176/47 | 74/108/38 | 0.42 | 0.42 | 1.01 (0.79–1.29) | 0.99 | 0.50 | 0.49 | 0.39 | 0.47 | |
| 2 | CD28 | Intron 1 | C | T | rs10932017 | 74/182/90 | 43/116/64 | 0.48 | 0.45 | 1.10 (0.87–1.4) | 0.47 | 0.44 | 0.42 | 0.43 | 0.45 | |
| 2 | CD28 | Intron 1 | A | T | rs2013278 | 39/145/148 | 25/98/97 | 0.34 | 0.34 | 1.00 (0.78–1.29) | 0.96 | 0.36 | 0.23 | 0.26 | ||
| 2 | CD28 | 3′UTR | G | T | rs3181113 | 313/35/0 | 211/11/1 | 0.05 | 0.03 | 1.76 (0.92–3.37) | 0.11 | 0.03 | 0.03 | 0.04 | 0.02 | |
| 2 | CD28 | 3′UTR | T | A | rs11681201 | 22/117/210 | 12/87/128 | 0.23 | 0.24 | 1.08 (0.82–1.42) | 0.64 | 0.13 | 0.20 | 0.15 | 0.18 | |
| 2 | ICOS | Prom −1817 | T | C | rs4452124 | 263/76/5 | 182/38/2 | 0.13 | 0.09 | 1.37 (0.93–2.02) | 0.14 | 0.08 | 0.06 | 0.08 | 0.07 | |
| 2 | ICOS | Intron 1 | C | T | rs4335928 | 0/79/269 | 4/52/170 | 0.11 | 0.13 | 1.20 (0.84–1.71) | 0.38 | 0.11 | 0.10 | 0.10 | 0.13 | 0.12 |
| 2 | ICOS | Intron 1 | C | T | rs4675377 | 20/146/179 | 13/92/121 | 0.27 | 0.26 | 1.04 (0.8–1.37) | 0.80 | 0.17 | 0.18 | 0.22 | 0.26 | |
| 12 | VDR | BsmI Intron3 | T | C | rs1544410 | 142/146/56 | 86/99/38 | 0.38 | 0.39 | 1.08 (0.84–1.37) | 0.60 | 0.12–0.25 | 0.44 | 0.38 | 0.12–0.23 | 0.38 |
| 4 | TLR2 | S450S | T | C | rs3804100 | 302/38/1 | 197/25/2 | 0.06 | 0.06 | 1.11 (0.68–1.82) | 0.77 | 0.41† | 0.05 | 0.07 | 0.39† | 0.07 |
| 19 | KIR 2DS3 | Abs | Pres | 252/80/0 | 158/60/0 | 0.12 | 0.14 | 1.16 (0.81–1.67) | 0.46 | 0.38 | 0.32 | 0.21 | 0.41 | |||
| 10 | TCF7L2 | Intron 3 | C | T | rs7903146 | 182/134/31 | 115/90/21 | 0.28 | 0.29 | 1.05 (0.81–1.36) | 0.78 | 0.29 | 0.25 | 0.31 | 0.41 | 0.30 |
| 8 | SLC30A8 | R325W | C | T | rs13266634 | 187/131/30 | 116/86/24 | 0.27 | 0.30 | 1.11 (0.86–1.45) | 0.46 | 0.30 | 0.25 | 0.29 | 0.25 | 0.31 |
| 10 | HHEX | HHEX region | A | G | rs7923837 | 48/160/136 | 33/112/81 | 0.37 | 0.39 | 1.10 (0.86–1.4) | 0.50 | 0.38 | 0.38 | 0.37 | 0.34 | 0.40 |
| 10 | HHEX | HHEX region | G | A | rs1111875 | 115/179/54 | 73/108/45 | 0.41 | 0.44 | 1.11 (0.87–1.41) | 0.42 | 0.40 | 0.44 | 0.39 | 0.36 | 0.41 |
| 11 | LOC387761 | Intron 5 | G | A | rs7480010 | 189/139/21 | 115/92/19 | 0.26 | 0.29 | 1.15 (0.88–1.5) | 0.32 | 0.30 | 0.25 | 0.30 | 0.34 | 0.32 |
| 11 | EXT2 | Intron 14 | A | G | rs3740878 | 200/124/21 | 119/96/11 | 0.24 | 0.26 | 1.12 (0.85–1.47) | 0.48 | 0.27 | 0.30 | 0.27 | 0.24 | 0.27 |
| 12 | ERBB2 | Intron 7 | A | C | rs2292239 | 46/191/109 | 35/100/90 | 0.41 | 0.38 | 1.14 (0.89–1.45) | 0.32 | 0.36 | 0.30 | 0.41 | ||
| 18 | PTPN2 | Intron 7 | T | C | rs1893217 | 236/101/8 | 149/62/14 | 0.17 | 0.20 | 1.22 (0.9–1.66) | 0.22 | 0.17 | 0.19 | 0.21 | ||
| 12 | SH2B3 | Exon 3 | C | T | rs3184504 | 85/168/89 | 54/111/58 | 0.49 | 0.49 | 1.01 (0.8–1.29) | 0.97 | 0.49 | 0.41 | 0.56 | ||
| 12 | C12Orf30 | Intron 15 | A | G | rs17696736 | 99/174/72 | 72/105/47 | 0.46 | 0.44 | 1.07 (0.84–1.36) | 0.62 | 0.43 | 0.35 | 0.49 | ||
| 16 | CLEC16A | Intron 19 | A | G | rs12708716 | 160/147/33 | 100/90/30 | 0.31 | 0.34 | 1.13 (0.88–1.46) | 0.37 | 0.35 | 0.29 | 0.31 | ||
Allele frequencies from the Diabetes in Adolescents and the Very Young (DAVY) study (31).
Allele frequency in Koreans. Abs, absent; MAF, minor allele frequency; Pres, present.
Recently described polymorphisms in the TCF7L2, HHEX, and SLC30A8 loci that predispose to type 2 diabetes (38) were also tested, because islet dysfunction could precipitate diabetes; no difference in these markers was observed between progressors and nonprogressors (Table 1).
Skewed allele frequencies were observed across most CTLA4 SNPs, in addition to CT60, and in the neighboring CD28 and ICOS genes. Building on a recent population genetics study of the CD28 costimulatory locus (28), we computationally reconstructed haplotypes across CTLA4 in progressor and nonprogressor individuals. As depicted in Fig. 1, the CTLA4.h1 haplotype carries all high-risk CTLA4 alleles (CT60 G, +49 G, JO31 G), whereas CTLA4.h2 regroups most low-risk alleles and is part of a very homogeneous extended haplotype that spans the whole costimulatory locus from CD28 to the ICOS promoter region (28). Among DPT-1 individuals, CTLA4.h1 haplotype was enriched in progressors and in nonprogressors, leading to an inversion of the h1-to-h2 frequency ratio relative to the general population (Fig. 1B). CTLA4.h2 appeared to be more underrepresented in DPT-1 subjects compared with a U.S. Caucasian control cohort (DCGS) than any of its individual allelic SNP components (Fig. 1B). Similarly, a recent study of the costimulatory locus in patients with celiac disease showed that extended haplotypes in the region demonstrated stronger association with disease susceptibility than individual SNPs (39).
FIG. 1.
CTLA4 haplotype representation in DPT-1 individuals. A: Allelic composition of major CTLA4 haplotypes computationally reconstructed in DPT-1 individuals. For reference, corresponding frequencies in various population groups are indicated (CEPH-HGDP DNA panel [28]). B: Skewing (OR) of CTLA4 SNPs/haplotype frequency in DPT-1 individuals when compared with ethnicity-matched control cohorts. C: Frequencies of major CTLA4 haplotypes in DPT-1 and control cohorts.
Thus, these results suggest that the strongest non-HLA susceptibility alleles impact type 1 diabetes pathogenesis at an early stage, conditioning whether tolerance is broken and autoimmunity sets in, but have less or no influence on the course of disease and the probability that this autoimmunity will lead to terminal β-cell destruction.
HLA.
Class II genes at the HLA locus represent the strongest genetic determinant of type 1 diabetes susceptibility (1,2). We chose to analyze the distribution of HLA alleles and their combinations in a stepwise fashion, to avoid dilution of effect by multiple genotypic combinations. Tables 2 and 3 represents the distribution of HLA-DQB alleles among 638 individuals randomized into the DPT-1 trial (association with single DQA alleles showed no strong signal by themselves or only that expected from their linkage disequilibrium to DQB alleles; see below). Because DPT-1 was based on first-degree relatives of type 1 diabetic patients, allele frequencies in such families were bound to be enriched in susceptibility alleles, thus precluding the use of frequency data in healthy control subjects as a comparator. Thus, we inferred allele frequencies in the parental population of DPT-1 individuals based on the HLA allele frequencies of progressors (i.e., type 1 diabetic patients) and the known transmission disequilibrium biases of alleles to type 1 diabetes probands (33–35). As an additional comparison, parental data from the T1DGC were retrieved (25). In comparison with these frequencies, which represent a null-hypothesis baseline for no association, a strong enrichment for DQB1*0201 was observed in progressors and in nonprogressor individuals, with a weaker trend in DQB*0302. The frequency of *0201 alleles was the same in both groups, but DQB1*0302 was enriched in progressors relative to nonprogressors (progressor 42.6% vs. nonprogressor 34.7%; odds ratio [OR] 1.39; P = 0.0047 or P ∼ 0.042 after correcting for multiple sampling). The reverse held true for DQB1*0301, as this protective allele was significantly less frequent in progressors than in nonprogressors (progressor 8.6% vs. nonprogressor 14.3%; OR 0.57; P = 0.0026, corrected P ∼ 0.023). None of the other alleles appeared differentially represented in progressors versus nonprogressors, with frequencies comparable with that of the parental population. These results suggest that type 1 diabetes susceptibility loci in the MHC can impact several levels of the disease process. Not only can they increase the probability of autoimmunity, but some haplotypes also appear to condition the chance of progression from pre-diabetes to diabetes.
TABLE 2.
HLA DQβ alleles and progression to diabetes
| DQβ Alleles |
N |
% |
Parental frequency |
P value* | OR (95% CI)* | Median (y)† | |||
|---|---|---|---|---|---|---|---|---|---|
| Progressor | Nonprogressor | Progressor | Nonprogressor | T1DGC | DPT-1 | ||||
| 0201 | 158 | 245 | 31.7 | 31.5 | 23.2 | 22.1 | 0.93 | 1.01 (0.79–1.29) | 12.3 |
| 0301 | 43 | 111 | 8.6 | 14.3 | 13 | 13.7 | 2.59E–03 | 0.57 (0.39–0.82) | 11.9 |
| 0302 | 212 | 270 | 42.6 | 34.7 | 32.8 | 28.7 | 4.70E–03 | 1.39 (1.11–1.76) | 12.6 |
| 0303 | 4 | 9 | 0.8 | 1.2 | 3.5 | 1.4 | 0.54 | 0.69 (0.21–2.26) | 13.1 |
| 0402 | 11 | 15 | 2.2 | 1.9 | 1.9 | 2.4 | 0.73 | 1.15 (0.52–2.52) | 11.2 |
| 0501 | 33 | 54 | 6.6 | 6.9 | 7.1 | 7.3 | 0.83 | 0.95 (0.61–1.49) | 13.1 |
| 0502 | 5 | 10 | 1.0 | 1.3 | 1.1 | — | 0.65 | 0.78 (0.26–2.29) | 6.7 |
| 0603 | 11 | 19 | 2.2 | 2.4 | 3.1 | 6.5 | 0.79 | 0.9 (0.43–1.91) | 12.0 |
| 0604 | 16 | 27 | 3.2 | 3.5 | 4.7 | 2.6 | 0.80 | 0.92 (0.49–1.73) | 16.6 |
For progressor versus nonprogressor comparisons.
Age of onset in progressors.
TABLE 3.
HLA DQβ alleles and progression to diabetes among 0302-, 0301-, or 0201-positive individuals
| DQβ Alleles |
N |
% |
P value* | OR (95% CI)* | Median (y)† | Survival P value | ||
|---|---|---|---|---|---|---|---|---|
| Progressor | Nonprogressor | Progressor | Nonprogressor | |||||
| 0302/0201 | 87 | 121 | 48.1% | 50.6% | 0.60 | 0.9 (0.61–1.33) | 12.07 | 0.27 |
| 0302/0301 | 21 | 30 | 11.6% | 12.6% | 0.77 | 0.91 (0.5–1.66) | 11.77 | 0.93 |
| 0302/0302 | 31 | 31 | 17.1% | 13.0% | 0.72 | 1.39 (0.81–2.38) | 13.05 | 0.05 |
| 0302/0402 | 6 | 5 | 3.3% | 2.1% | 0.44 | 1.6 (0.48–5.34) | 11.04 | 0.38 |
| 0302/0501 | 18 | 17 | 9.9% | 7.1% | 0.30 | 1.44 (0.72–2.88) | 12.89 | 0.72 |
| 0302/0603 | 6 | 11 | 3.3% | 4.6% | 0.51 | 0.71 (0.26–1.96) | 11.85 | 0.74 |
| 0302/0604 | 5 | 11 | 2.8% | 4.6% | 0.33 | 0.59 (0.2–1.73) | 16.36 | 0.55 |
| 0301/0201 | 5 | 36 | 12.5% | 35.6% | 6.4E–03 | 0.26 (0.09–0.72) | 14.16 | 5.5E-03 |
| 0301/0301 | 3 | 10 | 7.5% | 9.9% | 0.65 | 0.73 (0.19–2.84) | 11.57 | 0.58 |
| 0301/0501 | 4 | 11 | 10.0% | 10.9% | 0.88 | 0.91 (0.27–3.04) | 10.72 | 0.69 |
| 0301/0604 | 5 | 6 | 12.5% | 5.9% | 0.19 | 2.26 (0.65–7.88) | 11.36 | 0.41 |
| 0301/0302 | 21 | 30 | 52.5% | 29.7% | 0.01 | 2.62 (1.23–5.56) | 11.77 | 2.8E-03 |
| 0201/0201 | 20 | 25 | 14.6% | 11.4% | 0.38 | 1.33 (0.71–2.51) | 11.23 | 0.13 |
| 0201/0301 | 5 | 36 | 3.6% | 16.4% | 2.5E–04 | 0.19 (0.07–0.51) | 14.16 | 2.3E-04 |
| 0201/0302 | 87 | 121 | 63.5% | 55.0% | 0.11 | 1.42 (0.92–2.21) | 12.07 | 0.29 |
| 0201/0402 | 4 | 7 | 2.9% | 3.2% | 0.89 | 0.92 (0.26–3.19) | 13.71 | 0.74 |
| 0201/0501 | 8 | 6 | 5.8% | 2.7% | 0.14 | 2.21 (0.75–6.52) | 10.38 | 0.17 |
| 0201/0603 | 4 | 3 | 2.9% | 1.4% | 0.30 | 2.18 (0.48–9.87) | 12.71 | 0.50 |
| 0201/0604 | 4 | 8 | 2.9% | 3.6% | 0.71 | 0.8 (0.24–2.7) | 14.09 | 0.95 |
For progressor versus nonprogressor comparisons within a given HLA-DQB positive group.
Age of onset in progressors.
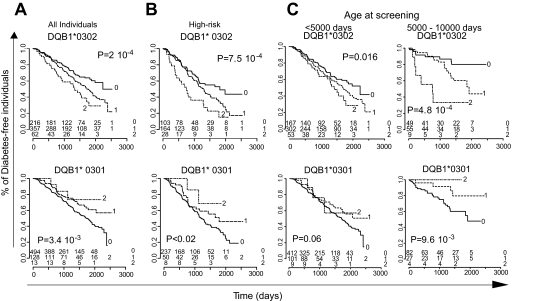
We also performed log-rank survival analyses to compare the kinetics of progression to type 1 diabetes of individuals carrying different HLA alleles. As seen in Fig. 2A, the time–to–type 1 diabetes onset was markedly influenced by the presence of *0302 alleles, in a dose-dependent manner: Median onset with no *0302 allele was 6.12 years, with a single *0302 allele was 4.71 years, and with two alleles was 3.65 years (P = 2 × 10−4). Conversely, *0301 alleles delayed the progression to overt type 1 diabetes, also in a dose-dependent manner. These findings held true when only high-risk individuals were considered (Fig. 2B). Because it is known that type 1 diabetes in very young individuals has a specific genetic architecture (31), we investigated whether DQB alleles impacted differently on type 1 diabetes incidence based on the age of the individual, splitting at 5,000 days, which roughly corresponds to the pubertal period (Fig. 2C). Individuals homozygous for DQB*0302 showed an increased incidence of type 1 diabetes and faster kinetics of progression irrespective of age, whereas the incidence of type 1 diabetes significantly dropped after 5,000 days in subjects lacking *0302. On the other hand, the protective effect of DQB*0301 was mostly visible in the >5,000 days group.
FIG. 2.
Diabetes-free survival and HLA-DQB1 genotypes. Diabetes-free survival in all (n = 638) (A) or high-risk (n = 295) (B) individuals screened at age <10,000 days according to their HLA-DQB1*0302 or HLA-DQB1*0301 genotypes. Significance of differences in survival is evaluated by log-rank test. The number of diabetes-free individuals in each category and at distinct time point is shown on the bottom of the figure. C: Disease-free survival in individuals screened at age <5,000 or between 5,000 and 10,000 days, stratified based on their HLA-DQB*0302 or *0301 genotypes.
We then investigated whether combinations of DQβ alleles might differentially impact on progression. Table 3 assesses the representation of the second DQB1 allele among progressor and nonprogressor individuals already positive for DQB1*0302, *0301, or *0201. For 0302-positive individuals, none of the additional alleles had meaningful impact in either direction (save for the enhancement of progression in DQB1*0302 homozygotes, consistent with Fig. 2). On the other hand, the *0301/*0201 combination was significantly underrepresented in progressors, whether compared with all *0301-positive or with all *0201-positive individuals (P ∼ 0.02 and 0.0016, respectively), suggesting a strong epistatic interaction between these two alleles (or with the other loci linked to these variants within the MHC). In contrast, the *0301 allele had no impact in *0302-positive individuals.
Because nonrandom pairing exists between DQA* and DQB* alleles in strong linkage disequilibrium, we compared the representation of specific DQA alleles in individuals bearing DQB1*0302, *0301, and *0201 (Supplementary Tables 2–4). In individuals heterozygous for the DQB allele of interest, we used direct DQA allele counting instead of reconstructing two-loci haplotypes in double heterozygous (which can only be estimated, because their true gametic phase is unknown), which provided sensitivity for DQA-DQB trans-complementation effects. For DQB1*0302 individuals, the diversity in DQA allele representation was essentially restricted to heterozygous individuals, given the complete linkage disequilibrium between DQA*0301 and DQB1*0302, and no significant modulation of the progression was observed. On the other hand, the impact of DQB1*0201 was somewhat modified by the presence of certain DQA alleles: DQA*0201 was protective and DQA*0501 promoted progression but only in DQB*0201 homozygotes. No significant impact of DQA was observed in DQB*0301-positive individuals, although expected trends toward an enrichment of DQA*0301 in progressors and DQA*0501 in nonprogressors were seen.
Treatment effects.
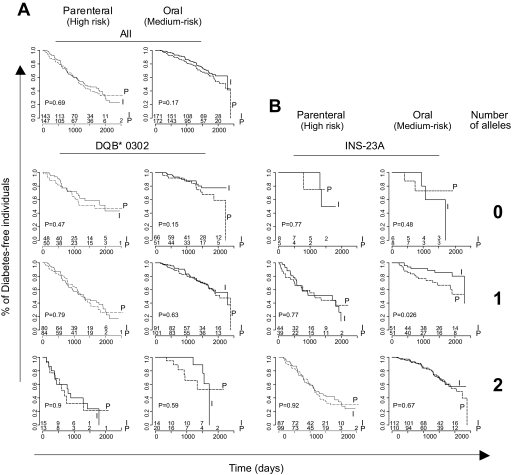
Insulin had no significant effect overall in DPT-1, except for individuals with high anti-IAAs treated with oral insulin (19,24), but we investigated whether stratification by genotype might show a different outcome. No difference was found on stratification in DQB1*0302 (Fig. 3) or *0301 (not shown) subgroups. For the INS-23 polymorphism, a significantly enhanced effect of the oral treatment was seen in individuals heterozygous for the susceptibility allele; this result should be interpreted with caution, however, because it was not reproduced in homozygotes, and the nominal degree of significance (P = 0.028) would not resist proper correction for multiple sampling. On the other hand, it is interesting that the group of INS-23A heterozygotes is that which shows high IAA titers (see below), and thus likely overlaps with the high-IAA subgroup which showed some treatment effect in post hoc analyses (24). Comparative survival analysis showed very similar hazards ratio for treatment effect among high-IAA (>75th percentile) and among INS-23A heterozygotes (OR 0.41 [95% CI 0.2–0.85] and 0.36 [0.15–0.87], respectively).
FIG. 3.
Response to treatment according to HLA-DQB1 and INS-23 genotype. A: All 638 individuals were stratified according to the number of HLA-DQB1*0302 alleles and treatment group. Significance of differences in survival is evaluated by log-rank test. I, intervention group; P, placebo/observation. B: Individuals were stratified based on the number of INS-23A risk alleles.
Type 1 diabetes susceptibility loci and initial autoantibody levels.
We then used linear regression to investigate whether any of the genetic markers investigated showed an association with the level of autoantibodies in at-risk individuals. Antibody titers at screening were used for that purpose, instead of summing the counts of positive antibodies that might mask specific effects of a given polymorphism on distinct antibody reactivities (acknowledging that fluctuations in the titers could be observed over the course of a few months and that the elapsed time since seroconversion was unknown).
Only the INS, ICOS, and HLA variants showed an association with IAA titers (Table 4). Genotypes at INS-23 appeared to be the most strongly associated with differences in baseline IAA levels (P = 3.7 × 10−5, or ∼1.5 × 10−3 after correction for multiple sampling), which is consistent with previous reports (40–42). The effect was dominant: individuals heterozygous or homozygous for the high-risk allele both demonstrated a 2.4-fold increase in median IAA levels at baseline. A weak association was observed with DQB1*0302; of note, most of the INS-23 association with IAA titers was found in DQB*0302-negative individuals. The DQB1*0201 susceptibility allele was negatively associated with IAA levels. These results suggest complex mechanistic interactions of HLA susceptibility haplotypes, with a different effect on IAA for the *0302 and *0201 susceptibility alleles.
TABLE 4.
Initial IAA titers and type 1 diabetes susceptibility loci genotypes
| Gene | Polymorphism | Allele 0 | Allele 1 | Median IAA level by genotype |
Linear regression P values |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 0 and 1 | 1 and 2 | 0-1-2 | 0 vs. 1 and 2 | 0 and 1 vs. 2 | ||||
| HLA-DQB1 | DQB1*0302 | Counts | 115 | 183 | 156 | 150 | 180.2 | 0.034 | 0.67 | 1.36E–02 | |
| HLA-DQB1 | DQB*0201 | Counts | 176 | 164 | 82 | 169 | 135 | 0.044 | 0.40 | 1.80E–03 | |
| *0302/0201 | Abs | Pres | 137.5 | 210 | 0.013 | ||||||
| HLA-DQB1 | DQB*0301 | Counts | 168 | 124 | 82.5 | 153 | 124 | 0.16 | 0.714 | 0.14 | |
| Lymphotoxin-α | +10 | G | A | 153 | 150 | 144 | 150 | 150 | 0.75 | 0.62 | 0.91 |
| Insulin | −23 Hphl | T | A | 61 | 111 | 192 | 104 | 164.5 | 3.74E–05 | 1.05E–04 | 0.014 |
| PTPN22 | R620W | C | T | 145 | 177 | 220 | 150 | 178 | 0.37 | 0.56 | 0.42 |
| IL-2 Ra (CD25) | Intron 1 | G | A | 166 | 173 | 103 | 168 | 149.5 | 0.46 | 0.11 | 0.80 |
| IL-2 Ra (CD25) | Intron 1 | T | C | 161.5 | 166 | 120 | 165 | 150 | 0.76 | 0.30 | 0.78 |
| IL-2 Ra (CD25) | CD25 region | C | A | 148 | 162.5 | 87 | 150 | 156 | 0.35 | 0.88 | 0.45 |
| IL-2 Ra (CD25) | CD25 region | T | A | 146 | 166.5 | 98 | 150 | 165 | 0.65 | 0.43 | 0.66 |
| IFIH1 | A946T | C | T | 124 | 154.5 | 149 | 147 | 150 | 0.35 | 0.56 | 0.29 |
| Fc receptor-like 3 | Prom −169 | A | G | 177.5 | 141.5 | 142 | 151.5 | 142 | 0.55 | 0.94 | 0.39 |
| C6orf118 | M256I | C | A | 153 | 153 | 121.5 | 153 | 150 | 0.62 | 0.63 | 0.73 |
| CAPSL | R75Q | A | G | 131.5 | 164 | 141 | 150 | 157.5 | 0.74 | 0.71 | 0.31 |
| CTLA4 | Prom −1577 | G | A | 183 | 135 | 139.5 | 156 | 136 | 0.044 | 0.21 | 0.06 |
| CTLA4 | Prom −318 | T | C | 18 | 152.5 | 150 | 147 | 150 | 0.53 | 0.33 | 0.08 |
| CTLA4 | T49A | G | A | 148.5 | 146.5 | 147 | 148 | 147 | 0.73 | 0.95 | 0.49 |
| CTLA4 | 3′UTR 6230 (CT60) | G | A | 187.5 | 135 | 139.5 | 156 | 136 | 0.037 | 0.22 | 0.041 |
| CTLA4 | JO31 | C | A | 174 | 146 | 148 | 162 | 146 | 0.16 | 0.26 | 0.24 |
| CD28 | Intron 1 | C | T | 131 | 165.5 | 141.5 | 153 | 159.5 | 0.63 | 0.57 | 0.15 |
| CD28 | Intron 1 | A | T | 171 | 147 | 147 | 150 | 147 | 0.41 | 0.56 | 0.40 |
| CD28 | 3′UTR | G | T | 157.5 | 132 | 540 | 150 | 133.5 | 0.15 | 0.38 | 0.11 |
| CD28 | 3′UTR | T | A | 194 | 159 | 141 | 168 | 149 | 0.051 | 0.07 | 0.22 |
| ICOS | Prom −1817 | T | C | 148 | 160 | 203 | 150 | 166.5 | 0.09 | 0.26 | 0.13 |
| ICOS | Intron 1 | C | T | 63 | 182.5 | 141.5 | 179 | 153 | 0.17 | 0.11 | 0.42 |
| ICOS | Intron 1 | C | T | 382.5 | 168 | 135.5 | 178.5 | 144.5 | 4.08E–03 | 0.044 | 1.54E–03 |
| VDR | BsmI Intron3 | T | C | 153 | 131 | 116 | 150 | 128 | 0.87 | 0.62 | 0.78 |
| TLR2 | S450S | T | C | 150 | 142 | NA | 150 | 142 | 0.59 | — | — |
| KIR 2DS3 | Abs | Pres | 142 | 150 | 212 | 146 | 164.5 | 0.66 | 0.37 | 0.94 | |
| TCF7L2 | Intron 3 | C | T | 138 | 166.5 | 225.5 | 149 | 173.5 | 0.26 | 0.24 | 0.43 |
| SLC30A8 | R325W | C | T | 177 | 147 | 162.5 | 147 | 150 | 0.63 | 0.55 | 0.91 |
| HHEX | HHEX region | A | G | 170.5 | 142 | 174 | 149 | 147 | 0.98 | 0.76 | 0.84 |
| HHEX | HHEX region | G | A | 145 | 181.5 | 92 | 156 | 168 | 0.97 | 0.08 | 0.40 |
| LOC387761 | Intron 5 | G | A | 169 | 131 | 180 | 150 | 133 | 0.85 | 0.27 | 0.76 |
| EXT2 | Intron 14 | A | G | 218 | 231.5 | 182 | 218 | 188 | 0.17 | 0.22 | 0.17 |
| ERBB2 | Intron 7 | A | C | 210 | 120 | 177 | 137 | 141 | 0.72 | 0.14 | 0.19 |
| PTPN2 | Intron 7 | T | C | 148 | 177 | 126 | 156 | 170 | 0.84 | 0.80 | 0.73 |
| SH2B3 | Exon 3 | C | T | 169 | 156 | 134 | 160 | 144.5 | 0.70 | 0.86 | 0.40 |
| C12Orf30 | Intron 15 | A | G | 141 | 177 | 124 | 162 | 157.5 | 0.52 | 0.69 | 0.17 |
| CLEC16A | Intron 19 | A | G | 142 | 159 | 147.5 | 149.5 | 156 | 0.83 | 0.92 | 0.72 |
UTR, untranslated region.
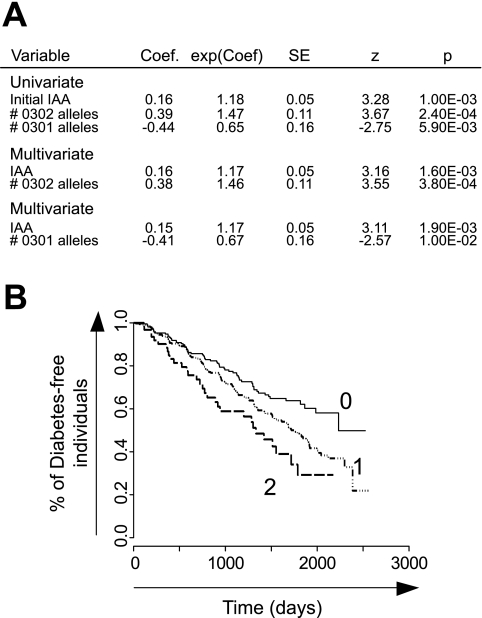
Redondo et al. (43) observed the same association between DQB1*0302 and anti-insulin titers but came to the conclusion that progression to type 1 diabetes in the DPT-1 cohort was only indirectly correlated with HLA-DQ status and that the risk of progression to overt diabetes was actually correlated with the number of autoantibodies present at randomization, HLA-DQ status being only a modulator of this number (similarly counting the number of positive antibodies in our restricted subset also showed that HLA impacted the progression to type 1 diabetes mostly in individuals with 0–2 autoantibodies; data not shown). Because our analysis, which was restricted to the individuals actually enrolled in the trial, argued for more complex HLA effects in relation to IAA levels (and not mere positivity) and type 1 diabetes progression, we investigated whether these parameters were truly independent by building uni- and multivariate Cox proportional hazards models, including DQB1*0302, *0301, and baseline IAA levels. As shown in Fig. 4A, baseline IAA and the number of DQB1*0302 alleles were independently associated with faster progression toward type 1 diabetes. This effect of HLA alleles, independent of IAA, is graphically illustrated by the multivariate Cox analysis of the predicted time-to-progression for individuals with different numbers of *0302 alleles, with IAA levels conditioned on their average value (Fig. 4B).
FIG. 4.
A: Cox proportional hazard modeling was applied to evaluate the individual contributions of DQB1 status and initial IAA levels to progression toward type 1 diabetes. B: Diabetes-free survival according to DQB1*0302 status fitted on averaged IAA levels in the Cox model.
For anti–glutamic acid decarboxylase (GAD) 65 antibodies, only a weak association with polymorphisms in CD25 (Supplementary Table 5) was found, compatible with an additive effect (e.g., median titers 0.185, 0.215, and 0.467 for 0, 1, and 2 rs706778 A alleles; NS when corrected for multiple sampling).
DISCUSSION
The goal of this study was to identify among known or suspected type 1 diabetes susceptibility loci those impinging on conversion from the pre-diabetes to the diabetic state. A clear association was observed with some, but interestingly not all, HLA class II alleles, an influence that went beyond their relation to initial IAA levels. On the other hand, none of the extra-HLA polymorphisms differed significantly in frequency between progressors versus nonprogressors, a number of them (notably INS-23, PTNP22 R620W, and several CTLA4 markers) showing the typical elevated frequencies of susceptible alleles in DPT-1 individuals, irrespective of eventual progression to overt diabetes. These results, obtained in individuals <10,000 days old at screening, still held true when considering the whole cohort irrespective of age (not shown).
The implication of these results is that most non-HLA type 1 diabetes susceptibility loci described so far affect the initial breakdown of immunological tolerance and the initiation of autoimmunity, rather than the later failures of immunoregulation that lead to terminal islet destruction. It is quite plausible that the INS-23A polymorphism would affect tolerance: Together with the length polymorphism of the VNTR element further upstream in the INS promoter region, with which it is in tight linkage disequilibrium, it is thought to lower thymic expression of insulin and lead to less effective induction of T-cell tolerance to insulin (3,4). The significant association between the INS susceptibility alleles and higher titers of anti-insulin antibodies, which confirm prior observations associating INS-23 to IAA incidence in Scandinavian cohorts (40–42), is also consistent with this notion. PTPN22 encodes a regulatory phosphatase that modulates T-cell receptor signaling, and one might hypothesize that the variant modifies signaling in immature thymocytes and hence tolerance induction, or the activation of autoreactive T-cells in the periphery. CTLA4 and CD25, because of their involvement in regulatory T-cells, might have been thought a priori to impact on diabetes progression, but this proved not to be the case. Here also, one might invoke an effect on T-cell activation at the initiation of autoimmune T-cell infiltration.
HLA.
Only HLA-DQ alleles showed a noticeable effect on type 1 diabetes progression in DPT-1 individuals, partially in correlation with enhancement of IAA levels, but also with effects independent of IAA titers. The data confirm an earlier report of an association of IAA and IA-2 positivity with the presence of DQB*0302 in Scandinavian type 1 diabetic patients (40). Redondo et al. (43) have also reported an analysis of HLA haplotypes and genotypes in relation to autoantibodies and disease progression in the DPT-1 cohort, albeit with a different strategy that encompassed all ICA+ individuals genotyped for HLA (n = 2,046), potentially diluting a genetic effects on progression in high-risk individuals by the inclusion of many low-risk individuals, and focused on compound genotypes rather than individual alleles. These authors also observed the relationship between progression and DQB1*0302 and *0301 and found that 57% of DQB*0302-homozygous individuals were positive for two or more antibodies, compared with only 30% of DQA*0501/DQB*0201 homozygous (P = 3 × 10−9), yet both genotypes had roughly the same 5-year type 1 diabetes risk when considering their larger cohort (36 vs. 34%). Redondo et al. (43) concluded that a relationship between DQB genotypes and number of different autoantibodies at screening could account for these effects on progression (HLA being irrelevant in individuals with two or more autoantibodies in their data). The quantitative survival analysis performed here, based on measured titers rather than on positive/negative calls, indicates that the picture is more complex, demonstrating the limitations of imposing cut-offs when dissecting a quantitative trait.
The two main susceptibility alleles (DQB1*0302 and *0201) appear to have different impacts in several respects: *0302 has a direct effect on the risk of progression, whereas *0201 does not; *0302 has a moderate association to IAA titers, whereas *0201 does not; *0201 is associated with higher GAD65 titers (40), whereas *0302 is not; the impact of *0201 varies with the DQA chain with which it is paired (in cis or trans), whereas *0302 does not; *0201 has a strong epistatic interaction with *0301, resulting in protection of *0201/*0301 heterozygotes, whereas *0302 does not. These observations are consistent with the notion that *0302 and *0201 provide mechanistically different contributions to disease pathogenesis beyond a mere modulation of antibody numbers (44–47).
How could HLA be involved at different stages of the autoimmune pathogenesis? Early effects on tolerance, for instance by allowing the emergence of a T-cell repertoire with reactivity against islet peptides (insulin?) might be expected from their role in selecting T-cells and presenting self-antigens. In the NOD mouse, the H2g7 MHC alleles associated to type 1 diabetes are sufficient to select an autoreactive repertoire (48). More puzzling is the additional contribution of DQB1*0302 to further progression. The enrichment in heterozygous *0302 individuals among progressors might mirror a multistage process wherein the initial trigger is amplified through the presentation of later-stage “epitope-spreading” antigens, at which *0302 would be particularly efficient. Alternatively, HLA class II alleles might mediate sensitivity to environmental insults (e.g., infections or food-borne antigens) after the establishment of a “respectful” insulitis, perhaps tipping the balance toward immune activation and full-blown islet destruction. Finally, it is plausible that the negative epistasis between DQB1*0201 and DQB1*0301 reflects the ability of MHC class II molecules to form trans-encoded αβ dimers. As usual for loci in the HLA region, this discussion must be cautioned by the strong and complex linkage disequilibrium structure in the region. Although these effects may be ascribed to the DQB alleles themselves, it is also possible that some of them arise from loci in linkage disequilibrium with the DQB alleles, for instance class I genes (49).
Pharmacogenetics?
Can one, from this analysis, draw conclusions that would guide the design of other prevention studies, attempting to improve the power of the trials by using genetic data to refine the selection and better define groups of at-risk individuals? Future trials aimed at evaluating prophylactic interventions might require more stringent selection criteria against low-risk or protective HLA genotypes such as DQB*0301 (DQB*0602 was already an exclusion criteria in DPT-1), which might lead to artifactual treatment efficacy results if unbalanced among the study arms (50). The selection of DQB1*0302 individuals would improve the power (power calculations show that a ∼20% reduction in total group size could be achieved by selecting only *0302-positive individuals), but such a selection would clearly leave out an important fraction of type 1 diabetic patients.
Although there was no significant effect of oral insulin administration over the entire pool of DPT-1 medium-risk subjects, post hoc analysis did reveal a slight treatment effect in the subgroup with the highest initial IAA titers (19,24). We found that stratification by INS-23 genotype also uncovered a significant effect of oral insulin treatment in heterozygotes (with the limitations on validity of any such post hoc analysis). Given the association between INS-23 genotype and IAA levels, one might expect that the two observations are linked, and subgroup analysis confirmed this to be true. In other trials of oral insulin, it may be of interest to select candidates on the basis of INS-23 and IAA titers, to test the significance and reproducibility of these observations.
Supplementary Material
Acknowledgments
This work has been supported by National Institutes of Health Grant P01-AI-056299, the William T. Young Chairs in Diabetes Research, and the Joslin Diabetes and Endocrinology Research Center funded cores. The DPT-1 was supported through cooperative agreements by the NIDDK, the National Institute of Allergy and Infectious Diseases, the National Institute of Child Health and Human Development, the American Diabetes Association, and the Juvenile Diabetes Research Foundation.
We thank Drs. C. Greenbaum and the DPT-1 Publications Committee for inspiring discussions, the NIDDK Central DNA Repository for making samples available, W. Besse for help with genotyping, and all of the DPT-1 participants.
Published ahead of print at http://diabetes.diabetesjournals.org on 12 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Eisenbarth GS: Update in type 1 diabetes. J Clin Endocrinol Metab 92 :2403 –2407,2007 [DOI] [PubMed] [Google Scholar]
- 2.Maier LM, Wicker LS: Genetic susceptibility to type 1 diabetes. Curr Opin Immunol 17 :601 –608,2005 [DOI] [PubMed] [Google Scholar]
- 3.Vafiadis P, Bennett ST, Todd JA, Nadeau J, Grabs R, Goodyer CG, Wickramasinghe S, Colle E, Polychronakos C: Insulin expression in human thymus is modulated by INS VNTR alleles at the IDDM2 locus. Nat Genet 15 :289 –292,1997 [DOI] [PubMed] [Google Scholar]
- 4.Pugliese A, Zeller M, Fernandez A Jr, Zalcberg LJ, Bartlett RJ, Ricordi C, Pietropaolo M, Eisenbarth GS, Bennett ST, Patel DD: The insulin gene is transcribed in the human thymus and transcription levels correlated with allelic variation at the INS VNTR-IDDM2 susceptibility locus for type 1 diabetes. Nat Genet 15 :293 –297,1997 [DOI] [PubMed] [Google Scholar]
- 5.Bottini N, Musumeci L, Alonso A, Rahmouni S, Nika K, Rostamkhani M, MacMurray J, Meloni GF, Lucarelli P, Pellecchia M, Eisenbarth GS, Comings D, Mustelin T: A functional variant of lymphoid tyrosine phosphatase is associated with type I diabetes. Nat Genet 36 :337 –338,2004 [DOI] [PubMed] [Google Scholar]
- 6.Ueda H, Howson JMM, Esposito L, Heward J, Snook H, Chamberlain G, Rainbow DB, Hunter KMD, Smith AN, Di Genova G, Herr MH, Dahlman I, Payne F, Smyth D, Lowe C, Twells RCJ, Howlett S, Healy B, Nutland S, Rance HE, Everett V, Smink LJ, Lam AC, Cordell HJ, Walker NM, Bordin C, Hulme J, Motzo C, Cucca F, Hess JF, Metzker ML, Rogers J, Gregory S, Allahabadia A, Nithlyananthan R, Tuomilehto-Wolf E, Tuomilehto J, Bingley P, Gillespie KM, Undlen DE, Renningen KS, Guja C, Ionescu-Tirgoviste C, Savage DA, Maxwell AP, Carson DJ, Patterson CC, Franklyn JA, Clayton DG, Peterson LB, Wicker LS, Todd JA, Gough SCL: Association of the T-cell regulatory gene CTLA4 with susceptibility to autoimmune disease. Nature 423 :506 –511,2003 [DOI] [PubMed] [Google Scholar]
- 7.The Wellcome Trust Case Control Consortium: Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature 447 :661 ,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Todd JA, Walker NM, Cooper JD, Smyth DJ, Downes K, Plagnol V, Bailey R, Nejentsev S, Field SF, Payne F, Lowe CE, Szeszko JS, Hafler JP, Zeitels L, Yang JH, Vella A, Nutland S, Stevens HE, Schuilenburg H, Coleman G, Maisuria M, Meadows W, Smink LJ, Healy B, Burren OS, Lam AA, Ovington NR, Allen J, Adlem E, Leung HT, Wallace C, Howson JM, Guja C, Ionescu-Tirgoviste C, Simmonds MJ, Heward JM, Gough SC, Dunger DB, Wicker LS, Clayton DG: Robust associations of four new chromosome regions from genome-wide analyses of type 1 diabetes. Nat Genet 39 :857 –864,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hakonarson H, Grant SF, Bradfield JP, Marchand L, Kim CE, Glessner JT, Grabs R, Casalunovo T, Taback SP, Frackelton EC, Lawson ML, Robinson LJ, Skraban R, Lu Y, Chiavacci RM, Stanley CA, Kirsch SE, Rappaport EF, Orange JS, Monos DS, Devoto M, Qu HQ, Polychronakos C: A genome-wide association study identifies KIAA0350 as a type 1 diabetes gene. Nature 448 :591 –594,2007 [DOI] [PubMed] [Google Scholar]
- 10.Kishimoto H, Sprent J: A defect in central tolerance in NOD mice. Nat Immunol 2 :1025 –1031,2001 [DOI] [PubMed] [Google Scholar]
- 11.Zucchelli S, Holler P, Yamagata T, Roy M, Benoist C, Mathis D: Defective central tolerance induction in NOD mice: genomics and genetics. Immunity 22 :385 –396,2005 [DOI] [PubMed] [Google Scholar]
- 12.Liston A, Lesage S, Gray DH, O'Reilly LA, Strasser A, Fahrer AM, Boyd RL, Wilson J, Baxter AG, Gallo EM, Crabtree GR, Peng K, Wilson SR, Goodnow CC: Generalized resistance to thymic deletion in the NOD mouse: a polygenic trait characterized by defective induction of Bim. Immunity 21 :817 –830,2004 [DOI] [PubMed] [Google Scholar]
- 13.Steele C, Hagopian WA, Gitelman S, Masharani U, Cavaghan M, Rother KI, Donaldson D, Harlan DM, Bluestone J, Herold KC: Insulin secretion in type 1 diabetes. Diabetes 53 :426 –433,2004 [DOI] [PubMed] [Google Scholar]
- 14.Sosenko JM, Palmer JP, Greenbaum CJ, Mahon J, Cowie C, Krischer JP, Chase HP, White NH, Buckingham B, Herold KC, Cuthbertson D, Skyler JS: Patterns of metabolic progression to type 1 diabetes in the Diabetes Prevention Trial–Type 1. Diabetes Care 29 :643 –649,2006 [DOI] [PubMed] [Google Scholar]
- 15.Anderson MS, Bluestone JA: The NOD mouse: a model of immune dysregulation. Annu Rev Immunol 23 :447 –485,2005 [DOI] [PubMed] [Google Scholar]
- 16.Tang Q, Bluestone JA: Regulatory T-cell physiology and application to treat autoimmunity. Immunol Rev 212 :217 –237,2006 [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez A, Katz JD, Mattei MG, Kikutani H, Benoist C, Mathis D: Genetic control of diabetes progression. Immunity 7 :873 –883,1997 [DOI] [PubMed] [Google Scholar]
- 18.Yamanouchi J, Rainbow D, Serra P, Howlett S, Hunter K, Garner VE, Gonzalez-Munoz A, Clark J, Veijola R, Cubbon R, Chen SL, Rosa R, Cumiskey AM, Serreze DV, Gregory S, Rogers J, Lyons PA, Healy B, Smink LJ, Todd JA, Peterson LB, Wicker LS, Santamaria P: Interleukin-2 gene variation impairs regulatory T cell function and causes autoimmunity. Nat Genet 39 :329 –337,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diabetes Prevention Trial-Type 1 Diabetes Study Group: Effects of insulin in relative of patients with type 1 diabetes mellitus. N Engl J Med 346 :1685 –1691,2002 [DOI] [PubMed] [Google Scholar]
- 20.Atkinson MA, Maclaren NK, Luchetta R: Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes 39 :933 –937,1990 [DOI] [PubMed] [Google Scholar]
- 21.Bowman MA, Campbell L, Darrow BL, Ellis TM, Suresh A, Atkinson MA: Immunological and metabolic effects of prophylactic insulin therapy in the NOD-scid/scid adoptive transfer model of IDDM. Diabetes 45 :205 –208,1996 [DOI] [PubMed] [Google Scholar]
- 22.Keller RJ, Eisenbarth GS, Jackson RA: Insulin prophylaxis in individuals at high risk of type I diabetes. Lancet 341 :927 –928,1993 [DOI] [PubMed] [Google Scholar]
- 23.Fuchtenbusch M, Rabl W, Grassl B, Bachmann W, Standl E, Ziegler AG: Delay of type I diabetes in high risk, first degree relatives by parenteral antigen administration: the Schwabing Insulin Prophylaxis Pilot Trial. Diabetologia 41 :536 –541,1998 [DOI] [PubMed] [Google Scholar]
- 24.Skyler JS, Krischer JP, Wolfsdorf J, Cowie C, Palmer JP, Greenbaum C, Cuthbertson D, Rafkin-Mervis LE, Chase HP, Leschek E: Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial–Type 1. Diabetes Care 28 :1068 –1076,2005 [DOI] [PubMed] [Google Scholar]
- 25.Erlich H, Valdes AM, Noble J, Carlson JA, Varney M, Concannon P, Mychaleckyj JC, Todd JA, Bonella P, Fear AL, Lavant E, Louey A, Moonsamy P: HLA DR-DQ haplotypes and genotypes and type 1 diabetes risk: analysis of the Type 1 Diabetes Genetics Consortium families. Diabetes 57 :1084 –1092,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Julier C, Hyer RN, Davies J, Merlin F, Soularue P, Briant L, Cathelineau G, Deschamps I, Otter JI, Froguel P, Boitard C, Bell JI, Lathrop GM: Insulin-IGF2 region on chromosome 11p encodes a gene implicated in HLA-DR4-dependent diabetes susceptibility. Nature 354 :155 –159,1991 [DOI] [PubMed] [Google Scholar]
- 27.Knight JC, Keating BJ, Kwiatkowski DP: Allele-specific repression of lymphotoxin-alpha by activated B cell factor-1. Nat Genet 36 :394 –399,2004 [DOI] [PubMed] [Google Scholar]
- 28.Butty V, Roy M, Sabeti P, Besse W, Benoist C, Mathis D: Signatures of strong population differentiation shape extended haplotypes across the human CD28, CTLA4, and ICOS costimulatory genes. Proc Natl Acad Sci U S A 104 :570 –575,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qu HQ, Montpetit A, Ge B, Hudson TJ, Polychronakos C: Toward further mapping of the association between the IL2RA locus and type 1 diabetes. Diabetes 56 :1174 –1176,2007 [DOI] [PubMed] [Google Scholar]
- 30.Smyth DJ, Cooper JD, Bailey R, Field S, Burren O, Smink LJ, Guja C, Ionescu-Tirgoviste C, Widmer B, Dunger DB, Savage DA, Walker NM, Clayton DG, Todd JA: A genome-wide association study of nonsynonymous SNPs identifies a type 1 diabetes locus in the interferon-induced helicase (IFIH1) region. Nat Genet 38 :617 –619,2006 [DOI] [PubMed] [Google Scholar]
- 31.Rodacki M, Svoren B, Butty V, Besse W, Laffel L, Benoist C, Mathis D: Altered natural killer cells in type 1 diabetic patients. Diabetes 56 :177 –185,2007 [DOI] [PubMed] [Google Scholar]
- 32.Lowe CE, Cooper JD, Brusko T, Walker NM, Smyth DJ, Bailey R, Bourget K, Plagnol V, Field S, Atkinson M, Clayton DG, Wicker LS, Todd JA: Large-scale genetic fine mapping and genotype-phenotype associations implicate polymorphism in the IL2RA region in type 1 diabetes. Nat Genet 39 :1074 –1082,2007 [DOI] [PubMed] [Google Scholar]
- 33.Guja C, Guja L, Nutland S, Rance H, Sebastien M, Todd JA, Ionescu-Tirgoviste C: Type 1 diabetes genetic susceptibility encoded by HLA DQB1 genes in Romania. J Cell Mol Med 8 :249 –256,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lie BA, Ronningen KS, Akselsen HE, Thorsby E, Undlien DE: Application and interpretation of transmission/disequilibrium tests: transmission of HLA-DQ haplotypes to unaffected siblings in 526 families with type 1 diabetes. Am J Hum Genet 66 :740 –743,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kawasaki E, Noble J, Erlich H, Mulgrew CL, Fain PR, Eisenbarth GS: Transmission of DQ haplotypes to patients with type 1 diabetes. Diabetes 47 :1971 –1973,1998 [DOI] [PubMed] [Google Scholar]
- 36.Stephens M, Smith NJ, Donnelly P: A new statistical method for haplotype reconstruction from population data. Am J Hum Genet 68 :978 –989,2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Motohashi Y, Yamada S, Yanagawa T, Maruyama T, Suzuki R, Niino M, Fukazawa T, Kasuga A, Hirose H, Matsubara K, Shimada A, Saruta T: Vitamin D receptor gene polymorphism affects onset pattern of type 1 diabetes. J Clin Endocrinol Metab 88 :3137 –3140,2003 [DOI] [PubMed] [Google Scholar]
- 38.Moore AF, Florez JC: Genetic susceptibility to type 2 diabetes and implications for antidiabetic therapy. Annu Rev Med 59 :95 –111,2008 [DOI] [PubMed] [Google Scholar]
- 39.Brophy K, Ryan AW, Thornton JM, Abuzakouk M, Fitzgerald AP, McLoughlin RM, O'Morain C, Kennedy NP, Stevens FM, Feighery C, Kelleher D, McManus R: Haplotypes in the CTLA4 region are associated with coeliac disease in the Irish population. Genes Immun 7 :19 –26,2006 [DOI] [PubMed] [Google Scholar]
- 40.Graham J, Hagopian WA, Kockum I, Li LS, Sanjeevi CB, Lowe RM, Schafer JB, Zarghami M, Day HL, Landin-Olsson M, Palmer JP, Janer-Villanueva M, Hood L, Sundkvist G, Lernmark A, Breslow N, Dahlquist G, Blohme G, Diabetes Incidence in Sweden Study Group, Swedish Childhood Diabetes Study Group: Genetics effects on age-dependent onset and islet cell autoantibody markers in type 1 diabetes. Diabetes Care 26 :226 –229,2003. 12502685 [Google Scholar]
- 41.Hermann R, Laine AP, Veijola R, Vahlberg T, Simell S, Lahde J, Simell O, Knip M, Ilonen J: The effect of HLA class II, insulin and CTLA4 gene regions on the development of humoral beta cell autoimmunity. Diabetologia 48 :1766 –1775,2005 [DOI] [PubMed] [Google Scholar]
- 42.Nielsen LB, Mortensen HB, Chiarelli F, Holl R, Swift P, de Beaufort C, Pociot F, Hougaard P, Gammeltoft S, Knip M, Hansen L: Impact of IDDM2 on disease pathogenesis and progression in children with newly diagnosed type 1 diabetes: reduced insulin antibody titres and preserved beta cell function. Diabetologia 49 :71 –74,2006 [DOI] [PubMed] [Google Scholar]
- 43.Redondo MJ, Babu S, Zeidler A, Orban T, Yu L, Greenbaum C, Palmer JP, Cuthbertson D, Eisenbarth GS, Krischer JP, Schatz D: Specific human leukocyte antigen DQ influence on expression of antiislet autoantibodies and progression to type 1 diabetes. J Clin Endocrinol Metab 91 :1705 –1713,2006 [DOI] [PubMed] [Google Scholar]
- 44.Ziegler AG, Standl E, Albert E, Mehnert H: HLA-associated insulin autoantibody formation in newly diagnosed type I diabetic patients. Diabetes 40 :1146 –1149,1991 [DOI] [PubMed] [Google Scholar]
- 45.Thomson G, Valdes AM, Noble JA, Kockum I, Grote MN, Najman J, Erlich HA, Cucca F, Pugliese A, Steenkiste A, Dorman JS, Caillat-Zucman S, Hermann R, Ilonen J, Lambert AP, Bingley PJ, Gillespie KM, Lernmark A, Sanjeevi CB, Ronningen KS, Undlien DE, Thorsby E, Petrone A, Buzzetti R, Koeleman BP, Roep BO, Saruhan-Direskeneli G, Uyar FA, Gunoz H, Gorodezky C, Alaez C, Boehm BO, Mlynarski W, Ikegami H, Berrino M, Fasano ME, Dametto E, Israel S, Brautbar C, Santiago-Cortes A, Frazer DL, She JX, Bugawan TL, Rotter JI, Raffel L, Zeidler A, Leyva-Cobian F, Hawkins BR, Chan SH, Castano L, Pociot F, Nerup J: Relative predispositional effects of HLA class II DRB1-DQB1 haplotypes and genotypes on type 1 diabetes: a meta-analysis. Tissue Antigens 70 :110 –127,2007 [DOI] [PubMed] [Google Scholar]
- 46.Hagopian WA, Sanjeevi CB, Kockum I, Landin-Olsson M, Karlsen AE, Sundkvist G, Dahlquist G, Palmer J, Lernmark A: Glutamate decarboxylase-, insulin-, and islet cell-antibodies and HLA typing to detect diabetes in a general population-based study of Swedish children. J Clin Invest 95 :1505 –1511,1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aly TA, Ide A, Jahromi MM, Barker JM, Fernando MS, Babu SR, Yu L, Miao D, Erlich HA, Fain PR, Barriga KJ, Norris JM, Rewers MJ, Eisenbarth GS: Extreme genetic risk for type 1A diabetes. Proc Natl Acad Sci U S A 103 :14074 –14079,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stratmann T, Martin-Orozco N, Mallet-Designe V, McGavern D, Losyev G, Dobbs C, Oldstone MBA, Yoshida K, Kikutani H, Mathis D, Benoist C, Haskins K, Teyton L: Susceptible MHC alleles, not background genes, select an autoimmune T cell reactivity. J Clin Invest 112 :902 –914,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nejentsev S, Howson JM, Walker NM, Szeszko J, Field SF, Stevens HE, Reynolds P, Hardy M, King E, Masters J, Hulme J, Maier LM, Smyth D, Bailey R, Cooper JD, Ribas G, Campbell RD, Clayton DG, Todd JA, Burton PR, Clayton DG, Cardon LR, Craddock N, Deloukas P, Duncanson A, Kwiatkowski DP, McCarthy MI, Ouwehand WH, Samani NJ, Todd JA, Donnelly P, Barrett JC, Burton PR, Davison D, Donnelly P, Easton D, Evans D, Leung HT, Marchini JL, Morris AP, Spencer CC, Tobin MD, Cardon LR, Clayton DG, Attwood AP, Boorman JP, Cant B, Everson U, Hussey JM, Jolley JD, Knight AS, Koch K, Meech E, Nutland S, Prowse CV, Stevens HE, Taylor NC, Walters GR, Walker NM, Watkins NA, Winzer T, Todd JA, Ouwehand WH, Jones RW, McArdle WL, Ring SM, Strachan DP, Pembrey M, Breen G, St. Clair D, Caesar S, Gordon-Smith K, Jones L, Fraser C, Green EK, Grozeva D, Hamshere ML, Holmans PA, Jones IR, Kirov G, Moskvina V, Nikolov I, O'Donovan MC, Owen MJ, Craddock N, Collier DA, Elkin A, Farmer A, Williamson R, McGuffin P, Young AH, Nicol FI, Ball SG, Balmforth AJ, Barrett JH, Bishop DT, Iles MM, Maqbool A, Yuldasheva N, Hall AS, Braund PS, Burton PR, Dixon RJ, Mangino M, Stevens S, Tobin MD, Thompson JR, Samani NJ, Bredin F, Tremelling M, Parkes M, Drummond H, Lees CW, Nimmo ER, Satsangi J, Fisher SA, Forbes A, Lewis CM, Onnie CM, Prescott NJ, Sanderson J, Mathew CG, Barbour J, Khalid MM, Todhunter CE, Mansfield JC, Ahmad T, Cummings FR, Jewell DP, Webster J, Brown MJ, Clayton DG, Lathrop GM, Connell J, Dominiczak A, Samani NJ, Braga CA, Burke B, Dobson R, Gungadoo J, Lee KL, Munroe PB, Newhouse SJ, Onipinla A, Wallace C, Xue M, Caulfield M, Farrall M, Barton A, Bruce IN, Donovan H, Eyre S, Gilbert PD, Hider SL, Hinks AM, John SL, Potter C, Silman AJ, Symmons DP, Thomson W, Worthington J, Clayton DG, Dunger DB, Nutland S, Stevens HE, Walker NM, Widmer B, Todd JA, Frayling TM, Freathy RM, Lango H, Perry JR, Shields BM, Weedon MN, Hattersley AT, Hitman GA, Walker M, Elliott KS, Groves CJ, Lindgren CM, Rayner NW, Timpson NJ, Zeggini E, McCarthy MI, Newport M, Sirugo G, Lyons E, Vannberg F, Hill AV, Bradbury LA, Farrar C, Pointon JJ, Wordsworth P, Brown MA, Franklyn JA, Heward JM, Simmonds MJ, Gough SC, Seal S, Stratton MR, Rahman N, Ban M, Goris A, Sawcer SJ, Compston A, Conway D, Jallow M, Newport M, Sirugo G, Rockett KA, Kwiatkowski DP, Bryan C, Bumpstead SJ, Chaney A, Downes K, Ghori J, Gwilliam R, Hunt SE, Inouye M, Keniry A, King E, McGinnis R, Potter S, Ravindrarajah R, Whittaker P, Withers D, Deloukas P, Leung HT, Nutland S, Stevens HE, Walker NM, Todd JA, Easton D, Clayton DG, Burton PR, Tobin MD, Barrett JC, Evans D, Morris AP, Cardon LR, Cardin NJ, Davison D, Ferreira T, Pereira-Gale J, Hallgrimsdottir IB, Howie BN, Marchini JL, Spencer CC, Su Z, Ying TY, Vukcevic D, Donnelly P, Bentley D, Brown MA, Cardon LR: Localization of type 1 diabetes susceptibility to the MHC class I genes HLA-B and HLA-A. Nature 450 :887 –892,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Skyler JS: Prediction and prevention of type 1 diabetes: progress, problems, and prospects. Clin Pharmacol Ther 81 :768 –771,2007 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.