Abstract
OBJECTIVE—Poor glycemic control, elevated triglycerides, and albuminuria are associated with vascular complications in diabetes. However, few studies have investigated combined associations between metabolic markers, diabetic kidney disease, retinopathy, hypertension, obesity, and mortality. Here, the goal was to reveal previously undetected association patterns between clinical diagnoses and biochemistry in the FinnDiane dataset.
RESEARCH DESIGN AND METHODS—At baseline, clinical records, serum, and 24-h urine samples of 2,173 men and 2,024 women with type 1 diabetes were collected. The data were analyzed by the self-organizing map, which is an unsupervised pattern recognition algorithm that produces a two-dimensional layout of the patients based on their multivariate biochemical profiles. At follow-up, the results were compared against all-cause mortality during 6.5 years (295 deaths).
RESULTS—The highest mortality was associated with advanced kidney disease. Other risk factors included 1) a profile of insulin resistance, abdominal obesity, high cholesterol, triglycerides, and low HDL2 cholesterol, and 2) high adiponectin and high LDL cholesterol for older patients. The highest population-adjusted risk of death was 10.1-fold (95% CI 7.3–13.1) for men and 10.7-fold (7.9–13.7) for women. Nonsignificant risk was observed for a profile with good glycemic control and high HDL2 cholesterol and for a low cholesterol profile with a short diabetes duration.
CONCLUSIONS—The self-organizing map analysis enabled detailed risk estimates, described the associations between known risk factors and complications, and uncovered statistical patterns difficult to detect by classical methods. The results also suggest that diabetes per se, without an adverse metabolic phenotype, does not contribute to increased mortality.
Patients with type 1 diabetes are susceptible to severe microvascular complications such as proliferative retinopathy and chronic kidney disease, which are often accompanied by cardiovascular disease and premature death (1,2). Currently, the risk assessment and diagnostics rely on the urine albumin excretion, serum creatinine, and lipid profile (3,4). In many cases, however, the biochemical measurements are treated as independent factors without explicit attention to the overall metabolic imbalance behind the complications. Although the risk factors for cardiovascular disease and diabetes complications have been verified statistically in large clinical studies (5–7), the overall picture on the mutual relationships and their relevance for risk assessment remains fragmented.
The metabolic syndrome (8) is one attempt to describe the co-occurrence of vascular complications and insulin resistance, but so far its applicability to type 1 diabetes and its exact definition remain controversial (9,10). Moreover, gradually developing conditions, such as cardiovascular disease, do not present a physiologically clear border between health and disease, so quantitative risk assessment tools are needed to augment and even replace discrete differential categorizations (11). Hence, we are developing new approaches to attain more accurate phenotypes without excessive cost, both through high-throughput analytics (12,13) and computational methods (14,15).
In this work, we develop an analysis framework based on the self-organizing map and statistically verified visualizations (13,16,17) for a large clinical study. Our goal is to characterize typical phenotypes (or metabolic profiles) that can be associated with high or low mortality during several years of follow-up. We do not question the role of albuminuria as the primary marker of increased risk, but we aim for a more comprehensive metabolic description of the conditions that manifest as albumin excretion. We show how numerous biochemical variables from serum and urine can be integrated under one statistical model while maintaining interpretability of the results, and we also demonstrate how nonlinear multivariate statistics can reveal complex phenotypes that are difficult to detect by classical methods.
RESEARCH DESIGN AND METHODS
Patients with type 1 diabetes were recruited by the Finnish Diabetic Nephropathy Study (FinnDiane), a nationwide multicenter effort to identify genetic and clinical risk factors for diabetic nephropathy. Diagnostic criteria for type 1 diabetes included age of onset <35 years and transition to insulin treatment within a year of onset. Four patients were excluded because of insufficient biochemical data. The design was cross-sectional (n = 4,197), but with longitudinal records of albuminuria and clinical events before baseline and with all-cause mortality data available after an average of 6.5 years of follow-up from baseline (25,714 patient-years). The study protocol was in accordance with the Declaration of Helsinki and approved by the local ethics committee in each of the participating centers.
Biochemical data came both from centrally organized measurements (90% of values) and from local healthcare centers and hospitals (10%). When both were available, the centrally measured value was used. The pattern of missing values was regular (online appendix 1, available at http://dx.doi.org/10.2337/db08-0332), but no significant sampling bias was detected.
Clinical definitions.
Data on medication, cardiovascular status, and diabetes complications were registered by a standardized questionnaire, which was completed by the patient's attending physician according to the medical file. Vitality was obtained from the national registry maintained by the Population Register Center of Finland.
The classification of renal status was made centrally according to urinary albumin excretion rate (AER) in at least two of three consecutive overnight or 24-h urine samples. Patients on renal replacement therapy (dialysis or transplantation) were classified as having end-stage renal disease (ESRD). Absence of nephropathy was defined as normoalbuminuria (AER <20 μg/min or <30 mg/24 h), whereas overt diabetic kidney disease (DKD) was obtained by pooling macroalbuminuria (AER ≥200 μg/min or ≥300 mg/24 h) and ESRD. The intermediary range was defined as microalbuminuria (20 ≤ AER < 200 μg/min or 30 ≤ AER < 300 mg/24 h). Timed albumin excretion from the latest 24-h urine collection for each patient was also available, and it was used as a biochemical variable in parallel with the longitudinal records of AER.
The metabolic syndrome was defined as a score of ≥3 according to the modified National Cholesterol Education Program Adult Treatment Panel III criteria (10,13,18), where every patient with type 1 diabetes has an initial score of 1 for hyperglycemia. Diabetic retinopathy (DRP) was defined as present if a patient had undergone laser treatment of the retina. Macrovascular disease was defined as a pooled end point of coronary heart disease, myocardial infarction, stroke, and peripheral vascular disease. Blood pressure was measured twice with 2-min intervals in the sitting position after a 10-min rest.
Biochemical measurements.
Serum lipid and lipoprotein concentrations were measured from fasting blood samples at the research laboratory of Helsinki University Central Hospital, Division of Cardiology (Helsinki). Total cholesterol and triglycerides were determined enzymatically using an autoanalyzer (Cobas Mira or Mira Plus; ABX Diagnostics, Montpellier, France). Total HDL and HDL3 cholesterol were determined enzymatically using an assay reader (HTS 7000 Plus Bio; Perkin Elmer, Wellesley, MA). HDL2 cholesterol was calculated by subtracting HDL3 cholesterol from total HDL cholesterol. LDL cholesterol was calculated according to the Friedewald formula. Serum apolipoprotein A-I, A-II, and B concentrations were determined by immunoassays (Orion Diagnostica, Espoo, Finland). Serum and 24-h urine creatinine (enzymatic), 24-h urine albumin (immunoturbidimetric), C-reactive protein (radioimmunoassay), and C-peptide (radioimmunoassay) were quantified at the Helsinki University Central Hospital Laboratory (Helsinki). Adiponectin and mannan binding lectin were measured as previously described (19,20). Twenty-four–hour urine urea (enzymatic), Na, and K (ion selective electrode) were measured on a Cobas Integra analyzer (Hoffmann-La Roche, Basel, Switzerland) by Medix Laboratories (Espoo, Finland). A1C was determined by standardized assays at local healthcare centers and hospitals.
Statistical analyses.
To characterize metabolic profiles and compare them with the current clinical classifications, a self-organizing map was constructed based on the biochemical variables only. Before analysis, variables that were highly correlated (e.g., 24-h urine creatinine and urea) were pruned if considered biologically redundant, which left 14 variables in the final training set (online appendix 1). The input data were rank-transformed (shifted and scaled rank indexes from −1 to 1) for both sexes separately to ensure that each variable had an identical value distribution irrespective of sex-specific differences in absolute values. Simultaneously, the impact of outliers and extreme values was attenuated. Missing values were ignored in the transformation but imputed from the preprocessed data by least-squares linear regression before analysis.
The self-organizing map is an unsupervised pattern recognition method (13,16) and was used here for automated comparisons between the multivariate biochemical profiles. The resulting map layout of patients becomes such that those who have similar profiles are as close to each other as possible, whereas those who have different profiles are placed far apart on the map (online appendix 2). Here, a 7 × 10 grid of hexagonal map units was chosen with a Gaussian neighborhood function (60 patients per unit). The self-organizing map algorithm was initialized based on the two first principal components of the input data and finished by the batch training procedure.
After the patients’ positions were computed, the map was colored according to the patients’ characteristics (clinical variables) within different regions (13). To verify that the results were statistically reliable, 10,000 random colorings were computed by permuting the data values to obtain empirical P values for each of the variables separately. The null distributions from the permutation analysis were also the basis of the color scale in each figure, so that the categorical and continuous variables, possibly with some data missing, could be compared visually while maintaining the statistical interpretation. In addition, 95% CIs were obtained by bootstrapping and are listed in the text when available. The aforementioned techniques were also applied to the biochemical variables, but because they were the inputs for the self-organizing map, the null hypothesis was no longer valid. The respective P values and CIs were thus omitted.
Relative risk of dying (Fig. 4) was estimated by first computing the colorings for age and for the frequency of deaths observed on each map unit and then adjusting the estimated mortality against the expected mortality in the respective age segment in the Finnish population, for men and women separately, during the follow-up period. The unadjusted death percentages and follow-up times are available in online appendix 3. The population history was collected by Statistics Finland (http://www.stat.fi).
FIG. 4.
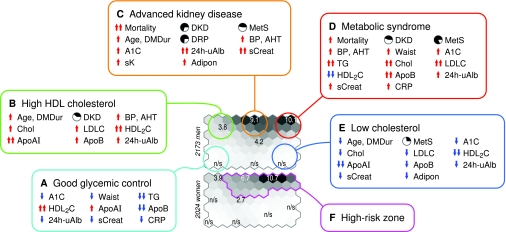
Metabolic phenotypes and risk of premature death. The relative risk of death for men and women was estimated against the expected sex-specific mortality in Finland. A–E: Five model phenotypes were constructed based on observations from Figs. 1–3. The models do not represent distinct clusters in the dataset, but they summarize the characteristics of patients around the highlighted area to make the discussion in relation to Figs. 2 and 3 easier. F: A high-risk region was highlighted for detailed comparisons of DKD categories (results are listed in text).
The self-organizing map analysis was performed with the MeliKerion software package for the Matlab/Octave programming environment (online appendix 2). A web-based interface for the software is also available (http://www.computationalmedicine.fi/software). In-house scripts were used for the data preprocessing and additional analyses. The computational analyses for the study took ∼2 h on a workstation with a single-core 3.2 GHz CPU.
RESULTS
Initial analyses showed that the self-organizing map was heavily influenced by the physiological differences between men and women. To avoid confounding effects, the sex-specific features were eliminated by rank transformation (see research design and methods). Figure 1 illustrates the sex distribution on the self-organizing map of the preprocessed data and shows no spatial separation between men and women.
FIG. 1.
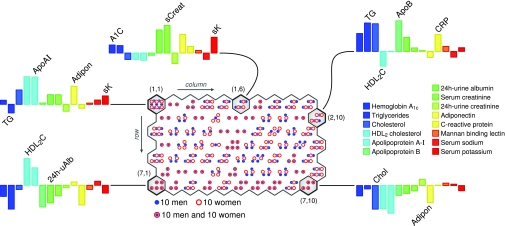
Multivariate metabolic profiles of patients with type 1 diabetes. The figure depicts the distribution of men and women on the self-organizing map that was constructed based on the listed biochemical variables. The self-organizing neural network algorithm places those patients that have similar biochemical profiles close to each other and those that have differing profiles far apart on the map. The bar plots illustrate the averaged profile for patients that reside on a given hexagonal region. •, Sets of 10 men; ○, sets of 10 women: this was done to avoid excessive clutter from individual markers for each patient.
The patients’ locations on the map were determined by their similarity to the self-organizing map profiles. The profiles, in turn, were derived from the average properties of the local population in the corresponding neighborhood. During the training process, this reciprocal relationship stabilized to a set of models, a number of which are depicted in Fig. 1. For instance, patients in the southwest corner (row 7, column 1) were characterized by a profile with good glycemic control (low A1C), high HDL2 cholesterol, low apolipoprotein B, and low C-reactive protein. Patients in the opposite corner in the northeast (2,10) were summarized by a profile of high levels of triglycerides and low HDL2 cholesterol.
By examining several profiles from various parts of the map the global features begin to emerge. For example, serum creatinine is higher on the northern regions (1,1; 1,6; 2,10) if compared with the south (7,1; 7,10) and apolipoprotein A-I is higher in the west (1,1; 7,1) compared with the east (2,10; 7,10). Although with this many variables, the bar profiles are not the most visually effective way to investigate the global properties, and thus the map coloring approach was applied to better reveal the statistical patterns.
Vascular complications and premature death.
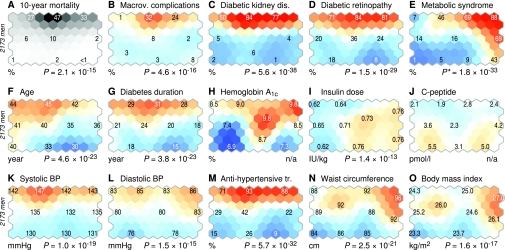
The patients that are located on a given hexagonal unit determine the color for the respective area of the self-organizing map (13). Figure 2 depicts the spatial distributions of clinical categories for the male patients. Not surprisingly, the highest (47% [38–57%]) 10-year mortality is observed near the highest DKD prevalence (84% [80–89%]) and is coupled with a history of macrovascular complications (Fig. 2A–C). Women have lower mortality (36% [26–46%]) and DKD prevalence (71% [65–77%]), but the coupling with macrovascular complications remains (online appendix 4). The highest percentages of DRP coincide with DKD and high mortality in the north, with 84% (79–88%) prevalence for men and 77% (72–83%) for women; on the southern half of the map, less than one-third of patients have DRP (Fig. 2D). The metabolic syndrome does not predict death accurately because the highest percentages (men, 88% [83–93%]; women, 86% [81–91%]) occur in the northeastern corner (Fig. 2E), away from the areas with the highest mortality. A detailed breakdown of the DKD grades and the metabolic syndrome scores is available in online appendix 3.
FIG. 2.
Self-organizing map colorings of clinical features for men with type 1 diabetes. The map in Fig. 1 can be colored according to the characteristics of the local residents within each hexagonal unit. The color scale indicates the deviation from population mean with respect to the random fluctuations that could be expected by chance. The numbers on selected units tell the local prevalence (binary variables) or mean value (continuous variables) for that particular region. For plot A, which illustrates time-adjusted mortality, the random fluctuations could not be estimated using the standard procedure, hence the pseudocolors are different to avoid direct comparisons with the rest of the colorings. The P values below the plots indicate the probability of observing equivalent regional variability for random data. *The metabolic syndrome included variables that were also self-organizing map inputs, hence the P value is only suggestive. Colorings for women are available in online appendix 4.
Age and diabetes duration (Fig. 2F and G) contribute to the development of diabetes complications but do not fully explain the deaths: Although the patients on the northwestern regions of the map are older (men, 44.3 years [42.9–45.9 years]; women, 43.2 years [41.4–45.1 years]), they have average mortality. The same regions are associated with lower insulin doses (men, 0.62 IU/kg [0.59–0.65 IU/kg]; women, 0.60 IU/kg [0.57–0.63 IU/kg]) and lower A1C, especially toward the south (Fig. 2H and I). C-peptide levels are negligible (<10 pmol/l) in every map region (Fig. 2J).
Systolic blood pressure is highest in the north (men, 147 mmHg [145–151 mmHg]; women, 142 mmHg [139–146 mmHg]) and is linked to high mortality (Fig. 2K and L). Patients in the same regions are on medication, with up to 93% (90–96%) of men and 85% (81–90%) of women under antihypertensive treatment (Fig. 2M). Abdominal obesity is most prevalent in the northeastern parts of the map (Fig. 2N and O), with wide waist (men, 96 cm [94–98 cm]; women, 87 cm [85–89 cm]) and large BMI (men, 27.0 kg/m2 [26.5–27.5 kg/m2]; women, 26.6 kg/m2 [26.1–27.3 kg/m2]). Neither of the anthropometric markers predict death accurately.
Biochemical features of diabetes complications.
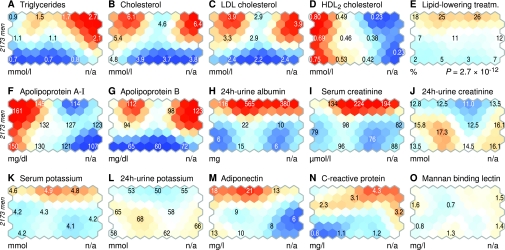
The complex nature of lipoproteins and lipids is explicitly revealed by the self-organizing map (Fig. 3A–D; online appendix 5). The metabolic syndrome region in the northeast is characterized by the highest triglyceride concentration (men, 2.7 mmol/l; women, 2.2 mmol/l) and high LDL and total cholesterol but low HDL2 cholesterol (men, 0.23 mmol/l; women, 0.32 mmol/l). Closer to the western corner, HDL2 is restored (men, 0.49 mmol/l; women, 0.67 mmol/l) with elevated LDL (men, 3.9 mmol/l; women, 3.7 mmol/l). Patients on the southern half have lower triglycerides and cholesterol, except for HDL2, which exhibits an ascending east-west pattern. Lipid-lowering treatment is more common in the north but fails to explain the changes in lipoprotein fractions (Fig. 3E).
FIG. 3.
Colorings of biochemical variables on the self-organizing map. The map colorings were produced with the same procedure as in Fig. 2. However, empirical P values are not available for the biochemical variables that were included in the self-organizing map training data. Colorings for women are available in online appendix 5.
The number of VLDL/IDL/LDL particles (apolipoprotein B in Fig. 3G) is the highest in the metabolic syndrome corner (men, 123 mg/dl; women, 126 mg/dl), with an overall pattern similar to cholesterol and triglycerides. Apolipoprotein A-I is related to HDL2 cholesterol, as expected (Fig. 3F).
24-h urine albumin (Fig. 3H) is consistent with the DKD classification (Fig. 2C), and the highest serum creatinine concentrations (men, 221 μmol/l; women, 162 μmol/l) are found near the high-mortality regions (Figs. 1A and 3H). This advanced DKD phenotype in the north is also associated with decreased 24-h urine creatinine (men, 11.0 mmol; women, 8.2 mmol), elevated serum potassium (men, 4.9 mmol/l; women, 4.7 mmol/l) and decreased 24-h urine potassium (men, 50 mmol; women, 42 mmol) in Fig. 3J–L.
The highest adiponectin levels can be observed in the north and northwest (men, 20 mg/l; women, 26 mg/l). Albuminuria coincides with high adiponectin only on the western quadrant; the metabolic syndrome corner in the northeast shows a relative reduction in adiponectin (Fig. 3H) but higher serum C-reactive protein levels (men, 4.3 mg/l; women, 7.6 mg/l) in Fig. 3N. Mannan-binding lectin concentrations exhibit no clear patterns (Fig. 3O).
High-risk metabolic phenotypes.
The observations on clinical and biochemical characteristics were summarized by five model phenotypes. The five models do not represent patient clusters; they show a condensed version of the colorings in Figs. 2 and 3 (see also online appendix 6). Figure 4A depicts a phenotype with low A1C, high HDL2 cholesterol, and low triglyceride concentration, with very few complications. Accordingly, the population-adjusted risk of premature death remains nonsignificant. In the north, total cholesterol, apolipoprotein B and 24-h urine albumin are higher, along with higher age in Fig. 4B. The risk of premature death is 3.8-fold (2.7–5.0) for men and 3.9-fold (2.4–5.7) for women on the map units 2,2 and 1,2.
DKD is the defining factor for a high-risk phenotype that is characterized by high serum creatinine, albuminuria, and elevated adiponectin (Fig. 4C). For men, the 10-year mortality peaks in this region (Fig. 2A), but after adjusting by age, the 9.1-fold (7.3–10.4) risk is no longer the highest on the map. For women, the highest 10.7-fold (7.9–13.7) risk coincides with mortality (online appendix 4) but is closer to the northeast corner.
The highest 10.1-fold (7.3–13.1) risk of death in male patients is observed at the metabolic syndrome corner in Fig. 4D. By definition, the phenotype is characterized by wide waist, high triglycerides, and low HDL2 cholesterol. Although the prevalence of DKD is high in these patients, they exhibit the highest total cholesterol and apolipoprotein B concentrations, in contrast to the lower values in Fig. 4C.
Diabetes duration is the shortest in the southeastern regions, where mortality is low and relative risk nonsignificant (Fig. 4E). Nevertheless, the phenotype shares low HDL2 cholesterol with the northeast corner, which contributes to the observed prevalence of the metabolic syndrome. The two corners are separated by the triangle of A1C, total cholesterol, and triglycerides, which are elevated in the north.
A number of patients in the high-risk zone (Fig. 4F) have normoalbuminuria despite otherwise adverse biochemical profiles. Aside from 24-h urine albumin and serum creatinine, antihypertensive treatment is the most significant discriminating factor, with 23% of normoalbuminuric men (14% of women) but >90% of those with DKD (P = 1.1 × 10−52 for men, P = 3.8 × 10−54 for women) on medication. The next best discriminator is diabetes duration: Normoalbuminuric men in the high-risk zone have 13 years shorter duration (P = 1.9 × 10−12) and women have 11 years shorter duration (P = 2.3 × 10−16) than the “metabolic peers” with DKD.
DISCUSSION
The life expectancy for a patient with type 1 diabetes depends heavily on the development of kidney disease and the concurrent incidence of macrovascular events (1,7,21,22). Our analyses with the self-organizing map confirmed this and provided carefully adjusted estimates for the relative risk of premature death. In addition, we also revealed direct associations between the metabolic features and several clinical outcomes.
Urine albumin is biologically highly variable, which has raised questions about its role as an early marker of complications (23). Also, there is continuous discussion on the exact cutoffs that define the clinical DKD categories, which in turn have significant impact on the treatment decisions at an individual level (24). Could albuminuria be supplemented by other sources of information? Much work is being concentrated at finding new biomarkers for kidney disease, and some candidates have been found (25–27), although many of them are compared against existing albuminuria and hence do not directly lead to better accuracy in subclinical risk assessment. Genetic susceptibility has also been investigated (28–32), but conclusive results are still lacking, and it is uncertain whether genetic testing will have clinical applications in the near future. Considering the slow development of these complications, the substantial individual and environmental variation, and difficulties in exact phenotyping, it may be too optimistic to expect any single factor to be decisive in the traditional reductionist fashion (33). Our attention has therefore shifted back to the known biomarkers and risk factors but with a more comprehensive approach.
Multivariate exploratory analysis has been previously applied to dyslipidemia, the metabolic syndrome, and type 2 diabetes (34–36), but most of these studies were focused on the associations between variables (linear factorization) rather than on the individuals. Here, we described the dataset by five model phenotypes after examining the diversity of the patients’ biochemical profiles and combining the results with existing clinical knowledge. The phenotypes do not represent patient clusters because that would imply detectable boundaries between the metabolic states (see online appendix 6). Instead, the data reflect different stages of gradual damage in a continuous and time-dependent manner, which prevents clear categorizations in a cross-sectional population-based study.
The self-organizing map has been applied previously in the analysis of spectroscopic data (13,14), in molecular conformation analysis (37), in studies of gene-metabolite interactions (38), and in multivariate assessment of insulin resistance (17), among others. Although the sensitivity was demonstrated also in this study, the nonlinear algorithm may also lead to the typical problems of multivariate modeling such as overfitting and false positives (39). Here, the number of samples was large compared with the number of explanatory variables (4,197 patients vs. 14 variables), which already makes the model less prone to exaggerate weak results. Modest-sized maps with smoothed estimates were chosen, and to be absolutely sure, only the biochemical variables were used as inputs; this way, the statistical significance of the observed variability for the clinical features on the self-organizing map could be verified.
The self-organizing map is an indeterministic method in the sense that a small change in the input data may change the shapes of observed patterns significantly. We therefore recommend testing with different map sizes and subsets of the input data to determine the biologically relevant interpretations. The problem is not so much in the robustness of the algorithm, but with the fact that nonbiological effects such as sampling bias, differences in data collection methods and laboratory protocols over time may produce data-driven artifacts in clinical studies with a long history.
The highest relative risk of premature death (10.1-fold for men and 10.7-fold for women) was associated with a metabolic profile that shared features from DKD (high 24-h urine albumin and serum creatinine) and from the metabolic syndrome (high triglycerides, low HDL cholesterol, and wide waist). Albuminuria was the common risk factor, as expected, but the two sides were also distinguished by adiponectin, which was higher on the map units dominated by microvascular complications (Figs. 2 and 3, northwest vs. northeast), and C-reactive protein, which was higher in the metabolic syndrome corner.
Earlier studies already showed that female sex and renal insufficiency are associated with higher adiponectin levels (40) and that DKD is associated with increased C-reactive protein levels (41). Similar results have been obtained with respect to microangiopathy (19,42) and long diabetes duration (43). Unlike C-reactive protein, adiponectin is considered to be inversely related to obesity and dyslipidemia, and low levels have been linked to cardiovascular disease (44,45). By considering only the correlation between the metabolic syndrome and DKD, one could conclude that this is not true in type 1 diabetes. The self-organizing map analysis, however, was able to show that the link between adiponectin and the metabolic syndrome (or abdominal obesity and low HDL cholesterol) is, in fact, present, although concealed by the overall increase of adiponectin due to diabetes complications. Also, C-reactive protein seemed to have a more complex role than classical analyses would suggest: The highest levels were not observed for DKD specifically but for patients with high scores of the metabolic syndrome.
The incomplete overlap between DKD and metabolic syndrome was also visible from the lipid-centered perspective. The northwest and northeast quadrants of the colorings in Figs. 2 and 3 indicated that the lipid profile of a typical patient with advanced DKD was located between a high HDL phenotype with microvascular complications and a high triglyceride–low HDL phenotype with the metabolic syndrome (Fig. 4B–D). Survival bias (ESRD plus metabolic syndrome underrepresented) may have been the cause for the reduction in cholesterol and triglyceride concentrations in Fig. 4C, because mortality was high. Altered nutrition and other effects due to ESRD are other likely causes, but the data are insufficient for a final conclusion.
The diverse picture of different lipid profiles in various manifestations of microvascular and macrovascular diseases sheds light on the heterogeneous results from previous studies (46–49). For instance, the role of HDL metabolism in the development of DKD has not yet been determined conclusively; this study suggests that low HDL2 cholesterol is coupled with abdominal obesity and increased insulin requirement but not necessarily with persistent albuminuria. On the other hand, high HDL2 cholesterol may represent a marker of good glycemic control and a low prevalence of complications at higher age (Fig. 2, southwest and western regions).
The clinical material was extensive from the cross-sectional phase, but the exact causes of death were not available for the prospective part. Nevertheless, the population registry in Finland is highly accurate, and the vitality status itself was reliable. From previous studies, it is known that most premature deaths related to type 1 diabetes are caused by cardiovascular events (2,9), which is plausible also in this study: A history of macrovascular disease coincided with the high-mortality regions (Fig. 2A and B).
On one hand, a clear relationship between DKD and mortality exists, but on the other hand, there seems to be a dual nature to the vascular complications, with a quartet of poor glycemic control, adverse lipid profile, abdominal obesity, and insulin resistance on one corner (50) and a high HDL, high adiponectin, leaner phenotype with microvascular complications on the other. When these two collide, the risk of death peaks according to our present and previous results (13). Many of these observations have already been reported, but here all the data were viewed within a single statistical framework that not only described the known risk factors in their metabolic context but also showed additional details that would have been laborious to detect by classical methods. We therefore expect that the multivariate approach, as applied here, will enable more rapid and thorough investigations of large clinical datasets, give grounds to novel hypotheses of the pathology of diabetes complications, and open new perspectives into the complex interactions between metabolic risk factors.
Supplementary Material
Acknowledgments
V.-P.M. has received support from the Center of Excellence Program of the Academy of Finland. K.K. has received support from the Center of Excellence Program of the Academy of Finland. M.A.-K. has received support from the Center of Excellence Program of the Academy of Finland. This study has received grants from the Folkhälsan Research Foundation, the Jenny and Antti Wihuri Foundation, the Wilhelm and Else Stockmann Foundation, the Liv och Hälsa Foundation, and the European Commission (QLG2-CT-2001-01669, LSHB-CT-2006-037681).
Published ahead of print at http://diabetes.diabetesjournals.org on 19 June 2008.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C: Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 294 :1782 –1787,2005 [DOI] [PubMed] [Google Scholar]
- 2.Morrish N, Wang S, Stevens L, Fuller J, Keen H, the WHO Multinational Study Group: Mortality and causes of death in the WHO multinational study of vascular disease in diabetes. Diabetologia 44 :S14 –S21,2001 [DOI] [PubMed] [Google Scholar]
- 3.Gross J, de Azevedo M, Silveiro S, Canani L, Caramori M, Zelmanovitz T: Diabetic nephropathy: diagnosis, prevention, and treatment. Diabetes Care 28 :164 –176,2005 [DOI] [PubMed] [Google Scholar]
- 4.Newman D, Mattock M, Dawnay A, Kerry S, McGuire A, Yaqoob M, Hitman G, Hawke C: Systematic review on urine albumin testing for early detection of diabetic complications. Health Technol Assess 9 :iii –vi, xiii–163,2005 [DOI] [PubMed] [Google Scholar]
- 5.Soedamah-Muthu S, Chaturvedi N, Toeller M, Ferriss B, Reboldi P, Michel G, Manes C, Fuller J: EURODIAB Prospective Complications Study Group risk factors for coronary heart disease in type 1 diabetic patients in Europe: the EURODIAB Prospective Complications Study. Diabetes Care 27 :530 –537,2004 [DOI] [PubMed] [Google Scholar]
- 6.D'Agostino R, Grundy S, Sullivan L, Wilson P, the CHD Risk Prediction Group: Validation of the Framingham coronary heart disease prediction scores: results of a multiple ethnic groups investigation. JAMA 11 :180 –187,2001 [DOI] [PubMed] [Google Scholar]
- 7.Colhoun H, Lee E, Bennett P, Lu M, Keen H, Wang S, Stevens L, Fuller J, the WHO Multinational Study Group: Risk factors for renal failure: the WHO Multinational Study of Vascular Disease in Diabetes. Diabetologia 44 :S46 –S53,2001 [DOI] [PubMed] [Google Scholar]
- 8.Eckel R, Grundy S, Zimmet P: The metabolic syndrome. Lancet 365 :1415 –1428,2005 [DOI] [PubMed] [Google Scholar]
- 9.Pambianco G, Costacou T, Orchard T: The prediction of major outcomes of type 1 diabetes: a 12 year prospective evaluation of three separate definitions of the metabolic syndrome, and their components and estimated glucose disposal rate: the Pittsburgh Epidemiology of Diabetes Complications Study experience. Diabetes Care 30 :1248 –1254,2007 [DOI] [PubMed] [Google Scholar]
- 10.Thorn LM, Forsblom C, Fagerudd J, Thomas M, Petterson-Fernholm K, Saraheimo M, Wadén J, Rönnback M, Rosengård-Bärlund M, Af Björkesten C, Taskinen MR, Groop PH, the FinnDiane Study Group: Metabolic syndrome in type 1 diabetes: association with diabetic nephropathy and glycemic control (the FinnDiane study). Diabetes Care 28 :2019 –2024,2005 [DOI] [PubMed] [Google Scholar]
- 11.Ala-Korpela M, Sipola P, Kaski K: Characterization and molecular detection of atherothrombosis by magnetic resonance: potential tools for individual risk assessment and diagnostics. Ann Med 38 :322 –336,2006 [DOI] [PubMed] [Google Scholar]
- 12.Mäkinen VP, Soininen P, Forsblom C, Parkkonen M, Ingman P, Kaski K, Groop PH, Ala-Korpela M, the FinnDiane Study Group: Diagnosing diabetic nephropathy by 1H NMR metabonomics of serum. MAGMA 19 :281 –296,2006 [DOI] [PubMed] [Google Scholar]
- 13.Mäkinen VP, Soininen P, Forsblom C, Parkkonen M, Ingman P, Kaski K, Groop PH, Ala-Korpela M, the FinnDiane Study Group: 1H NMR metabonomics approach to the disease continuum of diabetic complications and premature death. Mol Syst Biol 4 :167 ,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suna T, Salminen A, Soininen P, Laatikainen R, Ingman P, Mäkelä S, Savolainen M, Hannuksela M, Jauhiainen M, Taskinen M, Kaski K, Ala-Korpela M: 1H NMR metabonomics of plasma lipoprotein subclasses: elucidation of metabolic clustering by self-organising maps. NMR Biomed 20 :658 –672,2007 [DOI] [PubMed] [Google Scholar]
- 15.Vehtari A, Mäkinen VP, Soininen P, Ingman P, Mäkelä S, Savolainen M, Hannuksela M, Kaski K, Ala-Korpela M: A novel Bayesian approach to quantify clinical variables and to determine their spectroscopic counterparts in 1H NMR metabonomic data. BMC Bioinformatics 8 :S2 ,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kohonen T: Self-Organizing Maps. Heidelberg, Springer-Verlag,2000
- 17.Valkonen V, Kolehmainen M, Lakka H, Salonen J: Insulin resistance syndrome revisited: application of self-organizing maps. Int J Epidemiol 31 :864 –871,2002 [DOI] [PubMed] [Google Scholar]
- 18.National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III): Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 106 :3143 –3421,2002 [PubMed] [Google Scholar]
- 19.Frystyk J, Tarnow L, Hansen T, Parving HH, Flyvbjerg A: Increased serum adiponectin levels in type 1 diabetic patients with microvascular complications. Diabetologia 48 :1911 –1918,2005 [DOI] [PubMed] [Google Scholar]
- 20.Thiel S, Møller-Kristensen M, Jensen L, Jensenius JC: Assays for the functional activity of the mannan-binding lectin pathway of complement activation. Immunobiology 205 :446 –454,2002 [DOI] [PubMed] [Google Scholar]
- 21.Stadler M, Auinger M, Anderwald C, Kästenbauer T, Kramar R, Feinböck C, Irsigler K, Kronenberg F, Prager R: Long-term mortality and incidence of renal dialysis and transplantation in type 1 diabetes mellitus. J Clin Endocrinol Metab 91 :3814 –3820,2006 [DOI] [PubMed] [Google Scholar]
- 22.Torffvit O, Lövestam-Adrian M, Agardh E, Agardh C: Nephropathy, but not retinopathy, is associated with the development of heart disease in type 1 diabetes: a 12-year observation study of 462 patients. Diabet Med 22 :723 –729,2005 [DOI] [PubMed] [Google Scholar]
- 23.Caramori M, Fioretto P, Mauer M: The need for early predictors of diabetic nephropathy risk: is albumin excretion rate sufficient? Diabetes 49 :1399 –1408,2000 [DOI] [PubMed] [Google Scholar]
- 24.Thomas M, Viberti G, Groop PH: Screening for chronic kidney disease in patients with diabetes: are we missing the point? Nat Clin Pract Nephrol 4 :2 –3,2008 [DOI] [PubMed] [Google Scholar]
- 25.Saraheimo M, Forsblom C, Hansen T, Teppo A, Fagerudd J, Pettersson-Fernholm K, Thiel S, Tarnow L, Ebeling P, Flyvbjerg A, Groop PH, the FinnDiane Study Group: Increased levels of mannan-binding lectin in type 1 diabetic patients with incipient and overt nephropathy. Diabetologia 48 :198 –202,2004 [DOI] [PubMed] [Google Scholar]
- 26.Meier M, Kaiser T, Herrmann A, Knueppel S, Hillmann M, Koester P, Danne T, Haller H, Fliser D, Mischak H: Identification of urinary protein pattern in type 1 diabetic adolescents with early diabetic nephropathy by a novel combined proteome analysis. J Diabetes Complications 19 :223 –232,2005 [DOI] [PubMed] [Google Scholar]
- 27.Von Hertzen L, Forsblom C, Stumpf K, Petterson-Fernholm K, Adlercreutz H, Groop PH, the FinnDiane Study Group: Highly elevated serum phyto-oestrogen concentrations in patients with diabetic nephropathy. J Int Med 255 :602 –609,2004 [DOI] [PubMed] [Google Scholar]
- 28.Österholm A, He B, Pitkäniemi J, Albinsson L, Berg T, Sarti C, Tuomilehto J, Tryggvason K: Genome-wide scan for type 1 diabetic nephropathy in the Finnish population reveals suggestive linkage to a single locus on chromosome 3q. Kidney Int 71 :140 –145,2007 [DOI] [PubMed] [Google Scholar]
- 29.Boright A, Paterson A, Mirea L, Bull S, Mowjoodi A, Scherer S, Zinman B, the DCCT/EDIC Research Group: Genetic variation at the ACE gene is associated with persistent microalbuminuria and severe nephropathy in type 1 diabetes. Diabetes 54 :1238 –1244,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorn LM, Forsblom C, Fagerudd J, Pettersson-Fernholm K, Kilpikari R, Groop PH, FinnDiane Study Group: Clustering of risk factors in parents of patients with type 1 diabetes and nephropathy. Diabetes Care 30 :1162 –1167,2007 [DOI] [PubMed] [Google Scholar]
- 31.Al-Kateb H, Boright A, Mirea L, Xie X, Sutradhar R, Mowjoodi A, Bharaj B, Liu M, Bucksa J, Arends V, Steffes M, Cleary P, Sun W, Lachin J, Thorner P, Ho M, McKnight A, Maxwell A, Savage D, Kidd K, Kidd J, Speed W, Orchard T, Miller R, Sun L, Bull S, Paterson A, The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group: Multiple superoxide dismutase 1/splicing factor serine alanine 15 variants are associated with the development and progression of diabetic nephropathy: the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Genetics study. Diabetes 57 :218 –228,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vionnet N, Tregouët D, Kazeem G, Gut I, Groop PH, Tarnow L, Parving H, Hadjadj S, Forsblom C, Farrall M, Gauguier D, Cox R, Matsuda F, Heath S, Thévard A, Rousseau R, Cambien F, Marre M, Lathrop M: Analysis of 14 candidate genes for diabetic nephropathy on chromosome 3q in European populations: strongest evidence for association with a variant in the promoter region of the adiponectin gene. Diabetes 55 :3166 –3174,2006 [DOI] [PubMed] [Google Scholar]
- 33.Loscalzo J, Kohane I, Barabasi A: Human disease classification in the postgenomic era: a complex systems approach to human pathobiology. Mol Syst Biol 3 :124 ,2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stirnadel H, Lin X, Ling H, Song K, Barter P, Kesäniemi Y, Mahley R, McPherson R, Waeber G, Bersot T, Cohen J, Grundy S, Mitchell B, Mooser V, Waterworth D: Genetic and phenotypic architecture of metabolic syndrome-associated components in dyslipidemic and normolipidemic subjects: The GEMS Study. Atherosclerosis 197 :868 –876,2008 [DOI] [PubMed] [Google Scholar]
- 35.Shen B, Todaro J, Niaura R, McCaffery J, Zhang J, Spiro A, Ward K: Are metabolic risk factors one unified syndrome? Modeling the structure of the metabolic syndrome X. Am J Epidemiol 157 :701 –711,2003 [DOI] [PubMed] [Google Scholar]
- 36.Hanley A, Festa A, D'Agostino R, Wagenknecht L, Savage P, Tracy R, Saad M, Haffner S: Metabolic and inflammation variable clusters and prediction of type 2 diabetes: factor analysis using directly measured insulin sensitivity. Diabetes 53 :1773 –1781,2004 [DOI] [PubMed] [Google Scholar]
- 37.Hyvönen M, Hiltunen Y, El-Deredy W, Ojala T, Vaara J, Kovanen P, Ala-Korpela M: Application of self-organizing maps in conformational analysis of lipids. J Am Chem Soc 123 :810 –816,2001 [DOI] [PubMed] [Google Scholar]
- 38.Hirai M, Yano M, Goodenowe D, Kanaya S, Kimura T, Awazuhara M, Arita M, Fujiwara T, Saito K: Integration of transcriptomics and metabolomics for understanding of global responses to nutritional stresses in Arabidopsis thaliana. Proc Natl Acad Sci U S A 101 :10205 –10210,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lampinen J, Kostiainen T: Overtraining and model selection with the self-organizing map. Neural Networks 3 :1911 –1915,1999 [Google Scholar]
- 40.Saraheimo M, Forsblom C, Fagerudd J, Teppo A, Petterson-Fernholm K, Frystyk J, Flyvbjerg A, Groop PH, the FinnDiane Study Group: Serum adiponectin is increased in type 1 diabetic patients with nephropathy. Diabetes Care 28 :1410 –1414,2005 [DOI] [PubMed] [Google Scholar]
- 41.Saraheimo M, Teppo A, Forsblom C, Fagerudd J, Groop PH, the FinnDiane Study Group: Diabetic nephropathy is associated with low-grade inflammation in type 1 diabetic patients. Diabetologia 46 :1402 –1407,2003 [DOI] [PubMed] [Google Scholar]
- 42.Hadjadj S, Aubert R, Fumeron F, Pean F, Tichet J, Roussel R, Marre M, the SURGENE and DESIR Study Groups: Increased plasma adiponectin concentrations are associated with microangiopathy in type 1 diabetic subjects. Diabetologia 48 :1088 –1092,2005 [DOI] [PubMed] [Google Scholar]
- 43.Lindström T, Frystyk J, Hedman C, Flyvbjerg A, Arnqvist HJ: Elevated circulating adiponectin in type 1 diabetes is associated with long diabetes duration. Clin Endocrinol 65 :776 –782,2006 [DOI] [PubMed] [Google Scholar]
- 44.Lara-Castro C, Fu Y, Chung BH, Garvey WT: Adiponectin and the metabolic syndrome: mechanisms mediating risk for metabolic and cardiovascular disease. Curr Opin Lipidol 18 :263 –270,2007 [DOI] [PubMed] [Google Scholar]
- 45.Maahs D, Ogden L, Snell-Bergeon J, Kinney G, Wadwa R, Hokanson J, Dabelea D, Kretowski A, Eckel R, Rewers M: Determinants of serum adiponectin in persons with and without type 1 diabetes. Am J Epidemiol 166 :731 –740,2007 [DOI] [PubMed] [Google Scholar]
- 46.Chaturvedi N, Fuller J, Taskinen MR: Differing associations of lipid and lipoprotein disturbances with the macrovascular and microvascular complications of type 1 diabetes. Diabetes Care 24 :2071 –2077,2001 [DOI] [PubMed] [Google Scholar]
- 47.Lyons T, Jenkins A, Zheng D, Lackland D, McGee D, Garvey W, Klein R: Diabetic retinopathy and serum lipoprotein subclasses in the DCCT/EDIC cohort. Invest Ophthalmol Vis Sci 45 :910 –918,2004 [DOI] [PubMed] [Google Scholar]
- 48.Tolonen N, Forsblom C, Thorn LM, Wadén J, Rosengård-Bärlund M, Saraheimo M, Heikkilä O, Pettersson-Fernholm K, Taskinen M, Groop PH, The FinnDiane Study Group: Relationship between lipid profiles and kidney function in patients with type 1 diabetes. Diabetologia 51 :12 –20,2008 [DOI] [PubMed] [Google Scholar]
- 49.Groop PH, Thomas M, Rosengård-Bärlund M, Mills V, Rönnback M, Thomas S, Forsblom C, Taskinen M, Viberti G: HDL composition predicts new-onset cardiovascular disease in patients with type 1 diabetes. Diabetes Care 30 :2706 –2707,2007 [DOI] [PubMed] [Google Scholar]
- 50.Groop PH, Forsblom C, Thomas M: Mechanisms of disease: pathway-selective insulin resistance and microvascular complications of diabetes. Nat Clin Pract Endocrinol Metab 1 :100 –110,2005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.