INTRODUCTION
Although intraocular pressure (IOP) is no longer included in the definition of open-angle glaucoma (OAG), it remains as the only modifiable and a major risk factor for the development and progression of the disease.1, 2 Several studies have identified characteristics associated with elevated IOP in persons of African, European and Asian ancestries.3–7 Reported factors include older age,3, 4, 6–13 gender,3, 6, 8, 14, 15 race (African ancestry),11, 14 blood pressure (BP),1, 3, 6, 9, 11, 14–18 diabetes,1, 3, 6, 19–21 pulse rate,3, 6 body mass index (BMI),3, 4, 6 nuclear sclerosis,3, 5 iris color,5, 22 myopia,5, 7 use of alcohol,5, 6, 17 smoking6 and family history of glaucoma.5, 6, 13
To our knowledge there are no reports on the factors associated with IOP in the Latino population, which is the largest and fastest growing minority group in the US.23 Latinos of predominantly Mexican ancestry in Los Angeles have high rates of OAG, similar to those of African-Americans and significantly higher than non-Hispanic whites in the US.24 The Los Angeles Latino Eye Study (LALES), a population-based study of Latinos of mostly Mexican ancestry in Los Angeles County that was initiated to better understand health care and eye disease in Latinos,25 provided us with opportunity to evaluate the biological factors associated with higher IOP in this population.
MATERIALS AND METHODS
The University of Southern California's Institutional Review Board approved the study, and all procedures were in accord with the standards of the Declaration of Helsinki for research involving human subjects. Informed consent was obtained from all study participants. Details of the study design, sampling plan, and baseline data are reported elsewhere.25 In brief, the study population consisted of self-identified Latinos (primarily Mexican American), aged ≥40 years, living in six adjacent census tracts in the city of La Puente, California. All self identified Latinos aged 40 years and older (n=7789) were invited to complete an in-home questionnaire and a complete clinical and ocular examination. Of the eligible participants, 6357 (82%) completed both the in-home questionnaire and the clinical exam. Of these 6357 persons, 399 had a prior history of ocular hypotensive medication use, laser or incisional surgery and were excluded from the study. Of the remaining 5958 participants without a prior history of glaucoma treatment, 281 met the LALES criteria for definite or probable OAG and 215 met the criteria for ocular hypertension. These individuals were included in the analysis. In brief, definite or probable OAG was defined as the presence of an open angle and (1) evidence of characteristic or compatible glaucomatous optic disc damage on stereo fundus photography in at least one eye; and/or (2) congruent, characteristic, or compatible glaucomatous visual field abnormality. The IOP level was not considered in establishing the diagnosis of OAG. Ocular hypertension was diagnosed in individuals with an IOP of >21 mmHg and the absence of either of the above mentioned criteria for OAG.
The IOP data provided here is from a randomly selected eye of each participant. Three measurements of IOP were obtained using the Goldmann applanation tonometry (Haag-Streit, Bern, Switzerland) and averaged.
Risk Factor Assessment
Candidate risk factors were grouped into demographic, medical, and ocular factors. The demographic information obtained from the in-home questionnaire included age and gender. During the in-home interview, the following clinical and ocular history information was obtained from the participants: previous diagnosis of diabetes or hypertension, previous history of other cardiovascular disease such myocardial infarction, angina or cerebrovascular accident, history and extent of tobacco and alcohol use, current or previous use of any type of corticosteroids, family history of glaucoma in first degree relatives and history of eye trauma.
Medical characteristics obtained from clinical examination included weight and height for calculation of body mass index (BMI), pulse (beats/minute), random blood glucose level (using the Hemocue B-Glucose Analyzer), glycosylated hemoglobin (used the DCA 2000+ System), and systemic blood pressure. Body mass index was defined as weight (in kilograms) divided by the square of the height in meters (kg/m2). Participants were considered to have diabetes if (1) they self-reported a history of diabetes and were under treatment with oral hypoglycemic medications, insulin, or diet alone; or (2) if they had a hemoglobin A1C (HbA1C) level of 7.0% or higher or (3) if they had a random blood glucose of 200 mg% or higher. Each participant had two blood pressure and heart rate measurements taken while sitting. The mean of these two measurements were used in the statistical analysis.
The ocular characteristics were obtained from a comprehensive eye examination where all procedures were performed in a standardized manner by one of three LALES ophthalmologists and trained ophthalmic technicians. Details of the complete clinical protocol have been described previously.25 The pertinent ocular factors evaluated here included measurements of central corneal thickness (CCT), axial length, grading of nuclear cataract26 and iris color.27
Statistical Analyses
The means and standard deviations of IOP, stratified by both age and gender, were calculated. Additional means and standard deviations were calculated while stratifying by each categorical predictor. Analysis of variance technique (ANOVA) was done for each demographic, medical, and ocular predictor versus IOP. Partial r-square values were reported for continuous covariates. The variables that were significant from the univariate ANOVA analysis (p<0.05) were entered into a multivariable regression model and those that remained significant (p<0.05) were kept in the final model. All analysis was done using SAS version 9 (SAS Institute Inc., Cary, NC).
We also modeled the nature of the relationship between the various significant risk factors and IOP by plotting the LOWESS (locally weighted least squares) fit28 for age, systolic BP, BMI, and CCT after adjusting for all other variables. The predicted median IOP for a particular level of a risk factor was determined from the multivariable regression model after adjusting for other covariates. The estimated median IOP was then plotted against the variable of interest (e.g., systolic blood pressure) and a locally weighted regression line was fitted using S-PLUS (Insightful Corporation, Seattle, WA).
RESULTS
From the 6357 participants, the cohort for this study includes 5958 participants who had completed the interview and clinical examination and did not have a prior history of glaucoma treatment. The majority of participants were female (58%); the average age (± standard deviation) was 54.9 ± 10.9 years. The mean IOP for the entire study population was 14.5 ± 3.2 mm Hg.
The mean IOP values increased from 14.0 ± 2.8 mm Hg at the age of 40 to 49 years to 15.1 ± 3.7 mm Hg at 70 years of age or older (P < 0.0001,Table 1) Overall women had slightly higher IOPs than men (P < 0.0001). Table 2 provides the result of the univariate analysis for categorical variables associated with IOP as a comparison of means. Significant factors include female gender, history of hypertension and diabetes, nuclear sclerosis and brown iris color. The results of the univariate analysis for continuous variables associated with IOP are listed in Table 3. Factors associated with elevated IOP include older age, higher BMI, elevated systolic and diastolic BP, faster pulse rate, higher glycosylated hemoglobin (HbA1C) and thicker CCT.
Table 1.
Age and Gender Specific Distribution of Intraocular pressure (IOP) in participants of the Los Angeles Latino Eye Study
| Age (years) | Men | Women | Both | |||
|---|---|---|---|---|---|---|
| n | Mean (± SD) | n | Mean (± SD) | n | Mean (± SD) | |
| 40–49 | 949 | 13.9 (3.0) | 1341 | 14.1 (3.7) | 2290 | 14.0 (2.8) |
| 50–59 | 748 | 14.4 (3.4) | 1058 | 14.8 (3.0) | 1806 | 14.6 (3.2) |
| 60–69 | 474 | 14.5 (3.4) | 688 | 15.0 (3.5) | 1162 | 14.8 (3.4) |
| >70 | 304 | 14.9 (3.6) | 396 | 15.3 (3.7) | 700 | 15.1 (3.7) |
| All ages | 2475 | 14.3 (3.3) | 3483 | 14.6 (3.1) | 5958 | 14.5 (3.2) |
SD = standard deviation; IOP = intraocular pressure (mm Hg).
Table 2.
Univariate Associations with Intraocular Pressure (IOP) in participants of the Los Angeles Latino Eye Study
| Risk Factors † | N | IOP (mm Hg)* | P-value |
|---|---|---|---|
| Gender | |||
| Female | 3483 | 14.6 ± 3.1 | |
| Male | 2475 | 14.3 ± 3.3 | <0.0001 |
| Family History of Glaucoma | |||
| Yes | 467 | 14.4 ± 2.9 | |
| No | 5203 | 14.5 ± 3.2 | 0.77 |
| Hypertension | |||
| Yes | 2318 | 15.0 ± 3.3 | |
| No | 3640 | 14.1 ± 3.0 | <0.0001 |
| Diabetes | |||
| Yes | 1416 | 15.2 ± 3.3 | |
| No | 4542 | 14.2 ± 3.1 | <0.0001 |
| History of Tobacco | |||
| Never | 3624 | 14.5 ± 3.2 | |
| Ex-smoker | 1460 | 14.4 ± 3.2 | |
| Current | 848 | 14.7 ± 3.2 | 0.20 |
| History of Alcohol | |||
| Never | 2303 | 14.4 ± 3.2 | |
| Occasional | 1783 | 14.5 ± 3.1 | |
| Regular | 774 | 14.6 ± 3.3 | |
| Ex-Drinker | 1080 | 14.5 ± 3.2 | 0.66 |
| History of Steroid Use | |||
| Never | 3642 | 14.4 ± 3.1 | |
| Used in past | 188 | 14.5 ± 4.0 | |
| Current use | 59 | 14.3 ± 2.8 | 0.90 |
| Cardiovascular Disease | |||
| Yes | 442 | 14.4 ± 3.3 | |
| No | 5516 | 14.5 ± 3.2 | 0.86 |
| History of Eye Trauma | |||
| Yes | 1189 | 14.5 ± 3.0 | |
| No | 4769 | 14.5 ± 3.2 | 0.76 |
| Nuclear Cataract Grading‡ | |||
| Grade 0 | 1956 | 14.0 ± 2.9 | |
| Grade 0–I | 3362 | 14.6 ± 3.1 | |
| Grade I–II | 357 | 15.2 ± 3.6 | |
| Grade II–III | 83 | 15.3 ± 4.0 | |
| >III | 17 | 18.0 ± 9.0 | <0.0001 |
| Pseudophakics | 184 | 15.0 ± 4.0 | |
| Iris Color | |||
| Blue/Green/Hazel | 2080 | 14.2 ± 3.1 | |
| Light Brown | 3125 | 14.3 ± 3.3 | |
| Dark Brown | 747 | 14.6 ± 3.1 | 0.0009 |
Table 3.
Univariate Associations with Intraocular Pressure (IOP) in participants of the Los Angeles Latino Eye Study
| Risk Factors † | r2 (%)* | P-value |
|---|---|---|
| Age | 0.01 (1.0) | <0.001 |
| BMI (kg/m2) | 0.003 (0.3) | <0.0001 |
| Systolic BP (mm Hg) | 0.04 (4.0) | <0.0001 |
| Diastolic BP (mm Hg) | 0.02 (2.0) | <0.0001 |
| Pulse (beats/min) | 0.005 (0.5) | <0.0001 |
| CCT (µ) | 0.03 (3.0) | <0.0001 |
| HbA1C (%) | 0.02 (2.0) | <0.0001 |
| Axial Length (mm) | 0.0004 (0.04) | 0.13 |
Table 3 includes continuous variables.
Coefficient of determination.
BMI = body mass index, BP = blood pressure, CCT = central corneal thickness.
Factors found to be significant in the univariate analysis were included the final step-wise multivariable regression model. Because of the high inter-correlation between systolic BP, diastolic BP and hypertension, and HbA1C and diabetes, hypertension and HbA1C were removed from this model. Variables that continued to be associated with elevated IOP in the final model were age, female gender, diabetes, higher systolic and diastolic blood pressure, elevated BMI, nuclear sclerosis, brown iris color and thicker CCT (Table 4). All of the factors combined, only explained 10% of the variation in IOP. Higher systolic BP and thicker CCT were the most significant factors associated with IOP, each explaining approximately 4% of IOP variance. The final multivariable regression analysis was repeated after excluding the 281 individuals with the diagnosis of OAG; the variables associated with elevated IOP remained unchanged.
Table 4.
Multivariable Associations with Intraocular Pressure (IOP) in participants of the Los Angeles Latino Eye Study
| Risk Factors | Partial r2 (%)* | P-Value |
|---|---|---|
| Age | 0.002 (0.2) | <0.0001 |
| Gender | 0.005 (0.5) | 0.0001 |
| Diabetes | 0.008 (0.8) | <0.0003 |
| Nuclear Cataract Grading‡ | 0.003 (0.3) | <0.0002 |
| Iris Color | 0.001 (0.1) | 0.009 |
| BMI (kg/m2) | 0.001 (0.2) | 0.0009 |
| Systolic BP (mm Hg) | 0.04 (4.0) | <0.0001 |
| Diastolic BP (mm Hg) | 0.0009 (0.09) | 0.02 |
| CCT (µ) | 0.04 (4.0) | <0.0001 |
Coefficient of partial determination.
Lens Opacity Classification System II.
BMI = body mass index, BP = blood pressure, CCT = central corneal thickness.
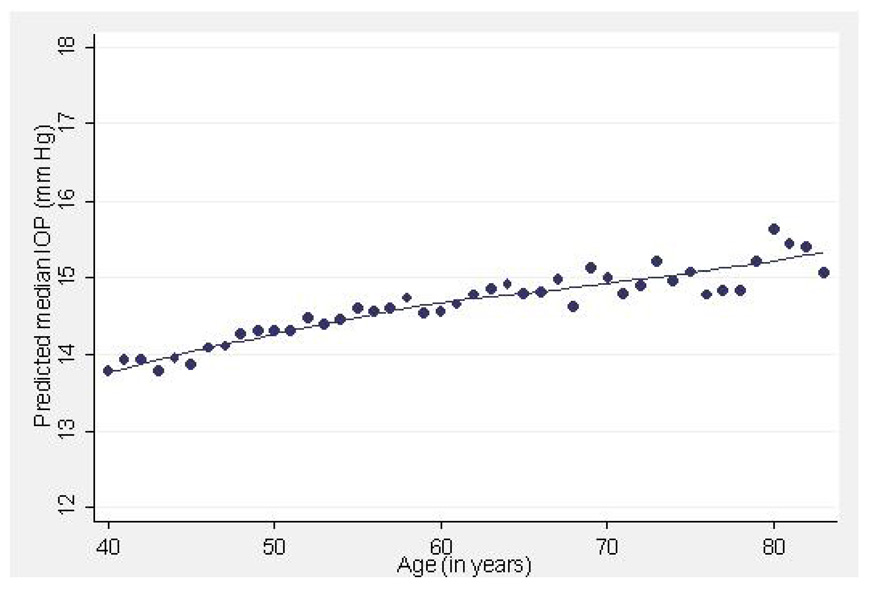
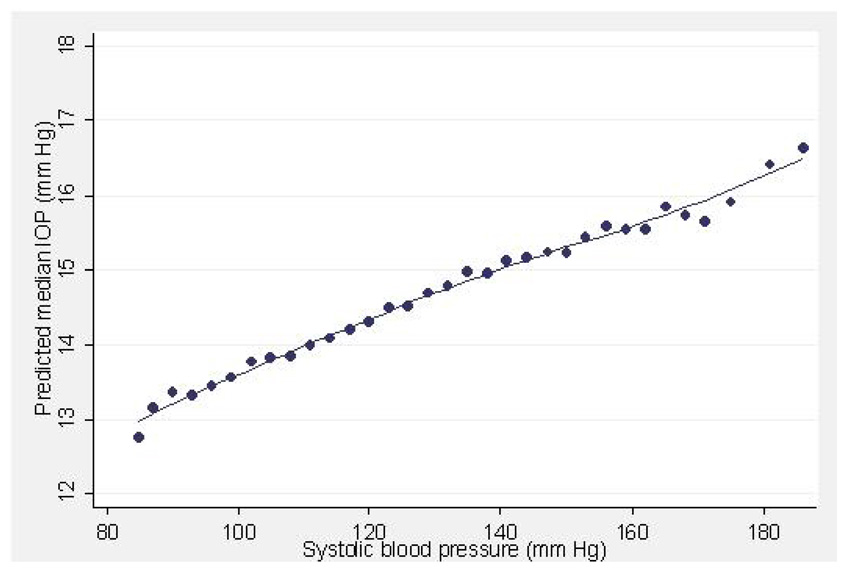
Figure 1–Figure 4 illustrate the relationship between IOP and age, systolic BP, BMI and CCT, respectively, after adjusting for all other variables. The plots for SBP and DBP (not shown) suggest that there is an essentially linear relationship between these variables and IOP after adjusting for other risk factors. A 10 year difference in age is associated with a 0.34 mm Hg difference in IOP (Figure 1). A 20 mm Hg higher systolic or diastolic BP is associated with a 0.7 and 0.9 mm Hg higher IOP respectively (Figure 2).
Figure 1.
Predicted median intraocular pressure (IOP, mmHg) in relation to age (years) in participants of the Los Angeles Latino Eye Study.
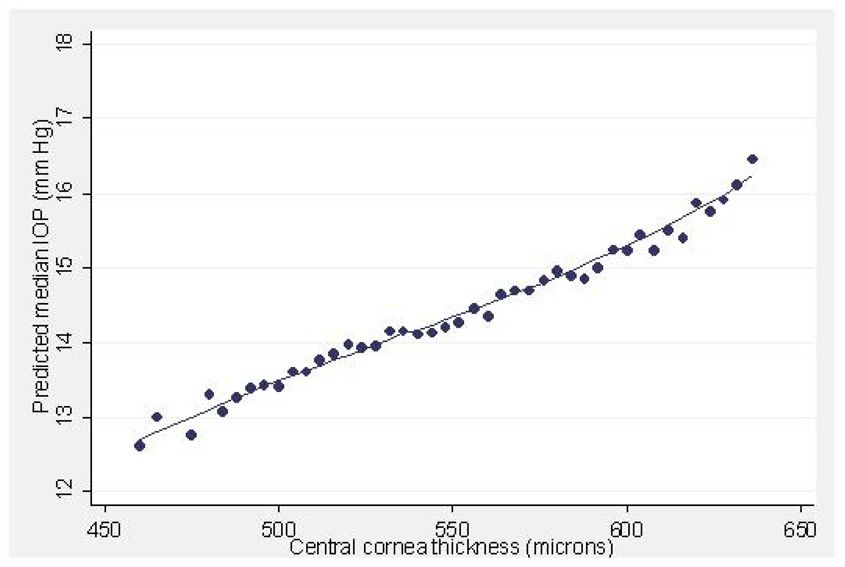
Figure 4.
Predicted median intraocular pressure (IOP, mmHg) in relation to central corneal thickness (µ) in participants of the Los Angeles Latino Eye Study.
Figure 2.
Predicted median intraocular pressure (IOP, mmHg) in relation to systolic blood pressure (mmHg) in participants of the Los Angeles Latino Eye Study.
DISCUSSION
We set out to identify biological factors associated with IOP in this Latino population without a prior history of glaucoma treatment.
Age
Several cross-sectional studies have shown a higher IOP with older age.6, 12, 22, 29 This has been attributed to significant reduction in outflow facility counteracting the reduced production of aqueous with age.30, 31 Other studies have found no correlation or even a negative correlation of IOP with age.3, 4, 17, 19, 32 It has been suggested that the rise in IOP that is observed in aging populations may be related to other factors such as a concomitant rise in blood pressure and/or BMI.4, 10, 18, 33 In our cross-sectional data IOP does vary with age and age remains a significant factor in the analysis even after adjusting for all other variables. Figure 1 plots the independent relationship between median IOP and age after controlling for other variables. There is slow rise in IOP with age. The variation is small, equaling slightly more than 1 mm Hg for those > 70 years of age compared to those 40–49 years of age (Table 1).
Gender
Results of previous studies evaluating gender differences in IOP are inconsistent.3–6, 15 We find a small but statistically significantly higher IOP in females than males although this difference is clinically insignificant. The relationship between gender and IOP is difficult to interpret. Women are believed to possibly have a more marked tendency for increase in IOP with age with peaking at a higher age than in men.34, 35 Other possible explanations for this include the higher level of obesity and hypertension in women or a longer life expectancy.
High Blood Pressure
High blood pressure is a well recognized risk factor for elevated IOP. Numerous studies have reported a consistent positive correlation between systolic blood pressure and IOP3, 4, 6, 9, 10, 12, 14–16, 18 although the association with diastolic BP is less consistent.3, 11, 14, 15, 18 Here we find a statistically significant association of elevated systolic and diastolic BP with IOP. Although arterial blood pressure and IOP both increase with age, this association in our data remains significant even when the data is adjusted for age. Figure 2 demonstrates the relationship between systolic BP and median IOP while controlling for all other variables. As systolic BP increases, IOP increases in linear fashion.
The mechanism responsible for elevated IOP with increasing BP is not well understood but increasing aqueous humor production by ultrafiltration as a result of elevated ciliary artery pressure or a generalized increased sympathetic tone and elevated serum corticosteroid levels resulting in simultaneous increase in BP and IOP together has been postulated.4, 10, 14 Regardless of the mechanism of IOP elevation, given the fact that BP is amendable to therapy and that IOP is a major risk factor for OAG, BP may potentially be a modifiable risk factor for OAG.
Diabetes Mellitus
Numerous large population-based studies have documented an association between diabetes, HbA1C and elevated IOP.3, 6, 10, 19 In this study our criteria for diagnosis of diabetes mellitus were stringent and based either on a history of currently being treated for DM and/or defined by a blood glucose and HbA1C testing. Those with a diagnosis of diabetes mellitus had a significantly higher IOP than those who did not. Although a physiologic explanation for this finding is unclear several theories have been proposed. The elevated blood glucose level in diabetes may induce an osmotic gradient and attract fluid into the intraocular space, resulting in elevated IOP.34 Alternatively, diabetes related autonomic dysfunction or genetic factors may play a role.36, 37
Although the positive association between diabetes and IOP is well documented, the relationship between diabetes and glaucoma is more controversial. Although several studies have shown diabetes to be a risk factor for the development of OAG,33, 38–40 others have found no association between diabetes and OAG.21, 41 Diabetes mellitus, in the Latino population, has been found to be an independent risk factor for the development of OAG,38 thus, diabetes, similar to BP, may be a modifiable risk factor for IOP and potentially for OAG. Interestingly, in our study those with higher HbA1C values also had a higher IOP (Table 3) suggesting that improved blood sugar control may indeed help improve IOP control. We removed HbA1C from our final regression model, however, due to its high inter-correlation with diabetes.
Body Mass Index
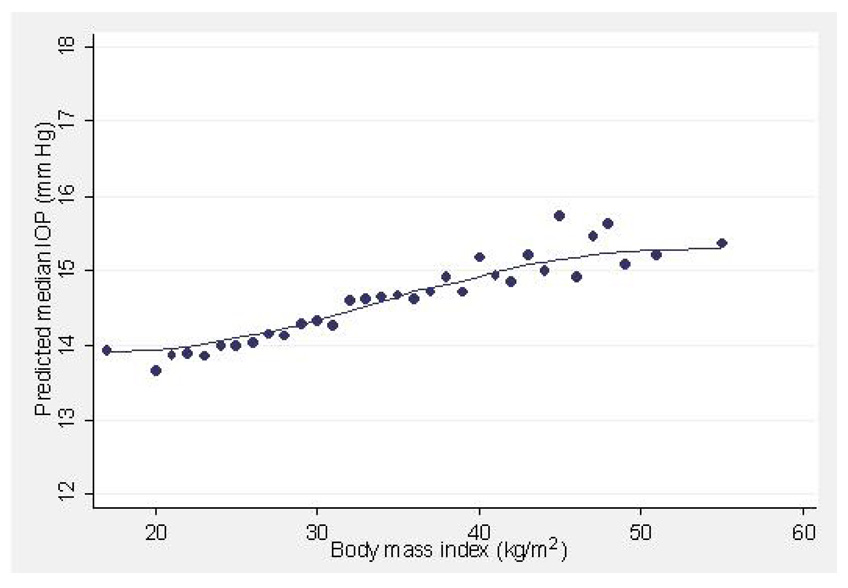
Body mass index, a measure of obesity was positively correlated with IOP. Although obesity is correlated with elevated blood pressure and diabetes, the association between BMI and higher IOP was found independently of these 2 variables. In interpreting these results, however, the difficulty of obtaining accurate applation tonometry in some obese individuals must be considered. Other cross-sectional studies have also found similar associations between IOP and BMI.3, 6, 10, 12, 42 It has been suggested that increased orbital pressure due to excess orbital fat may increase episcleral venous pressure and result in a decrease of outflow facility.4 Alternatively, the deposition of lipids may reduce outflow facility for the aqueous, resulting in higher IOP.14 Figure 3 plots the variation in IOP with increasing BMI. IOP is elevated by slightly more than one mmHg, as BMI increases by a massive amount (30 kg/m2). This association in clinical practice is likely insignificant.
Figure 3.
Predicted median intraocular pressure (IOP, mmHg) in relation to body mass index (kg/m2) in participants of the Los Angeles Latino Eye Study.
Iris Color
Interestingly, we found a significant positive association of IOP with darker iris pigmentation. Other studies have also found a similar association.5, 22, 43 Although pigment dispersion syndrome is thought to be related to the release of pigment and mechanical obstruction of the trabecular meshwork by pigment, in the absence of characteristics of pigment dispersion the relationship is difficult to interpret. Although we excluded cases with definite pigment dispersion, it is possible that subtle cases of the syndrome may have been missed. The differences in mean IOP between people with lighter colored irides and those with darker color irides, however, is very small and not clinically relevant.
Nuclear Lens Opacity Grade
The severity of nuclear sclerosis was also correlated with increasing IOP. Those with a II–III grade of nuclear sclerotic cataracts had on average a 1.3 mmHg increase in intraocular pressure compared to those with no cataracts, independently of other variables. A plausible explanation for this association is the mechanical effect of thicker lenses on the angle with subsequent compromise of aqueous outflow facility. IOP has been noted to reduce significantly after cataract extraction in several studies44, 45 and has been attributed to improvement of aqueous outflow facility by widening the drainage angle.46
Central Corneal Thickness
The relationship between CCT and IOP as measured by applanation tonometry has been well studied.47–51 Since Goldmann based his readings on the assumption that the mean normal corneal thickness is approximately 500 µm,52 the intraocular pressure readings obtained by the Goldmann applanation tonometry (GAT) for an eye with average CCTs has good accuracy. For significantly thinner or thicker corneas, however, Goldmann tonometry tends to underestimate or overestimate, respectively, the true intraocular pressure. Our results confirm this positive correlation of increasing IOP as measured by GAT with increasing CCT (Figure 4).
To the best of our knowledge, this is the first study to report on the factors associated with elevated IOP in the Latino population. Many of the variables that had been found to be significantly associated with IOP in previous studies in other populations were also found to be significant in this population. Age, female gender, higher systolic and diastolic BP, diabetes, larger BMI, darker colored irides, nuclear sclerosis and thicker CCTs were significant factors contributing to an elevated IOP. It should however be emphasized that the overall difference in IOP among individuals with specific characteristics is small and in some cases clinically insignificant. Nevertheless, recognition and identification of these variables allows for better understanding of IOP determinants and more importantly allows us to define specific subgroups of individuals who are at high risk of having increased IOP and possible progression to OAG.
Since elevated IOP is a major risk factor for the development of OAG it is reasonable for one to assume that the two conditions have similar risk profiles. Diabetes in the Latino population, for example, has been shown to be a risk factor for the development of OAG.38 This association, however, is independent of IOP. The relationship between OAG in the LALES population and other variables discussed here has not been elucidated and is currently under investigation.
The association of some variables such as systolic and diastolic blood pressure, diabetes and BMI with elevated IOP introduces the suggestion that perhaps individuals who are screened for these conditions should also be evaluated for elevated IOP and referred for further assessment and treatment if necessary. It is conceivable that by identifying such individuals, we may be able to decrease the burden of undetected eye disease in this fastest growing segment of the US population.
Acknowledgements/Disclosure
Support: National Institutes of Health Grants NEI U10-EY-11753 and EY-03040 and an unrestricted grant from the Research to Prevent Blindness, New York, NY. Rohit Varma is a Research to Prevent Blindness Sybil B. Harrington Scholar.
Financial Disclosure: The authors have no proprietary or commercial interest in any materials discussed in the manuscript.
Contributions: design and conduct of the study (RV, SA); collection, management, analysis, and interpretation of the data (FM, RV, SA, MYL); and preparation, review, or approval of the manuscript (RV, SA,FM, MYL).
Statement about Conformity: The study protocol was approved by the Institutional Review Board (IRB)/Ethics Committee at the University of Southern California and all study procedures adhered to the recommendations of the Declaration of Helsinki. Written consent was obtained from all participants.
Other Acknowledgements: The Los Angeles Latino Eye Study Group, University of Southern California, Los Angeles, CA.-Rohit Varma, MD, MPH; Sylvia H. Paz, MS; Stanley P. Azen, PhD; Lupe Cisneros, COA; Elizabeth Corona; Carolina Cuestas, OD; Denise R. Globe, PhD; Sora Hahn, MD; Mei-Ying Lai, MS; George Martinez; Susan Preston-Martin, PhD; Ronald E. Smith, MD; LaVina Tetrow, Mina Torres, MS; Natalia Uribe, OD; Jennifer Wong, MPH; Joanne Wu, MPH; Myrna Zuniga.
Battelle Survey Research Center, St. Louis, MO- Sonia Chico, BS; Lisa John, MSW; Michael Preciado, BA; Karen Tucker, MA.
Ocular Epidemiology Grading Center, University of Wisconsin, Madison, WI Ronald Klein, MD, MPH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leske MC. The epidemiology of open-angle glaucoma: a review. Am J Epidemiol. 1983;118:166–191. doi: 10.1093/oxfordjournals.aje.a113626. [DOI] [PubMed] [Google Scholar]
- 2.Sommer A. Intraocular pressure and glaucoma. Am J Ophthalmol. 1989;107:186–188. doi: 10.1016/0002-9394(89)90221-3. [DOI] [PubMed] [Google Scholar]
- 3.Klein BE, Klein R, Linton KL. Intraocular pressure in an American community. The Beaver Dam Eye Study. Invest Ophthalmol Vis Sci. 1992;33:2224–2228. [PubMed] [Google Scholar]
- 4.Shiose Y, Kawase Y. A new approach to stratified normal intraocular pressure in a general population. Am J Ophthalmol. 1986;101:714–721. doi: 10.1016/0002-9394(86)90776-2. [DOI] [PubMed] [Google Scholar]
- 5.Weih LM, Mukesh BN, McCarty CA, Taylor HR. Association of demographic, familial, medical, and ocular factors with intraocular pressure. Arch Ophthalmol. 2001;119:875–880. doi: 10.1001/archopht.119.6.875. [DOI] [PubMed] [Google Scholar]
- 6.Wu SY, Leske MC. Associations with intraocular pressure in the Barbados Eye Study. Arch Ophthalmol. 1997;115:1572–1576. doi: 10.1001/archopht.1997.01100160742012. [DOI] [PubMed] [Google Scholar]
- 7.Xu L, Li J, Zheng Y, et al. Intraocular pressure in Northern China in an urban and rural population: the Beijing eye study. Am J Ophthalmol. 2005;140:913–915. doi: 10.1016/j.ajo.2005.04.050. [DOI] [PubMed] [Google Scholar]
- 8.Bonomi L, Marchini G, Marraffa M, et al. Prevalence of glaucoma and intraocular pressure distribution in a defined population. The Egna-Neumarkt Study. Ophthalmology. 1998;105:209–215. doi: 10.1016/s0161-6420(98)92665-3. [DOI] [PubMed] [Google Scholar]
- 9.Bonomi L, Marchini G, Marraffa M, Bernardi P, Morbio R, Varotto A. Vascular risk factors for primary open angle glaucoma: the Egna-Neumarkt Study. Ophthalmology. 2000;107:1287–1293. doi: 10.1016/s0161-6420(00)00138-x. [DOI] [PubMed] [Google Scholar]
- 10.Carel RS, Korczyn AD, Rock M, Goya I. Association between ocular pressure and certain health parameters. Ophthalmology. 1984;91:311–314. doi: 10.1016/s0161-6420(84)34282-8. [DOI] [PubMed] [Google Scholar]
- 11.Hennis A, Wu SY, Nemesure B, Leske MC. Hypertension, diabetes, and longitudinal changes in intraocular pressure. Ophthalmology. 2003;110:908–914. doi: 10.1016/S0161-6420(03)00075-7. [DOI] [PubMed] [Google Scholar]
- 12.Klein BE, Klein R. Intraocular pressure and cardiovascular risk variables. Arch Ophthalmol. 1981;99:837–839. doi: 10.1001/archopht.1981.03930010837009. [DOI] [PubMed] [Google Scholar]
- 13.Leske MC, Connell AM, Wu SY, Hyman L, Schachat AP. Distribution of intraocular pressure. The Barbados Eye Study. Arch Ophthalmol. 1997;115:1051–1057. doi: 10.1001/archopht.1997.01100160221012. [DOI] [PubMed] [Google Scholar]
- 14.Bulpitt CJ, Hodes C, Everitt MG. Intraocular pressure and systemic blood pressure in the elderly. Br J Ophthalmol. 1975;59:717–720. doi: 10.1136/bjo.59.12.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dielemans I, Vingerling JR, Algra D, Hofman A, Grobbee DE, de Jong PT. Primary open-angle glaucoma, intraocular pressure, and systemic blood pressure in the general elderly population. The Rotterdam Study. Ophthalmology. 1995;102:54–60. doi: 10.1016/s0161-6420(95)31054-8. [DOI] [PubMed] [Google Scholar]
- 16.Foster PJ, Machin D, Wong TY, et al. Determinants of intraocular pressure and its association with glaucomatous optic neuropathy in Chinese Singaporeans: the Tanjong Pagar Study. Invest Ophthalmol Vis Sci. 2003;44:3885–3891. doi: 10.1167/iovs.03-0012. [DOI] [PubMed] [Google Scholar]
- 17.Leske MC, Warheit-Roberts L, Wu SY. Open-angle glaucoma and ocular hypertension: the Long Island Glaucoma Case-control Study. Ophthalmic Epidemiol. 1996;3:85–96. doi: 10.3109/09286589609080113. [DOI] [PubMed] [Google Scholar]
- 18.McLeod SD, West SK, Quigley HA, Fozard JL. A longitudinal study of the relationship between intraocular and blood pressures. Invest Ophthalmol Vis Sci. 1990;31:2361–2366. [PubMed] [Google Scholar]
- 19.Dielemans I, de Jong PT, Stolk R, Vingerling JR, Grobbee DE, Hofman A. Primary open-angle glaucoma, intraocular pressure, and diabetes mellitus in the general elderly population. The Rotterdam Study. Ophthalmology. 1996;103:1271–1275. doi: 10.1016/s0161-6420(96)30511-3. [DOI] [PubMed] [Google Scholar]
- 20.Klein BE, Klein R, Moss SE. Intraocular pressure in diabetic persons. Ophthalmology. 1984;91:1356–1360. doi: 10.1016/s0161-6420(84)34142-2. [DOI] [PubMed] [Google Scholar]
- 21.Tielsch JM, Katz J, Quigley HA, Javitt JC, Sommer A. Diabetes, intraocular pressure, and primary open-angle glaucoma in the Baltimore Eye Survey. Ophthalmology. 1995;102:48–53. doi: 10.1016/s0161-6420(95)31055-x. [DOI] [PubMed] [Google Scholar]
- 22.Hiller R, Sperduto RD, Krueger DE. Race, iris pigmentation, and intraocular pressure. Am J Epidemiol. 1982;115:674–683. doi: 10.1093/oxfordjournals.aje.a113350. [DOI] [PubMed] [Google Scholar]
- 23.U.S. Bureau of the Census; Current Population Reports. P25–1130: Population Projections of the United States by Age, Sex, Race, and Hispanic origin: 1995 to 2050. 1996
- 24.Varma R, Ying-Lai M, Francis BA, et al. Prevalence of open-angle glaucoma and ocular hypertension in Latinos: the Los Angeles Latino Eye Study. Ophthalmology. 2004;111:1439–1448. doi: 10.1016/j.ophtha.2004.01.025. [DOI] [PubMed] [Google Scholar]
- 25.Varma R, Paz SH, Azen SP, et al. The Los Angeles Latino Eye Study: design, methods, and baseline data. Ophthalmology. 2004;111:1121–1131. doi: 10.1016/j.ophtha.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 26.Chylack LT, Jr, Leske MC, McCarthy D, Khu P, Kashiwagi T, Sperduto R. Lens opacities classification system II (LOCS II) Arch Ophthalmol. 1989;107:991–997. doi: 10.1001/archopht.1989.01070020053028. [DOI] [PubMed] [Google Scholar]
- 27.Seddon JM, Sahagian CR, Glynn RJ, Sperduto RD, Gragoudas ES. Evaluation of an iris color classification system. The Eye Disorders Case-Control Study Group. Invest Ophthalmol Vis Sci. 1990;31:1592–1598. [PubMed] [Google Scholar]
- 28.Cleveland WS, Grosse E. Computational methods for local regression. Stat Comp. 1991;1:47–62. [Google Scholar]
- 29.Armaly MF. On the Distribution of Applanation Pressure. I. Statistical Features and the Effect of Age, Sex, and Family History of Glaucoma. Arch Ophthalmol. 1965;73:11–18. doi: 10.1001/archopht.1965.00970030013005. [DOI] [PubMed] [Google Scholar]
- 30.Brubaker RF, Nagataki S, Townsend DJ, Burns RR, Higgins RG, Wentworth W. The effect of age on aqueous humor formation in man. Ophthalmology. 1981;88:283–288. doi: 10.1016/s0161-6420(81)35037-4. [DOI] [PubMed] [Google Scholar]
- 31.Miyazaki M, Segawa K, Urakawa Y. Age-related changes in the trabecular meshwork of the normal human eye. Jpn J Ophthalmol. 1987;31:558–569. [PubMed] [Google Scholar]
- 32.Mapstone R, Clark CV. Prevalence of diabetes in glaucoma. Br Med J (Clin Res Ed) 1985;291:93–95. doi: 10.1136/bmj.291.6488.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitchell P, Smith W, Chey T, Healey PR. Open-angle glaucoma and diabetes: the Blue Mountains eye study, Australia. Ophthalmology. 1997;104:712–718. doi: 10.1016/s0161-6420(97)30247-4. [DOI] [PubMed] [Google Scholar]
- 34.Hollows FC, Graham PA. Intra-ocular pressure, glaucoma, and glaucoma suspects in a defined population. Br J Ophthalmol. 1966;50:570–586. doi: 10.1136/bjo.50.10.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiose Y. Intraocular pressure: new perspectives. Surv Ophthalmol. 1990;34:413–435. doi: 10.1016/0039-6257(90)90122-c. [DOI] [PubMed] [Google Scholar]
- 36.Clark CV, Mapstone R. The prevalence of diabetes mellitus in the family history of patients with primary glaucoma. Doc Ophthalmol. 1986;62:161–163. doi: 10.1007/BF00229127. [DOI] [PubMed] [Google Scholar]
- 37.Cristiansson J. Intraocular pressure in diabetes mellitus. Acta Ophthalmol (Copenh) 1961;39:155–167. doi: 10.1111/j.1755-3768.1961.tb00249.x. [DOI] [PubMed] [Google Scholar]
- 38.Chopra V, Varma R, Francis BA, Wu J, Torres M, Azen SP. Type 2 diabetes mellitus and the risk of open-angle glaucoma the Los Angeles Latino Eye Study. Ophthalmology. 2008;115:227–232. doi: 10.1016/j.ophtha.2007.04.049. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Klein BE, Klein R, Jensen SC. Open-angle glaucoma and older-onset diabetes. The Beaver Dam Eye Study. Ophthalmology. 1994;101:1173–1177. doi: 10.1016/s0161-6420(94)31191-2. [DOI] [PubMed] [Google Scholar]
- 40.Pasquale LR, Kang JH, Manson JE, Willett WC, Rosner BA, Hankinson SE. Prospective study of type 2 diabetes mellitus and risk of primary open-angle glaucoma in women. Ophthalmology. 2006;113:1081–1086. doi: 10.1016/j.ophtha.2006.01.066. [DOI] [PubMed] [Google Scholar]
- 41.de Voogd S, Ikram MK, Wolfs RC, et al. Is diabetes mellitus a risk factor for open-angle glaucoma? The Rotterdam Study. Ophthalmology. 2006;113:1827–1831. doi: 10.1016/j.ophtha.2006.03.063. [DOI] [PubMed] [Google Scholar]
- 42.Nomura H, Shimokata H, Ando F, Miyake Y, Kuzuya F. Age-related changes in intraocular pressure in a large Japanese population: a cross-sectional and longitudinal study. Ophthalmology. 1999;106:2016–2022. doi: 10.1016/S0161-6420(99)90417-7. [DOI] [PubMed] [Google Scholar]
- 43.Mitchell R, Rochtchina E, Lee A, Wang JJ, Mitchell P. Iris color and intraocular pressure: the Blue Mountains Eye Study. Am J Ophthalmol. 2003;135:384–386. doi: 10.1016/s0002-9394(02)01967-0. [DOI] [PubMed] [Google Scholar]
- 44.Cekic O, Batman C, Totan Y, Emre MI, Zilelioglu O. Changes in anterior chamber depth and intraocular pressure after phacoemulsification and posterior chamber intraocular lens implantation. Ophthalmic Surg Lasers. 1998;29:639–642. [PubMed] [Google Scholar]
- 45.Steuhl KP, Marahrens P, Frohn C, Frohn A. Intraocular pressure and anterior chamber depth before and after extracapsular cataract extraction with posterior chamber lens implantation. Ophthalmic Surg. 1992;23:233–237. [PubMed] [Google Scholar]
- 46.Meyer MA, Savitt ML, Kopitas E. The effect of phacoemulsification on aqueous outflow facility. Ophthalmology. 1997;104:1221–1227. doi: 10.1016/s0161-6420(97)30154-7. [DOI] [PubMed] [Google Scholar]
- 47.Brandt JD, Beiser JA, Kass MA, Gordon MO. Central corneal thickness in the Ocular Hypertension Treatment Study (OHTS) Ophthalmology. 2001;108:1779–1788. doi: 10.1016/s0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- 48.Ehlers N, Bramsen T, Sperling S. Applanation tonometry and central corneal thickness. Acta Ophthalmol (Copenh) 1975;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 49.Hahn S, Azen S, Ying-Lai M, Varma R. Central corneal thickness in Latinos. Invest Ophthalmol Vis Sci. 2003;44:1508–1512. doi: 10.1167/iovs.02-0641. [DOI] [PubMed] [Google Scholar]
- 50.Morad Y, Sharon E, Hefetz L, Nemet P. Corneal thickness and curvature in normal-tension glaucoma. Am J Ophthalmol. 1998;125:164–168. doi: 10.1016/s0002-9394(99)80086-5. [DOI] [PubMed] [Google Scholar]
- 51.Whitacre MM, Stein RA, Hassanein K. The effect of corneal thickness on applanation tonometry. Am J Ophthalmol. 1993;115:592–596. doi: 10.1016/s0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 52.Goldmann H, Schmidt T. [Applanation tonometry.] Ophthalmologica. 1957;134:221–242. doi: 10.1159/000303213. [DOI] [PubMed] [Google Scholar]