Abstract
OBJECTIVE—Vascular progenitors of bone marrow origin participate to neovascularization at sites of wound healing and transplantation. We hypothesized that the biological purpose of this bone marrow–derived vascular component is to contribute angiogenic and survival functions distinct from those provided by the local tissue-derived vasculature.
RESEARCH DESIGN AND METHODS AND RESULTS—To address this hypothesis, we investigated the functional impact of bone marrow–derived vascular cells on pancreatic islets engraftment using bone marrow–reconstituted Id1+/−Id3−/− mice, a model of bone marrow–derived vasculogenesis. We show that, in this model, bone marrow–derived vasculogenic cells primarily contribute to the formation of new blood vessels within islet transplants. In contrast, graft revascularization in a wild-type background occurs by tissue-derived blood vessels only. Using these distinct transplant models in which bone marrow–and tissue-derived vasculature are virtually mutually exclusive, we demonstrate that bone marrow–derived vasculogenic cells exhibit enhanced angiogenic functions and support prompt activation of islets survival pathways, which significantly impact on islets engraftment and function. Moreover, gene profiling of vascular and inflammatory cells of the grafts demonstrate that neovascularization by bone marrow–derived cells is accompanied by the activation of a genetic program uniquely tuned to downregulate harmful inflammatory responses and to promote tissue repair.
CONCLUSIONS—These studies uncover the biological significance of bone marrow–derived vasculogenic cells in the response to injury during transplantation. Enhancing the contribution of bone marrow–derived vasculogenic cells to transplantation sites may help to overcome both limited angiogenic responses of the adult tissue-derived vasculature and untoward effects of inflammation on transplant engraftment.
Tissue repair after wounding and/or cell transplantation requires the concerted regulation of angiogenic and self-limited local inflammatory responses (1). Several studies have shown that angiogenic responses involve both tissue-derived vascular cells and circulating bone marrow–derived vascular precursors (2–9). The potential of these progenitors to develop into endothelial and/or perivascular cells supporting tumorigenesis and tumor growth has been extensively documented (2–6,9–15). In the context of primary tissues, a much more limited incorporation of these cells into injured blood vessels has been observed, suggesting a role in vascular repair (2,7,8). Whether in this instance the angiogenic response of such bone marrow–derived vascular component is distinct from that supported by preexisting tissue-derived blood vessels and/or contributes a biological advantage is presently unknown. Furthermore, the potential of bone marrow–derived vascular cells to influence local inflammatory responses normally associated with angiogenesis has not been addressed.
Pancreatic islet transplants are highly sensitive to the efficiency of revascularization, because defects of this process result in rapid cell loss and/or altered response of the grafts to glucose (16–19). Thus, islet transplants provide an ideal model to assess the impact of bone marrow–derived vascular cells on both survival and function of a primary tissue. Hence, we hypothesized that bone marrow–derived vasculogenic cells, recruited at sites of pancreatic islet transplantation, contribute engrafting and survival functions distinct from those provided by the endothelium sprouting from the preexisting vasculature. To address this hypothesis, we used the bone marrow–reconstituted Id1+/−Id3−/− mouse, a model of bone marrow–derived vasculogenesis (9,20). Id1 and Id3 transcription factors regulate vascular and neuronal cell differentiation and proliferation (21). Accordingly, Id1−/−Id3−/− knockout mice exhibit severe vascular malformations, including absence of sprouting and branching of blood vessels (20). In contrast, Id1+/−Id3−/− mice, maintaining one functional Id1 allele, display no overt vascular defects. However, preexisting blood vessels in these mice fail to mount an efficient angiogenic response to support tumor transplant engraftment (9). Interestingly, reconstitution of the hemopoietic compartment of Id1+/−Id3−/− mice with wild-type bone marrow rescues tumor revascularization (9). Moreover, in these bone marrow chimeras, >90% of the endothelium forming new blood vessels in the tumor implants is derived from wild-type bone marrow, indicating that neovascularization is sustained almost entirely by bone marrow–derived endothelial precursors.
Here, we exploited this model for islet transplantation, and we demonstrate that the majority of new blood vessels formed at the site of islet transplantation in bone marrow–reconstituted Id1/Id3-deficient mice are of bone marrow origin. In contrast, revascularization of islet transplants in bone marrow–reconstituted wild-type recipients occurs predominantly by tissue-derived blood vessels. Using these transplantation models, we provide evidence that tissue- and bone marrow–derived vasculatures are not functionally equivalent, in that development of bone marrow–derived blood vessels is associated with enhanced angiogenesis and with the activation of survival and cellular pathways uniquely skewed toward protective inflammatory responses and tissue repair.
RESEARCH DESIGN AND METHODS
Animals and bone marrow reconstitution.
Id1+/−Id3+/− and wild-type mice were bred at The Scripps Research Institute (TSRI) pathogen-free facility, and Id1+/−Id3−/− mice were screened as described previously (20). C56BL/6-TgN(ACTBEGFP)1Osb, ROSA26 (The Jackson Laboratories), or wild-type mice were used as bone marrow donors. Bone marrow cells were flushed from femurs with RPMI-10% FCS, depleted of CD3+ T-cell using magnetic beads (Miltenyi Biotech), and injected intravenously (5–10 × 106) in 6- to 8-week-old lethally irradiated wild-type and Id1+/−Id3−/− mice (1,200 rads). After 6 weeks, bone marrow reconstitution was assessed by flow cytometry of peripheral blood to identify green fluorescent protein (GFP)+ cells or β-gal+ cells stained with fluorescein-β-d-galactopyranosyde (Molecular Probes).
Islet isolation and transplantation.
Islets were isolated by intraductal injection of 0.5 mg/ml liberase and purified on a Ficoll gradient. Islets were cultured overnight in RPMI-10% FCS, handpicked, and transplanted under the kidney capsule. Diabetic mice were generated by intraperitoneal injection of a single dose of 200 mg/kg streptozotocin (22) and transplanted with islets 1 week later. Upon ensuing of frank hyperglycemia (i.e., 200 mg/dl), mice were injected subcutaneously with insulin (Humulin L; Lilly) up to 3 days after transplant. In normoglycemic mice, the function of the graft was verified by the return to hyperglycemia on removal of the graft.
Histology.
To identify functional blood vessels, mice were injected intravenously with 200 μg fluorescein isothiocyanate–labeled isolectin-B4 (FITC-ISB4) (Molecular Probes) before euthanasia. Tissues were immunostained as described previously (8) using the antibodies listed in the online appendix available at http://dx.doi.org/10.2337/db08-0244. Apoptotic cells were detected by transferase-mediated dUTP nick-end labeling (TUNEL) using a digoxygenin-labeling kit (Chemicon). Sections were visualized at a Zeiss Axiovert microscope equipped with a scanning laser confocal attachment (Radiance-2000; Bio-Rad) or at a NIKON Eclipse-800 microscope, equipped with a Spot II CCD camera. Morphometric analysis were performed on ∼20–30 sections per graft collected at 100-μm intervals until exhaustion of the grafts using the Spot Advanced and ImageProPlus software.
Cell separation and flow cytometry.
After in vivo injection of FITC-ISB4, grafts were microdissected and dissociated at 37°C in Hanks’ balanced salt solution, 0.5 mg/ml liberase, and 50 μg/ml DNase I followed by nonenzymatic dissociation medium (Sigma). Cells were then incubated with primary and secondary antibodies as detailed in the online appendix. For cell isolation, single cells dissociated from biotin-ISB4–perfused grafts (n = 5) were labeled with primary antibodies followed by R-phycoeritrin–-conjugated secondary reagents and anti-phycoeritrin microbeads (Miltenyi Biotech) as detailed in the online appendix. Cells were positively selected on magnetic columns and analyzed at a FACScan (Becton Dickinson). CD31+ and F480+ cells were >90% pure, whereas purity of ISB4+ fractions was >75%.
Western blotting and pAkt[S473] enzyme-linked immunosorbent assay.
Cells were lysed in 10 mmol/l Tris, 100 mmol/l NaCl, 1 mmol/l EDTA, 1 mmol/l EGTA, 1 mmol/l NaF, 20 mmol/l Na4P2O7, 2 mmol/l Na3VO4, 1% Triton X-100, 0.1% SDS, 0.5% deoxycolate, 1 mmol/l phenylmethylsulfonyl fluoride, and Complete (Roche). Equal amounts of proteins were loaded on 4–12% SDS-PAGE gels, transferred to polyvinylidine fluoride membranes, and probed with mouse anti–promyelocitic leukemia antigen (anti-PML) (clone MAB3738; Chemicon), goat anti-adipsin (Santa Cruz Biotechnology), or anti-S100A8 (R&D Systems) antibodies. Antibody binding was revealed using an ECL-based detection system (Kirkegaard and Perry Labs). Phosphorylated pAkt[S473] and total Akt were measured by ELISA (Biosource) using 5 μg proteins.
RNA extraction and DNA microarrays.
RNA was extracted using RNeasy kit (Qiagen). Biotinylated cRNA was prepared using the Illumina RNA Amplification kit (Ambion), and microarray experiments were performed as detailed in the online appendix.
Statistical analysis.
Statistical analysis of the data between naïve and bone marrow–reconstituted Id1+/−Id3−/− mice or between bone marrow–reconstituted Id1+/−Id3−/− and wild-type mice was performed using the Student's t test, and data comparing more than two groups were validated by ANOVA followed by Bonferroni's post hoc test. Data with P values <0.05 were considered statistically significant.
RESULTS
Failure of pancreatic islet engraftment in the Id1/Id3-deficient mice and rescue by bone marrow reconstitution.
To evaluate whether bone marrow reconstitution of Id1+/−Id3−/− mice supports engraftment of pancreatic islets as seen for tumor grafts (9), Id1+/−Id3−/− and wild-type mice were reconstituted with wild-type bone marrow. To track bone marrow–derived cells, GFP transgene or ROSA26 mice were used as bone marrow donors. Untreated Id1+/−Id3−/− mice were used as controls. Fluorescence-activated cell sorter (FACS) analysis at 6 weeks after bone marrow transplantation demonstrated that >80% of the leukocytes in bone marrow–reconstituted Id1+/−Id3−/− and wild-type mice were GFP or β-gal+, i.e., of bone marrow–donor origin. Furthermore, colony-forming unit assays and FACS analysis demonstrated that all hemopoietic lineages were reconstituted to a normal range in both wild-type and Id1+/−Id3−/− hosts (not shown).
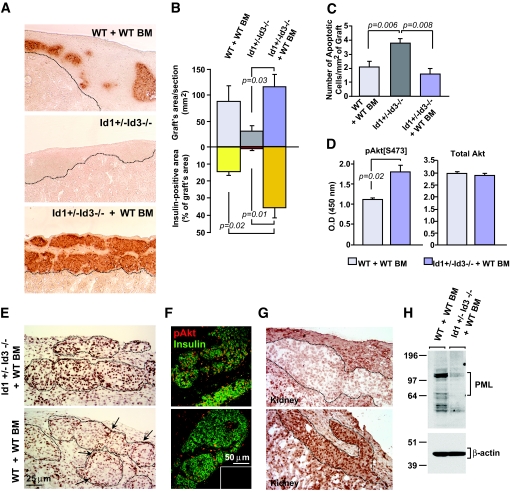
Six weeks after bone marrow engraftment, the mice were transplanted under the kidney capsule with 500 wild-type pancreatic islets. Immunostaining for insulin at 1 week after transplantation demonstrated that untreated Id1+/−Id3−/− mice harbored significantly smaller grafts than bone marrow–reconstituted wild-type mice (Fig. 1A). However, reconstitution with wild-type bone marrow rescued engraftment in Id1+/−Id3−/− mice (Fig. 1B). Furthermore, the insulin+ area of the grafts from bone marrow–reconstituted Id1+/−Id3−/− mice was >2.5-fold larger than that of grafts from bone marrow–reconstituted wild-type mice (Fig. 1B), suggesting differential islet cell survival. Morphometric analysis demonstrated approximately a twofold increase in the number of TUNEL+ apoptotic cells in islet grafts of untreated Id1+/−Id3−/− mice compared with bone marrow–reconstituted wild-type or Id1+/−Id3−/− mice (Fig. 1C). In contrast, the number of apoptotic cells in the grafts of bone marrow–reconstituted wild-type and Id1+/−Id3−/− mice was not significantly different. Even at earlier time points (i.e., 2 days), TUNEL+ islet cells were very rare in these two groups of mice (i.e., <0.1%), possibly because of efficient clearance of apoptotic cells.
FIG. 1.
Rescue of transplant engraftment in Id1+/−Id3−/− mice by reconstitution with wild-type bone marrow is associated with the activation of islet cell survival signals. A: Graft tissue sections at 1 week after transplantation stained by immunoperoxidase for insulin (brown). Scar tissue occupies the transplantation site in untreated Id1+/−Id3−/− mice (middle panel, area bordered by dotted line), indicating failure of islet engraftment. In contrast, grafts containing insulin+ cells are present in bone marrow–reconstituted wild-type and Id1+/−Id3−/− mice (top and bottom panels). B: Morphometric analysis of whole grafts (top graph) and insulin+ areas (bottom graph) measured in multiple sections collected at 100-μm intervals throughout the grafts as described in research design and methods. Each bar represents the mean ± SE of measurements from 80–120 tissue sections per group with n = 4 mice/group. C: Quantitative analysis of apoptotic cells detected by TUNEL at 1 week after transplantation. Values are means ± SE of measurements from n = 3 mice per group. Statistical significance by t test is indicated. Significance was also validated by ANOVA and Bonferroni post hoc test (online appendix). D: Quantitative determination of pAkt[S473] (left) and total Akt (right) detected by ELISA in lysates of islet clusters microdissected from the grafts. Values are means ± SE of triplicate samples from a pool of n = 2 grafts per group. E: Tissue sections stained for pAkt[S473] by immunoperoxidase. In bone marrow–reconstituted Id1+/−Id3−/− (top panel), virtually all cells within islet cell clusters (dotted areas) express high levels of pAkt[S473], whereas in wild-type mice (bottom panel), only cells at the periphery of the clusters (arrows) are strongly positive for pAkt[S473]. F: Confocal microscopy of tissues sections stained by two-color immunofluorescence for pAkt[S473] (red) and insulin (green). Most insulin+ cells are pAkt+ in bone marrow–reconstituted Id1+/−Id3−/− mice (top panel), whereas fewer insulin+ cells express pAkt in wild-type controls (bottom panel). Inset represents background staining by control IgGs. Immunostainings are representative of n = 3 grafts. G: Tissue sections stained for PML by immunoperoxidase. Weak expression of PML in nuclei and cytoplasm of islet cells from the grafts of bone marrow–reconstituted Id1+/−Id3−/− (top panel) mirrors pAkt[S473] expression pattern. H: Western blotting of PML and β-actin in protein lysates of islet cell clusters microdissected from the grafts demonstrates differential expression of PML in wild-type and Id1/Id3-deficient mice. (Please see http://dx.doi.org/10.2337/db08-0244 for a high-quality digital representation of this image.)
Signaling by Akt positively regulates cell survival and proliferation (23,24). Therefore, we investigated whether this pathway was differentially activated in grafts of bone marrow–reconstituted wild-type versus Id1+/−Id3−/− mice. Measurement of Akt activation by detection of pAkt[S473] in islet cell lysates revealed significantly higher levels of pAkt[S473] in bone marrow–reconstituted Id1+/−Id3−/− versus wild-type mice (Fig. 1D). Furthermore, graft immunostaining demonstrated a strikingly different pattern of pAkt[S473] expression in situ. Thus, whereas in bone marrow–reconstituted Id1+/−Id3−/− mice, a strong immunoreactivity for pAkt was observed throughout the graft, in wild-type controls, only cells at the periphery of islets were strongly positive for pAkt (Fig. 1E and F, arrows). In both grafts, pAkt[S473] highlighted primarily cell nuclei and, to a lesser extent, the cytoplasm, two known localizations of activated Akt (25). The preferential nuclear localization of pAkt in the transplants of bone marrow–reconstituted Id1+/−Id3−/− resembled that of tumors lacking the PML, a tumor suppressor gene regulating cell proliferation and apoptosis (26). Consistent with the function of PML as negative regulator of Akt activation and nuclear localization (26), immunostaining for PML in islet grafts demonstrated a pattern of expression that mirrored pAkt[S473], i.e., weak labeling of islet cells in bone marrow–reconstituted Id1+/−Id3−/− mice and strong labeling of islets in wild-type recipients (Fig. 1G). Immunoblotting of islet protein extracts confirmed downregulation of PML in bone marrow–reconstituted Id1+/−Id3−/− versus wild-type mice (Fig. 1H). In both grafts, no cycling endocrine cells were detected (not shown), indicating that pAkt expression and its preferential nuclear localization were linked to cell survival rather than proliferation. Thus, rescue of transplant engraftment by bone marrow reconstitution in Id1+/−Id3−/− mice is associated with decreased islet cell death and activation of survival signals.
Characterization of vasculature of the grafts.
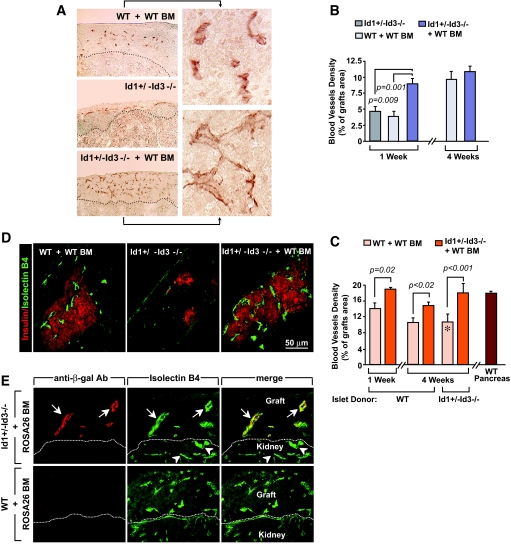
Whole grafts of bone marrow–reconstituted Id1+/−Id3−/− mice at 1 week after transplant displayed approximately a twofold higher density of platelet/endothelial cell adhesion molecule-1 (PECAM-1)+ blood vessels than those of either untreated Id1+/−Id3−/− or bone marrow–reconstituted wild-type mice (Fig. 2A and B). PECAM-1+ blood vessels in the grafts of bone marrow–reconstituted Id1+/−Id3−/− mice also appeared more branched than those of wild-type controls (Fig. 2A, insets). Compared with the endocrine component, the nonendocrine (i.e., connective and inflammatory) tissue was more abundant in wild-type recipients (Fig. 1A) and less vascularized. Morphometric analysis of blood vessels within the grafts endocrine component only showed an ∼25% increase of vascular density in bone marrow–reconstituted Id1+/−Id3−/− (n = 3) versus wild-type mice (n = 4) (Fig. 2C).
FIG. 2.
Bone marrow–derived vasculogenic cells form a dense network of functional blood vessels and differentially contribute to islet grafts revascularization in bone marrow–reconstituted Id1+/−Id3−/− and wild-type mice. A: Grafts tissue sections at 1 week after transplantation stained by immunoperoxidase for PECAM-1 demonstrates a higher density of blood vessels in bone marrow–reconstituted Id1+/−Id3−/− mice versus untreated Id1+/−Id3−/− and wild-type mice. Insets are enlarged areas of the displayed images. B and C: Morphometric analysis of PECAM-1+ blood vessel density in whole grafts (B) and islets only (C) at 1 and 4 weeks after transplant. In C, the vascular density of islets endogenous to pancreata from wild-type mice is shown for comparison (brown bar at right). Islet tissue was identified by hematoxylin counterstaining and calculated areas verified by immunostaining for synaptophysin in consecutive sections. Values marked by * were derived from one surviving graft in a group of three where the two other grafts failed and were therefore unavailable for morphometric determination. Values are means ± SE of measurements from 80–120 tissue sections per group. Statistical significance by t test is indicated. Significance was also validated by ANOVA and Bonferroni post hoc test (online appendix). D: Confocal microscopy of grafts tissue sections from mice at 4 weeks after transplantation injected intravenously with FITC-ISB4 (green) to identify functional blood vessels and stained by immunofluorescence for insulin (red). E: To track the bone marrow origin of vascular endothelial cells, Id1+/−Id3−/− and wild-type mice were reconstituted with ROSA26 bone marrow, expressing β-gal in all nucleated cells. Labeling of blood vessels by ISB4 (green) and immunostaining of β-gal+ cells (red) demonstrates that grafts blood vessels in bone marrow–reconstituted Id1+/−Id3−/− mice are of bone marrow origin (arrows). In contrast, bone marrow–derived β-gal+ endothelial cells are undetectable in the grafts of wild-type recipients. Images are representative of n = 4 grafts. (Please see http://dx.doi.org/10.2337/db08-0244 for a high-quality digital representation of this image.)
The lower vascular density in the grafts of wild-type recipients could not be attributed to irradiation, because the density of PECAM-1+ blood vessels was not significantly different in irradiated and nonirradiated recipients (i.e., = 13.3 ± 0.25 vs. 14.1 ± 1.3%, mean ± SE, n = 4). Furthermore, staining with the Meca-32 antibody, preferentially labeling arterioles and venules over capillary endothelia (27), demonstrated that the density of Meca-32+ blood vessels was only slightly increased in bone marrow–reconstituted Id1+/−Id3−/− versus wild-type mice (i.e., 12.9 ± 0.2 vs. 11.1 ± 0.4%, mean ± SE, n = 3). Thus, at 1 week after transplantation, the increased vascular density of islet grafts in bone marrow–reconstituted Id1+/−Id3−/− appears to be mainly accounted for by the development of a PECAM-1+ capillary network.
At 4 weeks after transplantation, a time when revascularization of islet grafts is completed (16,17), vascular density of whole grafts was similar in bone marrow–reconstituted Id1+/−Id3−/− and wild-type recipients (Fig. 2B); however, in the endocrine component of the grafts, it was still ∼25% higher in bone marrow–reconstituted Id1+/−Id3−/− (n = 3) compared with wild-type mice (n = 4) (Fig. 2C). When compared with islets endogenous to the pancreas, the vascular density of the grafts in bone marrow–reconstituted Id1+/−Id3−/− mice was remarkably similar at 1 week after transplantation, whereas in wild-type mice, it was always less than that of endogenous islets (Fig. 2C).
Bone marrow–reconstituted Id1+/−Id3−/− mice also efficiently revascularized islets isolated from Id1+/−Id3−/− donors (Fig. 2C). We hypothesized that because of the growth defects of Id1/Id3-deficient endothelial cells (9,20,21,28), these transplants would unlikely be supported by islet donor–derived endothelial cells that might survive islet isolation (29,30). Under these conditions, two of three grafts failed to engraft in wild-type recipients. In contrast, all transplants in bone marrow–reconstituted Id1+/−Id3−/− mice engrafted and, at 4 weeks after transplant, displayed approximately a twofold higher vascular density than the single transplant engrafted in wild-type mice (Fig. 2C).
In vivo injection of FITC-ISB4 demonstrated that blood vessels developing in bone marrow–reconstituted wild-type and Id1+/−Id3−/− mice, but not those in untreated Id1+/−Id3−/− mice, were patent (Fig. 2D). ISB4+ blood vessels coexpressed the endothelial marker PECAM-1 but not the pan-leukocyte antigen CD45 (not shown). FACS analysis of cells dissociated from the grafts showed that in wild-type mice, 78 and 89% of PECAM-1+CD45− cells displayed bound ISB4 at 1 and 4 weeks after transplantation, respectively (Supplementary Fig. S1C [online appendix]); whereas in bone marrow–reconstituted Id1+/−Id3−/− mice, a higher proportion of PECAM-1+CD45− endothelial cells were ISB4+ at either time point (i.e., 91 and 98% respectively; Supplementary Fig. S1D). These results indicate that although the majority of PECAM-1+ cells detected in situ outline functional vessels, some do not. This latter fraction, possibly part of developing vascular structures yet not blood-perfused, is higher at 1 than at 4 weeks after transplantation in either graft and in the grafts of wild-type recipients at either time point.
Immunostaining for β-gal in islet grafts from Id1+/−Id3−/− mice reconstituted with ROSA26 bone marrow demonstrated that the majority of ISB4+ blood vessels were β-gal+, indicating their bone marrow origin (Fig. 2E). In contrast, bone marrow–derived β-gal+ endothelial cells were virtually undetectable in wild-type recipients reconstituted with ROSA26 bone marrow. Ultrastructurally, bone marrow–derived blood vessels appear as small capillaries lined by a fenestrated endothelium and perivascular cells (Supplementary Fig. S2A) reminiscent of the capillaries of islets in the endogenous pancreas (31). In contrast, blood vessels in the grafts of bone marrow–reconstituted wild-type mice appeared larger and lined by a fenestrated endothelium loosely attached to the basal membrane (Supplementary Fig. S2B, asterisks).
These results demonstrate that enhanced survival and engraftment of islet transplants in bone marrow–reconstituted Id1+/−Id3−/− mice correlates with a predominant bone marrow–derived vasculogenic component and rapid development of a functional, dense capillary network. In contrast, new blood vessels in wild-type mice are formed primarily from the preexisting tissue-derived vasculature and expand less efficiently into blood-perfused capillaries.
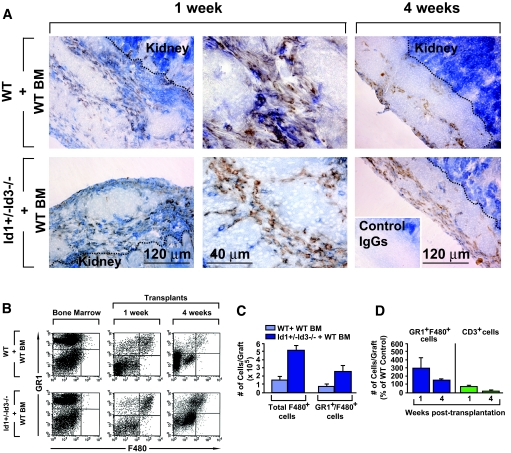
Graft revascularization by bone marrow–derived endothelium is associated with enhanced recruitment of GR1highF480+ inflammatory cells and with the activation of repair response genes.
At 1 week after transplantation, many CD45+β-gal+ inflammatory leukocytes were observed in the grafts of mice reconstituted with β-gal+ bone marrow (Supplementary Fig. S3). Peri-islet inflammatory cells were present in both Id1/Id3-deficient and wild-type recipients and comprised myeloid F480+ cells (Fig. 3A). FACS analysis demonstrated an ∼2.5-fold increase in F480+ and GR1highF480+ cells in the grafts, but not bone marrow compartment, of bone marrow–reconstituted Id1+/−Id3−/− mice versus wild-type controls (Fig. 3B and C; n = 4, P < 0.002). Numbers of T- and B-cells were similar in the two grafts at 1 week after transplantation, whereas grafts from Id1/Id3-deficient mice had fewer T-cells at 4 weeks after transplantation (Fig. 3D, n = 2).
FIG. 3.
Detection of inflammatory leukocytes in the islet grafts. A: Tissue sections from islet grafts of bone marrow–reconstituted wild-type and Id1+/−Id3−/− mice, at 1 and 4 weeks after transplantation, stained by two-color immunohistochemistry for the pan-leukocyte marker CD45 (blue) and the myeloid marker F480 (brown) or control IgGs (inset). A leukocytic inflammatory infiltrate, comprising myeloid cells is apparent in the grafts from both experimental groups. The dotted lines mark the border of the grafts with the kidney. The intense blue staining in the kidney is background due to color development by the alkaline phosphatase endogenous to the kidney epithelium. Images are representative of n = 3 grafts per experimental group. B: Flow cytometric analysis of leukocytes isolated from the grafts at 1 and 4 weeks after transplantation stained by two-color immunofluorescence for the myeloid markers GR1 and F480. An increased percentage of GR1highF480+ cells in the graft of bone marrow–reconstituted Id1+/−Id3−/− mice is evident compared with wild-type controls. Theses cells are not present in the bone marrow of either mouse. The dot plots are representative of n = 4 experiments. C and D: Quantitative analysis of GR1highF480+ and CD3+ cell subsets detected by flow cytometry in the graft bone marrow–reconstituted Id1+/−Id3−/− and wild-type mice at 1 and 4 weeks after transplantation. Bars are means ± SE of n = 4 independent determinations. (Please see http://dx.doi.org/10.2337/db08-0244 for a high-quality digital representation of this image.)
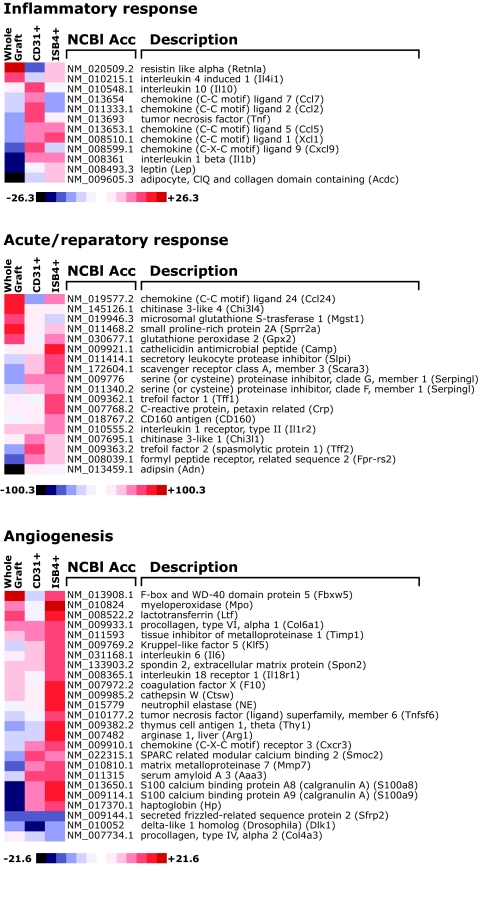
To gain insights into the genetic program activated within the distinct vascular and inflammatory transplant microenvironments in Id1/Id3-deficient and wild-type mice, we performed gene-screening experiments using RNA from whole grafts and from myeloid and endothelial (i.e., CD31+ and ISB4+) cells isolated from the grafts at 1 week after transplantation. Analysis of transcripts exhibiting more than twofold changes in bone marrow–reconstituted Id1+/−Id3−/− versus wild-type mice demonstrated differential expression of inflammation and angiogenesis-related genes (Table 1; Fig. 4). First, a significant decreased expression of complement components and adipokines, including adipsin, adiponectin, and leptin, was observed in whole grafts from bone marrow–reconstituted Id1+/−Id3−/− mice. Conversely, genes regulating the influx and function of neutrophils, monocytes, and eosinophils (e.g., CCL1, CCL2, CCL7, and Calgranulin A and B) were upregulated in CD31+ and ISB4+ samples of bone marrow–reconstituted Id1+/−Id3−/− mice. Most remarkably, in these samples, we observed an increased expression of genes marking macrophages polarized toward type II immune responses (e.g., resistitin-like molecule-α, interleukin [IL]-10, Chitinase 3-like molecules, CCL24, signaling lymphocytic activation molecule, and Arginase I) (32) and anti-inflammatory genes involved in the response to pathogens, oxidative stress, and wound healing (e.g., trefoil factors, serine peptidase inhibitors, cathelicidin, and glutathione peroxidase 2) (33,34). Angiogenesis-related genes were also upregulated in Id1/Id3-deficient versus wild-type mice and included adhesion receptors to extracellular matrix (ECM) proteins (Thy-1, Spondin 2, and SPARC [secreted protein, acidic, and rich in cysteine]), proangiogenic ECMs and remodeling enzymes (procollagen type VI, MMP7 [matrix metalloproteinase-7], and TIMP1 [tissue inhibitor of metalloproteinase]), and molecules regulating endothelial cell proliferation (CXCR3 and IL-6) (Fig. 4). Differential protein expression of select genes was confirmed by immunohistochemistry, immunoblotting, and/or ELISA (Supplementary Fig. S4). These findings demonstrate that compared with the tissue-derived vasculature, bone marrow–derived vascular cells are associated with an inflammatory component enriched for myeloid cells and functionally skewed toward protective immune responses and tissue repair.
TABLE 1.
Inflammation and angiogenesis-related genes differentially expressed in the grafts of bone marrow–reconstituted Id1/Id3-deficient versus wild-type mice
| Inflammatory response genes | Accession no. | Whole graft | CD31+ cells | ISB4+ cells | Function |
|---|---|---|---|---|---|
| Adiponectin | NM_009605.3 | −26 | −1.2 | 1.12 | Anti-inflammatory, antiangiogenic |
| Leptin | NM_008493.3 | −5.9 | 1 | 1.1 | Proinflammatory/Th1 immune responses |
| Resistin-like molecule α | NM_020509.2 | 24 | −3.1 | 2.2 | Proinflammatory/M2 polarized immune responses |
| Interleukin 10 | NM_010548.1 | 1 | 3.4 | 1 | Anti-inflammatory, Th2 immune responses |
| TNF-α | NM_013693 | 1.4 | 2.7 | 1 | Th1 immune responses, proangiogenic |
| Interleukin 1β | NM_008361 | −7.1 | 1.6 | 1.6 | Th1 immune responses, proangiogenic |
| Interleukin 4 induced 1 | NM_010215.1 | 2.4 | −1.1 | 1.2 | Downregulation of T-cell responses |
| CCL1 | NM_008510.1 | −2.0 | 1.9 | 2.2 | Eosinophil recruitment/Th2/Tc2/Treg responses |
| CCL2 (MCP-1) | NM_011333.1 | −1.3 | 2.6 | 1.6 | Monocyte recruitment/proangiogenic |
| CCL5 (RANTES) | NM_013653.1 | −1.9 | 2.0 | 1.7 | Monocyte/T-cell/eosinophil recruitment |
| CCL7 | NM_013654 | −1.1 | 2.1 | −1.5 | Monocyte recruitment |
| CXCL9 | NM_008599 | −2.6 | 2.6 | −1.4 | Monocyte/T-cell recruitment |
| Acute response/repair response genes | |||||
| Complement factor D (adipsin) | NM_013459.1 | −100 | 1.0 | 1.0 | Complement activation |
| Chitinase 3-Like 4 | NM_145126.1 | 24 | −1.5 | 2.9 | Th2/M2 polarized immune responses |
| Small proline-rich protein 2A | NM_011468.2 | 7.6 | 1.0 | 1.1 | Protection from ischemic injury |
| Microsomal glutathione S-transferase 1 | NM_019946.3 | 5.7 | 1.0 | −1.3 | Protection from oxidant stress |
| Glutathione peroxidase 2 | NM_030677.1 | 5.2 | −1.1 | 2.9 | Protection from oxidant stress |
| Interleukin 1 receptor, type II | NM_010555.2 | 1.2 | 1.4 | 2.7 | Decoy receptor, downregulation of IL-1 signaling |
| Trefoil factor 1 | NM_009362.1 | 1.2 | −1.2 | 3.2 | Anti-inflammatory, antiapoptotic, proangiogenic |
| Trefoil factor 2 | NM_009363.2 | −2.4 | 4.7 | 1.2 | Anti-inflammatory, induction of cell proliferation/migration |
| CCL24 | NM_019577.2 | 8.6 | −2.6 | 1.8 | Eosinophil recruitment/Th2/Tc2 responses |
| SLAM member 7 | AK089525 | −1.2 | 2.5 | 1.1 | Immune regulation, Th2 responses, healing |
| Scavenger receptor class A, member 3 | NM_172604.1 | −1.2 | 1.2 | 4.4 | Pathogen recognition, apoptotic cell clearance |
| C-reactive protein (pentraxin-related) | NM_007768.2 | 1.2 | 1.1 | 3.1 | Pathogen recognition, apoptotic cell clearance |
| Chitinase 3-like 1 | NM_007695.1 | 1 | 3.0 | 1.5 | Downregulation of IL-1/TNF signaling |
| Serine peptidase inhibitor (clade G) | NM_009776 | −0.1.8 | 2.0 | 2.1 | Complement component 1 inhibition, suppression of leukocyte transmigration |
| Serine peptidase inhibitor (clade F) | NM_011340.2 | −1.3 | 1.5 | 2.4 | Anti-inflammatory, antiapoptotic, antiangiogenic |
| Secretory leukocyte peptidase inhibitor | NM_011414.1 | −1.2 | 1.4 | 5.7 | Antiproteases, anti-inflammatory |
| Cathelicidin | NM_009921.1 | 1 | 1 | 13 | Antimicrobial, immunomodulatory |
| Formyl peptide receptor like 1 | NM_008039.1 | −3.7 | 2.4 | 1.39 | Neutrophil/monocyte/T-cell recruitment, regulation of neutrophil lifespan, proangiogenic |
| Angiogenesis-related genes | |||||
| Calgranulin A | NM_013650.1 | −4.4 | 1.8 | 6.5 | Myeloid cell recruitment/ proangiogenic |
| Calgranulin B | NM_009114.1 | −5.4 | 1.5 | 7.1 | Myeloid cell recruitment/ proangiogenic |
| Coagulation factor X | NM_007972.2 | 1.2 | 1.0 | 4.0 | Coagulation factor/proangiogenic |
| Thy0.1.2 | NM_009382.2 | 1.4 | −1.2 | 4.2 | Matrix interaction/proangiogenic |
| Procollagen type VI | NM_009933.1 | 2.8 | 1.8 | 2.2 | Expressed in tumor endothelium, ligand of TEM8 |
| Serum amyloid 3 | NM_011315 | −1.1 | 3.4 | 3.3 | Induction of MMPs/proangiogenic |
| Kruppel like factor 5 | NM_009769.2 | −1.1 | 1.3 | 3.2 | Vascular remodeling |
| Interleukin-6 | NM_031168.1 | 1 | 1.2 | 3.2 | VEGF induction/proangiogenic |
| Spondin 2 | NM_133903.2 | 1.3 | 1.2 | 3.2 | Cell spreading |
| TIMP1 | NM_011593 | 1.3 | 1.5 | 3.0 | Regulation of MMP/ECM remodeling |
| MMP7 | NM_010810 | −2.2 | −1.6 | 2.7 | Endothelium proliferation/ migration |
| SPARC-related protein | NM_022316.1 | −1.2 | 1.1 | 2.6 | Negative regulation of cell adhesion, ECM degradation |
| Procollagen type IV α3 | NM_007734.1 | 1 | −1.3 | −2.0 | Blood vessels regression, antiangiogenic |
| Haptoglobin | NM_017370.1 | −6.5 | −1.5 | 2.7 | Antioxidant, proangiogenic |
| Fas ligand | NM_010177.2 | −1.1 | 1 | 3.1 | Proangiogenic, proapoptotic |
| Interleukin 18 receptor | NM_008365.1 | 1.2 | 1 | 2.8 | Proinflammatory/proangiogenic |
| CXCR3 | NM_009910.1 | 0.8 | 1.9 | 2.4 | Endothelium proliferation/ monocyte homing |
| Myeloperoxidase | NM_010824 | 1.6 | −1.1 | 22 | Neutrophil phagocytic functions |
| Cathepsin G | NM_007800.1 | 0 | −1.2 | 5 | MMP/receptor activation, proangiogenic |
| Elastase 2 | NM_015779 | 0 | 1 | 4.4 | MMP/receptor activation, proangiogenic |
| Arginase I | NM_007482 | −1.1 | −1.4 | 6.4 | Polyamine synthesis, cell proliferation |
| Delta like 1 homolog | NM_010052 | −1.5 | −4.3 | −1.5 | Notch 1/4 ligand |
| Secreted frizzled-related protein 2 | NM_009144.1 | −2.7 | −3.5 | −2.3 | Notch pathway component |
| F-Box/Wd40 ubiquitin component | NM_013908.1 | 15 | −1.2 | 2.8 | Negative regulation of Notch pathway |
Data are fold change. Genes exhibiting at least a twofold change between Id1/Id3-deficient and wild-type mice in the cell fractions of the indicated grafts are displayed. Values equal to 1 indicate no change of gene expression in Id1/Id3-deficient versus wild-type mice, whereas positive and negative values indicate upregulation and downregulation of specific genes, respectively. MMP, matrix metalloproteinase-7; SLAM, signaling lymphocytic activation molecule; SPARC, secreted protein, acidic, and rich in cysteine; TIMP, tissue inhibitor of metalloproteinase-1; TNF-α, tumor necrosis factor-α.
FIG. 4.
Heatmaps of genes differentially expressed in whole grafts and cellular fractions of bone marrow–reconstituted Id1+/−Id3−/− mice over wild-type controls. Genes were grouped as inflammatory, repair, and angiogenesis-related genes. Fold changes between gene expression levels in samples obtained from bone marrow–reconstituted Id1+/−Id3−/− mice over those of wild-type controls are presented in the form of a heatmap (blue to red scale). Only genes displaying more than twofold changes are shown.
Islet transplants revascularized by bone marrow–derived vasculogenic cells readily reverse diabetes in vivo.
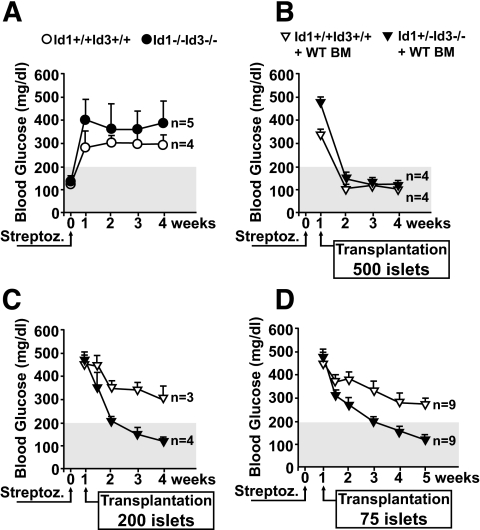
To evaluate the translational implications of the enhanced angiogenic and repairing functions associated with bone marrow–derived vasculogenic cells at sites of islet transplantation, we induced diabetes in the Id1/Id3-deficient strain by streptozotocin and then assessed the ability of islet transplants to reverse hyperglycemia. Streptozotocin-injected Id1+/−Id3−/− and wild-type mice became hyperglycemic by 72 h after injection and remained diabetic up to a 4-week follow-up period (Fig. 5A). After this time point, the experiment was ended because diabetic mice would not survive without treatment. Next, wild-type and Id1+/−Id3−/− mice were reconstituted with wild-type bone marrow, rendered diabetic by streptozotocin, and transplanted with either 500, 200, or 75 islets 1 week later. Daily monitoring of blood glucose demonstrated that transplants of 500 islets effectively restored normoglycemia in all mice within 1 week after transplantation (Fig. 5B). Interestingly, transplants of 200 and as few as 75 islets were also sufficient to rapidly restore normoglycemia in bone marrow–reconstituted Id1+/−Id3−/− mice but not in wild-type mice (Fig. 5C and D). Control mice (i.e., not transplanted) remained diabetic over the study period (blood glucose >400 mg/dl, n = 2; data not shown). Furthermore, removal of the grafts at 3 weeks after transplantation in cured mice led to recurrence of diabetes (blood glucose 283 ± 47 mg/dl, mean ± SE, n = 8), confirming the functionality of the grafts and the lack of functional recovery of the endogenous islets from streptozotocin-mediated destruction. In addition, insulin immunostaining of pancreata revealed that remnant insulin+ areas were not significantly different in streptozotocin-treated wild-type and Id1/Id3-deficient mice (not shown), indicating that there was no differential β-cell recovery within the 4 weeks after transplant period. Further experiments showed that serum insulin and adiponectin and response to insulin challenge were similar in wild-type and Id1+/−Id3−/− mice (Supplementary Fig. S5), indicating that differential peripheral insulin sensitivity did not account for the observed diabetes reversal by small islet transplants in bone marrow–reconstituted Id1+/−Id3−/− mice.
FIG. 5.
Functionality of islet grafts in diabetic recipients. A: Blood glucose levels in Id1+/−Id3−/− and wild-type Id1+/+Id3+/+ mice after streptozotocin injection. Shadowed area marks the limit of blood glucose values above which mice were considered frankly diabetic. B–D: Levels of blood glucose in mice rendered diabetic by streptozotocin and 1 week later transplanted with 500 (B), 200 (C), or 75 (D) wild-type islets per mouse. Values are means ± SE of measurements from the indicated number of mice.
These results demonstrate that the enhanced contribution of bone marrow–derived cells to islet revascularization has a significant impact on transplant engraftment and function, allowing even a limited number of islets to reverse diabetes in transplant recipients.
DISCUSSION
Bone marrow–derived vascular cells have been proposed as targetable cell types for drug or gene delivery and for vascular repair (2). However, conditions permissive to the substantial recruitment of these cells in nontumoral tissues remain to be defined. Furthermore, it is uncertain whether such vasculature would support normal tissue functions. Here, we show that functional bone marrow–derived blood vessels can develop within pancreatic islet grafts and that Id1/Id3 defective expression at transplantation sites is required for this phenomenon. Neovascularization by bone marrow–derived vasculogenic cells is associated with enhanced islets’ vascular density and improved graft survival and function, demonstrating a biological advantage over the tissue-derived vasculature. Moreover, the downregulation of genes involved in tissue damage and the activation of protective repairing responses observed in these grafts provide evidence for a role of bone marrow–derived cells in antagonizing pathogenic inflammation and promoting tissue healing.
The vascular contribution of bone marrow–derived vasculogenic cells varies greatly in tumors of different origin and grades and in transplanted versus spontaneous tumors (9,20,22,35). These observations predict that recruitment and development of these progenitors may also differ among quiescent tissues. We demonstrate herein that islet transplants supports vascular development of bone marrow–derived cells. Angiogenic factors of islets, such as vascular endothelial growth factor (36), may contribute to this effect. Yet, these factors may not be sufficient, because the same tissue engrafted in wild-type mice harbored virtually no bone marrow–derived blood vessels. Accordingly, previous reports have shown a few bone marrow–derived vascular cells in models of islet transplantation or islet injury (37,38). In addition, overexpression of proangiogenic factors and/or use of progenitors mobilizing agents, although enhancing local accumulation of various bone marrow–derived cell lineages, do not necessarily result in increased incorporation of bone marrow–derived vascular cells into blood vessels (39,40). Thus, in a wild-type environment, the development of bone marrow–derived vascular progenitors at sites of healing appears to be tightly regulated by unknown local homeostatic mechanisms. These tissue barriers are clearly overcome by downregulation of Id1 and Id3 at transplantation sites, providing a niche for bone marrow cells to home and/or expand. In this regard, it is noteworthy that Id1 in endothelial cells regulates expression of chondroitin sulfate proteoglycan and HIF1α, factors previously involved in the recruitment and/or development of bone marrow–derived endothelial cells (41,42). Hence, downregulation of Id1 and Id3 at transplantation sites (e.g., by retrovirus-mediated small interfering RNAs delivered to the local vasculature) may be envisaged as a therapeutic strategy to facilitate homing/development of bone marrow cells with high vasculogenic potential, thereby improving engraftment and function of cell transplants.
Although our studies uncover distinct engrafting and angiogenic functions of bone marrow–derived versus tissue-derived vasculogenic cells in islet transplants, they do not address to what extent these properties are contributed by the vascular cells and/or by associated inflammatory leukocytes. Vascular and inflammatory cells regulate each other during tissue healing (1). Further studies are warranted to determine how these complex cellular networks influence engraftment. Nevertheless, our gene-screening experiments provide important clues on the cell types and molecular pathways possibly involved. Thus, a hallmark of the grafts supported by bone marrow–derived blood vessels is the increased expression of genes regulating the influx, activation, and angiogenic function of neutrophils and monocytes. Consistent with an increased frequency of these myeloid subsets, these grafts harbored a higher number of GR1highF480+ cells. In addition, fewer T-cells were observed in those transplants. Interestingly, there is evidence that GR1+ leukocytes regulate the angiogenic switch in tumors (43) and that T-cells control vascular pruning and remodeling (44). Hence, GR1highF480+ cells may contribute to the enhanced angiogenic response of the bone marrow–derived vasculature, whereas the low number of T-cells recruited and/or surviving locally may be permissive to the expansion of that vascular network in the islet grafts. The increased expression of genes marking the activation of M2 polarized macrophages and antioxidative pathways point to other biological responses that may also positively affect engraftment. Importantly, this M2-polarized gene profile was not detected in peripheral macrophages (not shown), indicating that it was not dictated by the Id1/Id3-deficient environment per se. This pattern provides strong evidence that bone marrow–derived vascular cells are associated with and/or may support protective repairing rather than harmful inflammatory responses at sites of tissue injury. Finally, noteworthy for its direct implications on transplant survival is the downregulation of Factor D (adipsin) in the grafts from bone marrow–reconstituted Id1/Id3-deficient mice. Adipsin is the limiting factor for the activation of the alternative pathway of complement, reportedly involved in ischemia/reperfusion injury (45). The downregulation of this factor suggests that the microenvironment contributed by bone marrow–derived vasculogenic cells modulates the susceptibility of islet tissue to damage by complement.
Islet transplantation has the potential to replace pancreatic endocrine function in type 1 diabetics. However, the large β-cell mass required to treat individual patients has precluded the wide use of this approach. Our studies provide in vivo evidence that neovascularization by bone marrow–derived vasculogenic cells confers a significant survival advantage to islet transplants, allowing fewer islets to promptly reestablish normoglycemia in diabetic recipients. It will be important to determine in future studies whether bone marrow–derived endothelial progenitors can similarly enhance islet engraftment at other transplantation sites (e.g., the liver) and in models of allotransplantation in autoimmune diabetic mice. Notwithstanding, the syngeneic system presented here demonstrates that in the absence of a substantial input from this bone marrow vasculogenic component, the angiogenic response of the tissue vasculature appears insufficient to ensure survival throughout the graft, as inferred from the heterogeneous pAkt expression observed in situ. Notably, in the grafts of bone marrow–reconstituted Id1+/−Id3−/− mice, strong expression of nuclear pAkt inversely correlated with PML. This tumor suppressor gene opposes pAkt nuclear functions and negatively regulates responses to hypoxia and angiogenesis (26,46). Hence, PML downregulation within islet grafts is consistent with a coordinated activation of survival and proangiogenic signals.
In conclusion, these studies provide strong evidence that enhancing the contribution of bone marrow–derived vasculogenic cells is a promising therapeutic approach to improve recovery of pancreatic islets after transplant. Furthermore, the association of bone marrow–derived vascular cells with protective inflammatory responses shown here may have implications in transplant tolerance.
Supplementary Material
Acknowledgments
V.C. has received support from the Juvenile Diabetes Research Foundation (JDRF) and Larry L. Hillblom Foundation Research Grants. G.R.D. has received support from the JDRF and Larry L. Hillblom Foundation Research Grants. G.H. has received NIH/NIDDK Award 1 P30-DK-063491-03. B.E.T. has received Defense Advanced Research Projects Agency Grant W81XWH-04 C-0139. L.C. has received National Institutes of Health (NIH) Pilot Grant VU/U19 DK42502, a JDRF International Innovative Grant, and NIH Grant RO1-HL-075270. Confocal and electron microscopy was performed at the National Center for Microscopy and Imaging Research supported by NIH Grant P41-RR-004050 to Dr. M. Ellisman.
We thank Dr. G. Del Zoppo (TSRI) and Dr. H. Powell (University of California San Diego [UCSD]) for their advice on tissue histology. We thank J. Lapira and Dr. Roman Šášik at the UCSD Biomedical Genomics Microarray Facility laboratory for Illumina Beadarray processing and bioinformatics analysis.
Published ahead of print at http://diabetes.diabetesjournals.org on 2 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C Section 1734 solely to indicate this fact.
REFERENCES
- 1.Martin P, Leibovich SJ: Inflammatory cells during wound repair: the good, the bad and the ugly. Trends Cell Biol 15 :599 –607,2005 [DOI] [PubMed] [Google Scholar]
- 2.Kopp H, Ramos CA, Rafii S: Contribution of endothelial progenitors and proangiogenic hematopoietic cells to vascularization of tumor and ischemic tissue. Curr Op Hematol 13 :175 –181,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM: Isolation of putative progenitor endothelial cells for angiogenesis. Science 275 :964 –967,1997 [DOI] [PubMed] [Google Scholar]
- 4.Takahashi T, Kalka C, Masuda H, Chen D, Silver M, Kearney M, Magner M, Isner JM, Asahara T: Ischemia- and cytokine-induced mobilization of bone marrow-derived endothelial progenitor cells for neovascularization. Nat Med 5 :435 –438,1999 [DOI] [PubMed] [Google Scholar]
- 5.Shi Q, Rafii S, Wu MH, Wijelath ES, Yu C, Ishida A, Fujita Y, Kothari S, Mohle R, Sauvage LR, Moore MA, Storb RF, Hammond WP: Evidence for circulating bone marrow-derived endothelial cells. Blood 92 :362 –367,1998 [PubMed] [Google Scholar]
- 6.Lin Y, Weisdorf DJ, Solovey A, Hebbel RP: Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest 105 :71 –77,2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Asahara T, Masuda H, Takahashi T, Kalka C, Pastore C, Silver M, Kearne M, Magner M, Isner JM: Bone marrow origin of endothelial progenitor cells responsible for postnatal vasculogenesis in physiological and pathological neovascularization. Circ Res 85 :221 –228,1999 [DOI] [PubMed] [Google Scholar]
- 8.Crisa L, Cirulli V, Smith KA, Ellisman MH, Torbett BE, Salomon DR: Human cord blood progenitors sustain thymic T cell development and a novel form of angiogenesis. Blood 11 :3928 –3940,1999 [PubMed] [Google Scholar]
- 9.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S: Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med 7 :1194 –1201,2001 [DOI] [PubMed] [Google Scholar]
- 10.Bailey AS, Willenbring H, Jiang S, Anderson DA, Schroeder DA, Wong MH, Grompe M, Fleming WH: Myeloid lineage progenitors give rise to vascular endothelium. Proc Natl Acad Sci U S A 103 :13156 –13161,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuwana M, Okazaki Y, Kodama H, Satoh T, Kawakami Y, Ikeda Y: Endothelial differentiation potential of human monocyte-derived multipotential cells. Stem Cells 24 :2733 –2743,2006 [DOI] [PubMed] [Google Scholar]
- 12.De Palma M, Vennerim MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L: Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell 8 :211 –226,2005 [DOI] [PubMed] [Google Scholar]
- 13.Jin D, Shido KK, Kopp HG, Petit I, Shmelkov SV, Young LM, Hooper AT, Amano H, Avecilla ST, Heissig B, Hattori K, Zhang F, Hicklin DJ, Wu Y, Zhu Z, Dunn A, Salari H, Werb Z, Hackett NR, Crystal RG, Lyden D, Rafii S: Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med 12 :557 –567,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P: Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood 104 :2084 –2086,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jodele S, Chantrain CF, Blavier L, Lutzko C, Crooks GM, Shimada H, Coussens LM, Declerck YA: The contribution of bone marrow-derived cells to the tumor vasculature in neuroblastoma is matrix metallopro-teinase-9 dependent. Cancer Res 65 :3200 –3208,2005 [DOI] [PubMed] [Google Scholar]
- 16.Mattsson G, Jansson L, Carlsson P: Decreased vascular density in mouse pancreatic islets after transplantation. Diabetes 51 :1362 –1366,2002 [DOI] [PubMed] [Google Scholar]
- 17.Carlsson PO, Palm F, Andersson A, Liss P: Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes 50 :489 –495,2001 [DOI] [PubMed] [Google Scholar]
- 18.Zhang N, Su D, Qu S, Tse T, Bottino R, Balamurugan AN, Xu J, Bromberg JS, Dong HH: Sirolomus is associated with reduced islet engraftment and impaired islet cell function. Diabetes 55 :2429 –2436,2006 [DOI] [PubMed] [Google Scholar]
- 19.Lakey JR, Mirbolooki M, Shapiro AM: Current status of clinical islet cell transplantation. Methods Mol Biol 333 :47 –104,2006 [DOI] [PubMed] [Google Scholar]
- 20.Lyden D, Young AZ, Zagzag D, Yan W, Gerald W, O'Reilly R, Bader BL, Hynes RO, Zhuang Y, Manova K, Benezra R: Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401 :670 –677,1999 [DOI] [PubMed] [Google Scholar]
- 21.Ruzinova MB, Schoer RA, Gerald W, Egan JE, Pandolfi PP, Rafii S, Manova K, Mittal V, Benezra R: Effect of angiogenesis inhibition by Id loss and the contribution of bone-marrow-derived endothelial cells in spontaneous murine tumors. Cancer Cell 4 :277 –289,2003 [DOI] [PubMed] [Google Scholar]
- 22.Riley WJ, McConnell TJ, MacLaren NK, McLaughlin JV, Taylor G: The diabetogenic effects of streptozotocin in mice are prolonged and inversely related to age. Diabetes 30 :718 –723,1981 [DOI] [PubMed] [Google Scholar]
- 23.Kandel ES, Hay N: The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res 253 :210 –229,1999 [DOI] [PubMed] [Google Scholar]
- 24.Beith JL, Alejandro EU, Johnson JD: Insulin stimulates primary beta cell proliferation via Raf-1 kinase. Endocrinology 2008 149 :2251 –2260 [DOI] [PMC free article] [PubMed]
- 25.Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings BA: Role of translocation in the activation and function of protein kinase B. J Biol Chem 272 :31515 –31524,1997 [DOI] [PubMed] [Google Scholar]
- 26.Trotman LC, Alimonti A, Scaglioni PP, Koutcher JA, Cordon-Cardo C, Pandolfi P: Identification of a tumor suppressor network opposing nuclear Akt function. Nature 441 :523 –527,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Amerongen MJ, Molema G, Plantinga J, Moorlag H, van Luyn MJ: Neovascularization and vascular markers in a foreign body reaction to subcutaneously implanted degradable biomaterial in mice. Angiogenesis 5 :173 –180,2002 [DOI] [PubMed] [Google Scholar]
- 28.Sakurai D, Tsuchiya N, Yamaguchi A, Okaji Y, Tsuno NH, Kobata T, Takahashi K, Tokunaga K: Crucial role of inhibitor of DNA binding/differentiation in the vascular endothelial growth factor-induced activation and angiogenic processes of human endothelial cells. J Immunol 173 :5801 –5809,2004 [DOI] [PubMed] [Google Scholar]
- 29.Brissova M, Fowler M, Wiebe P, Shostak A, Shiota M, Radhika A, Lin PC, Gannon M, Powers AC: Intraislet endothelial cells contribute to revascularization of transplanted pancreatic islets. Diabetes 53 :1318 –1325,2004 [DOI] [PubMed] [Google Scholar]
- 30.Nyqvist D, Kohler M, Wahlstedt H, Berggren PO: Donor islet endothelial cells participate in formation of functional vessels within pancreatic islet grafts. Diabetes 54 :2287 –2293,2005 [DOI] [PubMed] [Google Scholar]
- 31.Aharinejad S, MacDonald IC, Schmidt EE, Bock P, Hagen D, Groom AC: Scanning and transmission electron microscopy and high resolution intravital video-microscopy of capillaries in the mouse exocrine pancreas, with special emphasis on endothelial cells. Anat Rec 237 :163 –177,1993 [DOI] [PubMed] [Google Scholar]
- 32.Gordon S: Alternative activation of macrophages. Nat Rev Immunol 3 :23 –35,2003 [DOI] [PubMed] [Google Scholar]
- 33.Taupin D, Podolsky DK: Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol 4 :721 –733,2003 [DOI] [PubMed] [Google Scholar]
- 34.Williams SE, Brown TI, Roghanian A, Sallenave JM: SLPI and elafin: one glove many fingers. Clin Sci 110 :21 –35,2006 [DOI] [PubMed] [Google Scholar]
- 35.Spring H, Schuler T, Arnold B, Hammerling GJ, Ganss R: Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci U S A 102 :18111 –18116,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christofori G, Naik P, Hanahan D: Vascular endothelial growth factor and its receptors, flt-1 and flk-1, are expressed in normal pancreatic islets and throughout islet cell tumorigenesis. Mol Endocrinol 9 :1760 –1770,1995 [DOI] [PubMed] [Google Scholar]
- 37.Contreras JL, Smyth CA, Eckstein C, Bilbao G, Thompson JA, Young CJ, Eckhoff DE: Peripheral mobilization of recipient bone marrow-derived endothelial progenitor cells enhances pancreatic islet revascularization and engraftment after intraportal transplantation. Surgery 134 :390 –398,2003 [DOI] [PubMed] [Google Scholar]
- 38.Hess D, Li L, Martin M, Hill D, Strutt B, Thyssen S, Gray DA, Bhatia M: Bone marrow-derived stem cells initiate pancreatic regeneration. Nat Biotechnol 21 :763 –770,2003 [DOI] [PubMed] [Google Scholar]
- 39.Zentilin L, Tafuro S, Zacchigna S, Arsic N, Pattarini L, Sinigaglia M, Giacca M: Bone marrow mononuclear cells are recruited to the sites of VEGF-induced neovascularization but are not incorporated into the newly formed vessels. Blood 107 :3546 –3554,2006 [DOI] [PubMed] [Google Scholar]
- 40.Aghi M, Cohen KS, Klein RJ, Scadden DT, Chiocca EA: Tumor stromal-derived factor-1 recruits vascular progenitors to mitotic neovasculature, where microenvironment influences their differentiated phenotypes. Cancer Res 66 :9054 –9064,2006 [DOI] [PubMed] [Google Scholar]
- 41.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner G: Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10 :858 –864,2004 [DOI] [PubMed] [Google Scholar]
- 42.Sassetti C, Van Zante A, Rosen SD: Identification of endoglycan, a member of the CD34/podocalyxin family of sialomucins. J Biol Chem 275 :9001 –9010,2000 [DOI] [PubMed] [Google Scholar]
- 43.Nozawa H, Chiu C, Hanahan D: Infiltrating neutrophils mediate the initial angiogenic switch in a mouse model of multistage carcinogenesis. Proc Natl Acad Sci U S A 103 :12493 –12498,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ishida S, Yamashiro K, Usui T, Kaji Y, Ogura Y, Hida T, Honda Y, Oguchi Y, Adamis AP: Leukocytes mediate retinal vascular remodeling during development and vaso-obliteration in disease. Nat Med 9 :781 –788,2003 [DOI] [PubMed] [Google Scholar]
- 45.Stahl GL, Xu Y, Hao L, Miller M, Buras JA, Fung M, Zhao H: Role for the alternative complement pathway in ischemia/reperfusion injury. Am J Pathol 162 :449 –455,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bernardi R, Guernah I, Jin D, Grisendi S, Alimonti A, Teruya-Feldstein J, Cordon-Cardo C, Simon MC, Rafii S, Pandolfi PP: PML inhibits HIF-1alpha translation and neoangiogenesis through repression of mTOR. Nature 442 :779 –785,2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.