Abstract
OBJECTIVE— The mechanism underlying pericyte loss during incipient diabetic retinopathy remains controversial. Hyperglycemia induces angiopoietin-2 (Ang-2) transcription, which modulates capillary pericyte coverage. In this study, we assessed loss of pericyte subgroups and the contribution of Ang-2 to pericyte migration.
RESEARCH DESIGN AND METHODS— Numbers of total pericytes and their subgroups were quantified in retinal digest preparations of spontaneous diabetic XLacZ mice. Pericytes were divided into subgroups according to their localization, their position relative to adjacent endothelial cells, and the expression of LacZ. The contribution of Ang-2 to pericyte migration was assessed in Ang-2 overexpressing (mOpsinhAng2) and deficient (Ang2LacZ) mice.
RESULTS— Pericyte numbers were reduced by 16% (P < 0.01) in XLacZ mice after 6 months of diabetes. Reduction of pericytes was restricted to pericytes on straight capillaries (relative reduction 27%, P < 0.05) and was predominantly observed in LacZ-positive pericytes (−20%, P < 0.01). Hyperglycemia increased the numbers of migrating pericytes (69%; P < 0.05), of which the relative increase due to diabetes was exclusively in LacZ-negative pericytes, indicating reduced adherence to the capillaries (176%; P < 0.01). Overexpression of Ang-2 in nondiabetic retinas mimicked diabetic pericyte migration of wild-type animals (78%; P < 0.01). Ang-2 deficient mice completely lacked hyperglycemia-induced increase in pericyte migration compared with wild-type littermates.
CONCLUSIONS— Diabetic pericyte loss is the result of pericyte migration, and this process is modulated by the Ang-Tie system.
Pericytes are heterogeneous with regard to their origin, distribution, phenotype, and function (1,2). Resident retinal pericytes derive from mesoderm and neural crest during development. A bone marrow origin of pericytes and transdifferentiation of endothelial cells into pericytes have also been demonstrated during postnatal vascular repair and in adult angiogenesis (3–5). Furthermore, pericytes can transdifferentiate into macrophage- and fibroblast-like cells (6,7). Pericytes are the capillary counterparts to smooth muscle cells (SMCs) on arterioles. In contrast to SMCs, they are completely embedded within the capillary basement membrane and extend processes, varying in length, arrangement, and form, indicating their mobility in functional blood vessels (8–11). One remarkable common feature of pericytes and SMCs is their contractile phenotype (12). However, the key molecules actin and myosin for vessel constriction are unequally distributed within the pericyte population (13–15), leading to differences in their contractive potential, depending on their localization within the capillary tree (12). Pericytes can control endothelial cell proliferation and angiogenesis, both under physiological and pathological conditions (16–22). Smooth muscle actin (SMA), desmin, proteoglycan NG2, platelet-derived growth factor receptor β (PDGFB-R), the aminopeptidase N, and the regulator of G-signaling 5 (RGS-5) are common pericyte markers, but none of them is sufficient to recognize every pericyte (15,19,23–29). Another experimental tool for studying pericytes is the XLacZ mouse. In this mouse, LacZ is expressed in vascular SMCs and pericytes at distinct levels of the vascular tree, but cell proliferation and migration are associated with transgene downregulation. To our own observations, LacZ is expressed only in a subset of retinal pericytes. The diversity of origin, differences in functional capacity, and the lack of a pan-pericyte marker suggest that the pericyte population is not uniform in a given organ; hence, the response of retinal pericytes on chronic hyperglycemia may differ in certain subpopulations.
Diabetic retinopathy is morphologically characterized by pathological changes in the retinal capillaries. The primary and predominant characteristics are the loss of pericytes and the progressive occlusion of capillaries (8,30). It is presumed that prevention of the earliest events in the pathogenesis of diabetic retinopathy, such as pericyte loss, will prevent the subsequent development of diabetic retinopathy. The underlying mechanisms responsible for pericyte loss in diabetic retinopathy are complex and not completely elucidated.
Apoptosis and destructive signaling pathways within pericytes are generally discussed to be the reasons for retinal pericyte loss under hyperglycemic conditions (31,32), but growing evidence suggests that pericytes are actively depleted by alternative mechanisms. Pericyte coverage of capillaries is modulated by a variety of growth factors systems, such as angiopoietins and their tyrosine kinase receptor Tie-2. Angiopoietin-2 (Ang-2) is expressed in the retina and is upregulated in the diabetic retina, before pericyte loss. We recently demonstrated that a 50% reduction of the Ang-2 gene dose prevents pericyte loss in the diabetic retina, suggesting an important role of the Ang-2/Tie-2 system in diabetic pericyte loss. Constitutive overexpression of Ang-2 in photoreceptor cells reduces pericyte coverage in the deep capillary layers of the retina, and injection of recombinant Ang-2 into the vitreous of nondiabetic rats induced a dose-dependent pericyte loss within days, indicating the importance of Ang-2 in the reduction of pericyte coverage (33,34). Increased levels of Ang-2 in diabetic animals and in vitreous fluid of patients with proliferative diabetic retinopathy support the role of the Ang-2/Tie-2 system in the pathogenesis of diabetic retinopathy (35,36), but the underlying mechanisms remain to be investigated.
In light of published evidence for the heterogeneity of pericytes and the association of pericyte loss with the Ang-2/Tie-2 system, we hypothesized that diabetic pericyte loss is the result of mechanisms other than apoptosis in situ and that factors such as Ang-2 may actively modulate this process.
In this study, we used transgenic mouse models for quantification of pericyte loss in experimental diabetic retinopathy to investigate the response of retinal pericytes to chronic hyperglycemia and the effect of Ang-2 on retinal pericyte migration in gain- and loss-of-function models.
RESEARCH DESIGN AND METHODS
All experiments in this study were performed according to the guidelines of the statement of animal experimentation issued by the Association for Research in Vision and Ophthalmology (ARVO) and were approved by the Institutional Animal Care and Use Committee. Animals were housed in groups in cages with free access to standard food and water under a 12-h light and 12-h dark rhythm.
Spontaneous diabetic Ins2Akita heterozygous mice, purchased from Jackson Laboratory (Charles River Laboratories, Sulzfeld, Germany) were bred with homozygous XLacZ mice, which express the reporter gene LacZ under the control of a smooth muscle and pericyte-specific promoter (29) to generate spontaneous diabetic heterozygous XLacZ mice (Ins2Akita+/−XLacZ+/−). Age-matched nondiabetic heterozygous XLacZ mice (Ins2Akita−/−XLacZ+/−) served as control. Genotype was determined by PCR 4 weeks after birth as described previously (37). Only male mice were used in this study, since the diabetes onset is earlier and hyperglycemia is more severe compared with female mice. Glucose levels and body weight were monitored consecutively every other week, and glycated hemoglobin concentration was determined by affinity chromatography at the end of the study (MicromatII; Bio-Rad Laboratories, Munich, Germany). Insulin was occasionally given to individual diabetic mice to prevent critical weight loss. After 6 months of experimental diabetes, eyes were enucleated under deep anesthesia and immediately frozen at −80°C for further analysis.
To study the impact of Ang-2 on pericyte migration, retinas of transgenic mOpsinAng2 and Ang2LacZ mice were analyzed. mOpsinhAng2 mice overexpress human Ang-2 in the photoreceptor layer of the retina. Ang2LacZ mice carry a targeting vector that replaces part of the coding region of Ang-2, with the LacZ gene encoding β-galactosidase with the intention of creating a null allele of Ang-2. Heterozygous LacZ knock-in results in a 50% reduction of functional Ang-2 gene dose. Generation and genotyping of mOpsinAng2 and Ang2LacZ mice have been described previously (34,38). Wild-type litters served as controls. Diabetes was induced by a single injection of streptozotocin (STZ) (purchased from Roche Diagnostics, Mannheim, Germany; 150 mg/kg i.p.) in randomly selected animals of transgenic and WT (wild-type) mice. STZ-injected animals were considered diabetic when blood glucose levels reached stable levels >250 mg/dl. Diabetic and nondiabetic mice were killed 6 months after diabetes induction, and eyes were collected under deep anesthesia and immediately frozen at −80°C.
LacZ staining.
Eyes of XLacZ mice were stained for β-galactosidase activity according to an established protocol (33). In brief, eyes were first fixed (phosphate buffer 100 mmol/l, pH 7.3, containing 1.5% [vol/vol] formaldehyde/0.2% [vol/vol] glutaraldehyde) and then incubated in a staining solution containing 0.1% X-gal (Roche Diagnostics), 5 mmol/l K4Fe(CN)6, and 5 mmol/l K3Fe(CN)6 overnight at 37°C. After washing in PBS, retinas were isolated and subjected to the retinal digestion procedure (see below).
Immunofluorescence analysis.
To examine the relationship of migrating pericytes with the underlying endothelium, retinal whole mounts fixed in 4% paraformaldehyde were permeabilized in 1% BSA and 0.5% Triton X-100 at room temperature for 1 h and incubated with a rabbit anti-NG2 chondroitin sulfate proteoglycan polyclonal antibody (Chemicon International, Hampshire, U.K.; dilution 1:200) and tetramethylrhodamine isothiocyanate (TRITC)-labeled isolectin B4 from Bandeiraea simplicifolia (Sigma-Aldrich, München, Germany; dilution 1:50) overnight at 4°C. Fluorescein isothiocyanate–conjugated swine anti-rabbit IgG (DakoCytomation, Hamburg, Germany; dilution 1:20) was used for the detection of NG2 primary antibody. Photos were taken using a confocal microscope (Leica TCS SP2 Confocal Microscope; Leica).
Retinal digest preparations.
Vascular preparations of whole-mount retinas of XLacZ, mOpsinhAng2, and Ang2LacZ mice were performed using a trypsin digestion technique as previously described (8,33). Briefly, retinas were digested in a solution containing 3% trypsin resolved in 0.2 mol/l Tris-HCl solution at 37°C for 3 h. The retinal vasculature was isolated by dropping water and dried on objective slides. Subsequently, retinal digest samples were stained with periodic acid Schiff base (39) and hematoxylin.
Morphological quantification.
To determine numbers and morphologies of retinal pericytes, retinal digest preparations of spontaneous diabetic and nondiabetic XLacZ mice (n = 7–8) were analyzed. First, total numbers of pericytes were counted in 10 randomly selected areas (magnification 400×) in the middle sector of the retina, using an image analyzing system (CUE-2; Olympus Opticals, Hamburg, Germany). Both areas, close to the optic nerve and to the external border of the retina, were excluded from analysis. Numbers were standardized relative to the capillary density (numbers of cells per mm2 of capillary area). Subsequently, according to their localization within the capillary tree and their position relative to adjacent endothelial cells, pericytes were divided into three subgroups. Pericytes were located either at capillary branches (B-PC) or on straight capillaries (S-PC) with broad contact to the underlying endothelium, or on straight capillaries with less area of contact to the microvasculature (M-PC). Pericytes with triangular nuclei, migrating from capillaries into the extravascular interstitium, were defined as M-PCs, since at least one lateral side of the triangular nuclei was longer than the basis in contact to the capillary. Migrating pericytes usually extended processes to neighboring capillaries. Finally, according to the LacZ staining, each group described above was divided into two subgroups, e.g., B-PC into B-PC+ (i.e., pericytes at branches stained positive for LacZ) and B-PC− (i.e., LacZ-negative pericytes at branches). The numbers of pericytes in 10 randomly selected fields of the retina were standardized relative to the capillary density (number of cells per mm2 of capillary area). All samples were evaluated in a masked fashion.
To examine the influence of the Ang-2/Tie-2 system on pericyte loss in diabetic retinopathy, we quantified the numbers of migrating pericytes (M-PCs) in retinas of nondiabetic and diabetic mOpsinhAng2 (n = 7–8 animals per group) and Ang2LacZ mice (n = 6) in comparison to nondiabetic and diabetic WT litters (n = 8).
Statistical analysis.
Quantitative data are given as means ± SD. Student's t test was used to make comparison between groups. A value of P < 0.05 was considered statistically significant.
RESULTS
Metabolic data of XLacZ mice, mOpsinhAng2, and Ang2LacZ mice.
Diabetic XLacZ mice developed elevated blood glucose levels after the fourth postnatal week. Two weeks later, most diabetic males reached stable blood glucose levels >500 mg/dl. Final blood glucose was significantly elevated in diabetic XLacZ mice (nondiabetic versus diabetic XLacZ mice: 147.1 ± 17.9 and 599.8 ± 0.7 mg/dl; P < 0.001) compared with nondiabetic XLacZ mice. At the end of the study, diabetic XLacZ mice showed a 32% reduced body weight compared with nondiabetic XLacZ mice (nondiabetic versus diabetic XLacZ mice: 35.9 ± 4.7 and 24.3 ± 3.3 g; P < 0.001). Glycated hemoglobin levels were 2.2-fold increased in diabetic XLacZ mice compared with nondiabetic mice (nondiabetic versus diabetic XLacZ mice: 5.8 ± 0.6 and 12.8 ± 1.8%; P < 0.01, Table 1).
TABLE 1.
Metabolic data of nondiabetic and diabetic XLacZ, mOpsinhAng2, and Ang2LacZ mice
| Nondiabetic XLacZ | Diabetic XLacZ | |
|---|---|---|
| n | 11 | 8 |
| Glucose level (mg/dl) | 147.1 ± 17.9 | 599.8 ± 0.7* |
| Body weight (g) | 35.9 ± 4.7 | 24.3 ± 3.3* |
| A1C (%) | 5.8 ± 0.6 | 12.8 ± 1.8* |
| Nondiabetic mOpsinhAng2 | Diabetic mOpsinhAng2 | |
| n | 7 | 8 |
| Glucose level (mg/dl) | 184.6 ± 19.0 | 575.0 ± 40.9† |
| Body weight (g) | 34.5 ± 4.6 | 28.0 ± 3.9* |
| A1C (%) | 5.7 ± 0.5 | 10.7 ± 2.1† |
| Nondiabetic Ang2LacZ | Diabetic Ang2LacZ | |
| n | 6 | 6 |
| Glucose level (mg/dl) | 165.5 ± 26.1 | 582.7 ± 42.5§ |
| Body weight (g) | 34.2 ± 3.5 | 24.3 ± 4.5* |
| A1C (%) | 5.4 ± 0.1 | 11.1 ± 1.9† |
Metabolic data of nondiabetic and diabetic mice. Diabetic XLacZ (*P < 0.001), mOpsinhAng2 (*P < 0.01, †P < 0.001), and Ang2LacZ mice (*P < 0.05, †P < 0.01, §P < 0.001) were compared with their nondiabetic litters.
After 6 months of hyperglycemia, diabetic mOpsinhAng2 mice showed significantly elevated levels of blood glucose (nondiabetic versus diabetic mOpsinhAng2 mice: 184.6 ± 19.0 and 575.0 ± 40.9 mg/dl, P < 0.001) and A1C (nondiabetic versus diabetic mOpsinhAng2 mice: 5.7 ± 0.5 and 10.7 ± 2.1%, P < 0.001) compared with their nondiabetic litters. Body weights of diabetic mOpsinhAng2 mice were significantly reduced (nondiabetic versus diabetic mOpsinhAng2 mice: 34.5 ± 4.6 and 28.0 ± 3.9 g, P < 0.01, Table 1).
In diabetic Ang2LacZ mice, final blood glucose levels and A1C were also significantly increased (nondiabetic versus diabetic Ang2LacZ: 165.5 ± 26.1 and 582.7 ± 42.5 mg/dl blood glucose, P < 0.001, and 5.4 ± 0.1 and 11.1 ± 1.9% A1C, P < 0.01), and chronic hyperglycemia led to weight loss of 29% in diabetic Ang2LacZ mice (nondiabetic versus diabetic Ang2LacZ: 34.2 ± 3.5 and 24.3 ± 4.5 g, P < 0.05, Table 1).
Differential pericyte loss in a pericyte subpopulation.
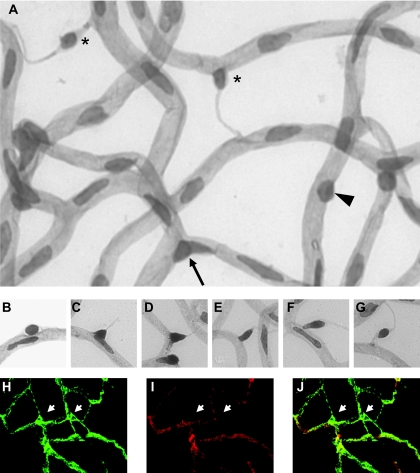
Pericytes show variable phenotypes. As demonstrated in Fig. 1A, pericytes were located either at capillary branches or on straight capillaries. Pericytes on straight capillaries showed small, round, or elongated nuclei. Notably, one type of pericyte on straight capillaries appeared to have less contact to the endothelium. Figure 1B–G illustrate a pericyte migration continuum process from broad to less contact to the underlying endothelium, which we frequently found at different stages in our studies. Pericytes in Fig. 1D–G were defined as migrating pericytes. To examine the relationship between pericyte and the adjacent endothelium during the migration process and to exclude artifacts of the digestion procedure, NG2 (green)/lectin (red) double stainings of retinal whole mounts were performed (Fig. 1H–J). In contrast to pericytes located on capillaries, migrating pericytes and their extensions to neighboring vessels were solely positive for NG2, suggesting that these structures derive from pericytes and that this pericyte phenotype is neither the consequence of vasoregression nor due to the digestion procedure.
FIG. 1.
Illustration of pericyte subpopulations according to their location and contact to capillaries (A) and of pericyte migration process (B–G) in retinal digest preparations. Arrow in A: B-PCs, pericytes at branches; arrowhead in A: S-PCs, pericytes on straight capillaries with broad contact to endothelium; asterisks in A: M-PCs, migrating pericytes on straight capillaries with less contact to endothelium. The continuum of pericyte migration process is depicted in Fig. 1B–G. According to our morphological definition, pericytes were considered as migrating pericytes (M-PCs), since at least one lateral side of the triangular nuclei was longer than the basis in contact to the capillary (Fig. 1D–G). Migrating pericytes usually extended processes to neighboring capillaries. Confocal microscopy of NG2 (green)/lectin (red) stained retinal whole mounts identified pericytes (arrows in H–J) and their processes to be dissociated from the underlying endothelium (Fig. 1H–J). H: NG2–fluorescein isothiocyanate staining; I: lectin-TRITC staining; J: merged image of H and I. Original magnification: 400×. (Please see http://dx.doi.org/10.2337/db08-0325 for a high-quality digital representation of this figure.)
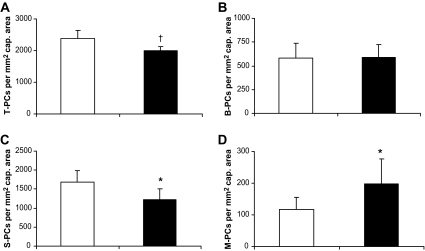
To assess the effect of elevated blood glucose on pericyte loss, we analyzed pericyte coverage in retinal digest preparations of diabetic XLacZ mice after 6 months of experimental hyperglycemia. We found a 16% reduction of total pericyte numbers after 6 months of hyperglycemia in diabetic XLacZ mice compared with their nondiabetic littermates (nondiabetic versus diabetic: 2,374 ± 257 and 2,002 ± 136 per mm2 of capillary area, P < 0.01, Fig. 2A). Analysis of pericyte subgroups revealed that pericytes on vessel branches (B-PCs) were completely unaffected by chronic hyperglycemia (nondiabetic versus diabetic: 580 ± 157 and 586 ± 139 per mm2 of capillary area, Fig. 2B). We found that the loss of pericytes in diabetic retinas was limited to pericytes on straight capillaries (S-PCs). Numbers of S-PCs were reduced by 27% (nondiabetic versus diabetic: 1,676 ± 316 and 1,218 ± 282 per mm2 of capillary area; P < 0.05, Fig. 2C), and this reduction completely explained the reduction in total pericyte numbers in diabetic animals. Hyperglycemia-induced reduction of pericytes was due to a loss of pericytes located on straight capillaries, accompanied by increased numbers of pericytes detaching preferably from straight parts of retinal capillaries. Hyperglycemia led to increased numbers of pericytes that were involved in morphologically defined migration processes (M-PC). The numbers of M-PCs increased by >69% in diabetic XLacZ retinas after 6 months of hyperglycemia compared with nondiabetic XLacZ retinas (nondiabetic versus diabetic: 117 ± 38 and 198 ± 79 per mm2 of capillary area; P < 0.05, Fig. 2D).
FIG. 2.
Quantitation of pericyte subpopulations in nondiabetic and diabetic XLacZ mice. Total numbers of pericytes (T-PC, A), numbers of pericytes at branches (B-PC, B), pericytes on straight capillaries with broad contact to endothelium (S-PC, C), and migrating pericytes (M-PC, D) in nondiabetic and diabetic XLacZ mice (□, nondiabetic; ▪, diabetic) after 6 months of hyperglycemia are shown. *P < 0.05, †P < 0.01.
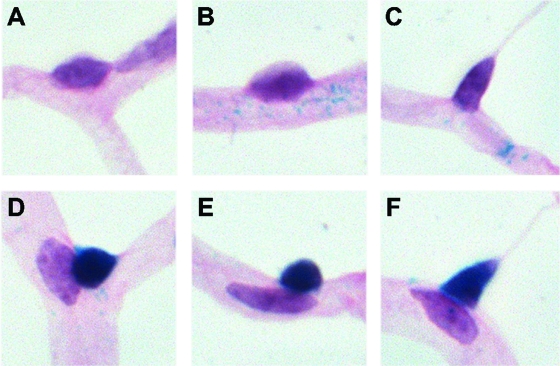
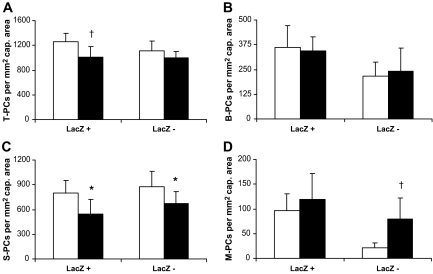
Furthermore, we analyzed pericyte loss in different pericyte subpopulations according to LacZ expression in XLacZ mice. In each subgroup mentioned above, there were LacZ-positive and -negative pericytes, as shown in Fig. 3A–F. Of total pericytes, 52% were stained LacZ positive, both in nondiabetic and diabetic retinas, but only LacZ-positive pericytes were significantly reduced. LacZ-positive pericytes showed a reduction of 20% (nondiabetic versus diabetic: 1,258 ± 134 and 1,008 ± 172 per mm2 of capillary area; P < 0.01, Fig. 4A). Numbers of LacZ-negative pericytes were only insignificantly changed (nondiabetic versus diabetic: 1,115 ± 158 and 994 ± 110 per mm2 of capillary area; P > 0.05, Fig. 4A). There was no change in the numbers of LacZ-positive and LacZ-negative B-PCs (Fig. 4B). The reduction of S-PCs was due to a significant loss of LacZ-positive and LacZ-negative pericytes (Fig. 4C). LacZ-positive S-PCs were reduced by 32% (nondiabetic versus diabetic: 799 ± 152 and 543 ± 181 per mm2 of capillary area; P < 0.05) and LacZ-negative S-PCs were reduced by 23% in diabetic animals (nondiabetic versus diabetic: 878 ± 188 and 674 ± 145 per mm2 of capillary area; P < 0.05). Increased numbers of M-PCs rose from pericytes that were negative for LacZ expression. LacZ-negative M-PCs increased over 2.5-fold (nondiabetic versus diabetic: 21 ± 10 vs. 79 ± 43 per mm2 of capillary area; P < 0.01) in diabetic retinas (Fig. 4D).
FIG. 3.
Illustration (A–F) of pericyte subpopulations considering their location and LacZ expression in XLacZ retinas. Pericytes at branches (A and D), pericytes on straight capillaries with broad contact to endothelium (B and E), and migrating pericytes (C and F) were divided into LacZ-positive (e.g., T-PC+, D–F) and LacZ-negative (e.g., T-PC−, A–C) stained pericytes. Original magnification 400×. (Please see http://dx.doi.org/10.2337/db08-0325 for a high-quality digital representation of this figure.)
FIG. 4.
Quantitation of pericyte subpopulations considering their location and LacZ expression in nondiabetic and diabetic XLacZ retinas. Numbers of total pericytes (T-PC, A) and numbers of B-PCs (B), S-PCs (C), and M-PCs (D) were quantified after 6 months of hyperglycemia (□, nondiabetic; ▪, diabetic). *P < 0.05, †P < 0.01.
Influence of Ang-2 modulation on pericyte migration.
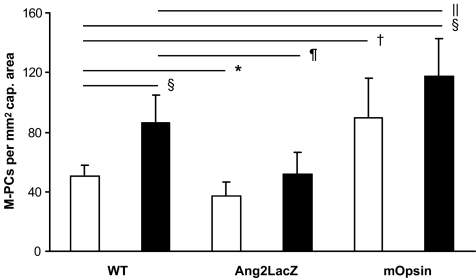
The Ang-Tie system is crucially involved in pericyte recruitment and attachment. Ang-2 is upregulated by hyperglycemia. Therefore, we examined the effect of Ang-2 changes on pericyte migration (Fig. 5).
FIG. 5.
Quantitation of migrating pericytes in nondiabetic and diabetic retinas with modulated Ang-2 expression (□, nondiabetic; ▪, diabetic). *P < 0.05, †P < 0.01, and §P < 0.001 compared with nondiabetic wild-type litters; ‖P < 0.05, ¶P < 0.01 compared with diabetic wild-type litters. Ang2LacZ, downregulation of Ang-2 in Ang2LacZ mice; mOpsin, overexpression of Ang-2 in mOpsinhAng2 mice.
As expected, diabetic WT mice showed significantly increased numbers of migrating pericytes up to 70% compared with nondiabetic WT mice (nondiabetic WT versus diabetic WT: 51 ± 7 and 86 ± 19 pericyte per mm2 of capillary area; P < 0.001). Therefore, the extent of increased pericyte migration in streptozotocin-induced diabetic animals was comparable to that observed in spontaneous diabetic XLacZ mice. Overexpression of Ang-2 in nondiabetic retinas was sufficient to mimic retinal pericyte migration of diabetic WT litters with increased numbers of migrating pericytes of 78% (nondiabetic WT versus nondiabetic mOpsinhAng2: 51 ± 7 and 90 ± 26 pericyte per mm2 of capillary area; P < 0.01). Furthermore, overexpression of Ang-2 in diabetic mice increased the numbers of migrating pericytes by 2.3-fold compared with nondiabetic WT mice (nondiabetic WT versus diabetic mOpsinhAng2: 51 ± 7 and 118 ± 25 pericyte per mm2 of capillary area; P < 0.0001) and by 37% compared with diabetic WT litters (diabetic WT versus diabetic mOpsinhAng2: 86 ± 19 and 118 ± 25 pericyte per mm2 of capillary area; P < 0.05). Quantitation of pericyte migration in nondiabetic Ang-2–deficient mice showed a significant reduction of 36% (nondiabetic WT versus nondiabetic Ang2LacZ: 51 ± 7 and 37 ± 10 pericyte per mm2 of capillary area; P < 0.05) compared with nondiabetic WT mice. Of note, diabetic mice deficient in Ang-2 completely lacked a hyperglycemia-induced increase in pericyte migration (diabetic WT versus diabetic Ang2LacZ: 86 ± 19 vs. 52 ± 15 pericyte per mm2 of capillary area; P < 0.01) compared with diabetic WT litters. Nevertheless, hyperglycemia slightly enhanced pericyte migration in Ang2LacZ mice (nondiabetic Ang2LacZ vs. diabetic Ang2LacZ: 37 ± 10 vs. 52 ± 15 pericyte per mm2 of capillary area).
DISCUSSION
In this study, we describe a novel mechanism of pericyte loss in experimental diabetic retinopathy. We demonstrated that pericyte loss in diabetic retinopathy is restricted to a subset of pericytes located on straight capillaries. Hyperglycemia-induced loss of pericytes, predominantly on straight capillaries, is accompanied by increased numbers of pericytes, migrating from the same location into the perivascular position. Increased numbers of migrating pericytes are LacZ negative. Furthermore, we show that Ang-2 is crucial for pericyte migration, since Ang-2–deficient mice completely lack hyperglycemia-induced pericyte migration, whereas overexpression of Ang-2 mimics increased pericyte migration observed in diabetic retinas.
Experimental long-term hyperglycemia enhances pericyte detachment, leading to reduced pericyte coverage of retinal capillaries. Growing evidence suggests that pericyte detachment and migration from underlying vessels into the perivascular parenchym is a general feature of pericytes in response to different kinds of stress inducers. In brain capillaries, pericytes migrate from capillaries as a result of ischemia, hypoxia, or injury (40,41). In response to traumatic brain injury, ∼40% of capillary pericytes migrated from their microvascular location and remained in a perivascular position, while remaining pericytes on capillaries displayed signs of degeneration. In tracheal capillaries, blockage of VEGF signaling resulted in migration of pericytes from the regressing capillaries onto surviving vessels, leaving behind empty basement membrane tubes, comparable to those observed in diabetic retinopathy (42). These data show that pericyte attachment is actively modulated depending on environmental conditions.
In the diabetic retina, pericyte loss is one of the earliest and most characteristic morphological changes. Our previous data revealed the importance of the Ang-2/Tie-2 system in the regulation of vascular morphological changes in the diabetic retina. In diabetic rat retina, Ang-2 is upregulated before pericyte loss becomes evident, and increased expression of Ang-2 correlates with the depletion of perivascular cells (33,35). Intravitreal injection of Ang-2 results in pericyte dropout, and Ang-2 haploinsufficiency appears to protect against diabetes-induced pericyte dropout. Now, we show that Ang-2 regulates pericyte migration in experimental diabetic retinopathy. In loss of function experiments, we show that Ang-2 is required for hyperglycemia-induced migration of pericytes, since Ang-2–deficient mice lack increased migration of pericytes compared with diabetic WT mice. In addition, gain of function experiments showed that overexpression of Ang-2 enhances pericyte migration in nondiabetic and diabetic animals. The extent of pericyte migration, induced by Ang-2 overexpression in nondiabetic retinas, was similar to the one observed in WT diabetic animals, highlighting the importance of the Ang-Tie system in cellular crosstalk and its involvement in the earliest stages of diabetic retinopathy. Nevertheless, we found a trend for a hyperglycemia-induced increase in pericyte migration in Ang-2–overexpressing and –deficient retinas, suggesting that other factors than Ang-2 may be of some importance for pericyte migration. Recent observations in microvascular endothelial cells revealed that high glucose increases the expression of Ang-2 through modification of corepressor mSim3A by methylglyoxal, an important intracellular advanced glycation end product (AGE) (43). Because methylglyoxal is the predominant intracellular AGE in the diabetic retina, this study links biochemical changes of diabetes to increased activation of the Ang-2/Tie-2 system and therefore to our observation that hyperglycemia enhances detachment and migration of pericytes from microvasculature, resulting in the typical pericyte loss of diabetic retinopathy.
Moreover, our data demonstrate that hyperglycemia-induced loss of pericytes is not equal in retinal pericyte subpopulations, such as it is restricted to pericytes located on straight capillaries of the retinal microvasculature. Straight capillaries are the only sites from which pericytes migrate. According to our observations, hyperglycemia reduces the number of S-PCs by ∼460 cells per mm2 of capillary area after 6 months of hyperglycemia and simultaneously increases the numbers of migrating pericytes by ∼80 cells per mm2 of capillary area. The gap between the numbers of lost and migrating pericytes is partly explained by the digestion process, since this eliminates pericytes that are completely dissociated. Furthermore, the retinal digest preparations represent snapshots of the permanently remodeling vasculatures. The quantitative contribution of pericyte migration to pericyte loss in experimental diabetic retinopathy and the destiny of dissociated pericytes remain to be established.
LacZ in XLacZ mice labels ∼52% of all pericytes and is therefore a restricted pericyte marker, such as others. Nevertheless, quantitation of total pericyte numbers and the expression of LacZ showed that only LacZ-positive pericytes are significantly reduced in diabetic retinas. Therefore, quantification of LacZ-expressing pericytes may provide a simplified method for the quantification of pericyte loss in experimental diabetic retinopathy. Furthermore, we showed that increased numbers of migrating pericytes rise from pericytes that are LacZ negative. This is in agreement with published data showing that proliferation and migration of LacZ-expressing cells are associated with transgene downregulation and vessel injury leads to changes in marker expression in pericyte subpopulations (29).
The causes of early pericyte loss in diabetic retinopathy have not been fully delineated. Numerous studies demonstrated that increased levels of glucose and AGEs trigger pericyte death in cell culture and that pericyte apoptosis is increased in human diabetic retinas as well as in experimental diabetic retinopathy (44–47). However, pericyte apoptosis fails to explain the total extent of pericyte loss and its time course in experimental diabetic retinopathy. In animal models of diabetic retinopathy, pericyte apoptosis has been detected at later stages, after more than 6 months of hyperglycemia (31). Not compatible with this observation is that significant pericyte loss is already detectable after 3 months of experimental diabetes (33). Pericyte migration, as an alternative or additional mechanism of pericyte loss in diabetic retinopathy, offers a possible explanation for the discrepancy between total extent of pericyte loss and published data of pericyte apoptosis in the diabetic retina.
In summary, our data provide morphological evidence that pericyte migration represents a novel mechanism of pericyte loss in the diabetic retina. We further show that this mechanism is regulated by signaling via the Ang-2/Tie-2 pathway. The exact mechanism underlying this process and the destiny of resting and migrating pericytes remain to be investigated.
Acknowledgments
This study was supported by grants from the Deutsche Forschungsgemeinschaft (Graduiertenkolleg 880 to F.P. and F.v.H.).
The authors thank Petra Bugert and Nadine Dietrich for technical support. We thank Prof. B. Yard, Dr. H. Köppel, and E. Riedl for fruitful discussions. We thank Regeneron Pharmaceuticals (Tarrytown, NJ) for providing transgenic Ang2LacZ knock-in mice.
Published ahead of print at http://diabetes.diabetesjournals.org on 16 June 2008.
F.P. and Y.F contributed equally to this work.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- 1.Sims DE: Recent advances in pericyte biology: implications for health and disease. Can J Cardiol 7 :431 –443,1991 [PubMed] [Google Scholar]
- 2.Sims DE: Diversity within pericytes. Clin Exp Pharmacol Physiol 27 :842 –846,2000 [DOI] [PubMed] [Google Scholar]
- 3.DeRuiter MC, Poelmann RE, VanMunsteren JC, Mironov V, Markwald RR, Gittenberger-de Groot AC: Embryonic endothelial cells transdifferentiate into mesenchymal cells expressing smooth muscle actins in vivo and in vitro. Circ Res 80 :444 –451,1997 [DOI] [PubMed] [Google Scholar]
- 4.Etchevers HC, Vincent C, Le Douarin NM, Couly GF: The cephalic neural crest provides pericytes and smooth muscle cells to all blood vessels of the face and forebrain. Development 128 :1059 –1068,2001 [DOI] [PubMed] [Google Scholar]
- 5.Rajantie I, Ilmonen M, Alminaite A, Ozerdem U, Alitalo K, Salven P: Adult bone marrow-derived cells recruited during angiogenesis comprise precursors for periendothelial vascular mural cells. Blood 104 :2084 –2086,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sundberg C, Ivarsson M, Gerdin B, Rubin K: Pericytes as collagen-producing cells in excessive dermal scarring. Lab Invest 74 :452 –466,1996 [PubMed] [Google Scholar]
- 7.Thomas WE: Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Rev 31 :42 –57,1999 [DOI] [PubMed] [Google Scholar]
- 8.Cogan DG, Toussaint D, Kuwabara T: Retinal vascular patterns. IV. Diabetic retinopathy. Arch Ophthalmol. 66 :366 –378,1961 [DOI] [PubMed] [Google Scholar]
- 9.Kuwabara T, Cogan DG: Retinal vascular patterns. VI. Mural cells of the retinal capillaries. Arch Ophthalmol 69 :492 –502,1963 [DOI] [PubMed] [Google Scholar]
- 10.Allt G, Lawrenson JG: Pericytes: cell biology and pathology. Cells Tissues Organs 169 :1 –11,2001 [DOI] [PubMed] [Google Scholar]
- 11.Bergers G, Song S: The role of pericytes in blood-vessel formation and maintenance. Neuro-oncology 7 :452 –464,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peppiatt CM, Howarth C, Mobbs P, Attwell D: Bidirectional control of CNS capillary diameter by pericytes. Nature 443 :700 –704,2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joyce NC, Haire MF, Palade GE: Contractile proteins in pericytes. II. Immunocytochemical evidence for the presence of two isomyosins in graded concentrations. J Cell Biol 100 :1387 –1395,1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joyce NC, Haire MF, Palade GE: Contractile proteins in pericytes. I. Immunoperoxidase localization of tropomyosin. J Cell Biol 100 :1379 –1386,1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nehls V, Drenckhahn D: Heterogeneity of microvascular pericytes for smooth muscle type alpha-actin. J Cell Biol 113 :147 –154,1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer HC, Steiner M, Bauer H: Embryonic development of the CNS microvasculature in the mouse: new insights into the structural mechanisms of early angiogenesis. Exs 61 :64 –68,1992 [DOI] [PubMed] [Google Scholar]
- 17.Betsholtz C: Insight into the physiological functions of PDGF through genetic studies in mice. Cytokine Growth Factor Rev 15 :215 –228,2004 [DOI] [PubMed] [Google Scholar]
- 18.Egginton S, Zhou AL, Brown MD, Hudlická O: The role of pericytes in controlling angiogenesis in vivo. Adv Exp Med Biol 476 :81 –99,2000 [DOI] [PubMed] [Google Scholar]
- 19.Gerhardt H, Betsholtz C: Endothelial-pericyte interactions in angiogenesis. Cell Tissue Res 314 :15 –23,2003 [DOI] [PubMed] [Google Scholar]
- 20.Ozerdem U, Stallcup WB: Early contribution of pericytes to angiogenic sprouting and tube formation. Angiogenesis 6 :241 –249,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakui S, Yokoo K, Muto T, Suzuki Y, Takahashi H, Furusato M, Hano H, Endou H, Kanai Y: Localization of Ang-1, -2, Tie-2, and VEGF expression at endothelial-pericyte interdigitation in rat angiogenesis. Lab Invest 86 :1172 –1184,2006 [DOI] [PubMed] [Google Scholar]
- 22.Antonelli-Orlidge A, Smith SR, D'Amore PA: Influence of pericytes on capillary endothelial cell growth. Am Rev Respir Dis 140 :1129 –1131,1989 [DOI] [PubMed] [Google Scholar]
- 23.Hughes S, Chan-Ling T: Characterization of smooth muscle cell and pericyte differentiation in the rat retina in vivo. Invest Ophthalmol Vis Sci 45 :2795 –2806,2004 [DOI] [PubMed] [Google Scholar]
- 24.Armulik A, Abramsson A, Betsholtz C: Endothelial/pericyte interactions Circ Res 97 :512 –523,2005 [DOI] [PubMed] [Google Scholar]
- 25.Bondjers C, He L, Takemoto M, Norlin J, Asker N, Hellström M, Lindahl P, Betsholtz C: Microarray analysis of blood microvessels from PDGF-B and PDGF-Rbeta mutant mice identifies novel markers for brain pericytes. FASEB J 20 :1703 –1705,2006 [DOI] [PubMed] [Google Scholar]
- 26.Bondjers C, Kalén M, Hellström M, Scheidl SJ, Abramsson A, Renner O, Lindahl P, Cho H, Kehrl J, Betsholtz C: Transcription profiling of platelet-derived growth factor-B-deficient mouse embryos identifies RGS5 as a novel marker for pericytes and vascular smooth muscle cells. Am J Pathol 162 :721 –729,2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho H, Kozasa T, Bondjers C, Betsholtz C, Kehrl JH: Pericyte-specific expression of Rgs5: implications for PDGF and EDG receptor signaling during vascular maturation. FASEB J 17 :440 –442,2003 [DOI] [PubMed] [Google Scholar]
- 28.Ozerdem U, Monosov E, Stallcup WB: NG2 proteoglycan expression by pericytes in pathological microvasculature. Microvasc Res 63 :129 –134,2002 [DOI] [PubMed] [Google Scholar]
- 29.Tidhar A, Reichenstein M, Cohen D, Faerman A, Copeland NG, Gilbert DJ, Jenkins NA, Shani M: A novel transgenic marker for migrating limb muscle precursors and for vascular smooth muscle cells. Dev Dyn 220 :60 –73,2001 [DOI] [PubMed] [Google Scholar]
- 30.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U: Pericytes and the pathogenesis of diabetic retinopathy. Diabetes 51 :3107 –3112,2002 [DOI] [PubMed] [Google Scholar]
- 31.Kowluru RA, Odenbach S: Effect of long-term administration of alpha-lipoic acid on retinal capillary cell death and the development of retinopathy in diabetic rats. Diabetes 53 :3233 –3238,2004 [DOI] [PubMed] [Google Scholar]
- 32.Mizutani M, Kern TS, Lorenzi M: Accelerated death of retinal microvascular cells in human and experimental diabetic retinopathy. J Clin Invest 97 :2883 –2890,1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammes HP, Lin J, Renner O, Shani M, Lundqvist A, Betsholtz C, Brownlee M, Deutsch U: Angiopoietin-2 causes pericyte dropout in the normal retina: evidence for involvement in diabetic retinopathy. Diabetes 53 :1104 –1110,2004 [DOI] [PubMed] [Google Scholar]
- 34.Feng Y, vom Hagen F, Pfister F, Djokic S, Hoffmann S, Back W, Wagner P, Lin J, Deutsch U, Hammes HP: Impaired pericyte recruitment and abnormal retinal angiogenesis as a result of angiopoietin-2 overexpression. Thromb Haemost 97 :99 –108,2007 [PubMed] [Google Scholar]
- 35.Ohashi H, Takagi H, Koyama S, Oh H, Watanabe D, Antonetti DA, Matsubara T, Nagai K, Arai H, Kita T, Honda Y: Alterations in expression of angiopoietins and the Tie-2 receptor in the retina of streptozotocin induced diabetic rats. Mol Vis 10 :608 –617,2004 [PubMed] [Google Scholar]
- 36.Watanabe D, Suzuma K, Suzuma I, Ohashi H, Ojima T, Kurimoto M, Murakami T, Kimura T, Takagi H: Vitreous levels of angiopoietin 2 and vascular endothelial growth factor in patients with proliferative diabetic retinopathy. Am J Ophthalmol 139 :476 –481,2005 [DOI] [PubMed] [Google Scholar]
- 37.vom Hagen F, Feng Y, Hillenbrand A, Hoffmann S, Shani M, Deutsch U, Hammes HP: Early loss of arteriolar smooth muscle cells: more than just a pericyte loss in diabetic retinopathy. Exp Clin Endocrinol Diabetes 113 :573 –576,2005 [DOI] [PubMed] [Google Scholar]
- 38.Hackett SF, Wiegand S, Yancopoulos G, Campochiaro PA: Angiopoietin-2 plays an important role in retinal angiogenesis. J Cell Physiol 192 :182 –187,2002 [DOI] [PubMed] [Google Scholar]
- 39.Uhlmann K, Kovacs P, Boettcher Y, Hammes HP, Paschke R: Genetics of diabetic retinopathy. Exp Clin Endocrinol Diabetes 114 :275 –294,2006 [DOI] [PubMed] [Google Scholar]
- 40.Dore-Duffy P, Owen C, Balabanov R, Murphy S, Beaumont T, Rafols JA: Pericyte migration from the vascular wall in response to traumatic brain injury. Microvascular Research 60 :55 –69,2000 [DOI] [PubMed] [Google Scholar]
- 41.Gonul E, Duz B, Kahraman S, Kayali H, Kubar A, Timurkaynak E: Early pericyte response to brain hypoxia in cats: an ultrastructural study. Microvasc Res 64 :116 –119,2002 [DOI] [PubMed] [Google Scholar]
- 42.Baffert F, Le T, Sennino B, Thurston G, Kuo CJ, Hu-Lowe D, McDonald DM: Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol Heart Circ Physiol 290 :H547 –H559,2006 [DOI] [PubMed] [Google Scholar]
- 43.Yao D, Taguchi T, Matsumura T, Pestell R, Edelstein D, Giardino I, Suske G, Rabbani N, Thornalley PJ, Sarthy VP, Hammes HP, Brownlee M: High glucose increases angiopoietin-2 transcription in microvascular endothelial cells through methylglyoxal modification of mSin3A. J Biol Chem 282 :31038 –31045,2007 [DOI] [PubMed] [Google Scholar]
- 44.Chen BH, Jiang DY, Tang LS: Advanced glycation end-products induce apoptosis involving the signaling pathways of oxidative stress in bovine retinal pericytes. Life Sci 79 :1040 –1048,2006 [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Son JW, Lee JA, Oh YS, Shinn SH: Methylglyoxal induces apoptosis mediated by reactive oxygen species in bovine retinal pericytes. J Korean Med Sci 19 :95 –100,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li W, Yanoff M, Liu X, Ye X: Retinal capillary pericyte apoptosis in early human diabetic retinopathy. Chin Med J (Engl) 110 :659 –663,1997 [PubMed] [Google Scholar]
- 47.Yang R, Liu H, Williams I, Chaqour B: Matrix metalloproteinase-2 expression and apoptogenic activity in retinal pericytes: implications in diabetic retinopathy. Ann N Y Acad Sci 1103 :196 –201,2007 [DOI] [PubMed] [Google Scholar]