NAADP, first identified as a calcium messenger in sea urchin egg,1 is structural analog of nicotinamide adenine dinuclotide phosphate (NADP) where the amide group is being replaced with carboxylic group. Interestingly, this minor structural variations makes NAADP a potent Ca2+ store mobilizing messenger.1(a),2 Its mode of Ca2+ release is distinct from cyclic ADP-ribose (cADPR) and inositol 1,4,5-triphosphate. NAADP operates on Ca2+ stores, which have been identified as acidic lysosome-related organelle.3 Recent studies have demonstrated calcium-mobilizing efficiency of NAADP in a wide variety of cells, from plant to animal, including humans.4 However NAADP transduction signaling, the receptors it acts on and other regulatory functions is not clear. To fully understand the molecular basis underlying NAADP signaling process, it is necessary to deliver it intracellularly in a controlled fashion. In this context blocking the active functionalities in NAADP and unlocking them when necessary is promising. Cell permeant and photolabile or caged NAADP are the ideal molecular tool for investigating the localized signaling behavior of NAADP. To date there is only one report of caged NAADP, based on caging reagent 1-(2-nitrophenyl)diazoethane (NPE) synthesized by direct alkylation of NAADP at pH 1.3.5 The extreme acidic condition was used to avoid multiple caging of NAADP. Inspite of this precaution, significant caging of carboxylate group with NPE was observed. The unstable nature of most caging reagents at low pH hindered the development of other caged NAADP derivatives.5(b)
In the present study we report the chemo-enzymatic synthesis and biological investigation of a novel caged NAADP (4,5-dimethoxynitroacetophenone (DMNPE)-NAADP, 4, Scheme 1). The presence of electron-donating methoxy groups of the caging chromophore must lead to fast release of NAADP. Furuta et al. and Ellis-Davis et al. have reported that DMNB-caged compounds uncage faster than simple ortho-nitrobenzyl caged compounds.7 Our attempts to directly cage NAADP under a variety of conditions, all proved unsuccessful, yielding only the starting material and hydrolysis product, ADP-ribose phosphate. Initially, the highly labile and photosensitive diazo caging reagent 2 was prepared by the oxidation of 4,5-dimethoxy-2-nitroacetophenone with MnO2 in CHCl3. The diazo compound was then added to NADP under biphasic condition (H2O-CHCl3) with vigorous stirring resulting in the formation of caged NADP.8 Finally, enzyme-catalyzed base-exchange reaction between caged NADP and excess nicotinic acid resulted in the formation of DMNPE-caged NAADP (Scheme1). The caging reaction was monitored by Anion exchange high pressure liquid chromatography (HPLC). The final purification of DMNPE-caged NAADP was achieved by HPLC using AG MP-1 columns (see supporting information).
Scheme1.
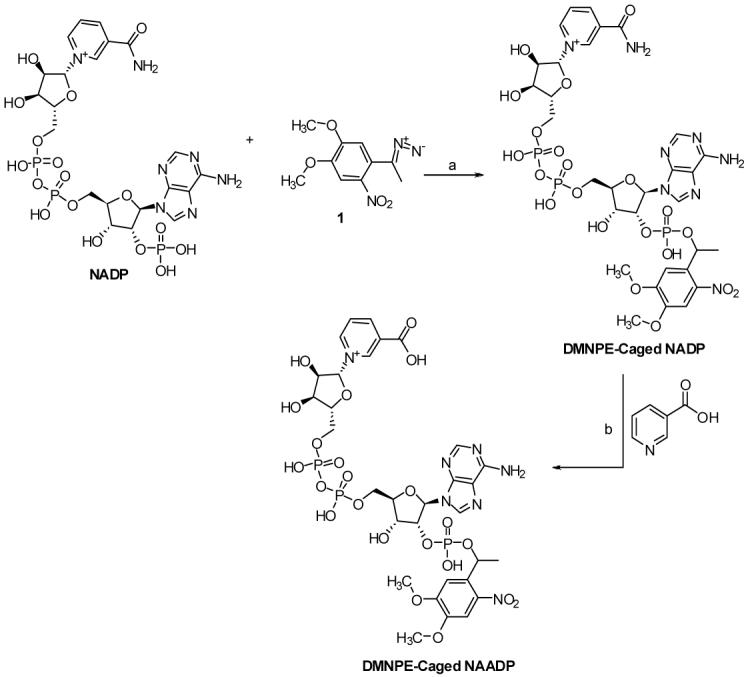
Chemical structure and synthesis of DMNPE-Caged-NAADP. (a) CHCl3-H2O, pH = 4; 60 %, 14 h. (b) ADP-ribocyclase, acetate buffer pH = 4.4, 5 h, 80%.
The structure of DMNPE-caged NAADP was confirmed by both 1H-NMR and 31P-NMR. The 1H-NMR of the purified compound shows two additional resonance peaks in the aromatic region that correspond to DMNPE phenyl group. Three resonance peaks, representing two methoxy groups and benzylic methyl groups are also visible. The 31P-NMR of DMNPE-caged NAADP showed only two peaks at -2.08 ppm and -11.31 ppm (Figure 1), which correspond to the 2′-phosphate and pyrophosphate groups. The 2′-phosphate is shifted upfield to about 2 ppm compared to that of NAADP, while the pyrophosphate signal is practically unchanged. This dramatic shift is consistent with the caging group being attached to the 2′-phosphate. As reported by Lee et al5(a) we also observed peak broadening, which might be due to the intermolecular hydrogen bond formation. The quantum yield (Φ) of the DMNPE-caged NAADP measured by comparison with a known standard, 4-methyl-7-nitroindolinyl (MNI)-glutamate was found to be 0.20 at pH 7. Both the DMNPE-caged NAADP and standard were photolysed separately to check that there was no interference from simultaneous photolysis. DMNPE-caged NAADP has an extinction coefficient (ε) of 8900 M-1 cm-1 at 350 nm. Therefore the brightness (ε × Φ) of the caged compounds was 1780 Int M-1 cm-1. This value is comparable to the other DMNPE caged compounds.
Figure 1.
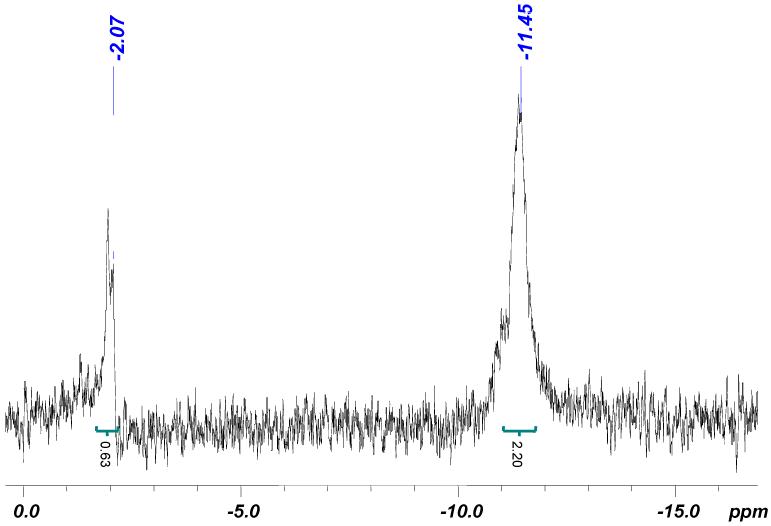
31P NMR spectrum of DMNPE-caged NAADP. The signal for 2′-phosphate is shifted upfield by about 2 ppm compared to NAADP.
The photolysis of DMNPE-caged NAADP to NAADP was confirmed by HPLC as depicted in Figure 2. The columns were packed with AG MP-1 resin and eluted with non-linear gradient of trifluoroacetic acid (150 mM). The HPLC trace of the DMNPE-caged NAADP showed a major peak with a retention time of 28 minutes. Upon photoirradiation, DMNPE-caged NAADP showed a new peak with retention time of 22 minutes that was identical to NAADP.
Figure.2.
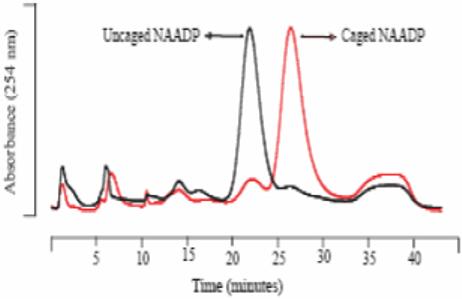
HPLC chromatogram of the DMNPE-caged and uncaged NAADP. As can be seen, the uncaging reaction is quite labile and leads to a quantitative production of NAADP (black trace).
The response of DMNPE-caged NAADP towards calcium in intact sea urchin eggs is shown in Figure 4. It is well-known that even nanomolar concentration of NAADP is sufficient to bind the receptor and block the calcium response.1,5 To avoid this desensitization of the receptor, caged NAADP was pretreated with alkaline phosphate5 to remove any trace amounts of free NAADP. Calcium response was monitored by Oregon Green, co-injected into the eggs along with the DMNPE-caged NAADP. Exposure of the eggs to UV light resulted in an increase in Ca2+ consistent with NAADP release. In another experiment, eggs were injected with caged NAADP containing trace amount of NAADP (10 nM). No calcium response was observed after UV irradiation. This conforms that the calcium response is indeed due to the NAADP released from the cage.
Figure 4.
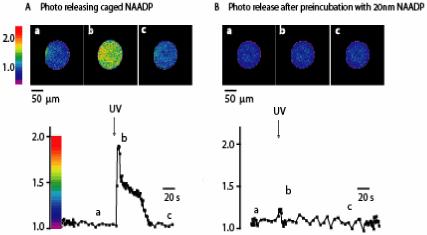
Effect of photoreleasing DMNPE-caged NAADP on Ca2+ signalling in the intact sea-urchin eggs. (A) Injecting sub-threshold amounts of NAADP (10 nm), prior to photoreleasing DMNPE-caged NAADP failed to illicit a response suggesting the inactivation of NAADP receptors. (B) Images are self-rations with colors representing the ratio according to the calibration scale. Traces are of average ratio over times. Eggs contained Oregon Green 488 BAPTA Dextran (10 μM) and DMNPE-caged NAADP (0.5 μM) as labeled. Caged was photoreleased as indicated by the arrows.
In conclusion, this report describes the novel chemo-enzymatic route of caged NAADP without the formation of multiple caging. Biological evaluation in sea-urchin eggs demonstrated fast uncaging of DMNPE-caged NAADP. The mild enzymatic route (pH > 4) will allow the caging of NAADP with novel caging chromophores such as two-photon active reagents.
Supplementary Material
Synthesis, experimental detail and characterization of DMNPE- caged NAADP. This material is avaliable free of charge via internet at http://pubs.acs.org.
ACKNOWLEDGMENT
This study was supported by a grant from Wellcome Trust, UK. We thank Prof. Hon-Cheung Lee for generous gift of ADP-ribosylcyclase.
REFERENCES
- 1.(a) Lee HC, Aarhus R. J. Biol. Chem. 1995;270:2152–2157. doi: 10.1074/jbc.270.5.2152. [DOI] [PubMed] [Google Scholar]; (b) Lee HC. Recent Prog. Hormone Res. 1996;52:357–299. [Google Scholar]; (c) Lee HC. Physiological Review. 1997;77:1133–1164. doi: 10.1152/physrev.1997.77.4.1133. [DOI] [PubMed] [Google Scholar]; (d) Galione A, Petersen OH. Mol. Interv. 2005;5:73–79. doi: 10.1124/mi.5.2.4. [DOI] [PubMed] [Google Scholar]
- 2.Cancela JM, Churchill GC, Galione A. Nature. 1999;398:74–76. doi: 10.1038/18032. [DOI] [PubMed] [Google Scholar]
- 3.Churchill GC, Okada Y, Thomas JM, Genazzani AA, Patel S, Galione A. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 4.(a) Bak J, Billington RA, Timar G, Dutton AC, Genazzani AA. Curr. Biol. 2001;11:987–990. doi: 10.1016/s0960-9822(01)00269-x. [DOI] [PubMed] [Google Scholar]; (b) Patel S, Churchill GC, Sharp T, Galione A. J. Biol. Chem. 2000;275:36495–36497. doi: 10.1074/jbc.C000458200. [DOI] [PubMed] [Google Scholar]
- 5.(a) Lee HC, Aarhus R, Gee KR, Kestner T. J. Biol. Chem. 1997;272:4172–4178. doi: 10.1074/jbc.272.7.4172. [DOI] [PubMed] [Google Scholar]; (b) Gee KR, Lee HC. Methods In Enzymology. 1998;291:403–415. doi: 10.1016/s0076-6879(98)91025-4. [DOI] [PubMed] [Google Scholar]
- 6.Hirayama Y, Iwamura M, Furuta T. Bioorg. Med. Chem. Lett. 2003;13:905–908. doi: 10.1016/s0960-894x(02)01074-0. [DOI] [PubMed] [Google Scholar]
- 7.Kantevari S, Hoang CJ, Ogrodnik J, Egger M, Niggli E, Ellis-Davies GC. ChemBioChem. 2006;7:174–180. doi: 10.1002/cbic.200500345. [DOI] [PubMed] [Google Scholar]
- 8.(a) Cohen BE, Stoddard BL, Koshland DE., Jr. Biochemistry. 1997;39:9035–9044. doi: 10.1021/bi970263e. [DOI] [PubMed] [Google Scholar]; (b) Wilcox M, Viola RW, Johnson KW, Billington AP, Carpenter BK, McCray JA, Guzikowski AP, Hess GP. J. Org. Chem. 1990;55:1585–1589. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Synthesis, experimental detail and characterization of DMNPE- caged NAADP. This material is avaliable free of charge via internet at http://pubs.acs.org.


