Abstract
The GTP-binding protein ADP-ribosylation factor (ARF) initiates clathrin-coat assembly at the trans-Goli network (TGN) by generating high-affinity membrane-binding sites for the AP-1 adaptor complex. Both transmembrane proteins, which are sorted into the assembling coated bud, and novel docking proteins have been suggested to be partners with GTP-bound ARF in generating the AP-1-docking sites. The best characterized, and probably the major transmembrane molecules sorted into the clathrin-coated vesicles that form on the TGN, are the mannose 6-phosphate receptors (MPRs). Here, we have examined the role of the MPRs in the AP-1 recruitment process by comparing fibroblasts derived from embryos of either normal or MPR-negative animals. Despite major alterations to the lysosome compartment in the MPR-deficient cells, the steady-state distribution of AP-1 at the TGN is comparable to that of normal cells. Golgi-enriched membranes prepared from the receptor-negative cells also display an apparently normal capacity to recruit AP-1 in vitro in the presence of ARF and either GTP or GTPγS. The AP-1 adaptor is recruited specifically onto the TGN and not onto the numerous abnormal membrane elements that accumulate within the MPR-negative fibroblasts. AP-1 bound to TGN membranes from either normal or MPR-negative fibroblasts is fully resistant to chemical extraction with 1 M Tris-HCl, pH 7, indicating that the adaptor binds to both membrane types with high affinity. The only difference we do note between the Golgi prepared from the MPR-deficient cells and the normal cells is that AP-1 recruited onto the receptor-lacking membranes in the presence of ARF1·GTP is consistently more resistant to extraction with Tris. Because sensitivity to Tris extraction correlates well with nucleotide hydrolysis, this finding might suggest a possible link between MPR sorting and ARF GAP regulation. We conclude that the MPRs are not essential determinants in the initial steps of AP-1 binding to the TGN but, instead, they may play a regulatory role in clathrin-coated vesicle formation by affecting ARF·GTP hydrolysis.
INTRODUCTION
Clathrin-coated vesicles that form on the trans-Golgi network (TGN)1 concentrate newly synthesized lysosomal hydrolases for transport from the Golgi system to the endosome compartment. In turn, the endosomes then deliver the hydrolases to the lysosome (von Figura and Hasilik, 1986; Kornfeld and Mellman, 1989). The formation of clathrin coats depends on the recruitment of the Golgi-specific adaptor protein complex AP-1 onto the cytosolic face of the TGN. Clathrin triskelia then assemble over the membrane-bound AP-1, forming a polyhedral lattice that is able to preferentially retain certain transmembrane molecules. While the assembling clathrin scaffold is thought to provide the driving force for the membrane-budding process, the topology of the adaptor complex allows AP-1 to perform two discrete but interrelated functions. One is to initiate the assembly of clathrin trimers into a membrane-bound lattice by binding to clathrin through a binding site located on the appendage domain of the β-subunit (Galluser and Kirchhausen, 1993; Shih et al., 1995; Traub et al., 1995). The other function is to selectively concentrate certain transmembrane proteins into the forming vesicles (Kornfeld and Mellman, 1989; Pearse and Robinson, 1990; Kirchhausen et al., 1997).
The protein-sorting function of the adaptor appears to be, at least in part, mediated by the μ1 subunit of the heterotetrameric AP-1 complex (Ohno et al., 1995, 1996). This subunit displays micromolar affinity for tyrosine-based sorting signals found on some proteins selectively incorporated into clathrin-coated vesicles. The μ subunit also appears to be able to interact with the dileucine-based class of protein-sorting signals (Bremnes et al., 1998; Rodionov and Bakke, 1998), although the β1 subunit of the AP-1 complex has also been reported to bind to dileucine-based sorting signals (Rapoport et al., 1998). The best known example of transmembrane proteins sorted into the clathrin coats found on the TGN are the mannose 6-phosphate receptors (MPRs). Two distinct MPRs have been identified, the ∼46-kDa cation-dependent (CD) MPR and the ∼275-kDa cation-independent (CI) mannose 6-phosphate/insulin-like growth factor II receptor (Kornfeld and Mellman, 1989). While there is little doubt that MPRs are actively sorted into AP-1-containing clathrin coats, there is some controversy in the literature over the precise role that these proteins play in the early events that initiate the clathrin coat-formation process.
A clue to the complexity of the clathrin coat assembly process came from studies showing the dramatic effect of brefeldin A (BFA) on the steady-state distribution of AP-1 in intact cells (Robinson and Kreis, 1992; Wong and Brodsky, 1992). This led to the identification of the small GTP-binding protein ADP-ribosylation factor (ARF) as a critical regulator of clathrin coat formation at the TGN (Stamnes and Rothman, 1993; Traub et al., 1993). The exchange of GTP for GDP and the concomitant membrane attachment of ARF1·GTP are the earliest steps of the coat-assembly process known. ARF1·GTP appears to regulate clathrin coat assembly by generating a high-affinity membrane-docking site for AP-1 on the TGN. ARF also controls the formation of the coat protein complex (COPI) coats at the transitional zone between the endoplasmic reticulum and the Golgi (Aridor et al., 1995) and on Golgi cisternae (Orci et al., 1993), as well as several other intracellular protein-sorting processes (Whitney et al., 1995; Simpson et al., 1996; Faundez et al., 1997; Ooi et al., 1998; Passreiter et al., 1998). These coat-recruitment events do not appear to be regulated differentially by distinct members of the ARF family, so specificity must be dictated by another mechanism. On the basis of these considerations, and on biophysical measurements showing that transmembrane proteins sorted into clathrin-coated pits have reduced lateral mobility but are not immobile (Fire et al., 1991), we formulated a model to explain how AP-1 is selectively recruited to the TGN in cells where ARF·GTP is present at multiple intracellular locations (Traub et al., 1993). We proposed that a putative docking protein resides at the TGN to precisely regulate the site of clathrin coat assembly. In the ground state, the docking protein exhibits minimal affinity for soluble AP-1 but, once activated by ARF·GTP, a high-affinity form of the docking protein is generated. AP-1 binds to the docking site via the core of the heterotetramer, composed of the amino-terminal trunks of the β1- and γ-subunits and the μ1 and ς1 chains (Traub et al., 1995). In the absence of nucleotide hydrolysis, the interaction between the putative docking protein and AP-1 is essentially irreversible (Traub et al., 1993; Zhu et al., 1998). The adaptor complex cannot be dislodged from membranes with either carbonate or 1 M Tris-HCl, pH 7.0, a treatment that releases adaptors from purified clathrin-coated vesicles (Keen et al., 1979). ARF·GTP therefore appears to be a stoichiometric component of the docking site for AP-1 (Zhu et al., 1998). In our model, the recruitment of clathrin onto membrane-bound AP-1 results in the formation of a clathrin-coated bud with a high local density of membrane-apposed adaptor molecules. Concentration of the adaptor ensures that on lateral movement of sorted transmembrane proteins, like the MPRs, into an assembling or preformed clathrin-coated pit, the molecules will become entrapped, and consequently concentrated, within budding vesicles even if the affinity of interaction between the sorting signal and the μ1 subunit is relatively weak. Our model, therefore, predicts that the movement of sorted membrane proteins, like the MPRs, into the coat lags behind the initial recruitment of AP-1.
An alternate model has been proposed that holds that it is the MPRs, together with ARF·GTP, that form the major membrane-docking sites for AP-1 at the TGN (Ludwig et al., 1995). This model is based on three main lines of experimental evidence. First, enormous overexpression of a construct bearing the cytosolic domain of the CI-MPR with a vaccinia virus expression system results in a twofold increase in AP-1 recruitment (Le Borgne et al., 1993). Similar overexpression of varicella-zoster virus glycoprotein I and major histocompatibility complex II molecules also enhances AP-1 recruitment to a similar extent (Alconada et al., 1996; Salamero et al., 1996), but expression of the cytosolic domain of the transferrin receptor in the same way has no affect on AP-1 binding (Le Borgne et al., 1993). Second, analysis of fibroblasts derived from embryos totally devoid of MPR expression indicates that these cells seem to have less AP-1 located at the TGN at steady state and less AP-1–containing clathrin-coated vesicles (Le Borgne and Hoflack, 1997). Further, the ability of the MPR-negative cells to recruit AP-1 in in vitro assays appears to be compromised (Le Borgne et al., 1993; Le Borgne et al., 1996). These alterations are corrected by stable reintroduction of either the CD- or the CI-MPR by transfection. Third, the translocation of AP-1 onto the TGN in vitro can be blocked by the addition of a glutathione S-transferase-fusion protein containing the 163-amino acid cytosolic domain of the CI-MPR (Le Borgne et al., 1993). The inhibitory effect of this protein fragment on adaptor recruitment is markedly potentiated after phosphorylation by casein kinase II.
Clearly, the MPRs are envisioned to play quite different roles in these two models. Because deciphering the precise role of the MPRs is crucial to our ability to both understand and study the early molecular events that initiate clathrin coat assembly at the TGN, we have independently analyzed the effect of genetic disruption of MPR expression on AP-1 binding. We find no evidence for these receptors controlling adaptor recruitment to the TGN.
MATERIALS AND METHODS
Antibodies
The following affinity-purified anti-peptide antibodies, directed against different subunits of the AP-1 complex, were used: the anti–γ-subunit antibody, AE/1, the anti–β1/β2-subunit antibody, GD/1, the anti–μ1- subunit antibody, RY/1 (Traub et al., 1995), and the anti–ς1-subunit antibody, DE/1 (Zhu et al., 1998). A monoclonal antibody (mAb) (clone 41) specific for the γ subunit of AP-1 was purchased from Transduction Laboratories (Lexington, KY). mAb 1D4B, which recognizes murine lysosome-associated membrane protein-1 (LAMP-1) and was developed by Dr. J.T. August, was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Biological Sciences, University of Iowa (Iowa City, IA). A hybridoma producing a clathrin heavy chain-specific antibody, mAb X22 (Brodsky, 1985), was generously provided by Dr. Frances Brodsky. Affinity-purified rabbit antibodies against the CI-MPR were prepared from the serum of rabbits immunized with the purified CI-MPR derived from bovine liver. For the affinity purification, the soluble form of the CI-MPR was purified from bovine serum on phosphopentamannosyl-agarose, eluted with mannose 6-phosphate, and then coupled to CNBr-activated Sepharose-CL4B. Anti–CI-MPR antibodies were eluted from the affinity matrix using 100 mM glycine-HCl, pH 2.5, followed by 100 mM triethylamine, pH 11.0. Fractions were immediately neutralized and then pooled for use. An antibody directed against denatured α-mannosidase II was generously supplied by Dr. Kelley Moreman (University of Georgia, Athens, GA). Horseradish peroxidase-conjugated secondary antibodies were obtained from Amersham Pharmacia Biotech (Piscataway, NJ), and the fluorochrome-conjugated secondary antibodies were purchased from Cappel (Durham, NC).
Cell Culture and Cellular Protein Determination
Spontaneously immortalized mouse embryo fibroblasts derived from either normal or CD-MPR– and CI-MPR–deficient (MPR-negative) animals (Ludwig et al., 1994; Mauxion et al., 1996) were kindly provided by Dr. Peter Lobel (UMDNJ-Robert Wood Johnson Medical School, Piscataway, NJ) and Dr. Bernard Hoflack (Institut Pasteur de Lille, Lille Cedex, France). The cells were grown in complete Dulbecco’s modified Earle’s medium supplemented with 10% FCS and antibiotics at 37°C in an atmosphere of 5% CO2. To determine the protein content of the MPR-positive and MPR-negative cells, confluent 60-mm dishes of each cell type were washed twice with ice-cold PBS, dissolved in 0.5 ml 0.1% SDS, and sonicated, and then the protein concentration of each cell lysate was determined using the Coomassie blue-based method (Bio-Rad Laboratories, Richmond, CA) with IgG as the standard. Duplicate dishes of each cell type were trypsinized and resuspended in PBS, and the cells were counted. The cell number determined was used to calculate the protein content per cell for the two cell lines.
Immunofluorescence Analysis and Subcellular Fractionation
For the examination of the steady-state distribution of AP-1 in vivo, normal and receptor-negative cells were cocultured on 12-mm round glass coverslips. The cells were fixed in 3.7% formaldehyde in PBS, permeabilized with 0.2% saponin in PBS supplemented with 10% normal goat serum, and then incubated with antibodies directed against clathrin, AP-1, the CI-MPR, or LAMP-1 as indicated in the legend to Figure 1. Bound antibodies were subsequently detected by incubation with fluorochrome-conjugated secondary antibodies as described elsewhere (Traub et al., 1996).
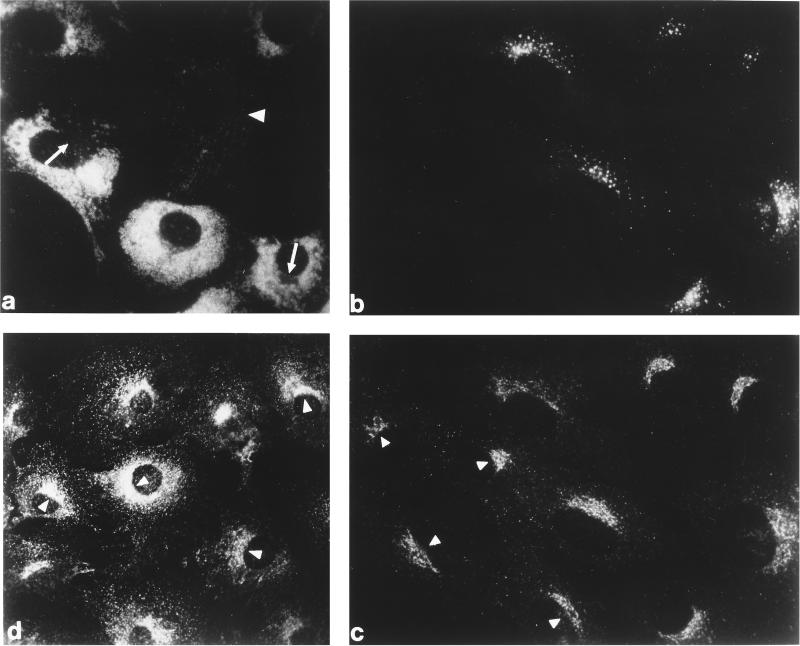
Figure 1.
Steady-state distribution of the AP-1 adaptor complex and clathrin in normal and MPR-negative cells in vivo. Normal and MPR-negative mouse embryo fibroblasts were cocultured on glass coverslips, fixed with formaldehyde, and then probed either with mAb 1D4B directed against mouse LAMP-1 (panel a), a mixture of affinity-purified rabbit antibodies against the CI-MPR (panel b), and a mAb (clone 41) recognizing the γ subunit of AP-1 (panel c). The MPR-negative cells are easily distinguished from the MPR-positive cells either by the presence of numerous swollen LAMP-1–positive structures (panel a) or by the lack of CI-MPR labeling (panel b). A control, MPR-containing cell is indicated (panel a, arrowhead) as is the region in some of the MPR-negative cells that is devoid of LAMP-1–positive structures and contains the bulk of the other organelles in these cells (panel a, arrows). Similar levels of AP-1 staining are observed in both the MPR-lacking (panel c, arrowheads) and the normal cells. A culture of the MPR-negative cells alone was processed for analysis with mAb X22, directed against the clathrin heavy chain (panel d). The concentration of clathrin over the juxtanuclear region of each cell is clearly visible.
Membrane fractions enriched in Golgi elements were prepared from the normal and MPR-negative cells using standard procedures (Balch et al., 1984). Small aliquots of the purified Golgi membrane fractions were quick frozen on dry ice and stored at −80°C. Rat liver cytosol was prepared exactly as described previously (Traub et al., 1993) and was also stored in small aliquots at −80°C. After thawing, the liver cytosol was gel filtered over a PD-10 column equilibrated in ice-cold assay buffer (25 mM HEPES-KOH, pH 7.0, 125 mM potassium acetate, 2.5 mM magnesium acetate, 0.25 M sucrose, 1 mM DTT) and any aggregated material was then removed by centrifugation at 245,000 × gmax in a TLA-100.3 rotor (Beckman, Fullerton, CA) for 20 min before use in the binding assays. The protein concentrations of the Golgi membrane fractions and the rat liver cytosol were determined with the Coomassie blue-based method using either IgG or BSA as standards. The recovery of total membrane protein in the Golgi-enriched fraction was approximately threefold greater from the MPR-negative cells compared with the normal cells.
Characterization of Fractions Isolated from Normal and MPR Cells
Equal amounts of protein from the total cell lysates or the Golgi-enriched membrane fractions were solubilized in 1× SDS sample buffer (62.5 mM Tris-HCl, pH 6.8, 10% sucrose, 2.3% SDS, 5% 2-mercaptoethanol, 0.005% bromophenol blue) and, after boiling briefly, were resolved on SDS-polyacrylamide gels, transferred onto nitrocellulose membranes, and probed with either the anti-murine LAMP-1 mAb, the affinity-purified anti-ς1 antibodies, the affinity-purified anti–CI-MPR antibodies, or the anti–α-mannosidase II antiserum as described previously (Traub et al., 1993; Zhu et al., 1998). Immunolabeled proteins were visualized using ECL (Amersham Pharmacia Biotech, Piscataway, NJ), and quantitation of the signals was performed by densitometry using a Personal Densitomiter equipped with ImageQuant software (Molecular Dynamics, Sunnyvale, CA). Galactosyltransferase activity in total cell lysates and the isolated Golgi-enriched fractions was measured using published procedures (Verdon and Berger, 1983) with ovomucoid as the acceptor and UDP-14C-galactose as the donor.
Assay of AP-1 Adaptor Binding to Golgi Membranes
AP-1 recruitment assays were performed as described in detail elsewhere (Traub et al., 1993, 1995; Zhu et al., 1998). Briefly, 200-μl reactions were prepared in assay buffer containing 50 μg/ml Golgi-enriched membranes, 5 mg/ml gel-filtered rat liver cytosol, 1 mM GTP, 100 μM GTPγS, and 4 μM recombinant myristoylated ARF1 as indicated in the individual figure legends. The bovine ARF1 used for these experiments has amino acids 3–7 replaced with the corresponding residues from yeast ARF2, facilitating near quantitative N-terminal myristoylation when expressed in Escherichia coli together with yeast N-myristoyltransferase (Liang et al., 1997). After mixing on ice, the tubes were incubated at 37°C for 15 min. Reactions were terminated by cooling rapidly on ice. After addition of an equal volume of cold assay buffer without sucrose, the membranes were pelleted at 16,000 × gmax at 4°C for 15 min and the supernatants were removed. For the Tris extraction experiments, the Golgi-enriched membrane pellets were each resuspended in 100 μl of 1.0 M Tris-HCl, pH 7.0, incubated on ice for 10 min (Keen et al., 1979), and then sedimented again to obtain the pellet and supernatant fractions. Proteins in the Tris supernatants were concentrated by methanol/chloroform precipitation (Wessel and Flugge, 1984) after 5 μg of BSA had been added to each tube as a carrier. All pellets were solubilized in 1× SDS-sample buffer, boiled briefly, and then prepared for immunoblot analysis as outlined above.
The cell-based morphological recruitment assays were performed as described in detail elsewhere (Traub et al., 1996). Briefly, the fibroblasts, cultured either alone or as a 1:1 mixture of the MPR-positive and MPR-negative cells on 12-mm glass coverslips, were permeabilized with 25 μg/ml digitonin in 25 mM HEPES-KOH, pH 7.2, 125 mM potassium acetate, 2.5 mM magnesium acetate, 1 mM DTT, and 1 mg/ml d-glucose on ice for 5 min. After thorough washing in the same buffer lacking the digitonin to deplete cytosolic components, the permeabilized cells were mixed with 5 mg/ml gel-filtered cytosol supplemented with 1 mM GDP or 100 μM GTPγS and then incubated at 37°C for 20 min. After chilling on ice and washing twice with ice-cold buffer, the cells were fixed and processed for indirect immunofluorescence analysis as indicated above.
RESULTS
AP-1 Is Recruited to the TGN in MPR-deficient Cells
In an attempt to resolve the current controversy regarding the contribution of MPRs to the generation of high-affinity AP-1 binding sites on the TGN, we have compared spontaneously immortalized fibroblasts derived from either normal (MPR-positive) or MPR-negative mouse embryos (Ludwig et al., 1994). Cells derived from the MPR-negative embryos are unable to target lysosomal enzymes correctly. These cells contain substantially more LAMPs than the normal MPR-positive cells as a consequence of the deficit in protein degradative capacity (Ludwig et al., 1994). The striking increase in LAMP-1 expression in the MPR-negative cells at steady state is clearly evident in cocultures of the MPR-positive and MPR-negative cells (Figure 1a). Unlike the MPR-positive cells, which display a normal distribution of lysosomes randomly scattered throughout the cell, the interior of the receptor-negative fibroblasts is filled with large numbers of LAMP-1–positive endolysosomal structures.
Despite this gross morphological alteration to the endolysosome compartment of the MPR-lacking cells, AP-1 is still found associated with the TGN at steady state. For these experiments, the MPR phenotype of the cells in coculture is shown with an antibody directed against the CI-MPR (Figure 1b). The juxtanuclear region of all the cells is labeled by an antibody that recognizes the γ subunit of the AP-1 complex, irrespective of the presence of MPRs (Figure 1c). It is now known that AP-1 also binds to endosomes (Le Borgne et al., 1996; Futter et al., 1998), but we find only a minor fraction of the AP-1 signal on peripheral endosomes in both cell types (Figure 1c). Thus, the bulk of membrane-associated AP-1 is found at the TGN in these cell lines. The steady-state distribution of clathrin in the MPR-negative cells also appears normal (Figure 1d). The characteristic perinuclear TGN staining is preserved which, superimposed upon the dispersed array of clathrin-containing structures at the cell surface, reveals a staining pattern essentially indistinguishable from that of clathrin in wild-type cells (Ahle et al., 1988). We do note that the AP-1 staining in the MPR-negative cells often appears more compact than that seen in the wild-type cells. This is most likely the consequence of the huge lysosome burden of the cells that restricts the distribution of the other organelles to a small perinuclear region (Figure 1a, arrows; see also Figure 5). Although these results are not quantitative, they clearly indicate that AP-1 is able to translocate onto the TGN in vivo in the absence of any MPR expression.
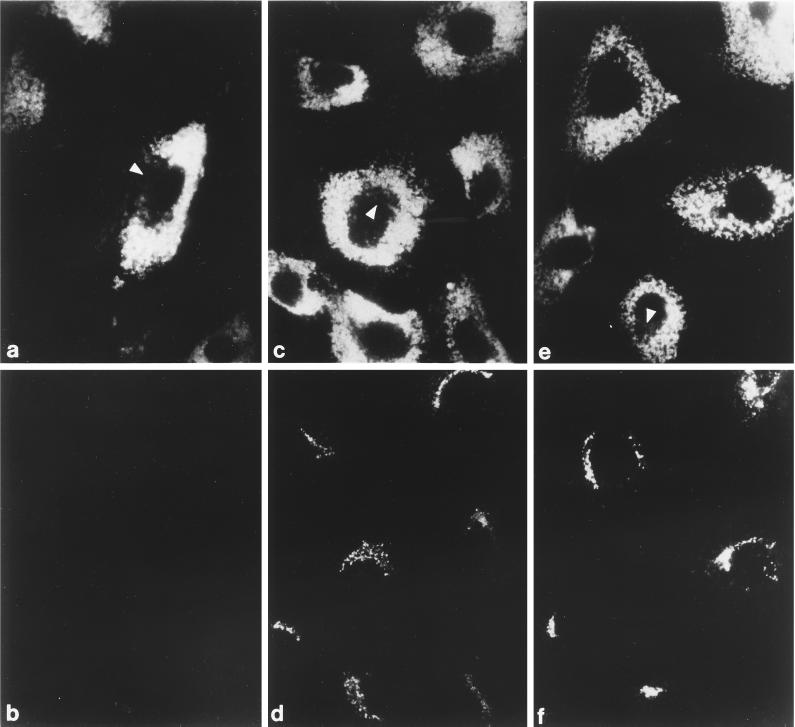
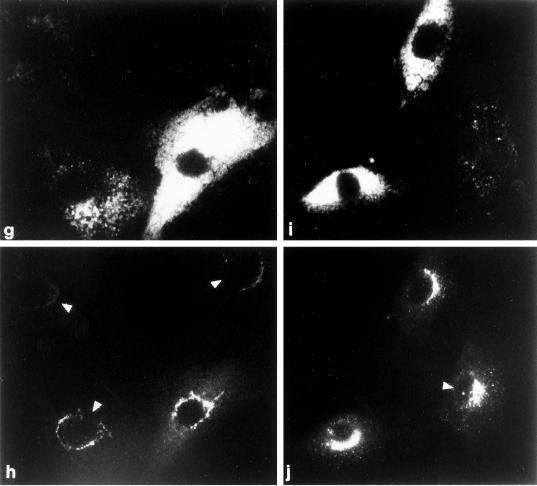
Figure 5.
Recruitment of AP-1 in permeabilized MPR-negative fibroblasts. (a–f) Digitonin-permeabilized receptor-negative cells were incubated at 37°C for 20 min with ∼5 mg/ml gel-filtered rat liver cytosol and either 1 mM GDP (panels a and b) or 100 μM GTPγS (panels c–f). After washing, the cells were fixed and then prepared for indirect immunofluorescence using a mixture of the anti–LAMP-1 mAb 1D4B (panels a and c) and affinity purified anti–γ-subunit antibody AE/1 (panels b and d) or mAb 1D4B (panel e) and affinity purified anti–β-subunit antibody GD/1 (panel f). The conditions for photography and printing of panels b, d, and f were identical. In some cells, the region that is devoid of LAMP-1–positive structures containing the bulk of the other perinuclear organelles is indicated (panels a, c, and e, arrowheads). (g–j) Cocultures of MPR-positive and MPR-negative fibroblasts were permeabilized, mixed with 5 mg/ml gel-filtered cytosol and 100 μM GTPγS, and incubated at 37°C for 20 min. After washing the cells were fixed and prepared for immunofluorescence analysis using a mixture of the anti–LAMP-1 mAb 1D4B (panels g and i) and affinity-purified anti–γ-subunit antibody AE/1 (panels h and j). Two representative images are shown, and the normal, MPR-positive cells are indicated by the arrowheads.
In other experiments, we compared the steady-state distribution of AP-1 in two mouse L (Rec−) cell lines (Gabel and Foster, 1986), designated L1 and L5 (Johnson and Kornfeld, 1992a). Both L1 and L5 cells lack the CI-MPR, but the L5 line is stably transfected with the bovine CD-MPR and, consequently, expresses roughly 50 times more of the ∼46-kDa receptor than the L1 line (Johnson and Kornfeld, 1992a). Despite this substantial difference in CD-MPR expression, we do not observe any major differences in the intracellular distribution of AP-1 in the two cell lines by immunofluorescence microscopy (our unpublished results).
Purification of Golgi-enriched Membranes from Normal and MPR-negative Cells
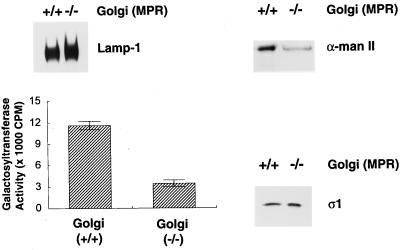
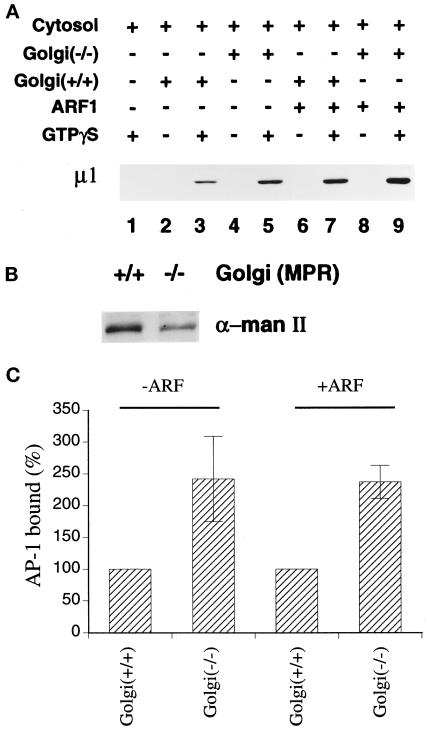
Next, Golgi-enriched membrane fractions from the normal and MPR-negative fibroblasts were prepared to assess adaptor recruitment in vitro (Traub et al., 1993; Zhu et al., 1998). Immunoblot analysis of total cell lysates confirms the accumulation of LAMP-1 in the receptor-deficient cells. At steady state, the MPR-negative cells contain approximately fourfold more LAMP-1 than the normal cells (Figure 2). The total amount of cellular AP-1, as indicated by presence of the ς1 subunit of the heterotetramer, is roughly equivalent in both cell types (Figure 2). In the preparations of Golgi-enriched membranes made from the normal and MPR-negative cell lysates, the density of LAMP-1 is more comparable (Figure 3). Although slightly more LAMP-1 is present in the MPR-negative Golgi-enriched membrane fractions, it is evident that the fractionation procedure removes much of the LAMP-positive structures present in the MPR-negative cell lysate. Analysis of MPR content with anti–CD-MPR and anti–CI-MPR antibodies reveals that, as expected, the MPR-negative Golgi fraction is devoid of both receptors (our unpublished results, see Figure 1b). To verify the Golgi content of the isolated Golgi-enriched membrane fractions, the presence of the medial-Golgi marker enzyme, α-mannosidase II, was determined on immunoblots (Figure 3). Quantitation indicates that when equivalent amounts of protein are analyzed, the MPR-positive Golgi fraction has roughly 2 to 2.5-fold (243 ± 34%, n = 3) more α-mannosidase II immunoreactivity than the MPR-negative fraction. The activity of galactosyltransferase, an enzyme located in the trans-Golgi, was also determined and, again, the MPR-positive Golgi fraction demonstrates approximately threefold (335 ± 5%, n = 3) more activity than the MPR-negative Golgi fraction. This decreased specific activity of the Golgi marker enzymes in the receptor-deficient cells is not simply due to less overall Golgi in these cells as the total yield of galactosyltransferase activity are similar for the two cell lines (our unpublished observations). Rather, the fraction appears to be contaminated with other membrane elements that accumulate abnormally in the receptor-deficient cells. Taken together, the data indicate that the normal Golgi-enriched fraction contains more Golgi per unit protein than the MPR-negative Golgi-enriched fraction, a finding that is used below to normalize the AP-1 recruitment on the two different types of Golgi preparations.
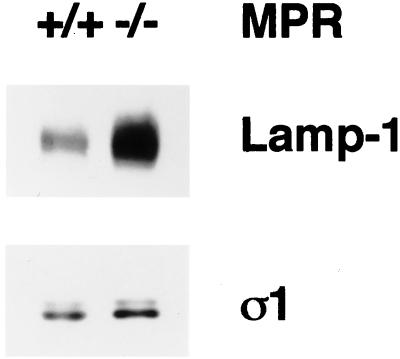
Figure 2.
Increased LAMP-1 content in MPR-negative cells. Aliquots of 10 μg protein of normal or MPR-negative fibroblast total cell lysates were boiled in 1× SDS-sample buffer, resolved on polyacrylamide gels, and transferred to nitrocellulose. The blot was probed with the anti-mouse LAMP-1 mAb 1D4B and the anti–AP-1 ς1 subunit antibody, DE/1. The nomenclature used to indicate the presence of the MPRs is +/+ for the normal cells, and −/− for the CD- and CI-MPR double-negative cells. Only the relevant portions of the blot are shown. The faint band seen above the ς1 subunit might represent ς1B (Takatsu et al., 1998).
Figure 3.
Characterization of Golgi-enriched membrane fractions. Aliquots of 10 μg protein from the Golgi-enriched membrane fractions derived from either normal (+/+) or the MPR-negative (−/−) cells were boiled in 1× SDS-sample buffer, resolved on polyacrylamide gels, and then transferred to nitrocellulose filters. The blots were probed with anti-mouse LAMP-1 antibody, mAb 1D4B, polyclonal anti–α-mannosidase II antiserum, or the anti–AP-1 ς1 subunit antibody, DE/1. Only the relevant portion of each blot is shown. Galactosyltransferase activity in 10-μg protein aliquots from the normal (+/+) or MPR-negative (−/−) Golgi-enriched fractions was determined. At this protein concentration, the enzyme activity is within the linear range of this assay.
Interestingly, when we assessed the amount of AP-1 that remained bound to the membranes during the purification, as an alternate criterion to evaluate the TGN content in each fraction, we found approximately equal amounts of AP-1 associated with both fractions (Figure 3). Unlike the oligosaccharide-processing enzymes, AP-1 is not an integral constituent of the Golgi and, instead, cycles on and off the membrane. The discrepancy in the amount of Golgi markers and AP-1 found in the MPR-negative preparations might be the result of alterations in AP-1 cycling kinetics due to the lack of the MPRs.
AP-1 Binds to MPR-negative TGN In Vitro
Golgi-enriched fractions prepared from the MPR-negative fibroblasts display an apparently normal capacity to recruit AP-1 in in vitro assays. The extent of AP-1 recruitment from rat liver cytosol onto the two different Golgi-enriched preparations was first determined in the absence of exogenous ARF. Under these conditions, the translocation of soluble adaptor onto the membrane is absolutely dependent upon the presence of GTPγS, and no AP-1 is found in the pellet of incubations of cytosol and GTPγS (Figure 4A, lane 1) or the membranes alone (lanes 2 and 4). When cytosol is mixed together with each membrane preparation and GTPγS, we find, unexpectedly, that the amount of AP-1 recruited onto the MPR-negative Golgi fraction is higher than that bound to the MPR-positive Golgi fraction (Figure 4A, lane 5 compared with lane 3). The difference in the mannosidase II reactivity of the particular membrane preparations used in Figure 4A is shown in panel B. Because the two preparations differ in Golgi membrane content, the data from six separate experiments were normalized for the Golgi marker content of the fractions. When this is done, it is apparent that the MPR-negative Golgi actually recruits at least 2 times more AP-1 than the MPR-positive Golgi in our assays (Figure 4C). This is consistent with the steady-state amount of AP-1 we found associated with the membranes after isolation (Figure 3). Supplementing the donor cytosol with 4 μM recombinant myristoylated ARF1 increases AP-1 recruitment onto both Golgi membrane fractions (Figure 4A, lane 7 and 9; Figure 4C). Again, when the data from this and three separate experiments are normalized for the Golgi marker content of each fraction, it is evident that the MPR-negative Golgi recruits approximately twofold more AP-1 than the MPR-positive Golgi under these conditions (Figure 4C).
Figure 4.
Recruitment of AP-1 onto MPR-positive and MPR-negative Golgi-enriched membranes. (A) Recruitment assays containing 50 μg/ml normal (+/+) or MPR-negative (−/−) Golgi-enriched membranes, 5 mg/ml gel-filtered rat liver cytosol, 4 μM recombinant myristoylated ARF1, and 100 μM GTPγS were prepared on ice as indicated. After incubation at 37°C for 15 min, the Golgi-enriched membrane pellets were recovered, resolved on polyacrylamide gels, and transferred to nitrocellulose. The blot was probed with the anti–μ1-subunit antibody, RY/1. Only the relevant portion of the blot is shown. (B) Aliquots of 10 μg protein of the Golgi-enriched membrane fractions derived from either normal (+/+) or the MPR-negative (−/−) cells used in panel A were analyzed by immunoblotting with the polyclonal anti–α-mannosidase II antiserum. (C) The μ1-subunit signal from six (−ARF) or four (+ARF) independent experiments was quantitated by densitometry and the average values (± SEM) are expressed relative to the Golgi marker content of the membranes. The values for the MPR-positive membranes were arbitrarily set to 100%.
Since a minor fraction of AP-1 is found on endosomes (Le Borgne et al., 1996; Futter et al., 1998) (Figure 1c) and the Golgi-enriched fractions prepared from the receptor-deficient cells do contain non-Golgi membrane elements, it was important to determine whether the AP-1 binding that we observe in the recruitment assays is predominantly onto the TGN rather than to non-Golgi structures. To do this, we used a cell-based recruitment assay utilizing digitonin-permeabilized MPR-negative fibroblasts, which allows recruited AP-1 to be localized at the morphological level. In this assay, AP-1 binding remains absolutely dependent on GTP. The adaptor complex does not translocate to the Golgi in the presence of cytosol and 1 mM GDP (Figure 5b). The mutant MPR phenotype of the cells is clearly demonstrated by double labeling with an anti–LAMP-1 monoclonal antibody (Figure 5, a, c, and e). When 100 μM GTPγS replaces the GDP, AP-1 efficiently binds to the perinuclear-situated TGN (Figure 5, d and f). The recruited AP-1 is detected by antibodies to either the γ subunit (panel d) or the β subunit (panel f) of the adaptor complex. If the recruitment assay is performed on a mixture of MPR-positive and MPR-negative cells, no dramatic differences in the extent of AP-1 recruitment onto the different cell types is evident (Figure 5, g–j). In all these experiments, very little AP-1 staining is observed outside of the perinuclear Golgi region (panels d, f, h, and j). These results rule out the possibility that AP-1 is being preferentially recruited onto endosomes or mistargeted onto the expanded endolysosome compartment in the mutant cells in an MPR-independent manner.
Extraction of Membrane-bound AP-1 with Tris-HCl
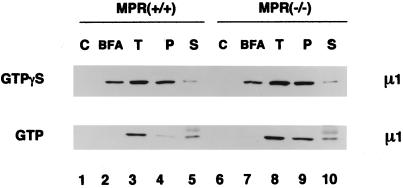
In our assay system, the AP-1 recruited onto rat liver Golgi membranes in the presence of GTPγS is bound with high affinity. The bound adaptor resists extraction with 1 M Tris-HCl, pH 7.0, a treatment that dissociates AP-1 recruited onto rat liver Golgi with GTP under physiological conditions (Traub et al., 1993; Zhu et al., 1998) and also removes adaptors from purified clathrin-coated vesicles. We therefore tested whether we could discern any difference in the Tris sensitivity of AP-1 bound to the MPR-positive versus the MPR-negative membranes. AP-1 was recruited onto each membrane preparation using cytosol supplemented with 4 μM recombinant ARF1. The exogenous ARF1 is added to facilitate efficient AP-1 recruitment in the presence of GTP (Zhu et al., 1998). Adaptor recruitment still remains completely dependent on guanine nucleotide as no AP-1 translocates onto either membrane in incubations lacking GTP or GTPγS (Figure 6, lanes 1 and 6). When GTPγS is used to drive adaptor recruitment, the AP-1 bound to either the normal or the MPR-negative Golgi membranes is resistant to Tris extraction, with almost all of the prebound adaptor being recovered in the pellet (lanes 4 and 9). This indicates that even without any MPR expression at the TGN, the AP-1 adaptor recruited by ARF1·GTPγS binds with high affinity. On the other hand, AP-1 recruited onto the normal Golgi membranes with ARF1·GTP, is almost completely sensitive to Tris extraction (lane 5), as we have shown previously for AP-1 bound to rat liver Golgi membranes (Zhu et al., 1998). Interestingly, the AP-1 prebound to membranes prepared from the MPR-negative cells with ARF·GTP is reproducibly more resistant to this Tris extraction procedure. Although roughly similar amounts of AP-1 are recruited onto the two membrane types with the ARF·GTP (lane 3 compared with lane 8), more than half of the bound AP-1 remains associated with the Tris-extracted Golgi pellet derived from the MPR-negative fibroblasts (lane 9 compared with lane 4). This finding represents the only difference that we have detected in AP-1 binding to the normal and MPR-negative Golgi membranes using our assays.
Figure 6.
Sensitivity of bound AP-1 to extraction with Tris-HCl. AP-1 recruitment assays containing cytosol, normal (+/+) or MPR-negative (−/−) Golgi-enriched membranes, and exogenous ARF1 were performed in the absence of nucleotides, or in the presence of either 100 μM GTPγS or 1 mM GTP, with or without 100 μg/ml BFA. After incubation, pellets from assays without added nucleotides (C), or with guanine nucleotide and BFA together (BFA), or with guanine nucleotide alone (T) were recovered and resuspended in SDS-sample buffer. A fourth pellet, identical to that in sample T, was resuspended in 100 μl of 1.0 M Tris-HCl, pH 7.0, on ice. After incubation on ice for 10 min, the membranes were collected again by centrifugation. The resulting supernatant was aspirated and protein was precipitated with methanol/chloroform after addition of 5 μg BSA as a carrier. The pellet (P) and supernatant (S) samples from the Tris extraction were resuspended in identical volumes of SDS-sample buffer and, together with the other pellets, analyzed by immunoblotting with the anti–AP-1 μ1-subunit antibody, RY/1. The diffuse band above the μ1 subunit seen in the lower panel (lanes 5 and 10) corresponds to nonspecific reactivity with the carrier BSA.
No perceptible difference in the inhibitory effect of BFA on AP-1 binding to the different membranes is evident (Figure 6). When added simultaneously, BFA inhibits AP-1 recruitment promoted by ARF·GTPγS on both membrane types, but the effectiveness of the inhibition is greatly enhanced when ARF·GTP is used (Figure 6, lane 2 compared with lane 3, and lane 7 compared with lane 8). This simply reflects the poorly hydrolyzable nature of GTPγS, as it is well established that the opposing effects of BFA and GTPγS can be titrated against each other. By adding lower concentrations of GTPγS, the inhibition of adaptor recruitment by BFA is increased (our unpublished observations).
DISCUSSION
A large body of evidence supports the idea that the MPRs are selectively sorted at the TGN and exit this structure in AP-1-containing clathrin-coated vesicles. Most compelling, perhaps, is the significant enrichment of MPRs in purified clathrin-coated vesicle preparations (Le Borgne and Hoflack, 1997; our unpublished observations) and elegant immunoelecton microscopic images showing that both the MPRs and AP-1 or clathrin colocalize on budding profiles at the TGN (Klumperman et al., 1993, 1998). There is also data showing that direct interaction between the cytosolic domain of the CI-MPR and AP-1 occurs and appears to depend on the tyrosine- and dileucine-based sorting signals (Glickman et al., 1989; Johnson and Kornfeld, 1992a,b; Sosa et al., 1993; Honing et al., 1997). AP-1–mediated sorting ensures that newly synthesized lysosomal hydrolases, bound to MPRs, are segregated from the molecules destined for the surface and are delivered efficiently to the lysosome compartment via an intracellular route. The direct interaction of the AP-1 adaptor with the cytosolic portion of the MPRs led to a model for clathrin coat assembly in which the recruitment of soluble AP-1 onto the MPRs at the TGN initiates the assembly of the clathrin-coated bud (Pearse and Robinson, 1990). This model did not account for the precise recruitment of AP-1 to the TGN when, at steady state, the bulk of the MPRs are found in the late endosome compartment (Griffiths et al., 1988). With the discovery that ARF controls the translocation of AP-1 onto the TGN (Stamnes and Rothman, 1993; Traub et al., 1993), the model was refined, predicting that ARF activates MPRs at the TGN, thereby generating a high-affinity membrane-binding site for the adaptor (Ludwig et al., 1995). Although this general model has received support from more recent studies (Alconada et al., 1996; Le Borgne et al., 1996; Mauxion et al., 1996; Salamero et al., 1996; Le Borgne and Hoflack, 1997), our experiments do not support the idea that the MPRs play a major role in bringing AP-1 to the TGN membrane.
Golgi-enriched membrane fractions prepared from the normal and MPR-negative fibroblasts display roughly similar capacities to recruit soluble AP-1 in vitro in the presence of ARF1. When GTPγS is added, the AP-1 bound to either membrane type is almost completely resistant to Tris extraction. Our interpretation of this is that no alteration in the binding affinity of AP-1 for the TGN membrane is discernible when the MPRs are absent. Although we have used different criteria to gauge the affinity of AP-1 binding, our results are clearly discordant with those of Hoflack and his colleagues, who have reported reduced AP-1 accumulation on the TGN of the MPR-negative cells at steady state (Le Borgne and Hoflack, 1997) and also a ∼70% reduction in AP-1 binding to the MPR-negative fibroblasts in vitro (Le Borgne et al., 1993, 1996; Le Borgne and Hoflack, 1997). The discrepancies between their observations and ours are most likely due to the different manner in which AP-1 recruitment is calculated. The CD-MPR and CI-MPR double-negative fibroblasts are literally filled with lysosomes (Ludwig et al., 1994) (Figures 1 and 5) that, being largely devoid of lysosomal hydrolases, are incapable of degrading and digesting the lumenal material into reutilizable constituents. We have found that these mutant fibroblasts contain, on average, about threefold more protein per confluent culture dish than the control fibroblasts. In our studies, we determined the amount of Golgi marker enzymes in each preparation and corrected for this by expressing the AP-1 bound to the membrane preparation as a function the Golgi membranes. In their studies, streptolysin O-permeabilized cells present in 24-well dishes were used, and it is stated that the data were normalized to the total protein concentration. If this is the case, the decrease in AP-1 binding they noted, rather than signifying the importance of a direct interaction between AP-1 and MPR trafficking motifs, might simply reflect the approximately threefold higher total protein content of the MPR-negative fibroblasts. In other words, even if both the MPR-negative and the normal cells displayed a similar capacity to recruit AP-1 in their in vitro recruitment assays, when normalized to the total protein content instead of the cell number, an underestimation of ∼60–70% in the amount of AP-1 bound to the MPR-negative cells is introduced. The MPR-negative cells do display a higher saturation density in culture, but when we normalized the protein concentration to the cell number, we found that the MPR-negative cells still have at least 2 times more protein per cell than the normal mouse fibroblasts. Stable transfection of MPR-encoding cDNAs would restore lysosomal enzyme trafficking in the MPR-negative cells. Over the course of establishing the stable lines, the lysosomes would be reconstituted, allowing for the subsequent metabolism of much of the accumulated undigested material. We expect that the end result would be a normalization of the protein content of the transfected cells so that, when used in their binding assay, the AP-1 recruitment would appear to have been normalized.
One difference in adaptor binding that we do observe is that AP-1 recruited onto the MPR-negative Golgi membranes by ARF·GTP is more resistant to Tris extraction than AP-1 recruited onto the MPR-positive Golgi membranes under the same conditions. Since we have previously found a good correlation between ARF·GTP hydrolysis and Tris sensitivity (Zhu et al., 1998), this finding may indicate that ARF·GTP is deactivated by nucleotide hydrolysis more slowly on the MPR-negative Golgi membranes, perhaps due to decreased ARF GAP activity. In this regard, it has been reported that the KDEL receptor, ERD2, interacts with ARF GAP, regulating the translocation of the GAP onto membranes and thereby modulating COPI coat assembly at the Golgi (Aoe et al., 1997). An interesting possibility is that the MPRs have a similar function in regulating clathrin coat assembly at the TGN. In this scenario, the ARF·GTP-activated high-affinity AP-1 docking site would remain active until a cargo molecule (the MPR) recruits the ARF GAP to the site, allowing GTP hydrolysis to occur. When MPRs are lacking, as in the MPR-negative membranes, the level of ARF GAP activity associated with the TGN would decline, resulting in less GTP hydrolysis and, consequently, increased resistance to extraction with Tris-HCl. We have used immunoblots to analyze the amount of ARF GAP (Cukierman et al., 1995) associated with the two types of Golgi-enriched membranes and find similar levels of ARF GAP in both fractions (our unpublished observations). Because ARF GAP can be recruited onto the Golgi membrane by ERD2 and perhaps other molecules as well, the measurement of total Golgi-associated ARF GAP may have obscured a selective decrease in this protein on the TGN.
The results of this study support our original model, that an ARF-activated docking apparatus facilitates the recruitment of AP-1 onto the TGN surface (Traub et al., 1993). Our results are also in accord with data in a recent report characterizing a novel protein, PACS-1, that regulates the trafficking of several proteins, including the CI-MPR, at the interface between the TGN and the endosome compartment (Wan et al., 1998). Using antisense cDNA to knockdown PACS-1 expression appears to cause the CI-MPR to accumulate within the endosomal system but has a negligible effect on the steady-state distribution of AP-1 at the TGN (Wan et al., 1998). These data resemble our results and, independently, prompted the conclusion that AP-1 assembly at the TGN does not seem to be directly controlled by sorted molecules such as the MPRs. Despite the fact that the putative docking molecules have not yet been identified and characterized, there is a growing consensus that these molecules do, in fact, appear to regulate clathrin coat initiation by delineating the membrane-binding site for the adaptors (Santini and Keen, 1996; Kirchhausen et al., 1997; Warren et al., 1997; Santini et al., 1998). Our model becomes more compelling as the number of trafficking processes regulated by ARF1 grows (Simpson et al., 1996; Faundez et al., 1997; Ooi et al., 1998; Passreiter et al., 1998). In particular, ARF has been linked to all protein export from the TGN (Traub and Kornfeld, 1997). Because both AP-1 and AP-3 coats appear to assemble at the TGN, and sorting by both these adaptor complexes seems to utilize tyrosine-based and dileucine-based trafficking signals (Johnson and Kornfeld, 1992a,b; Honing et al., 1996, 1998), dedicated docking sites would ensure that these coat-dependent sorting processes can occur simultaneously but independently at the TGN.
ACKNOWLEDGMENTS
We are grateful to our many colleagues who generously provided important reagents for this study. In particular, we are grateful to Bernard Hoflack and Peter Lobel for making the MPR-negative fibroblast cell line available to us. This work was supported in part by National Institutes of Health grant R01 CA-08759 to S.K., National Institutes of Health training grant HL-0708823, and by a grant from the Edward Mallinckrodt Jr. Foundation to L.M.T.
Footnotes
The abbreviations used are: ARF, ADP-ribosylation factor; BFA, brefeldin A; CI, cation-independent; CD, cation-dependent; GAP, GTPase-activating protein; GTPγS, guanosine 5′-O-(3-thiotriphosphate); LAMP, lysosome-associated membrane protein; MPR, mannose 6-phosphate receptor; TGN, trans-Golgi network; +/+, CD-MPR and CI-MPR positive; −/−, CD-MPR and CI-MPR negative.
REFERENCES
- Ahle S, Mann A, Eichelsbacher U, Ungewickell U. Structural relationships between clathrin assembly proteins from the Golgi and the plasma membrane. EMBO J. 1988;7:919–929. doi: 10.1002/j.1460-2075.1988.tb02897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alconada A, Bauer U, Hoflack B. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 1996;15:6096–6110. [PMC free article] [PubMed] [Google Scholar]
- Aoe T, Cukierman E, Lee A, Cassel D, Peters PJ, Hsu VW. The KDEL receptor, ERD2, regulates intracellular traffick by recruiting a GTPase-activating protein for ARF1. EMBO J. 1997;16:7305–7316. doi: 10.1093/emboj/16.24.7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum to Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, Dunphy WG, Braell WA, Rothman JE. Reconstitution of the transport of protein between successive compartments of the Golgi measured by the coupled incorporation of N-acetylglucosamine. Cell. 1984;39:405–416. doi: 10.1016/0092-8674(84)90019-9. [DOI] [PubMed] [Google Scholar]
- Bremnes T, Lauvrak V, Lindqvist B, Bakke O. A region from the medium chain adaptor subunit (μ) recognizes leucine and tyrosine-based sorting signals. J Biol Chem. 1998;273:8638–8645. doi: 10.1074/jbc.273.15.8638. [DOI] [PubMed] [Google Scholar]
- Brodsky FM. Clathrin structure characterized with monoclonal antibodies. I. Analysis of multiple antigenic sites. J Cell Biol. 1985;101:2047–2054. doi: 10.1083/jcb.101.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cukierman E, Huber I, Rotman M, Cassel D. The ARF1 GTPase-activating protein: zinc finger motif and Golgi complex location. Science. 1995;270:1999–2002. doi: 10.1126/science.270.5244.1999. [DOI] [PubMed] [Google Scholar]
- Faundez V, Horng JT, Kelly RB. ADP ribosylation factor 1 is required for synaptic vesicle budding in PC12 cells. J Cell Biol. 1997;138:505–515. doi: 10.1083/jcb.138.3.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fire E, Zwart DE, Roth MG, Henis YI. Evidence from lateral mobility studies for dynamic interactions of a mutant influenza hemagglutinin with coated pits. J Cell Biol. 1991;115:1585–1594. doi: 10.1083/jcb.115.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge I, Hopkins CR. In polarized MDCK cells basolateral vesicles arise from clathrin-γ-adaptin-coated domains on endosomal tubules. J Cell Biol. 1998;141:611–624. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel CA, Foster SA. Mannose 6-phosphate receptor-mediated endocytosis of acid hydrolases: internalization of β-glucuronidase is accompanied by a limited dephosphorylation. J Cell Biol. 1986;103:1817–1827. doi: 10.1083/jcb.103.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galluser A, Kirchhausen T. The β1 and the β2 subunits of the AP complexes are the clathrin coat assembly components. EMBO J. 1993;12:5237–5244. doi: 10.1002/j.1460-2075.1993.tb06219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glickman JN, Conibear E, Pearse BM. Specificity of binding of clathrin adaptors to signals on the mannose-6-phosphate/insulin-like growth factor II receptor. EMBO J. 1989;8:1041–1047. doi: 10.1002/j.1460-2075.1989.tb03471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Hoflack B, Simons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Honing S, Griffith J, Geuze HJ, Hunziker W. The tyrosine-based lysosomal targeting signal in lamp-1 mediates sorting into Golgi-derived clathrin-coated vesicles. EMBO J. 1996;15:5230–5239. [PMC free article] [PubMed] [Google Scholar]
- Honing S, Sandoval IV, von Figura K. A di-leucine-based motif in the cytoplasmic tail of LIMP-II and tyrosinase mediates selective binding of AP-3. EMBO J. 1998;17:1304–1314. doi: 10.1093/emboj/17.5.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S, Sosa M, Hille-Rehfeld A, von Figura K. The 46-kDa mannose 6-phosphate receptor contains multiple binding sites for clathrin adaptors. J Biol Chem. 1997;272:19884–19890. doi: 10.1074/jbc.272.32.19884. [DOI] [PubMed] [Google Scholar]
- Johnson KF, Kornfeld S. A His-Leu-Leu sequence near the carboxyl terminus of the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor is necessary for the lysosomal enzyme sorting function. J Biol Chem. 1992a;267:17110–17115. [PubMed] [Google Scholar]
- Johnson KF, Kornfeld S. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol. 1992b;119:249–257. doi: 10.1083/jcb.119.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen JH, Willingham MC, Pastan IH. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979;16:303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Kirchhausen T, Bonifacino JS, Riezman H. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr Opin Cell Biol. 1997;9:488–495. doi: 10.1016/s0955-0674(97)80024-5. [DOI] [PubMed] [Google Scholar]
- Klumperman J, Hille A, Veenendaal T, Oorschot V, Stoorvogel W, von Figura K, Geuze HJ. Differences in the endosomal distributions of the two mannose 6-phosphate receptors. J Cell Biol. 1993;121:997–1010. doi: 10.1083/jcb.121.5.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klumperman J, Kuliawat R, Griffith JM, Geuze HJ, Arvan P. Mannose 6-phosphate receptors are sorted from immature secretory granules via adaptor protein AP-1, clathrin, and syntaxin 6-positive vesicles. J Cell Biol. 1998;141:359–371. doi: 10.1083/jcb.141.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld S, Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Griffiths G, Hoflack B. Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Hoflack B. Mannose 6-phosphate receptors regulate the formation of clathrin-coated vesicles in the TGN. J Cell Biol. 1997;137:335–345. doi: 10.1083/jcb.137.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Borgne R, Schmidt A, Mauxion F, Griffiths G, Hoflack B. Binding of AP-1 Golgi adaptors to membranes requires phosphorylated cytoplasmic domains of the mannose 6-phosphate/insulin-like growth factor II receptor. J Biol Chem. 1993;268:22552–22556. [PubMed] [Google Scholar]
- Liang JO, Sung T-C, Morris AJ, Frohman MA, Kornfeld S. Different domains of mammalian ADP-ribosylation factor 1 mediate interaction with selected target proteins. J Biol Chem. 1997;272:33001–33008. doi: 10.1074/jbc.272.52.33001. [DOI] [PubMed] [Google Scholar]
- Ludwig T, Le Borgne R, Hoflack B. Roles for mannose 6-phosphate receptors in lysosomal enzyme sorting, IGF-II binding and clathrin-coat assembly. Trends Cell Biol. 1995;5:202–206. doi: 10.1016/s0962-8924(00)89000-5. [DOI] [PubMed] [Google Scholar]
- Ludwig T, Munier-Lehmann H, Bauer U, Hollinshead M, Ovitt C, Lobel P, Hoflack B. Differential sorting of lysosomal enzymes in mannose 6-phosphate receptor-deficient fibroblasts. EMBO J. 1994;13:3430–3437. doi: 10.1002/j.1460-2075.1994.tb06648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauxion F, Le Borgne R, Munier-Lehmann H, Hoflack B. A casein kinase II phosphorylation site in the cytoplasmic domain of the cation-dependent mannose 6-phosphate receptor determines the high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2171–2178. doi: 10.1074/jbc.271.4.2171. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyatake S, Saito T, Galluser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Ooi CE, Dell Angelica EC, Bonifacino JS. ADP-ribosylation factor 1 (ARF1) regulates recruitment of the AP-3 adaptor complex to membranes. J Cell Biol. 1998;142:391–204. doi: 10.1083/jcb.142.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Palmer DJ, Amherdt M, Rothman JE. Coated vesicle assembly in the Golgi requires only coatomer and ARF proteins from the cytosol. Nature. 1993;364:732–734. doi: 10.1038/364732a0. [DOI] [PubMed] [Google Scholar]
- Passreiter M, Anton M, Lay D, Frank R, Harter C, Wieland FT, Gorgas K, Just WW. Peroxisome biogenesis – involvement of ARF and coatomer. J Cell Biol. 1998;141:373–383. doi: 10.1083/jcb.141.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearse BMF, Robinson MS. Clathrin, adaptors and sorting. Annu Rev Cell Biol. 1990;6:151–171. doi: 10.1146/annurev.cb.06.110190.001055. [DOI] [PubMed] [Google Scholar]
- Rapoport I, Chen YC, Cupers P, Shoelson SE, Kirchhausen T. Dileucine-based sorting signals bind to the β-chain of AP-1 at a site distinct and regulated differently from the tyrosine-based motif-binding site. EMBO J. 1998;17:2148–2155. doi: 10.1093/emboj/17.8.2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS, Kreis TE. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of Brefeldin A and G protein activators. Cell. 1992;69:129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Rodionov DG, Bakke O. Medium chains of adaptor complexes AP-1 and AP-2 recognize leucine-based sorting signals from the invariant chain. J Biol Chem. 1998;273:6005–6008. doi: 10.1074/jbc.273.11.6005. [DOI] [PubMed] [Google Scholar]
- Salamero J, Le Borgne R, Saudrais C, Goud B, Hoflack B. Expression of major histocompatibility complex class II molecules in HeLa cells promotes the recruitment of AP-1 Golgi-specific assembly proteins on Golgi membranes. J Biol Chem. 1996;271:30318–30321. doi: 10.1074/jbc.271.48.30318. [DOI] [PubMed] [Google Scholar]
- Santini F, Keen JH. Endocytosis of activated receptors and clathrin-coated pit formation: deciphering the chicken or egg relationship. J Cell Biol. 1996;132:1025–1036. doi: 10.1083/jcb.132.6.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santini F, Marks MS, Keen JH. Endocytic clathrin-coated pit formation is independent of receptor internalization signal levels. Mol Biol Cell. 1998;9:1177–1194. doi: 10.1091/mbc.9.5.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih W, Galluser A, Kirchhausen T. A clathrin binding site in the hinge of the β2 chain of the mammalian AP complexes. J Biol Chem. 1995;270:31083–31090. doi: 10.1074/jbc.270.52.31083. [DOI] [PubMed] [Google Scholar]
- Simpson F, Bright NA, West MA, Newman LS, Darnell RB, Robinson MS. A novel adaptor-related protein complex. J Cell Biol. 1996;133:749–760. doi: 10.1083/jcb.133.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa MA, Schmidt B, von Figura K, Hille-Rehfeld A. In vitro binding of plasma membrane-coated vesicle adaptors to the cytoplasmic domain of lysosomal acid phosphatase. J Biol Chem. 1993;268:12537–12543. [PubMed] [Google Scholar]
- Stamnes MA, Rothman JE. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell. 1993;73:999–1005. doi: 10.1016/0092-8674(93)90277-w. [DOI] [PubMed] [Google Scholar]
- Takatsu H, Sakurai M, Shin H-W, Murakami K, Nakayama K. Identification and characterization of novel clathrin adaptor-related proteins. J Biol Chem. 1998;273:24693–24700. doi: 10.1074/jbc.273.38.24693. [DOI] [PubMed] [Google Scholar]
- Traub LM, Bannykh SI, Rodel JE, Aridor M, Balch WE, Kornfeld S. AP-2–containing clathrin coats assemble on mature lysosomes. J Cell Biol. 1996;135:1801–1804. doi: 10.1083/jcb.135.6.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Kornfeld S. The trans-Golgi network: a late secretory sorting station. Curr Opin Cell Biol. 1997;9:527–533. doi: 10.1016/s0955-0674(97)80029-4. [DOI] [PubMed] [Google Scholar]
- Traub LM, Kornfeld S, Ungewickell E. Different domains of the AP-1 adaptor complex are required for Golgi membrane binding and clathrin recruitment. J Biol Chem. 1995;270:4933–4942. doi: 10.1074/jbc.270.9.4933. [DOI] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon B, Berger EG. Galactosyltransferase. In: Bergmeyer HU, editor. Methods of Enzymatic Analysis. Vol. 3. Weinheim: Verlag Chemie; 1983. pp. 374–381. [Google Scholar]
- von Figura K, Hasilik A. Lysosomal enzymes and their receptors. Annu Rev Biochem. 1986;55:167–193. doi: 10.1146/annurev.bi.55.070186.001123. [DOI] [PubMed] [Google Scholar]
- Wan L, Molloy SS, Thomas L, Liu G, Xiang Y, Rybak SL, Thomas G. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell. 1998;94:205–216. doi: 10.1016/s0092-8674(00)81420-8. [DOI] [PubMed] [Google Scholar]
- Warren RA, Green FA, Enns CA. Saturation of the endocytic pathway for the transferrin receptor does not affect the endocytosis of the epidermal growth factor receptor. J Biol Chem. 1997;272:2116–2121. doi: 10.1074/jbc.272.4.2116. [DOI] [PubMed] [Google Scholar]
- Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Wong DH, Brodsky FM. 100-kDa proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties. J Cell Biol. 1992;117:1171–1179. doi: 10.1083/jcb.117.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Traub LM, Kornfeld S. ADP-ribosylation factor 1 transiently activates high-affinity adaptor protein complex AP-1 binding sites on Golgi membranes. Mol Biol Cell. 1998;9:1323–1337. doi: 10.1091/mbc.9.6.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]