Abstract
We reported previously that a conformation-specific antibody, Ab P2, to a 16-amino acid peptide (Glu-Gly-Tyr-Lys-Lys-Lys-Tyr-Gln-Gln-Val-Asp-Glu-Glu-Phe-Leu-Arg) of the cytoplasmic domain of the β-type platelet-derived growth factor receptor also recognizes the epidermal growth factor (EGF) receptor. Although the antibody is not directed to phosphotyrosine, it recognizes in immunoprecipitation the activated and hence phosphorylated form of both receptors. In P2 peptide, there are two tripeptide sequences, Asp-Glu-Glu and Tyr-Gln-Gln, that are also present in the EGF receptor. Our present studies using either EGF receptor C-terminal deletion mutants or point mutations (Tyr→Phe) and our previous studies on antibody inhibition by P2-derived peptides suggest that Gln-Gln in combination with Asp-Glu-Glu forms a high-affinity complex with Ab P2 and that such complex formation is dependent on tyrosine phosphorylation. Of the five phosphate acceptor sites in the EGF receptor, clustered in the extreme C-terminal tail, phosphorylation of three tyrosine residues (992, 1068, and 1086) located between Asp-Glu-Glu and Gln-Gln is necessary for Ab P2 binding. In contrast, the acceptor sites Tyr 1173 and 1148 play no role in the conformation change. Asp-Glu-Glu and Gln-Gln are located 169 amino acids apart, and it is highly likely that the interactions among three negatively charged phosphotyrosine residues in the receptor C terminus may result in the bending of the peptide chain in such a way that these two peptides come close to each other to form an antibody-binding site. Such a possibility is also supported by our finding that receptor dephosphorylation results in complete loss of Ab P2–binding activity. In conclusion, we have identified a domain within the cytoplasmic part of the EGF receptor whose conformation is altered by receptor phosphorylation; furthermore, we have identified the tyrosine residues that positively regulate this conformation.
INTRODUCTION
Receptor tyrosine kinases are multisited and multifunctional proteins with similar structural features that include a single hydrophobic transmembrane region of 20–25 amino acids that separates the large extracellular domain from the cytoplasmic region. The exoplasmic domain contains the ligand-binding site, whereas the intracellular domain contains the tyrosine kinase domain and the C-terminal tail that are important for signal transduction. The ligand-induced receptor activation results in the phosphorylation of its own tyrosine residues (autophosphorylation) as well as other intracellular substrates. Tyrosine autophosphorylation regulates the biological activity of the receptor by influencing its kinase activity and also by creating binding sites for several signal transduction molecules (reviewed in Ullrich and Schlessinger, 1990; Fantl et al., 1993). In the human epidermal growth factor (EGF)1 receptor (Mr 170,000), a glycoprotein of 1186 amino acids, three major autophosphorylation sites (Tyr 1068, 1148, and 1173), and two minor sites (Tyr 992 and 1086) have been identified (Downward et al., 1984; Hsuan et al., 1989; Margolis et al., 1989; Walton et al., 1990). These sites are clustered in the last 194 amino acids in the C-terminal tail of the receptor. In addition to being docking sites for Src homology-2 domain–containing proteins involved in signal transduction, the EGF receptor C-terminal tail is also important in receptor internalization, down-regulation, and endocytosis (Sorkin et al., 1992, 1996; Miloso et al., 1995; Nesterov et al., 1995). Furthermore, studies with deletion mutants lacking all five autophosphorylation sites indicate that the phosphorylated tyrosines at the extreme C terminus positively regulate biological and transforming activities of the EGF receptor (Helin et al., 1991).
We have reported previously the generation of a conformation-specific polyclonal antibody directed to an intracellular domain (amino acid residues 964–979; Glu-Gly-Tyr-Lys-Lys-Lys-Tyr-Gln-Gln-Val-Asp-Glu-Glu-Phe-Leu-Arg) of the 180-kDa β-type platelet-derived growth factor (PDGF) receptor (Bishayee et al., 1988). Although this antibody, Ab P2, is directed to an unphosphorylated peptide epitope, it recognizes in immunoprecipitation only the phosphorylated receptor. Our recent studies revealed that, in addition to PDGF receptor, Ab P2 also binds to the EGF receptor, and interestingly, its recognition is also phosphorylation dependent (Panneerselvam et al., 1995b). Although the immunoprecipitation of both receptors is inhibited by P2 peptide, no such inhibition is observed with phenyl phosphate, an analog of phosphotyrosine, suggesting that the antibody recognizes the phosphorylated protein and not phosphotyrosine itself. This indicates that the antibody-binding site is probably cryptic in nonactivated PDGF and EGF receptors and that receptor autophosphorylation uncovers this site. This implies a phosphorylation-induced conformational change of both receptors. In the P2 peptide, there are two sequences, Asp-Glu-Glu and Tyr-Gln-Gln, that are also present in the cytoplasmic domain of the EGF receptor at 979–981 and 1148–1150, respectively (Panneerselvam et al., 1995b). Thus, the cross-reactivity of Ab P2 with the phosphorylated EGF receptor is caused by the presence of either one or both of the tripeptides.
Considering that phosphorylation induces conformational changes in the intracellular domain of the EGF receptor and the receptor C-terminal tail plays a significant role in receptor functions, we investigated the role of individual phosphate acceptor sites in the regulation of this conformation. Using Tyr→Phe substitution mutants, we report here that phosphorylation of Tyr 992, 1068, and 1086 that are located between the tripeptides Asp-Glu-Glu and Tyr-Gln-Gln is highly critical in conformational change of the receptor as determined by Ab P2 binding; however, Tyr 1148 that is part of one of the tripeptides and Tyr 1173 play no role in the conformational change. We also report that, in addition to Asp-Glu-Glu that is located 21 amino acids downstream of the kinase domain of the receptor, Gln-Gln (amino acids 1149–1150) is involved in Ab P2 binding. This implies that two separate amino acid sequences in the EGF receptor interrupted by a span of >100 amino acids are brought closer to each other by phosphorylation of Tyr 992, 1068, and 1086.
MATERIALS AND METHODS
Materials
EGF was purified from mouse submaxillary glands and radiolabeled with 125I by the chloramine-T procedure (Das et al., 1984). Solid-phase EGF was prepared by coupling EGF to Affi-Gel 15 (Bio-Rad, Richmond, CA) (Cohen et al., 1980). Highly purified alkaline phosphatase coupled to agarose was purchased from Sigma Chemical (St. Louis, MO). Labeled ATP was prepared with 32Pi and the γ-Prep A kit (Promega, Madison, WI) according to the manufacturer’s directions. Specific radioactivity of [γ-32P]ATP was adjusted by adding unlabeled ATP (Sigma Chemical, St. Louis, MO) (Bishayee et al., 1986). Tran 35S-label was obtained from ICN Biomedical (Costa Mesa, CA).
EGF Receptor Mutants and Cell Culture
Human EGF receptor mutants were obtained using site-directed mutagenesis to substitute tyrosine residues with phenylalanine or to delete the coding sequence for the C-terminal deletion mutant Dc63. The generation and characterization of these mutants have been described previously (Velu et al., 1989; Helin et al., 1991; Sorkin et al., 1992; Soler et al., 1993). These human EGF receptor mutants were expressed in murine NIH 3T3 cells expressing ∼2500 endogenous EGF receptors. Receptor sites per cell were determined by 125I-EGF–binding assay. Briefly, 0.7 ng of 125I-EGF (3 × 105 cpm) in a total volume of 100 μl of Earle’s balanced salt solution containing 20 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), pH 7.5, 2.5 mg/ml bovine serum albumin, and unlabeled EGF (25 ng/ml for cells expressing <1 × 105 receptor sites per cell and 100 ng/ml for cells expressing >1 × 105 receptor sites per cell) were incubated at 20°C for 90 min with cells grown in 1-cm2 48-well plates. Nonspecific binding, which was 5–10% of the total binding, was measured by incubating the cells with labeled EGF in the presence of 200 nM unlabeled EGF. The EGF-binding sites/cell in different mutants are shown in Table 1. Transfected cells, except wild-type cells, were grown in DMEM containing 10% newborn calf serum, penicillin–streptomycin, and G418 (0.25–0.5 mg/ml); cells expressing wild-type receptor as well as parental NIH 3T3 cells were grown in the absence of G418. The human epidermoid carcinoma cells A431 were grown in DMEM containing 10% fetal bovine serum. Plasma membranes from these cells were prepared as described (Bishayee et al., 1986).
Table 1.
EGF receptor mutants used in Ab P2 binding studies
| Cell type | Receptor type | Number of receptors per cell (×105) |
|---|---|---|
| NIH3T3 | Untransfected | 0.025 |
| CL17 | Wild-type | 1.41 |
| MI31 | Y1173F | 0.56 |
| MI32 | Y1148F | 2.49 |
| MI33 | Y(1173-1148)F | 0.70 |
| MI34 | Y(1173-1148-1068)F | 0.77 |
| MI35 | Y(1148-1068)F | 0.25 |
| MI36 | Y(1173-1068)F | 0.45 |
| MI37 | Y1068F | 1.85 |
| MI38 | Y1086F | 0.25 |
| MI40 | Y992F | 0.61 |
| MI41 | Y(1173-1148-1086-1068-992)F | 0.50 |
| Dc63 | C-terminal deletion of 63 amino acids lacking tyrosine 1173 and 1148 | 0.39 |
The receptor sites per cell were determined by 125I-EGF binding according to the method described in MATERIALS AND METHODS. The amino acids are identified by their single letter codes: F for Phe, Y for Tyr.
Antibodies
Anti-peptide antibody Ab P2 is directed to amino acid residues 964–979 (Glu-Gly-Tyr-Lys-Lys-Lys-Tyr-Gln-Gln-Val-Asp-Glu-Glu-Phe-Leu-Arg) of the cytoplasmic domain of the human β-type PDGF receptor. This antibody recognizes human and murine PDGF receptor in immunoprecipitation and Western blotting (Bishayee et al., 1988). It was generated in rabbits using high-pressure liquid chromatography (HPLC)-purified peptide according to the method described previously (Bishayee et al., 1988). The monoclonal antibody (mAb) 425 raised against human A431 carcinoma cells and polyclonal antibody to denatured EGF receptor were gifts from Dr. M. Das and were developed as described (Murthy et al., 1986, 1987). mAb 425 is directed to a peptide chain of the extracellular domain of the human EGF receptor and recognizes only the native human receptor. The mouse monoclonal anti-phosphotyrosine antibody 1G2, used for purification of the tyrosine-phosphorylated proteins, was generated as described and coupled to activated sepharose (Bishayee et al., 1986).
Quantification of the 32P-labeled EGF Receptor
Isolated membranes from NIH 3T3 cells expressing different EGF receptor mutants were phosphorylated with [γ-32P]ATP in the presence of EGF under autophosphorylation conditions (Panneerselvam et al., 1995a,b). An aliquot of the labeled proteins purified by anti-phosphotyrosine monoclonal antibody (1G2) was subjected to SDS-PAGE. After autoradiography, the region of the dried gel corresponding to the EGF receptor band was cut out and counted using scintillation fluid. Adjacent regions of the dried gel were also counted to determine the background radioactivity, and this was subtracted from the count obtained with the EGF receptor band. Based on the specific radioactivity of [32P]ATP, moles of 32P (expressed in femtomoles) incorporated into the receptor were determined. The amount of receptor protein was then calculated by dividing the moles of 32P incorporated by the number of acceptor tyrosine residues (five for the wild-type receptor). This quantification is based on the assumption that there is no incorporation of 32P into Ser or Thr residues of the EGF receptor. The basis for such an assumption is that receptor phosphorylation was performed in the presence of EGF and the labeled receptor was purified by anti-phosphotyrosine antibody. As a result, it is expected that 32P is incorporated only into tyrosine and not on Ser or Thr residues of the receptor. This assumption appears to be valid because no 32P-labeled human EGF receptor could be detected in MI41, a murine NIH 3T3 cell line expressing a human EGF receptor mutant in which all five acceptor tyrosine residues have been substituted with phenylalanine (Soler et al., 1993).
Biosynthetic Labeling of the EGF Receptor
Twenty hours after subculturing, cell monolayers were washed with methionine- and cysteine-free DMEM containing 2% dialyzed newborn calf serum and were preincubated in the same medium at 37°C for 1 h. The cells were then incubated at 37°C for 10 h with Tran 35S-label (100 μCi/ml, 1190 Ci/mmol) in methionine- and cysteine-free DMEM with 2% dialyzed newborn calf serum. For labeling human epidermoid A431 carcinoma cells, 2% dialyzed fetal bovine serum was used in place of newborn calf serum. The labeled cells were washed three times with 20 mM HEPES, pH 7.4, containing 0.15 M NaCl and then were solubilized with 1% Nonidet P-40 (NP-40) in 20 mM HEPES, pH 7.4, 0.15 M NaCl, 10% glycerol, and protease inhibitors (aprotinin, leupeptin, and phenylmethylsulfonyl fluoride). An aliquot of the clarified supernatant obtained after centrifugation was phosphorylated with unlabeled ATP in the presence of 1 μM EGF under autophosphorylation conditions as described (Panneerselvam et al., 1995a,b), and the tyrosine-phosphorylated–labeled receptor was purified by the anti-phosphotyrosine monoclonal antibody 1G2.
Immunoprecipitation Technique
This was performed as described with some modifications (Panneerselvam et al., 1995b). Briefly, the 32P- or 35S-labeled receptor preparation was incubated with the indicated antibody at 4°C overnight in 15 μl (unless otherwise indicated) of 20 mM HEPES, pH 7.4, 0.15 M NaCl, 0.2% NP-40, 2.5 mg/ml bovine serum albumin, 1 mM vanadate, protease inhibitors, and 40 mM phenyl phosphate. The immune complexes were isolated by incubating the mixture at 4°C for 1 h with formaldehyde-fixed Staphylococcus aureus. After the bacterial pellets were washed to remove unbound radioactivity, the bound radioactivity was eluted by boiling the pellets with SDS-sample buffer and then subjected to SDS-PAGE (7% gel unless otherwise indicated). The gels containing the 32P-labeled receptors were dried and subjected to autoradiography at −80°C with Kodak (Rochester, NY) X-Omat AR film and Dupont (Wilmington, DE) intensifying screens. The gels containing 35S-labeled proteins were prepared for fluorography by immersion in acetic acid containing 2,5-diphenyloxazole, washed in water, dried, and exposed to x-ray films (Bishayee et al., 1988).
Phosphopeptide Analysis
For phosphopeptide mapping, 32P-labeled EGF receptor was purified by EGF–Affi-Gel chromatography (Biswas et al., 1985). Briefly, isolated membranes from cells expressing EGF receptors were solubilized with 1% NP-40 in 20 mM HEPES, pH 7.4, 0.15 M NaCl, 10% glycerol, and protease inhibitors (aprotinin, leupeptin, and phenylmethylsulfonyl fluoride). After centrifugation, the clarified supernatant was incubated at 4°C for 2 h with EGF–Affi-Gel (1 mg/ml). To remove the unbound proteins, we washed the gel beads three times with the binding buffer, and then the beads were incubated with [32P]ATP under autophosphorylation conditions (Panneerselvam et al., 1995a,b). After the gel beads were washed to remove free ATP, the bound EGF receptor was dissociated by heating with SDS-sample buffer and subjected to SDS-PAGE. After incubation overnight in fixing solution (25% methanol and 10% acetic acid in water), the wet gel was exposed to x-ray film. The region of the gel corresponding to the EGF receptor band was excised, soaked in 10% methanol for 2 h, and lyophilized. The dried gel was then digested with 0.5 ml of sequencing grade trypsin (20 μg/ml; Boehringer Mannheim, Indianapolis, IN) in 50 mM ammonium bicarbonate, pH 7.8. After 20 h at 37°C, fresh trypsin was added, and incubation was continued for another 20 h at 37°C. After centrifugation, the clear supernatant was dried in vacuum and dissolved in 0.1% trifluoroacetic acid in water. Equal counts of phosphopeptides derived from EGF receptor mutants were then subjected to reverse phase HPLC analysis using a DeltaPak 6μ C18 column (column size: 3.9 × 150 mm; Waters Associates, Milford, MA). Briefly, after injection, the column was washed with 10 ml of 0.1% trifluoroacetic acid in water, and then the phosphopeptides were eluted with a 0–60% acetonitrile gradient containing 0.1% trifluoroacetic acid with a flow rate of 1 ml/min (Margolis et al., 1989; Walton et al., 1990). Fractions (0.5 ml) were collected, and the Cerenkov counts were determined.
RESULTS
Phosphorylation-induced Conformational Change of the EGF Receptor Is Reversible
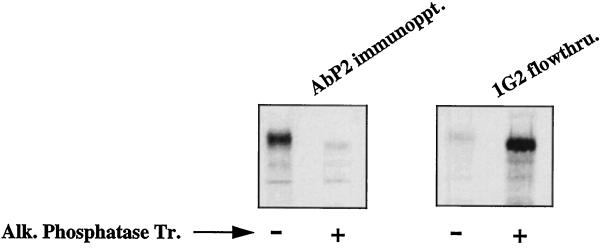
Our previous studies with Ab P2, a polyclonal antibody to a 16-amino acid unphosphorylated peptide epitope of the β-type PDGF receptor, indicated that it recognizes only the tyrosine-phosphorylated form of PDGF and EGF receptors (Bishayee et al., 1988; Panneerselvam et al., 1995b). However, the antibody is not directed to phosphotyrosine. This suggests that autophosphorylation induces a conformational change in the receptor, thus unmasking the antibody recognition site. It remains to be determined whether the phosphorylation-induced conformational change is permanent or the receptor reverts back to its original conformation after the phosphate groups are removed. In the interaction between phosphorylated receptor and Src homology-2–containing proteins, the removal of the phosphate(s) from the receptor or the phosphorylation of the substrate by the activated kinase results in the dissociation of the complex. Furthermore, the continuous presence of a ligand is required to maintain the receptor kinase in the active form. These results suggest that phosphorylation-induced conformational change is probably a reversible process. To test this possibility, we phosphorylated the 35S-labeled EGF receptor from the human epidermoid carcinoma cells A431 with unlabeled ATP in the presence of EGF and then purified the receptor by anti-phosphotyrosine antibody followed by wheat germ agglutinin. The purified receptor was either treated with solid-phase alkaline phosphatase or left untreated and then subjected to immunoprecipitation with Ab P2. As shown in Figure 1, alkaline phosphatase treatment resulted in the complete loss of Ab P2–binding activity of the receptor. Figure 1 (right) shows that alkaline phosphatase treatment indeed resulted in the removal of the phosphate groups from the receptor because the enzyme-treated receptor and not the untreated receptor failed to bind to the anti-phosphotyrosine antibody. The slight difference in the mobility of the EGF receptor band between the control and the alkaline phosphatase–treated samples is attributable to the fact that phosphorylated protein has slower mobility compared with that of unphosphorylated protein.
Figure 1.
The phosphorylation-induced conformational change is reversible. A431 cells were labeled with Tran 35S-label for 10 h. The EGF receptors in the detergent-solubilized cell lysates were phosphorylated with unlabeled ATP in the presence of 1 μM EGF as described in MATERIALS AND METHODS. After purification of the EGF receptors by anti-phosphotyrosine monoclonal antibody (1G2), the receptors were allowed to bind with wheat germ agglutinin–agarose. The bound receptor was eluted from the beads by 0.4 M N-acetylglucosamine in 20 mM HEPES, pH 7.4, 0.15 M NaCl, 0.2% NP-40, and protease inhibitors. An aliquot of the purified receptor was left untreated (−) or treated with alkaline phosphatase (50 units/ml) (+) coupled to agarose in the presence of 1 mg/ml bovine serum albumin. After incubation at 4°C for 1 h, both samples were centrifuged, and the supernatants were subjected to immunoprecipitation in the presence of 1 mM vanadate with Ab P2 (AbP2 immunoppt.; left) or anti-phosphotyrosine antibody 1G2 (right). 1G2 flowthru. denotes the labeled receptor that did not bind to 1G2. Alk. Phosphatase Tr., Alkaline phosphatase treatment.
Specific Tyrosine Residues Positively Regulate the Conformation of the Ab P2–binding Site
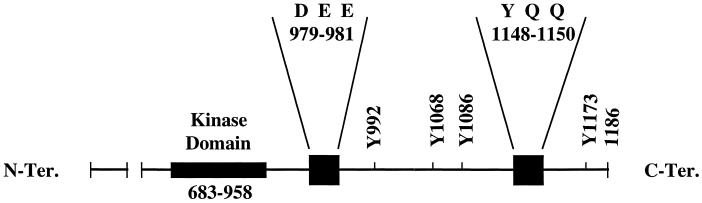
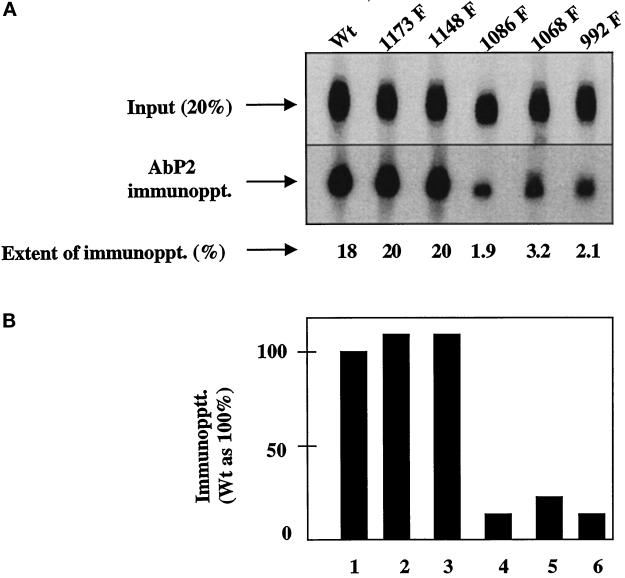
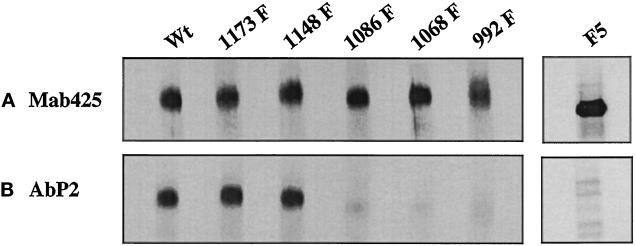
The antigenic peptide P2 that was used to develop the conformation-specific antibody (Ab P2) contains two tripeptide sequences (Tyr-Gln-Gln and Asp-Glu-Glu) that are also present in the cytoplasmic domain of the EGF receptor. Tyr-Gln-Gln and Asp-Glu-Glu are located in amino acid residues 1148–1150 and 979–981 of the human EGF receptor, respectively (Ullrich et al., 1984). Asp-Glu-Glu is 21 amino acids downstream of the kinase domain of the receptor. All five phosphate acceptor sites in the EGF receptor are clustered at the extreme C-terminal 124 amino acids. Three of these acceptor sites (Tyr 992, 1068, and 1086) are located between the two tripeptide sequences, whereas a fourth one (Tyr 1148) is part of one of the two tripeptide sequences. The locations of the tripeptides and the autophosphorylation sites with respect to the kinase domain are shown in Figure 2. We investigated whether phosphorylation of a specific tyrosine residue has any influence on the conformation of the Ab P2 recognition site. In this experiment, equal amounts of anti-phosphotyrosine monoclonal antibody–purified 32P-labeled EGF receptors from the wild-type and single-point mutant acceptors were subjected to immunoprecipitation with Ab P2. As shown in Figure 3, substitution of tyrosines at 1173 or 1148 had no effect on the conformation of the antibody recognition site because there is no change in the extent of immunoprecipitation of the mutant receptors compared with that of the wild type. However, substitution of any of the other three autophosphorylation sites resulted in a drastic reduction in immunoprecipitation by 80–90%.
Figure 2.
Locations of the phosphate acceptor sites and the tripeptides (Asp-Glu-Glu and Tyr-Gln-Gln) with respect to the kinase domain of the human EGF receptor. The amino acids are identified by their single-letter codes: D for Asp, E for Glu, Q for Gln, and Y for Tyr. C-Ter., C-terminal; N-Ter., N-terminal.
Figure 3.
Single Tyr→Phe substitution at 992, 1068, or 1086 drastically reduces the binding of Ab P2 to the 32P-labeled EGF receptor. Detergent-solubilized membranes from the wild type (Wt) or the single Y→F EGF receptor mutants were phosphorylated with labeled ATP (specific radioactivity of 350 cpm/fmol) in the presence of 1 μM EGF. After purification of the labeled receptor by 1G2–Sepharose, the 32P-labeled receptor was quantified as described in MATERIALS AND METHODS. For immunoprecipitation, 1.25 fmol of the EGF receptor was incubated with 5 μg of protein A–purified Ab P2 in a total volume of 15 μl under conditions described in MATERIALS AND METHODS. After isolation of the immune complexes with formaldehyde-fixed S. aureus, the labeled proteins were analyzed by SDS-PAGE and autoradiography, and the region containing the 170-kDa EGF receptor band was densitometrically scanned. (A) Top, 20% of labeled samples that have not been subjected to immunoprecipitation [Input (20%)]. Middle, the EGF receptor bands after immunoprecipitation with Ab P2 (AbP2 immunoppt.). Bottom, the extent of immunoprecipitation. (B) The results of the immunoprecipitation relative to that of the wild-type receptor.
In quantifying the 32P-labeled EGF receptor used in the above experiment, we assumed that all five acceptor tyrosines are equally phosphorylated. However, three of the five tyrosines in the EGF receptor are major autophosphorylation sites, whereas the other two are minor sites (Downward et al., 1984; Hsuan et al., 1989; Margolis et al., 1989; Walton et al., 1990) (also see Figure 5). This raises the possibility that the difference in the immunoprecipitation pattern seen in Figure 3 might be attributable to the use of different amounts of the EGF receptor. To eliminate such a possibility, we investigated the binding of Ab P2 with the 35S-labeled EGF receptor of single mutants. Detergent-solubilized lysates from 35S-labeled cells were phosphorylated with unlabeled ATP in the presence of EGF, and tyrosine-phosphorylated receptor was purified by anti-phosphotyrosine monoclonal antibody. Because different EGF receptor levels are expressed in the transfected cells (see Table 1), the receptor concentration was normalized by immunoprecipitation with an anti-EGF receptor monoclonal antibody, mAb 425, directed against a peptide epitope in the extracellular domain of the human EGF receptor (Figure 4A). In a parallel set of 35S-labeled proteins containing equal amounts of the EGF receptor, immunoprecipitation was performed with Ab P2. As with the 32P-labeled receptor, significantly decreased binding of the EGF receptor mutant in which Tyr 992, 1068, or 1086 was mutated into phenylalanine could be seen with Ab P2, whereas the substitution of Tyr 1148 or 1173 had no effect on the antibody binding (Figure 4B). We also tested the ability of Ab P2 to bind to the EGF receptor F5 mutant in which all five phosphate acceptor sites were mutated to phenylalanine (MI41) (Soler et al., 1993). For this purpose, the 35S-labeled cell lysates were incubated with unlabeled ATP in the presence of EGF, and the receptor was purified by wheat germ agglutinin and subjected to immunoprecipitation by the EGF receptor–specific antibody mAb 425 and by Ab P2. Although a very intense 170-kDa band could be seen in the mAb 425 immunoprecipitate (Figure 4A), no such band could be detected when the immunoprecipitation was performed with Ab P2 (Figure 4B). The faint bands that are visible in the Ab P2 immunoprecipitate were also seen when the receptor preparation was incubated with nonimmune serum (our unpublished results). This suggests that the F5 mutant lacking all five tyrosine acceptor sites is not recognized by Ab P2.
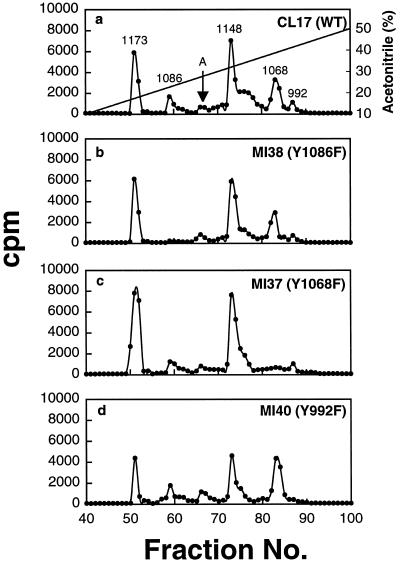
Figure 5.
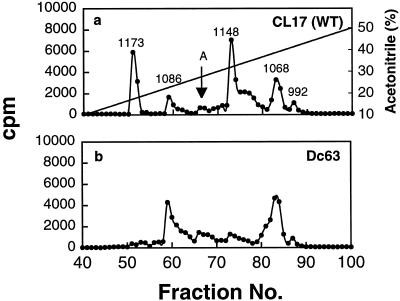
Phosphopeptide maps of the EGF receptors after trypsin digestion. (a–d) Receptors bound to EGF–Affi-Gel 15 were phosphorylated with [γ-32P]ATP under autophosphorylation conditions. After dissociation from the gel beads by heating with SDS-sample buffer, the 32P-labeled receptors were subjected to SDS-PAGE and digested twice with 20 μg of trypsin, and then 50,000 cpm were analyzed by a reverse phase HPLC C18 column as described in MATERIALS AND METHODS. Fractions (0.5 ml) were collected, and Cerenkov counts were determined. The arrow labeled A indicates the unidentified peak eluted between Tyr 1068 and 1148. WT, wild type.
Figure 4.
Interaction of Ab P2 with 35S-labeled EGF receptor is dependent on the phosphorylation of Tyr 992, 1068, and 1086. 35S-labeled EGF receptors from the wild type and the single Y→F substitution mutants were phosphorylated with unlabeled ATP in the presence of EGF, and the labeled receptors were purified with 1G2–Sepharose. (A) The relative concentrations of the EGF receptor in different cell types were quantified by precipitation with mAb 425 (Mab425), a monoclonal antibody to an external peptide epitope of the receptor. (B) Equal amounts of the EGF receptor from different mutants as shown in A were subjected to immunoprecipitation with Ab P2 (AbP2) followed by SDS-PAGE (3.5–10%) and fluorography. In a separate experiment, 35S-labeled cell lysates from the EGF receptor F5 mutant in which all five known phosphate acceptor sites were mutated to Phe were incubated with unlabeled ATP in the presence of EGF, and the labeled receptors were purified with wheat germ agglutinin. The purified receptor preparation was immunoprecipitated with mAb 425, Ab P2, or nonimmune serum and was analyzed by SDS-PAGE as described above.
Because the phosphorylation patterns of the mutants used in our studies were not characterized previously, we considered the possibility that single Tyr→Phe substitution at 992, 1068, or 1086 adversely affects the phosphorylation of the other two tyrosine residues. If this is the case, then the loss of antibody-binding activity of a single substitution mutant might be attributable to the lack of phosphorylation of not just one but all three tyrosine residues. To investigate such a possibility, we compared the HPLC elution profiles of the phosphopeptides from the mutant receptors with that of the wild-type EGF receptor. As shown in Figure 5a, six distinct phosphopeptide peaks could be detected with wild-type EGF receptor. On the basis of the elution characteristics reported by others (Downward et al., 1984; Hsuan et al., 1989; Margolis et al., 1989; Walton et al., 1990), five of the six peaks have been identified, and these are labeled by their tyrosine acceptor sites. Our peptide analysis confirmed previous reports that Tyr 1148 and 1173 are the major phosphate acceptor sites, whereas Tyr 1086 and 992 are the minor sites. Out of the total radioactivity present in these five peptides, 38% is in Tyr 1148, 26% is in Tyr 1173, 20% is in Tyr 1068, 10% is in Tyr 1086, and 6% is in Tyr 992. However, the identity of the sixth peak eluted between Tyr 1086 and Tyr 1148 and indicated by an arrow remains to be determined. The generation of this peptide is not caused by incomplete proteolysis of the receptor because exhaustive redigestion of this peptide with trypsin did not result in the loss of this peak (our unpublished results). In addition, we have also consistently observed this peptide in all the point mutants. It should also be mentioned that we could not detect any phosphorylation of the EGF receptor in the F5 mutant in which all five known phosphorylation sites were substituted with Phe (MI41), suggesting that the phosphorylation of this unidentified site depends on the phosphorylation of other tyrosine acceptor sites (our unpublished results). As seen in Figure 5, b-d, when the peptides from the single Tyr→Phe substitution were analyzed, all the peaks except the peak corresponding to the mutated tyrosine could be detected, suggesting that a single Tyr→Phe substitution has no negative effect on the phosphorylation of the other tyrosine residues.
The results shown in Figures 3–5 suggest that 1) the two tyrosine residues located at the extreme C-terminal tail of the EGF receptor do not regulate the conformation of the Ab P2 recognition site and 2) the antibody binding requires phosphorylation of all three tyrosine residues at 992, 1068, and 1086 because substitution at any of these three tyrosine residues results in drastic reduction in immunoprecipitation. The latter conclusion is further substantiated by the fact that only ∼15% of the phosphorylated receptor from the MI34 triple mutant Y(1173-1148-1068)F with Tyr 992 and 1086 as phosphate acceptor sites (Helin et al., 1991) could bind to the antibody compared with the binding of the wild-type receptor (our unpublished results).
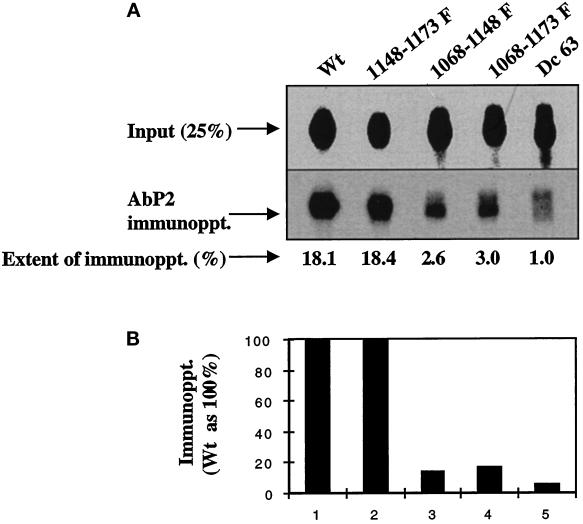
Because the results with the 32P- and 35S-labeled EGF receptors are similar, subsequent experiments were performed using the 32P-labeled receptors. Because the EGF receptor mutants MI31 (Y1173F) and MI32 (Y1148F) showed no change in antibody binding, we also investigated whether a double mutant (MI33) of the EGF receptor in which the tyrosines at 1173 and 1148 have been substituted with phenylalanine could be immunoprecipitated by the antibody. As shown in Figure 6, the extent of immunoprecipitation of the 32P-labeled EGF receptor from the double mutant Y(1173-1148)F was similar to that of the wild-type receptor, suggesting that these two tyrosines neither alone nor in combination play any role in antibody binding. On the other hand, there was an ∼85% reduction in the binding of the antibody with the receptor double mutants MI35 [Y(1148-1068)F] and MI36 [Y(1173-1068)F], confirming that phosphorylation of Tyr 1068 is important in regulating the conformation of the antibody-binding site.
Figure 6.
A deletion mutant with a truncation of the C-terminal 63 amino acids (Dc 63) does not bind to Ab P2. Anti-phosphotyrosine antibody (1.25 fmol; 1G2)-purified 32P-labeled EGF receptors from the wild-type or the mutated receptors were immunoprecipitated with 5 μg of Ab P2 under the conditions described in Figure 3. (A) Top, 25% of labeled samples that have not been subjected to immunoprecipitation [Input (25%)]. Middle, the EGF receptor bands after immunoprecipitation with Ab P2 (AbP2 immunoppt.). Bottom, the extent of immunoprecipitation. (B) The results of the immunoprecipitation relative to that of the wild-type receptor.
An EGF Receptor Mutant with Deletion of the C-Terminal 63 Amino Acids Does Not Bind to Ab P2
In addition to double mutants, we also tested whether Ab P2 could recognize an EGF receptor deletion mutant (Dc63) missing 63 amino acids from the extreme C-terminal tail and lacking both Tyr 1173 and 1148 (Velu et al., 1989). As shown in Figure 6 (lane 5), the antibody failed to recognize the 32P-labeled Dc63. Because Tyr 1173 and 1148 that are missing from the Dc63 mutant are not important in antibody binding (see above), such lack of recognition cannot be explained on the basis of the absence of these two tyrosines. This suggests that such lack of antibody binding is caused by the absence of a peptide sequence responsible for the interaction of the receptor with the antibody. We also considered an alternative possibility for the lack of antibody recognition by the Dc63 mutant. It is likely that because of the loss of 63 amino acids from the C-terminal tail of the receptor, the three remaining tyrosine acceptor sites in the deletion mutant are not phosphorylated as efficiently as are those in the wild-type receptor. To investigate such a possibility, we compared the phosphopeptide maps of the mutant with that of the wild-type receptor. As shown in Figure 7, out of five previously identified peaks in the wild-type receptor, only three peaks corresponding to Tyr 1086, 1068, and 992 could be observed in the mutant receptor. This eliminates the possibility that the loss of antibody-binding activity is caused by the failure of the phosphorylation of one or more of the three tyrosine residues at 992, 1068, and 1086 and points to the fact that an epitope responsible for the antibody binding is missing in the Dc63 mutant. It should be mentioned in this connection that the Dc63 mutant also lacks the tripeptide sequence Tyr-Gln-Gln (amino acid residues 1148–1150) that is part of the P2 peptide. This is further strengthened by our results showing that the loss of antibody binding is nearly complete with the deletion mutant, whereas residual 10–20% binding was consistently detected with other mutants (see Figures 3 and 6).
Figure 7.
The phosphopeptide maps of the tryptic digest from the EGF receptor deletion mutant Dc63. (a–d) The conditions of the experiment and the methods used are the same as those described in Figure 5.
DISCUSSION
Our results can be summarized as follows: 1) the phosphorylation-induced conformational change of the EGF receptor as detected by Ab P2 binding is reversible (Figure 1), 2) the two tyrosine residues at the extreme C-terminal tail of the receptor at amino acids 1173 and 1148 do not play any role in such conformational alteration, 3) the phosphorylation of all three tyrosines at 1086, 1068, and 992 is needed for the antibody to bind with the receptor, and 4) Gln-Gln (1149–1150 [Figure 6]) in combination with the tripeptide Asp-Glu-Glu (979–981 [Panneerselvam et al., 1995b]) forms the antibody-binding site in the EGF receptor.
On the basis of our antibody inhibition studies performed with different P2-derived peptides, we reported previously that the tripeptide Asp-Glu-Glu located near the kinase domain is responsible for the recognition of the EGF receptor by Ab P2 (Panneerselvam et al., 1995b). We showed that a short form of a peptide containing Tyr-Gln-Gln and missing Asp-Glu-Glu had no effect on the immunoprecipitation of the EGF receptor by Ab P2, whereas another peptide containing Asp-Glu-Glu and lacking Tyr-Gln-Gln (V-R peptide) could block the immunoprecipitation; however, a very high concentration of this peptide was needed for inhibition. We also demonstrated that, on the molar basis, the affinity of a K-R peptide that contains both Asp-Glu-Glu and Tyr-Gln-Gln was 10-fold higher compared with that of the V-R peptide (Panneerselvam et al., 1995b). These results rule out the possibility of two antigenic determinants and point to the fact that a single antibody-binding site comprised of both of these peptides creates a high-affinity complex with Ab P2. The involvement of Tyr-Gln-Gln in the recognition of Ab P2 is further supported by our studies with the deletion mutant Dc63. As shown in Figure 6, virtually no binding could be detected with the Dc63 mutant that lacks this tripeptide. It should be mentioned in this context that substitution of Tyr 1148 that is part of this tripeptide had no effect on the antibody binding (see Figures 3, 4, and 6), indicating that Gln-Gln and not Tyr-Gln-Gln in combination with Asp-Glu-Glu probably forms the antibody-binding site. Future studies with the EGF receptor mutant lacking only Gln-Gln or Asp-Glu-Glu will provide direct evidence of such a conclusion. In the PDGF receptor, these two peptides are separated by a single amino acid. However, in the human EGF receptor, these peptides are 169 amino acids apart. Thus, it is an open question how these two amino acid sequences in the EGF receptor come close to each other to form an antibody-binding site. Our present studies revealed that the antibody binding is highly dependent on the phosphorylation of all three tyrosine residues at 992, 1068, and 1086, located between these two peptides. Phosphorylation of even two (992 and 1086) out of the three tyrosines was not sufficient for antibody binding. This has been confirmed by studying the Ab P2–binding characteristics with an F3 mutant in which all three major autophosphorylation sites at 1173, 1148, and 1068 were substituted with phenylalanine (MI34); the extent of binding of the mutated receptor was 15% of that of the wild-type receptor (our unpublished results). Such phosphorylation-induced modification imparts very high negative charge to the peptide backbone. Thus, it is highly likely that the interactions among the negatively charged phosphotyrosine residues in the receptor molecule might result in the bending of the peptide chain surrounding these 169 amino acids in such a way that Gln-Gln and Asp-Glu-Glu come close to each other to form an antigenic determinant. The requirement for the phosphorylation of all three tyrosine residues lends further support for such a model. It will be of interest to investigate whether other modification of the tyrosine residues, such as nitration or sulfation, in the EGF receptor exerts a similar effect on Ab P2 binding. In the P2 peptide, Gln-Gln is the N terminus with respect to Asp-Glu-Glu, whereas the orientation is reversed in the EGF receptor, i.e., Gln-Gln is the C terminus. If the proper orientations of the peptides are obligatory for antibody recognition, then our model predicts that the conformational alteration of the receptor because of charge–charge interaction takes place in a highly ordered manner. The phosphorylation-induced bending not only brings Asp-Glu-Glu and Gln-Gln, which are 169 amino acids apart, closer to each other, it also results in changing the orientation of the peptides, Gln-Gln becoming the N terminus with respect to Asp-Glu-Glu as in the P2 peptide. Future studies with mutated EGF receptors with reverse orientation of the two peptides and lacking the intervening amino acids will help to address this issue.
We have consistently observed residual (10–20%) binding of F1 mutants (F992, F1068, and F1086) with the antibody. The reason for this binding activity is not clear at this time. However, it is possible that the receptor phosphorylated on the remaining sites might have weak affinity for the antibody. This possibility is strengthened by the fact that such residual binding could not be detected with an F5 mutant in which all five known acceptor sites were mutated to phenylalanine (MI41) (Figure 4).
Our phosphopeptide analysis revealed that only ∼6% of the EGF receptor population is phosphorylated on Tyr 992. This raises the question of how this minor phosphate acceptor site has such a profound effect on Ab P2 binding. Assuming that the fraction of the receptor population that is phosphorylated on Tyr 992 is also phosphorylated on all four other tyrosines, it is expected that not >30% (5 × 6%) of the 32P-labeled EGF receptor should be immunoprecipitated by a saturating concentration of the antibody. On the other hand, ∼18% of the labeled receptor from a population that is phosphorylated on two essential tyrosines (Tyr 1068 and 1086) along with Tyr 992 should bind to the antibody. As shown in Figures 3 and 6, 18–20% of the 32P-labeled receptor is capable of binding with the antibody. This finding suggests that the receptors that are phosphorylated on Tyr 992 are also phosphorylated at least on Tyr 1068 and 1086 and that the probability of the EGF receptor being phosphorylated only on Tyr 992 or on Tyr 992 together with Tyr 1148 and 1173 is very low.
Phosphorylation-induced conformational changes have been well documented with different receptor kinases. However, the susceptible epitopes and the tyrosine residue(s) involved in particular structural alteration mostly remain to be determined. In this respect, we have not only identified one such domain of at least 169 amino acids in the C-terminal tail of the EGF receptor but also identified the phosphate acceptor sites that are responsible for its conformational change. However, the significance of the phosphorylation-induced conformational change that we have observed on the receptor function and intracellular signaling remains to be elucidated. Because of the close proximity of the kinase domain to the phosphate acceptor sites, it is possible that such a conformational change may influence the kinase activity of the receptor. It should be mentioned in this context that the kinase activity of the triple mutant F3 (MI34) is much lower compared with that of the wild-type receptor (Helin and Beguinot, 1991; Helin et al., 1991; Sorkin et al., 1991). In addition, for another EGF receptor mutant, Dc123F, in which four tyrosines were deleted and the fifth (Tyr 992) was mutated to phenylalanine, the Vmax for kinase activity as determined by substrate phosphorylation was fourfold lower, and the Km for the substrate was threefold higher compared with that of the wild-type receptor (Alvarez et al., 1995). However, it is not known whether such a decrease in either the kinase activity or the Vmax is caused by the lack of phosphorylation of tyrosines that act as positive regulators of the Ab P2–binding site. Thus, it will be of interest to investigate the effect of Tyr→Phe substitution on the Vmax for autophosphorylation and for exogenous substrate phosphorylation. These studies are ongoing in the laboratory.
A number of anti-peptide antibodies that specifically recognize the activated receptor have been reported; however, all those antibodies are directed to phosphotyrosine-containing peptides (Campos-Gonzalez and Glenny, 1991; Bangalore et al., 1992; Epstein et al., 1992). In this respect, Ab P2 is one of the two conformation-specific anti-receptor antibodies directed to an unphosphorylated peptide that recognizes the activated receptor; the other anti-peptide antibody is directed to the insulin receptor (Herrera and Rosen, 1986). The importance of the conformation-specific antibodies as biological and diagnostic tools is underscored by a monoclonal antibody (40.10.09) to human placental uracil DNA glycosylase. This antibody recognizes both the native and the denatured forms of the enzyme in normal human cells; however, only the denatured form of the enzyme in Bloom’s syndrome patients is recognized by the antibody (Vollberg et al., 1987). Thus, this antibody that is capable of detecting a specific conformational abnormality of uracil DNA glycosylase in Bloom’s syndrome, an autosomal recessive human genetic disorder, has potential use in the early diagnosis of the disease. In this respect, because of the high-affinity interaction of Ab P2 with the EGF receptor phosphorylated on certain tyrosine residues, this antibody can also be used as a biological tool in studying the structure–function relationship of receptors and also in screening different EGF receptor mutants in which phosphorylation is affected. Furthermore, because this antibody recognizes the EGF receptor phosphorylated on specific tyrosine residues, Ab P2 has the potential as a diagnostic tool in detecting activated EGF receptors in human tumor biopsies.
ACKNOWLEDGMENTS
S.B. wishes to thank Dr. Stanley Cohen, University of Medicine and Dentistry of New Jersey-New Jersey Medical School, for his constant encouragement and support. This work was supported in part by a grant from the Foundation of the University of Medicine and Dentistry of New Jersey (to S.B.) and by grants from the Italian Association for Cancer Research and Consiglio Nazionale delle Ricerche (PF Biotecnologie) (to L.B.).
Abbreviations used:
- Ab
antibody
- EGF
epidermal growth factor
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- HPLC
high-pressure liquid chromatography
- mAb
monoclonal antibody
- NP-40
Nonidet P-40
- PDGF
platelet-derived growth factor
REFERENCES
- Alvarez CV, Shon K-J, Miloso M, Beguinot L. Structural requirements of the epidermal growth factor receptor for tyrosine phosphorylation of esp8 and esp15, substrates lacking Src SH2 homology domains. J Biol Chem. 1995;270:16271–16276. doi: 10.1074/jbc.270.27.16271. [DOI] [PubMed] [Google Scholar]
- Bangalore L, Tanner AJ, Laudano AP, Stern D. Antiserum raised against a synthetic phosphotyrosine-containing peptide selectively recognizes p185neu/erbB-2 and the epidermal growth factor receptor. Proc Natl Acad Sci USA. 1992;89:11637–11641. doi: 10.1073/pnas.89.23.11637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee S, Majumdar S, Scher CD, Khan S. Characterization of a novel anti-peptide antibody that recognizes a specific conformation of the platelet-derived growth factor receptor. Mol Cell Biol. 1988;8:3696–3702. doi: 10.1128/mcb.8.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishayee S, Ross AH, Womer R, Scher CD. Purified human platelet-derived growth factor receptor has ligand stimulated tyrosine kinase activity. Proc Natl Acad Sci USA. 1986;83:6756–6760. doi: 10.1073/pnas.83.18.6756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas R, Basu M, Sen-Majumdar A, Das M. Intrapeptide autophosphorylation of the epidermal growth factor receptor: regulation of kinase catalytic function by receptor dimerization. Biochemistry. 1985;24:3795–3802. doi: 10.1021/bi00335a056. [DOI] [PubMed] [Google Scholar]
- Campos-Gonzalez R, Glenny JR. Immunodetection of the ligand-activated receptor for epidermal growth factor. Growth Factors. 1991;4:305–316. doi: 10.3109/08977199109043916. [DOI] [PubMed] [Google Scholar]
- Cohen S, Carpenter G, King L., Jr Epidermal growth factor-receptor-protein kinase interactions: copurification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem. 1980;255:4834–4842. [PubMed] [Google Scholar]
- Das M, Biswas R, Knowles B, Bishayee S. Receptor modulating properties of an antibody directed against the epidermal growth factor (EGF)-receptor. Eur J Biochem. 1984;141:429–434. doi: 10.1111/j.1432-1033.1984.tb08209.x. [DOI] [PubMed] [Google Scholar]
- Downward J, Parker P, Waterfield MD. Autophosphorylation sites on the epidermal growth factor receptor. Nature. 1984;311:483–485. doi: 10.1038/311483a0. [DOI] [PubMed] [Google Scholar]
- Epstein RJ, Druker BJ, Roberts TM, Stiles CD. Synthetic phosphopeptide immunogens yield activation-specific antibodies to the c-ErbB-2 receptor. Proc Natl Acad Sci USA. 1992;89:10435–10439. doi: 10.1073/pnas.89.21.10435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantl WJ, Johnson DE, Williams LT. Signaling by receptor tyrosine kinases. Annu Rev Biochem. 1993;62:453–481. doi: 10.1146/annurev.bi.62.070193.002321. [DOI] [PubMed] [Google Scholar]
- Helin K, Beguinot L. Internalization and down-regulation of the human epidermal growth factor receptor are regulated by the carboxy-terminal tyrosines. J Biol Chem. 1991;266:8363–8368. [PubMed] [Google Scholar]
- Helin K, Velu T, Martin P, Vass WC, Allevato G, Lowy DR, Beguinot L. The biological activity of the human epidermal growth factor receptor is positively regulated by its C-terminal tyrosines. Oncogene. 1991;6:825–832. [PubMed] [Google Scholar]
- Herrera R, Rosen OM. Autophosphorylation of insulin receptor in vitro: designation of phosphorylation sites and correlation with receptor kinase activation. J Biol Chem. 1986;261:11980–11985. [PubMed] [Google Scholar]
- Hsuan JJ, Totty N, Waterfield MD. Identification of a novel autophosphorylation site (P4) on the epidermal growth factor receptor. Biochem J. 1989;262:659–663. doi: 10.1042/bj2620659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis BL, Lax I, Kris R, Dombalagian M, Honegger AM, Howk R, Givol D, Ullrich A, Schlessinger J. All autophosphorylation sites of epidermal growth factor (EGF) receptor and HER2/neu are located in their carboxyl-terminal tails: identification of a novel site in EGF receptor. J Biol Chem. 1989;264:10667–10671. [PubMed] [Google Scholar]
- Miloso M, Mazzotti M, Vass WC, Beguinot L. SHC and GRB-2 are constitutively activated by an epidermal growth factor receptor with a point mutation in the transmembrane domain. J Biol Chem. 1995;270:19557–19562. doi: 10.1074/jbc.270.33.19557. [DOI] [PubMed] [Google Scholar]
- Murthy U, Basu A, Rodeck U, Herlyn M, Ross AH, Das M. Binding of an antagonistic monoclonal antibody to intact and fragmented EGF receptor polypeptide. Arch Biochem Biophys. 1987;25:549–560. doi: 10.1016/0003-9861(87)90062-2. [DOI] [PubMed] [Google Scholar]
- Murthy U, Basu M, Sen-Majumder A, Das M. Perinuclear location and recycling of epidermal growth factor receptor. J Cell Biol. 1986;103:333–342. doi: 10.1083/jcb.103.2.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesterov A, Wiley HS, Gill GN. Ligand-induced endocytosis of epidermal growth factor receptors that are defective in binding adaptor proteins. Proc Natl Acad Sci USA. 1995;92:8719–8723. doi: 10.1073/pnas.92.19.8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panneerselvam K, Kanakaraj P, Raj S, Das M, Bishayee S. Characterization of novel epidermal-growth factor-receptor-related 200-kDa tyrosine kinase in tumor cells. Eur J Biochem. 1995a;230:951–957. doi: 10.1111/j.1432-1033.1995.tb20641.x. [DOI] [PubMed] [Google Scholar]
- Panneerselvam K, Reitz H, Khan SA, Bishayee S. A conformation-specific anti-peptide antibody to the β-type platelet-derived growth factor receptor also recognizes the activated epidermal growth factor receptor. J Biol Chem. 1995b;270:7975–7979. doi: 10.1074/jbc.270.14.7975. [DOI] [PubMed] [Google Scholar]
- Soler C, Beguinot L, Sorkin A, Carpenter G. Tyrosine phosphorylation of ras GTPase-activating protein does not require association with the epidermal growth factor receptor. J Biol Chem. 1993;268:21010–21019. [PubMed] [Google Scholar]
- Sorkin A, Helin K, Waters CM, Carpenter G, Beguinot L. Multiple autophosphorylation sites of the epidermal growth factor receptor are essential for receptor kinase activity and internalization: contrasting significance of tyrosine 992 in the native and truncated receptors. J Biol Chem. 1992;267:8672–8678. [PubMed] [Google Scholar]
- Sorkin A, Mazzotti M, Sorkina T, Scotto L, Beguinot L. Epidermal growth factor receptor interaction with clathrin adaptors is mediated by the Tyr974-containing internalization motif. J Biol Chem. 1996;271:13377–13384. doi: 10.1074/jbc.271.23.13377. [DOI] [PubMed] [Google Scholar]
- Sorkin A, Waters C, Overholser KA, Carpenter G. Multiple autophosphorylation site mutations of the epidermal growth factor receptor: analysis of kinase activity and endocytosis. J Biol Chem. 1991;266:8355–8362. [PubMed] [Google Scholar]
- Ullrich A, et al. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. Nature. 1984;309:418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Ullrich A, Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990;61:203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Velu TJ, Vass WC, Lowy DR, Beguinot L. Functional heterogeneity of proto-oncogene tyrosine kinases: the C terminus of the human epidermal growth factor receptor facilitates cell proliferation. Mol Cell Biol. 1989;9:1772–1778. doi: 10.1128/mcb.9.4.1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollberg TM, Seal G, Sirover MA. Monoclonal antibodies detect conformational abnormality of uracil DNA glycosylase in Bloom’s syndrome cells. Carcinogenesis. 1987;8:1725–1729. doi: 10.1093/carcin/8.11.1725. [DOI] [PubMed] [Google Scholar]
- Walton GM, Chen WS, Rosenfeld MG, Gill GN. Analysis of deletion of the carboxyl terminus of the epidermal growth factor reveals self-phosphorylation at tyrosine 992 and enhanced in vivo tyrosine phosphorylation of cell substrates. J Biol Chem. 1990;265:1750–1754. [PubMed] [Google Scholar]