Abstract
Purpose
Glaucoma is the leading cause of irreversible blindness worldwide. Most of the cases are primary open angle glaucoma (POAG). POAG is a genetically heterogenous disease; autosomal dominance is the most frequent type of monogenic inheritance. In this study, we identified the genotype of a MYOC mutation and investigated the phenotype of a Chinese juvenile-onset open angle glaucoma (JOAG) pedigree (GZ.1 pedigree).
Methods
Blood samples were obtained from 24 participants. We performed sequence and gene linkage analysis in the GZ.1 pedigree retrospectively. Comprehensive ophthalmologic examinations were performed for each family member. Pharmacological treatment or filtering surgery was performed as needed according to the intraocular pressure (IOP) of each individual.
Results
A Pro370Leu myocilin mutation located in exon 3 of MYOC was identified in 24 members of the GZ.1 pedigree. Sixteen patients had juvenile-onset primary open-angle glaucoma (JOAG), and the others participating in the project had no such genotype. Analysis of polymorphic microsatellite markers indicated that the disease in GZ.1 is autosomal dominant inheritance. The patients in GZ.1 are characterized by early age of onset (before 35 years of age), severe clinical presentations, and high intraocular pressure unresponsive to pharmacological treatment; requiring 89.5% of the patients to undergo filtering surgery. Fortunately, the success rate of surgery was high. None of the patients required further medical treatment and only one demonstrated low IOP fundus changes.
Conclusions
This is the first evidence of a founder effect for a Pro370Leu myocilin mutation in a Chinese POAG pedigree. The family with the Pro370Leu myocilin mutation presents with juvenile-onset glaucoma. After 10 years of follow-up, it is evident that the mutation is closely associated with the phenotype of the patients. Analysis of MYOC in JOAG patients may enable the identification of at-risk individuals and help prevent disease progression toward the degeneration of the optic nerve, and may also contribute to genetic counseling.
Introduction
Glaucoma, one of the leading causes of blindness, is a chronic neurodegenerative disease that will affect over 60 million people worldwide by 2010 [1]. The disease is characterized by painless, progressive, irreversible degeneration of the optic nerve and loss of visual field. Elevated intraocular pressure (IOP) resulting from the increased aqueous outflow resistance in the trabecular meshwork is a major risk factor. Thus, pharmacological and surgical treatments aim to facilitate aqueous outflow and are essential for normalizing IOP.
Primary open-angle glaucoma (POAG) is the most common form of glaucoma, especially in North America [2], representing more than half of all cases. Although the underlying etiology of POAG is unknown, there is evidence that gene mutations can be associated with this disease. According to an epidemiological survey, about 30%–56% of patients with POAG and ocular hypertension (OHT) have a family history, and the incidence in individuals with a first degree relative having glaucoma is about 7–10 times higher than in the general population [3].
Based on age at time of diagnosis, POAG is classified as either adult- or juvenile-onset, with 35 years of age being the boundary. Most cases of POAG follow a complex pattern of inheritance, while juvenile-onset primary open-angle glaucoma (JOAG) typically shows an autosomal dominant inheritance. The phenotype of POAG is also different from JOAG. Generally, the high intraocular pressure in POAG is stable with pharmacological treatment, but JOAG is usually a much more severe disease requiring surgery to avoid loss of sight [4].
Since the first POAG-correlated mutation gene (trabecluar meshwork-inducible glucocorticoid response/myocilin; TIGR/MYOC) was identified in 1997 [5,6], there have been three genes reported to be responsible for POAG, i.e., TIGR/MYOC, OPTN (optic neuropathy inducing gene) [7,8], and WDR36 (WD repeat domain 36 gene) [9], with TIGR/MYOC being the most frequently mutated gene [10-13]. In this case, the myocilin protein is mutated and its abnormal function increases the resistance of the aqueous humor outflow, leading to high IOP. This results in the degeneration of the optic nerve and visual field loss [14-16]. Studying the correlation between genotype and phenotype will contribute to an improved understanding of POAG.
Here, we report the analysis of MYOC mutations and describe clinical findings in a large Chinese autosomal dominant JOAG family (GZ.1). GZ.1 is a Pro370Leu mutation encompassing 56 family members with 19 of them exhibiting JOAG that is unresponsive to standard pharmacological treatments.
Methods
Subjects
The GZ.1 pedigree lives in Guangdong province, China and spans 5 generations with 56 members. The proband (III7) was tested in 1999 and diagnosed with JOAG, after which point we did an extended examination of family members. We discovered that affected individuals with documented bilateral glaucoma were present in each generation except generation V. The total number of JOAG cases is 14 (including deceased patients; I2 and II1). During follow up spanning the next 10 years, additional patients were diagnosed with JOAG; between 2000 and 2002, 2 individuals were diagnosed, and 3 more during the interval from 2003 to 2008.
In this study, we retrospectively analyze the genotype and phenotype of the GZ.1 pedigree. The study was done in accordance with the principles of the Declaration of Helsinki. Informed parental consent, informed patient consent, and approval by the Hospital Ethics Committee (Zhongshan Ophthalmic Centre, Sun Yat-sen University) were obtained before initiating the study.
Diagnostic criteria
The initial patient with primary open angle glaucoma (POAG) had an intraocular pressure (IOP) of 22 mmHg or higher (in absence of IOP lowering therapy), an open anterior chamber angle on gonioscopy, glaucomatous optic disc features, and visual field alteration consistent with assessed optic neuropathy. Diagnosis of juvenile-onset open angle glaucoma (JOAG) was given when patients were younger than 35 years of age at the time of POAG diagnosis. An IOP above 21 mmHg (without IOP lowing therapy) in the absence of damage to the optic nerve and loss of visual field is diagnosed as ocular hypertension (OHT).
Clinical examination
Comprehensive ophthalmologic examinations and general medical history were taken and documented by the same two experienced doctors (Zhongshan Ophthalmic Centre, Sun Yat-sen University). The protocol included the best-corrected visual acuity with Snellen charts, slit-lamp inspection of the anterior eye, IOP measurement by Goldmann application tonometry, anterior chamber angle evaluation by gonioscopy (Goldmann), and fundus examination by 78-diopter Hruby lens including vertical and horizontal optic cup disc ratio (C/D ratio) assessment. All subjects underwent automated visual field examination (tested with Humphrey, SITA fast strategy, program 30–2). The Optical Coherence Tomography (OCT) and color fundus photographs of the disc and macula were tested to aid with assessment of the patient’s visual condition and stage of illness.
Genomic DNA extraction
Peripheral blood leukocytes were obtained from all available family members, including 16 affected and 8 unaffected individuals. Genomic DNA was extracted from peripheral blood as recommended in the QIAamp DNA Blood Max Kit (QIAGEN, Hilden, Germany).
Microsatellite marker analysis
A genome-wide scanning was performed using a set of fluorescence-labeled microsatellite markers spanning the entire human genome at approximately 10 cM intervals with the ABI PRISM Linkage Mapping Set MD-10 (Applied Biosystems Inc.). DNA samples were subjected to polymerase chain reaction (PCR) amplification with a standard cycling profile of 30 cycles at 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 45 s. PCR reactions were performed in a 10 μl volume containing 0.4 mM of each primer, 200 μM dNTPs, and 1U Taq DNA polymerase. The PCR products were separated on 5% denaturing polyacrylamide gel in an Applied Biosystems 377 DNA sequencer (Applied Biosystems Inc.).
Linkage analysis
Linkage analysis was performed by calculating two-point lod scores using the MLINK routine from LINKAGE (ver. 5.1) software suite (provided in the public domain by the Human Genome Mapping Project Resources Center, Cambridge, UK). LOD scores were calculated with a presumed penetration rate of 95% and an allele frequency of 0.001 for the disease allele.
Mutation screening
To examine the probability of the existence of disease-associated mutations, exon-specific primers were designed for the myocilin gene (GenBank AB006686). PCR amplification was performed in a GeneAmp PCR System 9700 (Applied Biosystems Inc.), with 100 ng of genomic DNA, 10 pmol of primers and 1U of Taq DNA polymerase in a reaction volume of 20 μl. Primer sequences and their PCR product sizes are given in Table 1. Samples were subjected to a PCR amplification protocol beginning with a denaturation step at 94 °C for 2 min, followed by 30 cycles, each consisting of a denaturation step at 94 °C for 30 s, an annealing step at about 55 °C-58 °C for 30 s, and an extension step at 72 °C for 30 s, followed by a final extension at 72 °C for 8 min. The amplified exons were purified and sequenced on an automated DNA sequencer (model 377; Applied Biosystems Inc.). All PCR products were sequenced in both forward and reverse directions.
Table 1. Sequences of primers used for mutation screening of myocilin gene.
| Name | Primer sequence | Product Length (bp) |
|---|---|---|
| E1A | 5′-TATTTTCTAAGAATCTTGCTGG-3′ | 394 |
| 5′-TGGATTCATTGGGACTGG-3′ | ||
| E1B | 5′-GAAGCCTCACCAAGCCTC-3′ | 342 |
| 5′-GCCTGGTCCAAGGTCAAT-3′ | ||
| E1C | 5′-CTGGAGGCCACCAAAGCT-3′ | 448 |
| 5′-AGAAAGGGCAGGCAGGGA-3′ | ||
| E2 | 5′ CATAGTCAATCCTTGGGC-3′ | 392 |
| 5′-CTGCAGACCTGCTCTGACAA-3′ | ||
| E3A | 5′-TTTCTGAATTTACCAGGATG-3′ | 426 |
| 5′-GTCAATGTCCGTGTAGCC-3′ | ||
| E3B | 5′-CGGACAGTTCCCGTATTC-3′ | 431 |
| 5′-GCTTGGAGGCTTTTCACA-3′ | ||
| E3C | 5′-CAAGACCCTGACCATCCC-3′ | 412 |
| 5′-TGCCCCAAATCACAAGAA-3′ |
Clinical management
Topical medication was given to patients with an IOP higher than 21 mmHg. Patients whose IOP could not be controlled with medicine underwent combined trabeculectomy. After surgery, patients were followed closely until their IOP was stable. The assessment included: the best-correct visual acuity, IOP, the depth of the anterior chamber, filtering bleb morphous, C/D ratio, visual field, and OCT.
Results
Genotype of the GZ.1 pedigree
A MYOC Pro370Leu mutation was identified in 16 affected individuals of the GZ.1 pedigree, and the rate of occurrence of mutation was 100%. According to the distribution of the affected family members (Figure 1), the heredity of the GZ.1 pedigree is autosomal dominant.
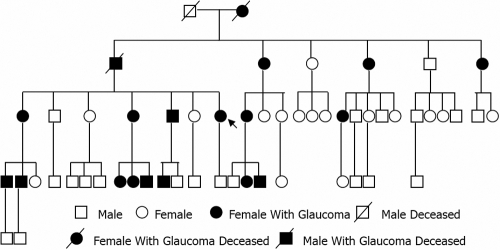
Figure 1.
Pedigree structure of the Chinese family with 19 affected subjects in 5 generations. Squares indicate male subjects; circles, female subjects; solid symbols, affected family members with JOAG; unfilled symbols, unaffected family members; diagonal line, deceased individual. Arrow indicates the proband (III:7). According to the distribution of the affected members, the heredity of the GZ.1pedigree is autosomal dominant.
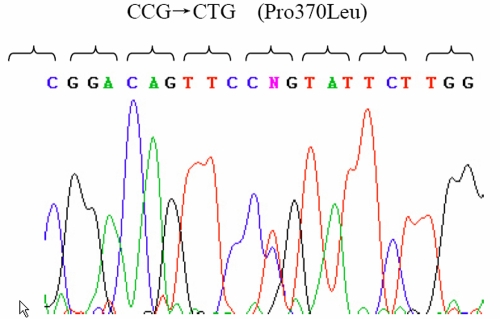
Linkage analysis and Haplotype analysis demonstrated that all affected individuals were heterozygous for this change (Figure 2). Two-point LOD scores of markers in this region are summarized in Table 2. A maximum LOD score of 5.46 was found for marker D1S2818 at 0.0. This marker is located in the close vicinity of MYOC. Mutation analysis of this gene showed a heterozygous C->T transversion at nucleotide 1,109 in exon 3, resulting in a substitution of Proline to Leucine (Pro370Leu; Figure 3). This mutation cosegregated in all affected individuals and was not observed in unaffected subjects.
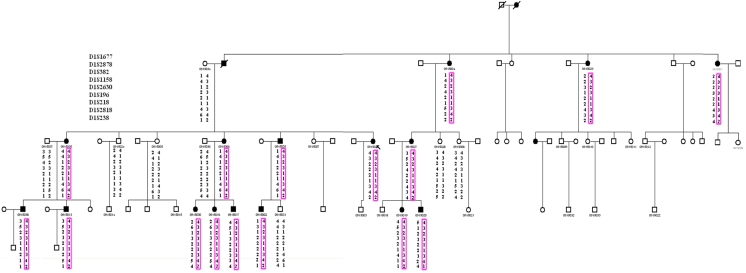
Figure 2.
Haplotype analysis of the GZ.1 pedigree using microsatellite markers encompassing MYOC on the long arm of chromosome 1. MYOC resides between markers D1S196 and D1S2818. Square cells refer to males and circle cells refer to females; Filled cells are JOAG; diagonal line, deceased; and arrow, proband. The analysis was performed in 24 informative family members including 16 affected and 8 unaffected individuals. Segregating haplotypes are shown in the rectangles.
Table 2. Two-Point LOD Scores of the 9 DNA Markers in the genome-wide scan of the GZ.1 pedigree.
| Marker |
Two-point LOD score values at recombination fraction (θ=) |
Zmax | θmax | |||||
|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | |||
| D1S1677 |
-4.21 |
1.37 |
1.38 |
1.00 |
0.45 |
0 |
1.38 |
0.2 |
| D1S 2878 |
4.82 |
3.98 |
3.05 |
2.03 |
0.93 |
0 |
4.82 |
0.0 |
| D1S 382 |
1.52 |
1.25 |
0.96 |
0.65 |
0.32 |
0 |
1.52 |
0.0 |
| D1S 1158 |
-1.51 |
1.39 |
1.31 |
0.98 |
0.54 |
0 |
1.39 |
0.1 |
| D1S 2630 |
1.85 |
1.62 |
1.09 |
0.51 |
0.09 |
0 |
1.85 |
0.0 |
| D1S 196 |
-2.37 |
0.50 |
0.52 |
0.34 |
0.11 |
0 |
0.52 |
0.2 |
| D1S 218 |
3.00 |
2.88 |
2.38 |
1.65 |
0.78 |
0 |
3.00 |
0.0 |
| D1S 2818 |
5.46* |
4.52 |
3.48 |
2.34 |
1.09 |
0 |
5.46 |
0.0 |
| D1S 238 | -5.5 | 0.84 | 0.80 | 0.59 | 0.32 | 0 | 0.84 | 0.1 |
Microsatellite markers with an average spacing of 10 cM were used in the initial genome-wide scan. The asterisk indicates the promising chromosome region on 1q21-q23 within a marker presented a suggestive LOD score value of Zmax=5.46 at θ=0.00.
Figure 3.
Detection of the Pro370Leu MYOC mutation by direct polymerase chain reaction DNA sequencing in the GZ.1 pedigree. Representative chromatogram contains sequence from the noncoding DNA strand. The location of the mutation within MYOC and the nature of the nucleotide change (C->T ) were shown as a double peak in the heterozygous condition.
Using a computer sequence alignment program (BLAST), amino acid sequences of MYOC obtained from GenBank were compared among human, rat, mouse, bovine and fugu. The comparison revealed that Pro370Leu occurred at a highly conserved position of the myocilin gene (Table 3).
Table 3. Comparison of amino acid sequences of myocilin between human, rat, mouse, bovin, fugu, and dare. The result revealed Pro370Leu occurred at highly conserved positions (bold P).
| Protein | Amino acid 370 |
|---|---|
| Myoc_human | ETVKAEKEIPGAGYHGQF P YSWGGYTD |
| Myoc_rat | ETVKAEKEIPGAGYHGQF P YAWGGYTD |
| Myoc_mouse | ETVKAEKEIPGAGYHGHF P YAWGGYTD |
| Myoc_bovin | ETLKAEKEIPGAGYHGQF P YSWGGYTD |
| Myoc_fugu | ESLAARLDLPHAGFHGQH P YSWGGYTD |
| Myoc_dare | ESIAARRDLPHAGFHGQF P YSWGGYTD |
Clinical phenotype of the GZ.1 pedigree
We studied an autosomal dominant family (GZ.1 pedigree) with 17 POAG patients; 31.48% of all family members. Among the affected individuals, 6 of are male and 11 are female. I2 and II1 were deceased at the time of our study, but their medical records provided adequate information concerning their ocular disease.
The onset of disease with all these patients was insidious. The average age at diagnosis was 30 years (ranging from 11 to 35 years), and the mean IOP before medical or surgical care at the time of last follow up was 45.52±6.39 mmHg (range from 35 to 56 mmHg). All of the patients exhibited severe degeneration of the optic nerve and visual field defects (Figure 4). According to our investigation, most of the JOAG patients were unresponsive to antiglaucoma medications; filtering surgery was often required for long-term IOP control. At the time of the study, 17 patients (34 eyes) were operated on to control IOP with combined trabeculectomy; none of them needed a second operation. All of the surgeries were done in the Zhongshan Ophthalmic Center, Sun Yat-sen University by experienced doctors. One OHT patients (IV21) was under treatment with medication.
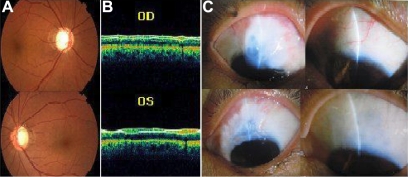
Figure 4.
Individual III5 (top) right eye, (bottom) left eye. A: Fundus images showing typical glaucomatous cupping of the optic disc, B: OCT images showing thinner nerve fiber layer, C: Thin-walled filtering blebs after filtering surgery.
After surgery, all participants obtained their target IOP; none of them requires any medications and all appear to be prone to filtering bleb scarring. It is gratifying that the postoperative IOP in both eyes of each patient has been controlled at around 10 mmHg without any severe complications, with the exception of one patient with low IOP fundus changes.
During the 10 years of follow up with the GZ.1 pedigree, we discovered an unusual phenomenon: with the passage of each generation, the age of onset tended to be younger, the pathogenetic condition tended to be more severe, and the post-operation IOP declined further. All of generation II and III patients exhibited type II filtering blebs, while all of generation IV patients exhibited type I filtering blebs (Figure 4). The condition of the patients at the last follow up can be seen in Table 4.
Table 4. Phenotype characteristic of the 17 patients with JOAG from GZ.1 pedigree at last follow up.
| Subject number | Age | Gender | BCVA | IOP (mmHg) | C/D | VF | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RE | LE | RE | LE | RE | LE | RE | LE | |||||
| V | H | V | H | |||||||||
| II2 | 74 | F | 0.3 | NLP | 17.1 | 17.5 | 0.8 | 0.8 | 0.9 | 0.9 | N/A | N/A |
| II4 | 65 | F | 0.3 | 0.5 | 7 | 22.6 | 0.6 | 0.7 | 0.9 | 0.8 | PC | IZD |
| II6 | 58 | F | 1.0 | 1.2 | 15.6 | 12.1 | 0.4 | 0.5 | 0.6 | 0.7 | N/A | N/A |
| III1 | 59 | F | 0.4 | 0.7 | 16.3 | 13.7 | 0.9 | 0.8 | 0.9 | 0.8 | N/A | N/A |
| III4 | 48 | F | 1.5 | 1.2 | 27.3 | 23 | 0.5 | 0.5 | 0.7 | 0.7 | N | N |
| III5 | 44 | M | LP | 1.2 | 19 | 18 | 1.0 | 1.0 | 0.8 | 0.7 | N/A | N/A |
| III7 | 38 | F | 1.5 | 0.1 | 14 | 15.4 | 0.4 | 0.4 | 1.0 | 0.9 | N/A | N/A |
| III8 | 55 | F | 1.0 | 0.6 | 13 | 16 | 0.5 | 0.6 | 0.95 | 0.85 | N/A | N/A |
| III19 | 35 | M | 1.5 | 1.2 | 15.2 | 17.9 | 0.2 | 0.2 | 0.2 | 0.2 | N/A | N/A |
| IV1 | 37 | M | 0.1 | LP | 16 | 19 | 0.9 | 1.0 | 1.0 | 1.0 | N/A | N/A |
| IV2 | 36 | M | 1.5 | 1.5 | 14 | 19 | 0.2 | 0.2 | 0.4 | 0.5 | N/A | N/A |
| IV8 | 26 | F | 1.0 | 1.2 | 11 | 19 | 1.0 | 1.0 | 0.9 | 0.9 | IZD | IZD |
| IV9 | 21 | F | 1.5 | 1.5 | 28 | 26 | 0.3 | 0.4 | 0.5 | 0.5 | N | N |
| IV10 | 19 | M | 1.5 | 1.2 | 36 | 37 | 0.9 | 0.8 | 0.55 | 0.6 | IZD | N |
| IV11 | 18 | M | 1.5 | HM | 8 | 7 | 0.5 | 0.4 | 0.3 | 0.3 | N/A | N/A |
| IV16 | 31 | F | 1.0 | 0.9 | 10 | 16 | 0.5 | 0.5 | 1.0 | 1.0 | N/A | N/A |
| IV17 | 32 | M | 0.5 | 0.6 | 8 | 8 | 0.8 | 0.8 | 0.8 | 0.8 | N/A | N/A |
The Pro370Leu mutation was present in all subjects in the table. All patients in the table had undergone trabeculectomy surgery to both eyes. Abbreviations in the table are: JOAG; juvenile open-angle glaucoma, BCVA; best-correct visual activity, RE; right eye, LE; left eye, IOP; intraocular pressure, C/D; cup disk ratio, V; vertical, H; horizontal, VF; visual field, F; female, M; male, OU; both eyes, N; normal, IZD; indicated inferior zone defect, NLP; no light perception, LP; light perception, and HM; hand movement, PC; poor cooperation, N/A; unavailable.
Discussion
MYOC was the first gene in which mutations were found to cause glaucoma. MYOC mutations account for most dominant juvenile glaucoma cases and for approximately 2% to 4% of unselected adult onset POAG [6]. More than 70 missense mutations in MYOC have been identified, with the majority of them being clustered in the conserved olfactomedin domain of exon 3 [17,18]. In this study, we report linkage of autosomal dominant open-angle glaucoma in a large Chinese family to a region on chromosome 1 between D1S218 and D1S2818. A missense mutation (Pro370Leu) in exon 3 of MYOC, one of the candidate genes mapped in this region, was identified in this family to cosegregate with the glaucoma phenotype. No other sequence changes were found in the entire coding region or splice junctions of MYOC in this family. All subjects with a diagnosis of POAG had this mutation. It is reasonable to decide, then, that this variant is pathogenic. The findings in the current study enrich the evidence for MYOC as a causative gene for POAG.
It has been observed that there might be some correlation between clinical phenotypes and different mutations in MYOC. A nonsense mutation Gln368STOP, the most frequently identified GLC1A mutation, is associated with late-onset POAG. The later age at diagnosis of 52 years and the lower mean peak IOP of 28 mmHg suggest that the Gln368STOP mutation gives rise to a more mild phenotype than mutations associated with juvenile open-angle glaucoma [19]. The Thr377Met mutation results in a more severe phenotype of the disease than the Gln368Stop mutation. Wiggs et al. [20] described a family in which the proband was diagnosed at age 42 years with an IOP at diagnosis of 24 mmHg. Shimizu et al. [21] described a family with a mean age at diagnosis of 38 years and a mean maximum IOP of 44 mmHg.
It is noteworthy that the Pro370Leu mutation has been reported to be widely distributed and found by multiple research groups in different locations, with data demonstrating juvenile-onset glaucoma in French, Japanese, North American, German, and Indian families [22-25]. JOAG is an uncommon autosomal dominant form of glaucoma. Onset occurs most often before the fourth decade of life and the phenotype is clinically more severe than late-onset POAG. Most of the reported pedigrees linked to Pro370Leu had the following characteristics: (1) development of POAG at a very early age, (2) high peak levels of IOP, and (3) poor response to medical treatment [22-25]. The phenotype of POAG associated with the Pro370Leu mutation was thoroughly assessed in our data set. The mutation carriers with glaucoma seen in this study had a form of early adult-onset glaucoma associated with elevated IOP. The age at diagnosis of POAG in mutation carriers ranged from 11 to 35 years (mean 30 years). Without medication, the patients with mutation presented greater median IOP of 45.52±6.39 mmHg. Most of the patients were unresponsive to antiglaucoma medications and filtering surgery was usually required for long-term IOP control. Thus the Pro370Leu mutation in this Chinese family presents a similar clinical phenotype as previously reported. However, there are some new clinical features; the age of onset became younger with the passage of each generation. Carriers were 44.5 years (range, 36–56 years) in generation II, while in generation III the mean age at diagnosis in 11 cases was 34.4 years (range, 30–44 years). Therefore, we believe that Pro370Leu might represent a severe and strong disease allele in Chinese peoples, exhibiting an earlier onset and more aggressive glaucoma phenotype.
So far, little has been known about the exact roles played by myocilin in the development of POAG. Current studies show that the mutant myocilin is not correctly folded into a functional conformation and accumulates into aggregates inside TM cells [26-28]. According to the structure of myocilin, Pro370Leu is located within the highly conserved OLF-domain of this protein, a major component of the extracellular matrix of the olfactory neuroepithelium [28]. Interestingly, this region contains most the reported mutations identified in patients with POAG. These factors together suggest that the domain is very important for the function of this protein.
Based on our evidence, we believe that genotyping will have predictive value, at least in cases analogous to the GZ.1 pedigree, where all of the affected patients have the Pro370Leu mutation, which can then serve to predict JOAG. The early detection of the at-risk individual, will allow the adoption of optimal measures to prevent the progress of the disease [29].
In conclusion, our data provide strong evidence of a founder effect for the Pro370Leu MYOC mutation in a Chinese family and show that the genetic analysis of this mutation could play a key role in the management of autosomal dominant JOAG in affected families from this country. The genetic analysis of MYOC in this family could not only be used to identify at-risk persons decades before the disease manifests phenotypically, but also to aid in genetic counseling. Next, we will use the genetic mutations in an animal model to explore further the mechanisms of disease.
ACKNOWLEGMENTS
We are most grateful to the family members for their enthusiastic participation in the project. This work was supported by grants from the Foundation for the Author of National Basic Research Program of China (973 Program, No.2007CB512200), National Excellent Doctoral Dissertation of China (No. 200363), the Natural Scientific Foundation of China (No.30700928) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (The Project-sponsored by SRF for ROCS, SEM [2006]331).
References
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Quigley HA, Vitale S. Models of open angle glaucoma prevalence and incidence in the United States. Invest Ophthalmol Vis Sci. 1997;38:83–91. [PubMed] [Google Scholar]
- 3.WuDunn D. Genetic basis of glaucoma. Curr Opin Ophthalmol. 2002;13:55–60. doi: 10.1097/00055735-200204000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Spaeth GL. Ophthalmic surgery principles and practice [M]. Philadelphia: Saunders, 1982, 18–31. [Google Scholar]
- 5.Sheffield VC, Stone EM, Alward WL, Drack AV, Johnson AT, Streb LM, Nichols BE. Genetic linkage of familial open angle glaucoma to chromosome 1-q21-q23. Nat Genet. 1993;4:47–50. doi: 10.1038/ng0593-47. [DOI] [PubMed] [Google Scholar]
- 6.Stone EM, Fingert JH, Alward WL, Nguyen TD, Polansky JR, Sunden SL, Nishimura D, Clark AF, Nystuen A, Nichols BE, Mackey DA, Ritch R, Kalenak JW, Craven ER, Sheffield VC. Identification of a gene that causes primary open angle glaucoma. Science. 1997;275:668–70. doi: 10.1126/science.275.5300.668. [DOI] [PubMed] [Google Scholar]
- 7.Sarfarazi M, Child A, Stoilova D, Brice G, Desai T, Trifan OC, Poinoosawmy D. Localization of the fourth locus (GLC1E) for adult-onset primary open-angle glaucoma to the 10p15-p14 region. Am J Hum Genet. 1998;62:641–52. doi: 10.1086/301767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rezaie T, Child A, Hitchings R, Brice G, Miller L, Coca-Prados M, Heon E, Krupin T, Ritch R, Kreutzer D, Crick RP, Sarfarazi M. Adult-onset primary open-angle glaucoma caused by mutations in optineurin. Science. 2002;295:1077–9. doi: 10.1126/science.1066901. [DOI] [PubMed] [Google Scholar]
- 9.Monemi S, Spaeth G, DaSilva A, Popinchalk S, Ilitchev E, Liebmann J, Ritch R, Heon E, Crick RP, Child A, Sarfarazi M. Identification of a novel adult-onset primary open-angle glaucoma (POAG) gene on 5q22.1. Hum Mol Genet. 2005;14:725–33. doi: 10.1093/hmg/ddi068. [DOI] [PubMed] [Google Scholar]
- 10.Stoilova D, Child A, Brice G, Desai T, Barsoum-Homsy M, Ozdemir N, Chevrette L. Novel TIGR/MYOC mutations in families with juvenile onset primary open angle glaucoma. J Med Genet. 1998;35:989–92. doi: 10.1136/jmg.35.12.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fingert JH, Heon E, Liebmann JM, Yamamoto T, Craig JE, Rait J, Kawase K. Analysis of myocilin mutations in 1703 glaucoma patients from five different populations. Hum Mol Genet. 1999;8:899–905. doi: 10.1093/hmg/8.5.899. [DOI] [PubMed] [Google Scholar]
- 12.Pang CP, Leung YF, Fan B, Baum L, Tong WC, Lee WS, Chua JK. TIGR/MYOC gene sequence alterations in individuals with and without primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2002;43:3231–5. [PubMed] [Google Scholar]
- 13.Hewitt AW, Bennett SL, Fingert JH, Cooper RL, Stone EM, Craig JE, Mackey DA. The optic nerve head in myocilin glaucoma. Invest Ophthalmol Vis Sci. 2007;48:238–43. doi: 10.1167/iovs.06-0611. [DOI] [PubMed] [Google Scholar]
- 14.Clark AF, Steely HT, Dickerson JE, Jr, English-Wright S, Stropki K, McCartney MD, Jacobson N. Glucocorticoid induction of the glaucoma gene MYOC in human and monkey trabecular meshwork cells and tissues. Invest Ophthalmol Vis Sci. 2001;42:1769–80. [PubMed] [Google Scholar]
- 15.Tawara A, Okada Y, Kubota T, Suzuki Y, Taniguchi F, Shirato S, Nguyen TD. Immunohistochemical localization of MYOC/TIGR protein in the trabecular tissue of normal and glaucomatous eyes. Curr Eye Res. 2000;21:934–43. doi: 10.1076/ceyr.21.6.934.6988. [DOI] [PubMed] [Google Scholar]
- 16.Fautsch MP, Bahler CK, Jewison DJ, Johnson DH. Recombinant TIGR/MYOC increases outflow resistance in the human anterior segment. Invest Ophthalmol Vis Sci. 2000;41:4163–8. [PubMed] [Google Scholar]
- 17.Rozsa FW, Shimizu S, Lichter PR, Johnson AT, Othman MI, Scott K, Downs CA. GLC1A mutations point to regions of potential functional importance on the TIGR/MYOC protein. Mol Vis. 1998;4:20. [PubMed] [Google Scholar]
- 18.Povoa CA, Malta RF, Rezende Mde M, de Melo KF, Giannella-Neto D. Correlation between genotype and phenotype in primary open angle glaucoma of Brazilian families with mutations in exon 3 of the TIGR/MYOC gene. 2006;69:289–97. doi: 10.1590/s0004-27492006000300002. Arq Bras Oftalmol [DOI] [PubMed] [Google Scholar]
- 19.Allingham RR, Wiggs JL, De La Paz MA, Vollrath D, Tallett DA, Broomer B, Jones KH, Del Bono EA, Kern J, Patterson K, Haines JL, Pericak-Vance MA. Gln368STOP myocilin mutation in families with late-onset primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 1998;39:2288–95. [PubMed] [Google Scholar]
- 20.Wiggs JL, Allingham RR, Vollrath D, Jones KH, De La Paz M, Kern J, Patterson K. Prevalence of mutations in TIGR/Myocilin in patients with adult and juvenile primary open-angle glaucoma. Am J Hum Genet. 1998;63:1549–52. doi: 10.1086/302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shimizu S, Lichter PR, Johnson AT, Zhou Z, Higashi M, Gottfredsdottir M, Othman M, Moroi SE, Rozsa FW, Schertzer RM, Clark MS, Schwartz AL, Downs CA, Vollrath D, Richards JF. Age-dependent prevalence of mutationsat the GLC1A locus in primary open-angle glaucoma. Am J Ophthalmol. 2000;130:165–77. doi: 10.1016/s0002-9394(00)00536-5. [DOI] [PubMed] [Google Scholar]
- 22.Suzuki Y, Shirato S, Taniguchi F, Ohara K, Nishimaki K, Ohta S. Mutations in the TIGR gene in familial primary open-angle glaucoma in Japan. Am J Hum Genet. 1997;61:1202–4. doi: 10.1086/301612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mukhopadhyay A, Acharya M, Mukherjee S, Ray J, Choudhury S, Khan M, Ray K. Mutations in MYOC gene of Indian primary open angle glaucoma patients. 2002;8:442–8. Mol Vis [PubMed] [Google Scholar]
- 24.Rose R, Karthikeyan M, Anandan B, Jayaraman G. Myocilin mutations among primary open angle glaucoma patients of Kanyakumari district, South India. Mol Vis. 2007;13:497–503. [PMC free article] [PubMed] [Google Scholar]
- 25.Campos-Mollo E, Sánchez-Sánchez F, López-Garrido MP, López-Sánchez E, López-Martínez F, Escribano J. MYOC gene mutations in Spanish patients with autosomal dominant primary open-angle glaucoma: a founder effect in southeast Spain. Mol Vis. 2007;13:1666–73. [PubMed] [Google Scholar]
- 26.Kim BS, Savinova OV, Reedy MV, Martin J, Lun Y, Gan L, Smith RS. Targeted Disruption of the Myocilin Gene (Myoc) Suggests that Human Glaucoma-Causing Mutations Are Gain of Function. Mol Cell Biol. 2001;21:7707–13. doi: 10.1128/MCB.21.22.7707-7713.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Joe MK, Sohn S, Hur W, Moon Y, Choi YR, Kee C. Accumulation of mutant myocilins in ER leads to ER stress and potential cytotoxicity in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;312:592–600. doi: 10.1016/j.bbrc.2003.10.162. [DOI] [PubMed] [Google Scholar]
- 28.Nguyen TD, Chen P, Huang WD, Chen H, Johnson D, Polansky JR. Gene structure and properties of TIGR, an olfactomedin-related glycoprotein cloned from glucocorticoid-induced trabecular meshwork cells. J Biol Chem. 1998;273:6341–50. doi: 10.1074/jbc.273.11.6341. [DOI] [PubMed] [Google Scholar]
- 29.Mackey DA, Craig JE. Predictive DNA testing for glaucoma: reality in 2003. 2003;16:639–45. doi: 10.1016/s0896-1549(03)00066-x. Ophthalmol Clin North Am [DOI] [PubMed] [Google Scholar]