Abstract
Synchronization of neuronal activity in the neocortex may underlie the coordination of neural representations and thus is critical for optimal cognitive function. Because cognitive deficits are the major determinant of functional outcome in schizophrenia, identifying their neural basis is important for the development of new therapeutic interventions. Here we review the data suggesting that phasic synaptic inhibition mediated by specific subtypes of cortical γ-aminobutyric acid (GABA) neurons is essential for the production of synchronized network oscillations. We also discuss evidence indicating that GABA neurotransmission is altered in schizophrenia and propose mechanisms by which such alterations can decrease the strength of inhibitory connections in a cell-type–specific manner. We suggest that some alterations observed in the neocortex of schizophrenia subjects may be compensatory responses that partially restore inhibitory synaptic efficacy. The findings of altered neural synchrony and impaired cognitive function in schizophrenia suggest that such compensatory responses are insufficient and that interventions aimed at augmenting the efficacy of GABA neurotransmission might be of therapeutic value.
Keywords: interneuron, schizophrenia, synchronization, GABA-A, GAD67
Introduction
Synchronization of neuronal firing is thought to increase the efficacy of communication between cortical areas,1,2 to be involved in the neural mechanisms underlying working memory,3,4 and to mediate the storage and retrieval of information from long-term memory.5 Interestingly, the recall of sequences of items may require a neural code dependent on the combined function of gamma and theta oscillations within a cortical area (see review in this issue6). Therefore, the alterations of neural synchrony revealed by electroencephalogram (EEG) studies of subjects with schizophrenia7–9 might contribute to the cognitive impairments characteristic of the illness. Synchronized neural activity can be irregular and not necessarily rhythmic; however, synchrony based on rhythmic neuronal activity is an energy-efficient mechanism that seems to predominate in the brain of many mammalian species.10 Consequently, knowledge of the physiological mechanisms that give rise to and regulate synchronized neural oscillations in cortical networks may be critical for the development and assessment of therapeutic interventions aimed at improving cognitive function in individuals with schizophrenia.
EEG rhythms originate from the synchronized activity of cortical pyramidal cells, which is reflected in changes of electrical potential in the extracellular space.11 Because more pyramidal neurons are synchronized, these changes become larger and when sufficiently large, they increase the amplitude of the EEG signal above noise.11 In cortical microcircuits, several physiological mechanisms may play a role in synchronizing the activity of large numbers of pyramidal cells. In particular, fast synaptic inhibition mediated by γ-aminobutyric acid (GABA) neurons appears to be efficient for generating network synchrony. Interestingly, convergent lines of evidence suggest that schizophrenia is associated with alterations of cortical GABA neurotransmission.12 Consequently, in this review, we focus on the role of GABA neurons in the generation of synchronized oscillations and on how their disturbances may contribute to impaired cortical synchrony in schizophrenia. Specifically, we review (1) the cellular mechanisms by which fast synaptic inhibition can produce neuronal synchronization, (2) the subtypes of GABA neuron that mediate inhibition in cortical circuits, (3) the differential involvement of specific interneuron subtypes in the mechanisms of synchronized oscillations, and (4) the potential ways in which cell type-specific alterations could disturb inhibitory synaptic strength and therefore neural synchrony in schizophrenia.
Cellular Mechanisms by Which Fast Synaptic Inhibition can Produce Neuronal Synchronization
GABA neuron-mediated fast synaptic inhibition occurs when an interneuron fires an action potential (or spike) that propagates down its axon and triggers GABA release from presynaptic vesicles into the synaptic cleft. In the cleft, GABA rapidly diffuses and binds to postsynaptic GABA-A receptors, membrane proteins that form a chloride ion channel which opens rapidly upon GABA binding. GABA-A channel opening allows chloride ions to flow along their concentration gradient, which is typically into the cell. The chloride current caused by synaptic GABA release is called an inhibitory postsynaptic current (IPSC). Because chloride is negatively charged, IPSCs usually hyperpolarize the postsynaptic membrane potential; ie, they increase the negative charge inside the cell. IPSCs produce inhibitory postsynaptic potentials (IPSPs) which make the membrane potential more negative or hyperpolarized. Because the spike threshold potential is always positive (or depolarized) relative to the resting membrane potential, hyperpolarizing IPSPs inhibit cell firing (figure 1A).
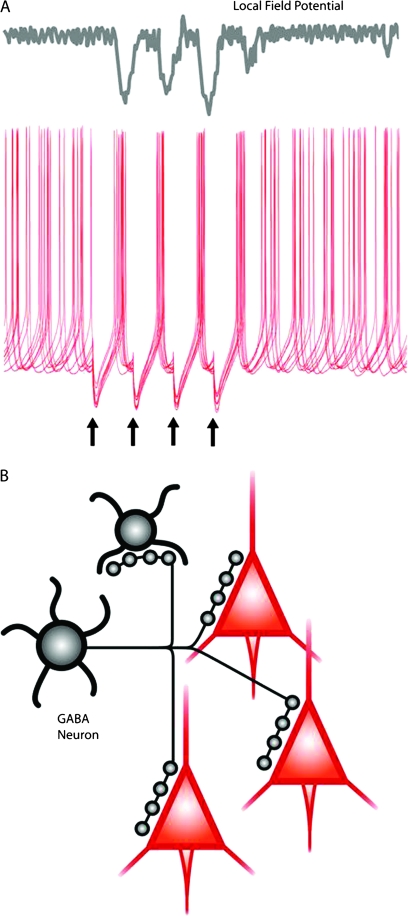
Fig. 1.
γ-aminobutyric acid (GABA) neurons are efficient at synchronizing neuronal activity in cortical networks. (A) Top, a local field potential (LFP) recorded with an extracellular electrode in the vicinity of the pyramidal neurons reflects the synchronization of pyramidal cell activity. Note the negative (downward) components of the LFP, roughly coincident with the periods of spike synchronization recorded simultaneously, as shown in the traces below. Bottom: superimposed traces of intracellular membrane potential recording, illustrating how the asynchronous firing of pyramidal neurons in response to continuous excitatory drive becomes transiently synchronized by phasic synaptic inhibition. Stimulation of an inhibitory input at the times indicated by the black arrows produces hyperpolarizing inhibitory postsynaptic potentials (IPSPs) that transiently inhibit spike firing and produce nearly synchronous spikes shortly after the IPSPs end. Data in part (A): unpublished results from G. Gonzalez-Burgos. (B) Diagram indicating that the axon of an individual GABA neuron makes multiple synaptic contacts onto multiple postsynaptic pyramidal cells and also onto other GABA neurons. The proportion of postsynaptic target cells (3 pyramidal:1 GABA) reflected in the diagram matches their relative numbers in real circuits. However, certain interneuron subtypes display marked target selectivity. For instance, chandelier neurons make synapses onto pyramidal cells but not onto other GABA neurons. In contrast, calretinin-containing interneurons synapse frequently onto other GABA neurons but rarely onto pyramidal cells (see figures 2,3 and main text).
IPSPs generated by a single GABA neuron may be sufficient to synchronize the firing of postsynaptic target cells.13 Shortly after the IPSPs terminate, the postsynaptic cells escape from the inhibitory effects and have a high probability of firing spikes.13 In other words, every time a GABA neuron fires, its postsynaptic cells are silenced for a brief time period that closely matches the IPSP duration and then they fire spikes in near synchrony (figure 1A). Synchronization by post-inhibition excitation may be more complex than a simple escape from inhibition because the hyperpolarizing IPSPs, in addition to directly inhibiting firing, may transiently change the intrinsic electrical properties of the postsynaptic neuron. Such changes may also increase the probability of firing post-inhibition through a process called rebound excitation. Whether post-inhibition excitation strictly involves rebound excitation depends on some properties of the postsynaptic cell membrane. For example, some neurons possess a special type of ion channel that is activated by hyperpolarization but that produces a depolarizing current. This hyperpolarization-activated current, called “h-current” or Ih, has a slow time course. Consequently, when activated by a hyperpolarizing IPSP, the lh remains transiently active after the IPSP is finished, increasing the probability that the postsynaptic cell will fire post-inhibition. This mechanism is called rebound excitation. Indeed, the Ih has been involved in several theoretical and experimental models of network oscillations, where it is typically associated with post-IPSP rebound spiking. In this review, we use post-inhibition excitation to refer to the process of spiking following escape from inhibition, independent of whether or not it actively involves rebound excitation mechanisms.
The ability of a single GABA neuron to transiently block pyramidal cell firing may be explained by the fact that the axon of each interneuron makes multiple synapses onto individual postsynaptic cells (figure 1B). Therefore, the IPSP produced by a single GABA neuron represents the sum of the effects of GABA released at multiple synaptic contacts on the postsynaptic cell. In addition, cortical GABA neurons make divergent connections onto many postsynaptic pyramidal cells (figure 1B). These properties are important given that the number of pyramidal neurons is ∼3 times larger than the number of GABA neurons. Therefore, in spite of their “numerical disadvantage,” the powerful and divergent inhibitory connections of individual GABA neurons enable them to be efficient inhibitors of neuronal activity in cortical networks and consequently to play a major role in the mechanisms underlying neural synchrony in the neocortex.
GABA-A receptor-mediated inhibition may indeed be sufficient to generate network oscillations. For example, in the face of complete pharmacological blockade of phasic excitatory output from pyramidal neurons, certain forms of electrical or chemical stimulation can produce highly synchronous rhythmic IPSPs across multiple pyramidal neurons14 suggesting that synchronized IPSP waves propagate throughout the cell network.14,15 If IPSPs produce post-inhibition excitation, then rhythmic IPSP waves would alternate with waves of excitation (figure 1A). Synchronization through this mechanism by the divergent connections from GABA neurons creates synchronized oscillations in the population of target cells, as found in computational network models with inhibition-based synchrony. Because a single GABA neuron can synchronize multiple postsynaptic cells (figure 1), synchronized inhibitory output from many GABA neurons could entrain the activity of large numbers of target cells in the network. In addition, synchronization of network activity by synaptic inhibition may be potentiated by the tendency for interneurons of the same type to act in concert, both by firing in synchrony and by furnishing convergent inputs onto the same target cells. Therefore, a key question is what mechanisms synchronize interneurons in the first place?
Computational simulations have demonstrated that interneurons can synchronize their activity efficiently through reciprocal inhibition, without involving pyramidal cells.14–18 Oscillations simulated in interneuron-only network models depend on the mechanism of post-inhibition excitation: GABA neurons fire synchronously as they escape from the inhibition generated by other GABA neurons. The synchronized rhythms observed experimentally in the absence of fast glutamate-mediated excitation14 may be mediated by mutual inhibition between GABA neurons, as shown in theoretical network models. Indeed, GABA-mediated chemical synaptic connections between GABA neurons are abundant in real cell networks, as demonstrated by morphological19–22 and electrophysiological studies in brain slices,23–28 indicating that cortical cell networks are properly wired for mutual inhibition.
As reviewed below, assessment of the electrical, biochemical, and morphological properties of GABA neurons reveals the existence of multiple subtypes. Importantly, mutual inhibition between GABA neurons is present in a cell subtype-specific manner.29 Cells of some subtypes are strongly interconnected via GABA synapses with other cells of the same subtype.26,27,30 Moreover, individual GABA neurons of certain subtypes are commonly self-innervating; ie, their axons make synapses onto their own dendrites,25,31–33 forming connections called autapses.34 In contrast, other subtypes of GABA neurons seem to specifically avoid making GABA synapses with each other but make connections with GABA neurons of other subtypes.26,30 Interestingly, GABA neurons of the same subtype, including those that are not interconnected by GABA-mediated chemical synapses, are frequently interconnected by a different kind of synapse, the so-called electrical synapses.29 Electrical synapses are not mediated by GABA release but depend on the existence of direct connections (called gap junctions) between cell membranes. Gap junctions allow direct electrical communication between cells, such that changes in membrane potential produced in one cell rapidly propagate to its electrically-connected neighbors. Because such rapid propagation tends to synchronize changes in membrane potential across neurons, electrical synapses are important for network synchrony.35 Electrical synapses therefore create networks of GABA neurons of the same subtype with a tendency to synchronize with each other when responding to similar temporal patterns of synaptic input. Although they boost the amplitude of synchronized oscillations, electrical synapses are neither necessary nor sufficient to generate oscillations in interneuron-only networks.36 However, gap junctions are also found between pyramidal cells, and as described below, such electrical synapses may play a critical role in network synchronization.
Simulations in computer-generated network models have demonstrated an important property of synchronization by mutual inhibition: the IPSC duration can determine the dominant frequency at which the network oscillates.18,37 Specifically, the oscillation frequency declines as the duration of the IPSCs interconnecting the GABA neurons increases.14,18,37 Strikingly similar findings are found experimentally, when the IPSC duration is prolonged via pharmacological manipulation.14,37,38 Therefore, GABA-A receptor–mediated inhibition of different durations could be associated with production of synchronized oscillations of different frequencies, including the gamma (30–80 Hz), beta (15–30 Hz), and theta (4–8 Hz) frequency bands. The effect of IPSC and consequently IPSP duration on network oscillations may be understood in terms of post-inhibition excitation: longer IPSPs decrease the oscillation frequency by extending the time window during which target cells are silenced before they begin spiking synchronously (figure 1A). By default, during simulations in interneuron-only model networks, the effects of changing IPSC duration take place in the interneuron-to-interneuron GABA synapses.14,18,37–39 The same effect is probably also true in the experimental studies of oscillations in which the pyramidal cell output was blocked and IPSC duration was manipulated pharmacologically.14,18,37,38 These observations suggest that interneuron-to-interneuron connections may be critical for setting oscillation frequency, whereas interneuron-to-pyramidal cell synapses distribute the synchronized activity to the pyramidal cell population.36 However, as summarized below, excitatory inputs from pyramidal cells onto GABA neurons may also contribute to rhythm generation, suggesting a more complex scenario.
In computational models of interneuron networks, production of oscillatory synchrony by mutual inhibition depends on providing GABA neurons with a continuous excitatory drive. It is therefore important to consider the sources of excitatory drive for interneurons during oscillations in real networks. In cortical circuits, the main source of neuronal excitation is the release of glutamate which usually activates receptors of 2 types, called N-methyl-D-aspartate (NMDA) and non-NMDA, in the postsynaptic membrane. Both subtypes of glutamate receptors form ion channels that open quickly upon glutamate binding. Glutamate receptor channels produce mixed sodium/potassium currents and a net flux of positive charges into the cell that depolarizes the membrane potential, shifting it closer to the cells’ spiking threshold and thus having an excitatory effect. The ionic current caused by synaptic release of glutamate is called the excitatory postsynaptic current (EPSC), and the associated change in membrane potential is known as the excitatory postsynaptic potential (EPSP). NMDA and non-NMDA receptors differ in a number of biophysical properties which suggest that they have different functional roles. One important difference is in the duration of the EPSC: the non-NMDA EPSC is significantly shorter in duration than the NMDA EPSC. This difference indicates that non-NMDA EPSCs are optimal for fast signaling and coincidence detection, whereas NMDA EPSCs are more suited for temporal summation. Fast excitatory signaling and coincidence detection are important for precise control of spike timing, which is critical for the physiological impact of synchronized oscillations. Non-NMDA receptors are composed of 2 subtypes called alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) and kainate (KA) receptors that also differ in important biophysical and pharmacological properties. KA receptors contribute to the EPSC in unique synapse types. In contrast, in most glutamate synapses, the EPSC is mediated by the simultaneous activation of AMPA and NMDA receptors. However, in certain synapse types, either NMDA or AMPA receptors predominate. For example, during early brain development, many synapses onto pyramidal neurons have few or no AMPA receptors and NMDA receptors predominate.40,41 On the other hand, the proportion of AMPA compared with NMDA receptors (or the AMPA/NMDA ratio) is higher in distal vs proximal compartments of hippocampal CA1 pyramidal cell dendrites.42,43
Interneurons in general receive robust excitatory input in the form of glutamate synapses, although certain subtypes of interneurons have a much higher excitatory synapse density.20 Furthermore, the type of glutamate receptors mediating EPSCs in interneurons may differ from those in pyramidal cells and also between different subtypes of GABA neurons. For instance, in some interneuron subtypes, NMDA receptors make a smaller contribution to the EPSC.44–48 In addition, in synapses onto interneurons, both AMPA and KA receptors may contribute significantly to the non-NMDA EPSC.49–51 In contrast, in pyramidal cells, postsynaptic KA receptor contribution to the non-NMDA EPSC is either absent or very small.49,52 Glutamate synapses onto interneurons are also stronger than those onto pyramidal cells. For example, glutamate synapses on interneurons have ∼4 times more AMPA receptor molecules per synapse53,54 and produce EPSCs with substantially larger amplitudes.45,55–60 Thus, background levels of excitatory network activity that do not produce significant pyramidal cell excitation may readily excite GABA neurons. If so, the arrival of stimuli-related signals to a given cortical area may readily recruit GABA neurons and initiate the mutual inhibition mechanisms that create synchronized rhythms in the network. Consequently, the induction and maintenance of synchronized network oscillations may depend not only on GABA-mediated inhibition but also on the recruitment of interneuron firing by glutamate excitation. Indeed, in several experimental models, induced network oscillations are abolished by blocking either GABA- or glutamate-mediated transmission.2,36
Other sources of interneuron excitation may also contribute to recruiting GABA neurons during oscillations. First, activation of metabotropic glutamate receptors (mGluRs), which do not possess the glutamate-gated ion channels present in the ionotropic receptors (NMDA, AMPA, or KA receptors), turns on one or more cascades of intracellular messengers that may indirectly depolarize the neuron's membrane potential.61 GABA neuron depolarization by mGluR activation may synergize with ionotropic EPSCs or may lead to sufficient interneuron depolarization to produce network rhythms.14,36,37,62 Second, neuromodulators are important in controlling interneuron excitability. For example, cholinergic receptor activation is critical for the induction of cortical rhythms in various in vitro models of oscillations36,62 and for oscillations and response synchronization in vivo during behavioral tasks.63,64 Cholinergic receptor stimulation alters the membrane potential of pyramidal cells, but its actions during network oscillations may depend critically on the depolarization of specific subtypes of GABA neurons, through activation of G protein–coupled muscarinic cholinergic receptors.36,65 Interneuron excitation is also significantly modulated by activation of dopamine receptors.46,66,67 Dopamine neuromodulation in neocortical circuits is critical for cognitive function68 and, interestingly, may play a significant role in regulating cortical network activity.69–71 A third potential source of GABA neuron depolarization may be the so-called tonic GABA currents. These currents, more prominent in interneurons than in pyramidal cells72, are mediated by activation of high affinity, extrasynaptic GABA-A receptors by ambient GABA.73 Because in some interneuron subtypes, activation of GABA-A receptors produces depolarizing instead of hyperpolarizing chloride currents,74,75 tonic GABA-A currents may be depolarizing as well. Depolarizing tonic GABA currents in interneurons could cooperate with the glutamatergic excitatory drive, increasing cellular excitability as observed in pyramidal neurons during early development.76
It is important to note that interneuron depolarization by metabotropic receptor activation (mGluR, dopamine, or acetylcholine receptors) or by tonic GABA-A currents has a slow time course, somewhat comparable with the continuous depolarization provided to interneurons in computational modeling studies. As highlighted elsewhere,38 the larger the magnitude of this tonic depolarization, the more likely it is that oscillations in the cortical network are controlled mainly by mutual inhibition between interneurons. On the other hand, oscillations may depend critically on the phasic excitation provided by EPSPs generated by pyramidal cells in the local network, as we will address below.
Subtypes of GABA Neuron That Mediate Inhibition in Cortical Circuits
GABA neurons exhibit substantial diversity, with at least 16 different subtypes found in the hippocampus.77 These observations raise the question of whether all subtypes contribute equally to the mechanisms underlying cortical rhythms or whether specific interneuron subtypes are differentially involved in oscillations. This question is particularly important given the evidence from postmortem studies that schizophrenia is associated with alterations, predominantly or exclusively, in certain subtypes of GABA neurons.12,78 Due to their substantial phenotypic diversity, classification of cortical interneurons is a complicated task which requires the combined analysis of their electrical, molecular, and morphological properties.77,79,80
Electrophysiologically, GABA cell subtypes are distinguished by their intrinsic electrical properties which reflect the manner in which the membrane potential of individual neurons responds to the direct application of hyperpolarizing currents and sustained depolarizing stimuli that produce action potential firing. All GABA neurons, independent of subtype, are distinguished from pyramidal cells by having a shorter duration of individual action potentials. In general, neuronal spikes are very brief, with a total duration of less than 3 msec. For various reasons, it is more precise to measure the duration of spikes at half amplitude rather than measuring their total duration. The spike duration at half amplitude is ∼1.0 to 1.5 msec for pyramidal cells and ∼0.3 to 0.7 msec for interneurons. However, spike duration alone is not sufficient to properly distinguish GABA cell subtypes. In contrast, subtypes of interneurons can be distinguished by the application of depolarizing stimuli above spike threshold for a few hundred milliseconds which results in the neuron firing multiple spikes in sequence. The temporal characteristics of such spike sequences, called firing patterns, differentiate GABA neuron subtypes. For example, so-called fast-spiking (FS) GABA neurons show a nearly constant time interval between spikes (figure 2). In contrast, in other GABA neurons, the inter-spike interval increases progressively, or adapts, throughout stimulus duration (figure 2); these cells comprise the non-FS or adapting cell subtype which can be broadly divided into 2 groups. Neurons in the first group display progressively longer inter-spike intervals and, for historical reasons, are commonly named regular-spiking non-pyramidal (RSNP) neurons. A second subtype of non-FS cells responds to the stimulus with an initial group of 3–5 spikes (or burst) fired with a short inter-spike interval, giving rise to their name of burst-spiking non-pyramidal (BSNP) neurons. The burst is typically followed by a progressive increase in inter-spike interval similar to that observed in RSNPs. For reasons related to the mechanisms of burst production, BSNPs are sometimes called low-threshold spiking (LTS) cells.
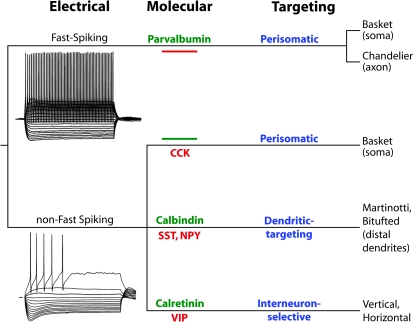
Fig. 2.
Highly simplified view of the γ-aminobutyric acid (GABA) neuron subtypes revealed by the combined analysis of electrical, molecular, and morphological properties. Electrophysiological properties divide interneurons into 2 major clusters, fast spiking (FS) and non-fast spiking (non-FS), based on the response of the neurons’ membrane potential to direct injection of current. FS neurons form a relatively homogeneous group of interneurons that contain the calcium-binding protein parvalbumin (PV), contain no neuropeptides, and make synapses onto the perisomatic compartment of the postsynaptic pyramidal cell. FS/PV basket cells synapse onto the proximal dendrites and soma of pyramidal cells and also onto other GABA neurons. FS/PV chandelier cells synapse onto the axon initial segment of pyramidal cells but not onto other GABA neurons. The non-FS cell cluster is largely heterogeneous, containing 2 electrical subgroups, the RSNP and the BSNP neurons, the latter are also called low-threshold spiking cells (see text for additional details). An important group of non-FS cells are the Martinotti neurons, which in most cases contain the calcium-binding protein calbindin (CB) and the neuropeptides somatostatin (SST) and neuropeptide Y (NPY). Martinotti cells make synapses onto the distal dendrites of pyramidal cells. The subgroup of non-FS cells that contain the calcium-binding protein calretinin (CR) also express vasoactive intestinal peptide (VIP) and make contacts onto other GABA neurons much more frequently than onto pyramidal cells and thus are called interneuron selective. The non-FS basket cells containing cholecystokinin (CCK) do not contain PV, CB, or CR and make synapses onto the perisomatic compartment of postsynaptic pyramidal cells.
Molecularly, GABA neurons are distinguished from pyramidal cells by the expression of specific sets of gene products.81 By definition, GABA neurons express the GABA-synthesizing enzyme glutamic acid decarboxylase (GAD), of which 2 isoforms, GAD65 and GAD67, are named according to their molecular weights.82 In addition, GABA neurons express other proteins in a cell subtype-specific manner. For instance, GABA neurons typically contain only one of 3 types of calcium-binding proteins that are thought to regulate the intracellular calcium concentration in neurons, (parvalbumin [PV], calbindin [CB], and calretinin [CR]). In the primate cortex, ∼50% of GABA neurons are CR positive, ∼25% are PV positive, and ∼25% are CB positive.83 These proportions contrast to those found in rodents, where PV cells constitute about 50% of all GABA neurons, and some overlap between PV and CB expression or CB and CR expression is present.79 The functional consequences of these differences in the proportions of GABA cell subtypes in rodent and primate brain are not well understood. GABA neurons may also contain neuropeptides that are thought to act as intercellular signaling molecules, including somatostatin (SST), neuropeptide Y (NPY), cholecystokinin (CCK), and vasoactive intestinal peptide (VIP). Neuropeptide expression is somewhat less cell subtype specific than calcium-binding protein expression but still distinguishes cell subtypes. For instance, most interneurons expressing NPY also contain SST but not other peptides, and many interneurons with CCK also contain VIP.84 Interestingly, PV-containing GABA neurons do not express any of the known neuropeptides; in addition, most CCK-containing neurons do not express PV, CB, or CR (figure 2).
The morphological details of interneuron axons (but not dendrites) also provide essential information to distinguish GABA neuron subtypes (figure 2). The axons of some interneuron subtypes arborize locally or spread horizontally to target neurons in the same layer where their cell body is located. Other GABA neuron axons project vertically, reaching target cells above and/or below the layer of the parent cell. The majority of “horizontal/within layer” GABA cell subtypes target the perisomatic compartment of the postsynaptic pyramidal neuron. This group includes the basket cells, that innervate the soma and proximal dendrites, and the chandelier or axo-axonic neurons, which uniquely make synapses onto the initial segment of the pyramidal cell axon (figure 2). On the other hand, the “vertical/across layers” cell subtypes target dendritic membrane compartments that are more distal from the pyramidal cell soma. This group includes the double-bouquet cells, Martinotti cells, bitufted cells, and several types of cells with mainly ascending or descending axonal trunks. An important exception is the neurogliaform interneurons, with axons that distribute mostly locally within the cortical layer of the parent cell, but that predominantly target pyramidal cell dendrites, as opposed to the perisomatic compartment. Importantly, many GABA neuron subtypes innervate pyramidal cells and also synapse onto other GABA neurons possibly mediating mutual inhibition.24,85 Anatomically, most morphological subtypes of interneurons seem to be present in all cortical areas. The abundance of some morphological subtypes, however, seems to vary between layers within an area.
Differential Involvement of Specific Interneuron Subtypes in the Mechanisms of Synchronized Oscillations
The relationship between GABA neuron subtypes and in vivo network oscillations has been studied by determining if their firing is coupled to specific phases of the oscillation cycle. In a remarkable series of studies, hippocampal interneurons of different subtypes were recorded during in vivo synchronized oscillations at different frequency bands.86–89 These experiments revealed that PV cell firing is robustly coupled to in vivo gamma oscillations89 but that the firing of dendrite-targeting SST oriens lacunosum-moleculare (OLM) cells does not display gamma modulation.89 In contrast, both PV and SST neuron firing is coupled to the cycle of hippocampal theta oscillations in vivo.77,86,87 Interestingly, both gamma90–93 and theta94,95 oscillations can be induced in brain slices, with a cell type-specificity of spike time coupling similar to that found in vivo.90–95 Thus, both gamma and theta rhythms can be generated in local circuits isolated in vitro, by mechanisms resembling those operating in vivo. These findings, however, do not exclude the possibility that under certain conditions oscillations require long-distance interactions between neurons located in different brain regions and mediated by connections that are silent in brain slices. For example, thalamocortical interactions are thought to be critical for oscillations recorded from neocortex in vivo.96 In addition, classic models of hippocampal theta oscillations suggest that inputs from the medial septum-diagonal band of Broca are required for theta rhythms because septal neurons act as theta oscillation pacemakers.97 Several inadequacies of this classic model, however, have been noted elsewhere97, including the fact that theta oscillatory activity of septal neurons requires hippocampal inputs.98 However, septal neurons isolated in vitro can generate theta rhythmic activity if they receive tonic depolarization through KA receptor activation.99
The analysis of spike timing not only suggests a division of labor among interneuron subtypes vis-à-vis the gamma and theta oscillation mechanisms but also suggests that certain interneuron subtypes, for instance, the FS/PV interneurons, contribute to oscillations in more than one frequency band. Whereas PV neurons are thought to play a primary role in gamma oscillations,89–93 some studies show that the activity of other cell types may also be important for oscillations in the gamma band. For example, the firing of bistratified cells is strongly coupled to the cycle of hippocampal gamma oscillations in vivo.89 Bistratified cells are a subtype of GABA neuron that targets pyramidal cell dendrites77 but are different from the dendrite-targeting SST OLM cells. Interestingly, the coupling of bistratified neuron spikes to the gamma cycle is stronger than the coupling of spikes in perisomatic-targeting PV interneurons.89 Furthermore, during gamma oscillations in vitro, interneuron-selective interneurons (see figure 2), which are CR positive77, show oscillatory firing that is as tightly coupled to the oscillation cycle as is the firing of perisomatic-targeting PV cells.90,100 On the other hand, the firing of PV and SST cells is coupled to the theta oscillation cycle with similar strength,86–88 suggesting that both cell populations contribute inhibition during theta oscillations.
However, the association of spike timing with oscillation cycle phase does not reveal the mechanistic links between the activity of specific interneuron types and the production of rhythms of different frequency. The only conclusive findings are for interneuron subtypes that remain silent or display firing that is not coupled with the oscillation cycle. In such cases, it can be concluded that a given interneuron subtype is not involved in generating that oscillation frequency. It is possible that interneuron firing during oscillations is involved in other mechanisms than the regulation of population oscillation frequency and coherence. For instance, inhibition potentially may be important for phase precession during theta oscillations6,97 (ie, the fine regulation of the spike timing of individual neurons relative to the population rhythm) and not in generating the oscillation. Phase coding may indeed operate also during oscillations in the gamma frequency band.101 Finally, important differences could exist between the interneuron populations present in the neocortex and the hippocampus and thus extrapolation of their role in hippocampal oscillations to the neocortex could be problematic.
As mentioned above, oscillation frequency might be determined by the IPSC duration; indeed synaptic coupling by short- or long-lasting IPSCs generates gamma36,39,102 or theta39,103 band synchrony, respectively. An interesting possibility is therefore that a given interneuron class is preferentially associated with high- or low-frequency oscillations, depending on the duration of the IPSCs elicited in postsynaptic neurons. Several mechanisms shape IPSC duration,73 including the presence of different subtypes of GABA-A receptors that produce currents of different duration when exposed to GABA for an identically brief time. GABA-A receptors are pentameric membrane proteins in which the GABA-gated chloride channel is formed typically with a 2α:2β:1γ subunit combination. The most abundant subunit combinations in the adult brain, α1β2γ2 and α2β3γ2, constitute, respectively, ∼60% and ∼20% of all GABA receptors, the remainder probably contain α3, α4, α5, or α6 (only found in cerebellum) subunits or a δ in place of γ.104 The α1-GABA-A receptor channels produce currents with a duration that is 6–7 times shorter than that associated with receptors containing other α subunits.73,105 Thus, differences in GABA-A receptor properties provide a molecular basis for the production of fast and slow IPSCs, if different GABA-A receptors predominate in mediating the response to GABA at certain synapses. Indeed, several studies of hippocampal synapses are consistent with α1 vs α2 subunit predominance. For example, in PV-negative synapses onto CA1 hippocampal pyramidal cells, α1 subunits are absent,106 but α2 subunits are very abundant.107 In PV-positive synapses onto the soma, α1 subunits are numerous106, whereas α2 subunits are present in very low levels.107 Electrophysiological studies of inhibitory synaptic transmission in hippocampal slices are consistent with such subunit localization.102,108 Interestingly, α2 subunits are predominant also at the PV-positive synapses made by chandelier neurons onto the pyramidal cell axon initial segment.109
In neocortical circuits, the α1- and α2-GABA-A receptor localization has been studied only in the brain of very young rats,110,111 which may not reflect the adult state, because early in development α1 subunit levels are low and other α subunits (especially α2) are more abundant.112–114 In developing rat cortex,111 FS basket cells elicit in pyramidal neurons fast IPSPs apparently mediated by α1 subunit–containing GABA-A receptors. On the other hand, non-FS basket cells (possibly CCK containing) elicit somewhat slower IPSPs through α2-GABA-A receptors, whereas dendrite-targeting bitufted and Martinotti cells elicit even slower IPSPs through α5 subunit–containing receptors.111 Mediation of IPSPs by α5-GABA-A receptors was somewhat surprising, given that α5 subunits are mostly localized outside of synapses. However, a proportion of α5 subunits may be localized synaptically,115 providing a basis for α5-GABA-A receptor-mediated IPSPs. Currently, the functional and pharmacological properties of most of the connections made by GABA neuron subclasses onto other cells in the network are not well understood.
Interneurons of each subclass have more than one type of postsynaptic target. For instance, FS/PV basket cells innervate not only pyramidal cells but also other FS cells and non-FS GABA neurons.26,27,30,116 Thus, understanding the mechanisms of oscillations requires knowledge of which connections in the neocortical circuit produce fast vs slow IPSCs. FS neurons are interconnected by fast IPSCs29,36,102 which is consistent with the involvement of FS cells in the mechanisms of gamma oscillations suggested by in vivo spike timing analysis and by theoretical interneuron-only networks with fast reciprocal IPSCs.2,36 In contrast, spike timing analysis suggests that LTS/SST cells play a unique role in theta oscillations: the firing of dendrite-targeting SST cells in the hippocampus is not coupled to the gamma oscillation cycle,89 and SST cells are silent during 200-Hz ripple oscillations86 (however, see Spampanato and Mody117). In contrast, SST cell firing is strongly coupled to the theta cycle.86 One possibility is that, by analogy with gamma oscillations generated in networks of cells interconnected by fast IPSCs, theta rhythms originate from slow reciprocal inhibition between LTS/SST cells. Indeed, in computational simulations, interneuron networks with such slow reciprocal IPSCs synchronize readily in the theta frequency band.39,103 However, GABA synapses between LTS neurons (including the SST cells) are very rare.26,30 Thus, production of theta rhythms by slow mutual inhibition between SST-containing neurons is unlikely in real cortical networks.
The functional role of a particular GABA neuron synaptic connection is determined by the IPSC duration in interaction with the electrical properties of the postsynaptic cell. Intrinsic electrical properties confer upon single neurons several features that may be important for network oscillations. Among these is the property called electrical resonance, which is studied by stimulating neurons with oscillatory sinusoidal currents instead of the constant currents typically employed to characterize the neurons’ firing patterns (figure 2). If cells with significant electrical resonance are stimulated with sinusoidal currents of different frequency, the membrane potential response is enhanced for a particular frequency band called the preferred input frequency. Oscillatory inputs within this frequency band will be more efficient than other frequencies in generating spikes. During network oscillations, individual cells display rhythmic waves of inhibitory and excitatory synaptic currents14,92,93 and near-sinusoidal waves of change in membrane potential.92,118,119 If such waves of synaptic current are within the preferred frequency band of cells with electrical resonance, then such neurons may generate stronger outputs and thus contribute more strongly to rhythmic network activity. Interestingly, pyramidal cells and dendrite-targeting OLM cells show electrical resonance in the theta frequency band120–122 and FS neurons have preferred input frequency in the gamma band.122
Can circuit models explain the involvement of both PV and SST neurons in theta activity suggested by in vivo studies?86 LTS/SST Martinotti cells synapse onto pyramidal cell dendrites85 and also onto interneurons,85 most likely of the FS/PV class, because LTS-to-FS GABA synapses are abundant.26,30 Thus, inhibition from LTS/SST cells may modulate FS/PV activity such that these cell populations fire during opposite phases of the theta oscillation cycle.86 The low probability of pyramidal cell firing at the theta phase when FS/PV cells are more likely to fire77,86,94 is consistent with a scenario in which pyramidal cell firing is inhibited by FS/PV cells that are rhythmically inhibited by LTS/SST cells. The latter may in addition modulate pyramidal cell firing through dendritic spikes generated by h-current–dependent rebound excitation, a modulation that in certain conditions could lead to nested theta-gamma oscillations. Indeed, as summarized elsewhere in this issue,6 theta and gamma oscillations very commonly occur simultaneously. When the rhythms of different frequencies occur simultaneously, interneurons of a given subtype, such as the FS/PV cells, may contribute mostly to set one particular frequency component of the rhythm. The simultaneous theta-gamma oscillations, however, do not seem to be independent, given that typically the amplitude of the gamma oscillation displays theta modulation.6
In interneuron-only networks, fast IPSCs at synapses between FS/PV cells are necessary,2,36 but not sufficient, to generate gamma oscillations because FS neurons must be provided with some excitatory drive. As summarized above, there are several potential mechanisms and sources of excitatory drive for interneurons. Several observations suggest that fast excitatory input from pyramidal cells may be involved in gamma synchronization: (1) the spike timing during gamma oscillations suggests monosynaptic recruitment of FS/PV cells by nearby pyramidal neurons100; (2) synaptic currents observed during gamma oscillations suggest that FS neurons receive phasic excitatory input from nearby pyramidal cells93; (3) genetically-engineered knock down of synaptic AMPA receptors in FS/PV cells impairs gamma oscillations48; and (4) the hippocampal CA1 network, in which pyramidal cells have few local axon collaterals and contact interneurons only in stratum oriens,123 is relatively unable to generate gamma rhythms intrinsically,124 and CA3 pyramidal cell input onto FS/PV GABA neurons in CA1 is probably required for production of CA1 gamma oscillations.124 Pyramidal cells providing excitation onto FS/PV neurons during the gamma cycle may in turn be rhythmically inhibited by FS/PV cells. As suggested elsewhere,2 the extra delays imposed by involving synaptic excitation might mean that beta and low-frequency band gamma oscillations depend on pyramidal-interneuron feedback loops, whereas gamma oscillations in a higher frequency band are more dependent on interactions within interneuron networks. In any case, to be consistent with the gamma cycle period, in FS/PV cell-pyramidal neuron feedback loops, not only inhibition but also excitation must be fast because of the extra delays associated with synaptic excitation.2,36 We and others have found that in FS cells, EPSCs/EPSPs indeed have very fast time course.44,45,59,125,126 Interestingly, a genetically engineered reduction in the number of AMPA receptors selectively at excitatory synapses onto FS/PV interneurons, leaving the number of NMDA receptors unaltered,48 indeed reduced the amplitude of fast, AMPA-mediated EPSCs in PV cells and produced a significant reduction in the amplitude of gamma oscillations.48
Deficits in glutamate receptor–mediated excitation of interneurons may contribute to disturbed interneuron-dependent network synchrony in schizophrenia, given the reported deficits in AMPA,127 NMDA,128 and KA129 receptor-mediated signaling in the illness. As mentioned above, KA receptors make a larger contribution to the non-NMDA EPSC in interneurons than in pyramidal cells. Whereas AMPA receptors appear to be universally present in mature glutamate synapses, the levels of NMDA receptors vary depending on the type of input and/or the type of interneuron. Whether NMDA receptors contribute significantly to inputs onto interneurons is important in the context of current models suggesting that schizophrenia is associated with NMDA receptor hypofunction. For instance, if in normal cortical circuits inputs onto a given neuronal type have a small contribution from NMDA receptors, such inputs are less likely to be directly affected by NMDA hypoactivation. As previously discussed,130 NMDA hypofunction could occur in inputs onto pyramidal cells, onto GABA neurons (particularly of the FS/PV population), or in both. Interestingly, electron microscopy studies reported that NMDA receptors are absent, or are found in very small numbers, in many glutamate synapses onto hippocampal PV neurons.131 Similarly, physiological experiments found a small NMDA contribution in EPSCs onto FS/PV neurons in rat hippocampus45 and in the neocortex of mice,132,133 rats,44,47 and monkeys.46 In particular, unitary EPSCs elicited in FS neurons by nearby pyramidal cells have a much smaller contribution from NMDA receptors (∼25% of the total charge) than unitary EPSCs elicited in LTS GABA neurons (∼ 80% of the total EPSC charge).134 Because AMPA EPSCs decay significantly faster than NMDA EPSCs, a low NMDA contribution suggests that excitation of FS/PV neurons is primarily an AMPA-mediated fast signaling process. EPSCs with fast decay are important for the production of synchronized oscillations in network models that involve feedback interactions between interneurons and pyramidal cells.135 These data are thus consistent with the hypothesis that FS neurons participate in gamma oscillations dependent on excitatory-inhibitory feedback loops.
These findings also raise questions about the mechanisms by which NMDA receptor antagonists affect the phenotype of PV GABA neurons.136,137 Blockade of NMDA receptors indeed must significantly alter network activity because the long-lasting NMDA EPSCs are critical for recurrent excitation between pyramidal cells.138–140 NMDA-driven recurrent excitation between pyramidal cells may underlie sustained neuronal firing, a potential neural substrate for the maintenance of items of information in working memory buffers.141,142 Recurrent excitation, however, requires tight inhibitory control to prevent runaway excitation.143 Interestingly, in vivo recordings indeed show that excitation is tightly balanced with inhibition.144 Such feedback inhibitory control may be provided by FS/PV neurons which, by targeting the perisomatic membrane compartment of pyramidal cells (figure 2), exert powerful inhibition of spike initiation. If NMDA antagonists depress recurrent excitation between pyramidal cells, they may indirectly reduce the activity of the GABA neurons providing feedback inhibition. GABA neuron hypoactivity likely produces changes in neuronal phenotype, such as a decrease in GAD67 levels, because GAD67 expression is activity dependent (see Lewis and Gonzalez-Burgos130). Such possibility is supported by data showing that reduced levels of network activity in vivo produce a concomitant reduction of GAD67 and PV expression, as well as a decrease in the IPSC strength, at least in developing somatosensory cortex.145 A critical issue is therefore the relative contribution of NMDA receptors to recurrent excitation between pyramidal cells vs recruitment of feedback inhibition. As mentioned above, several lines of evidence suggest that NMDA contribution to EPSCs is smaller in FS/PV neurons than in other GABA neuron subtypes.134 However, a detailed comparison of NMDA contribution to inputs onto different neuron subtypes has not been performed. It is possible that heterogeneity is present, with some types of input onto FS/PV cells showing small or no NMDA contribution and other inputs showing a significant contribution, but still smaller than that found in non-FS interneuron subtypes or in pyramidal cells.133 Interestingly, the NMDA contribution to inputs onto FS/PV neurons may vary with developmental stage47 and with brain region.146
Are gamma oscillations in real neuronal networks in vivo produced by mechanisms similar to those in interneuron-only networks (hence named “interneuron gamma” or ING)? Or by mechanisms that actively involve interneuron-pyramidal cell interactions (so-called “pyramidal interneuron gamma” or PING)? A crucial issue is the role of the recurrent inhibitory connections mediating mutual inhibition between interneurons. In ING, these connections generate a rhythm even if the interneuron depolarizing drive is constant and not rhythmic. However, if pyramidal cells that are synchronized by interneurons provide the interneuron depolarization, such excitatory drive will be rhythmic and heterogeneous, instead of constant. Heterogeneity in the excitatory drive onto interneurons could make network oscillations unstable.36 Interestingly, the recent finding that reciprocal inhibition between real FS/PV neurons occurs via shunting mechanisms,74 stimulated novel computational modeling work that, as reviewed elsewhere,2,36 suggests that shunting inhibition provides robustness against heterogeneity in the excitatory input. However, whether shunting inhibition indeed operates during oscillations in real networks awaits direct experimental demonstration.
In PING, the excitatory-inhibitory circuit created by reciprocal interneuron-pyramidal cell connections is an oscillator, in the absence of mutual inhibition between interneurons.135 As suggested elsewhere,2 PING-like mechanisms somehow may be involved in lower frequency band gamma oscillations, whereas high-frequency gamma band may depend more on ING-like mechanisms. The factors that could underlie such potential “switch” of mechanisms underlying gamma oscillations are not clear. In PING models, the presence of pyramidal cells reciprocally connected with the interneurons makes oscillation frequency far more stable and less sensitive to the IPSC time course,38 which in ING strongly determines oscillation frequency. More realistic PING models, however, would have to include interneuron-to-interneuron connections, given that such connectivity is very robust in actual cortical circuits. An additional feature of many PING-like models is that the synaptic weight simulating pyramidal-to-pyramidal excitatory connections is either very weak or absent.135 Whereas this is compatible with connectivity in the CA1 hippocampus, it does not match the architecture of the CA3 and neocortical circuits in which pyramidal cell axon collaterals are very dense and establish frequent connections with nearby pyramidal cells. One additional means for propagation of excitatory activity during oscillations are axo-axonal electrical synapses between pyramidal neurons.147,148 These connections may be an important source of synchronization and of synchronized phasic excitatory input onto interneurons during persistent gamma rhythms.149
Potential Ways in Which Cell Type–Specific Alterations of GABA Neurons could Disturb Inhibitory Synaptic Strength in Schizophrenia
Multiple studies have consistently found that, in subjects with schizophrenia, the levels of the mRNA for GAD67 are reduced in the dorsolateral prefrontal cortex (DLPFC),150 a neocortical region that is important for cognitive function. This deficit appears to be accompanied by a decrease in the cognate protein.151 In contrast, the levels of GAD65 mRNA and protein are not changed in schizophrenia, nor is the density of GAD65-immunoreactive axon terminals.152
At the cellular level, the density of neurons with detectable levels of GAD67 mRNA is significantly decreased in DLPFC of subjects with schizophrenia.153,154 However, in neurons with detectable levels of GAD67 mRNA, the expression level per neuron does not differ from control subjects.154 These observations indicate that in subjects with schizophrenia, most GABA neurons express normal levels of GAD67 mRNA, but about 25%–35% of GABA neurons do not express this transcript at a detectable level.153,154
Schizophrenia is also associated with changes in the levels of mRNA for the calcium-binding protein PV.155 The expression of PV mRNA per neuron is significantly decreased, but neither the density of neurons with detectable levels of PV mRNA nor the density of PV-immunoreactive neurons156,157 appears to be changed in subjects with schizophrenia. Interestingly, dual label in situ hybridization studies showed that ∼50% of PV mRNA+ neurons lacked detectable levels of GAD67 mRNA in the DLPFC of subjects with schizophrenia.155 These findings indicate that in schizophrenia PV neuron density is normal but that some of these cells have reduced, but detectable, levels of PV mRNA and markedly reduced GAD67 mRNA expression. PV neurons include the chandelier subtype whose axons form linear arrays of terminals (termed cartridges) that synapse exclusively on the axon initial segments of pyramidal neurons.
Most of the differences described above appear to be specific to the disease process of schizophrenia because they are not found in subjects with psychotic major depressive disorder or in monkeys exposed chronically to antipsychotic medications in a fashion that mimics the clinical treatment of schizophrenia.154,155,158–160
Several lines of evidence suggest that the deficit in GAD67 mRNA expression may represent a primary factor in GABA neuron dysfunction in schizophrenia. For example, abnormal histone protein methylation has been reported to alter the regulation of gene transcription at the promoter region of GAD1, the gene encoding GAD67, leading to decreased levels of GAD67 mRNA.161 In addition, some studies suggest that allelic variants in GAD1 are associated with both an increased risk for schizophrenia and lower levels of GAD67 mRNA.161 Because reduced levels of GAD67 mRNA in schizophrenia have been reported to be associated with lower levels of GAD67 protein and lower GAD enzymatic activity,151 it is likely that GABA synthesis is impaired in certain subtypes of cortical GABA neurons in schizophrenia, resulting in decreased cytosolic GABA concentration in synaptic boutons. As a consequence, synaptic vesicle filling may be deficient, resulting in lower intravesicular GABA concentrations. Even if the mechanisms that couple interneuron firing with vesicular GABA release remain intact, lower vesicular GABA concentration would decrease the peak level of the GABA concentration transient in the synaptic cleft. The resulting IPSC would have smaller amplitude and as a consequence shorter duration because it takes less time for a smaller IPSC to decay to baseline.
In the face of such causes (less GAD67 mRNA and protein) and consequences (less GABA synthesis, lower vesicular GABA concentration, and smaller IPSCs), a synapse has several potential mechanisms to compensate and restore synaptic function.78 On the presynaptic side, a potential substrate for adaptive changes is the plasma membrane GABA transporter GAT1. Under normal conditions, GAT1 prevents spillover of GABA between adjacent synapses and thus contributes to synapse independence and the spatiotemporal specificity of GABA transmission.162–164 However, under conditions of a pathological decrease in the peak concentration of synaptic cleft GABA, spillover is probably unlikely. Moreover, if a homeostatic mechanism couples extracellular GABA levels with the amount of GAT1 in the plasma membrane, then reduced extracellular GABA due to decreased GABA release may lead to reduced GAT1 in the plasma membrane of synaptic terminals. Interestingly, reducing GAT1-mediated GABA uptake prolongs the IPSC and IPSP duration,162–164 potentially compensating for the shorter inhibitory effect of a smaller IPSC. In normal GABA release conditions, reducing GAT1-mediated uptake does not increase the peak amplitude of the IPSC.162,164 This may be explained if the normal GABA concentration transient largely saturates the synaptic receptors, independent of GAT1 effects. Under conditions of decreased GABA content per vesicle, however, the cleft GABA transient may be subsaturating. Under such conditions, decreasing GABA uptake may increase the fraction of GABA-A receptors that are activated and thereby increase IPSC amplitude.
A compensatory increase in IPSC amplitude under conditions of reduced synaptic cleft GABA concentrations may also be achieved by increasing the number of postsynaptic GABA-A receptors. Such a compensation mechanism would likely require that synaptic cleft GABA levels and/or GABA-A receptor activation somehow regulate the insertion or removal of GABA-A receptors from the postsynaptic membrane.
The well-described association between decreased levels of GAD67 and PV mRNAs in schizophrenia also raises the question of whether the decline in PV expression represents a compensatory mechanism. PV is a slow but efficient Ca2+ buffer that accelerates the decay of Ca2+ transients,165,166 decreasing the residual Ca2+ levels that accumulate in nerve terminals and facilitate GABA release during repetitive firing.165 Reduced PV levels increase the facilitation of GABA release during repetitive synaptic activity, as observed in synapses from PV-deficient mice.167 In cerebellar neurons, PV deficiency reduces an asynchronous component of GABA release produced when PV liberates part of the Ca2+ bound during stimulation.165 However, in synapses made by hippocampal168 and neocortical125 FS/PV neurons, asynchronous GABA release is absent, and thus such an effect of PV deficiency cannot occur. Therefore, the main effect of PV reduction in neocortical and hippocampal synapses appears to be the facilitation of GABA release when FS neurons fire repetitively. Interestingly, in PV deficient mice, such increase in repetitive release is associated with a very large increase in the power of hippocampal gamma oscillations.167
Studies conducted to date suggest that many of these compensatory mechanisms may occur at the synapses formed by the PV-containing chandelier neurons (figure 2) in subjects with schizophrenia (figure 3). For example, GAT1 immunoreactivity is significantly reduced in the distinctive axon terminals (cartridges) of chandelier neurons,169 whereas in the postsynaptic axon initial segments of pyramidal neurons immunoreactivity for the GABA-A α2 subunit is markedly increased.159 Thus, the combined presynaptic reductions of PV and GAT1 and the upregulation of postsynaptic GABA-A receptor proteins may be compensatory changes triggered by reduced GAD67 activity and lower amounts of GABA released at chandelier cell synapses. Acting synergistically, these changes may help to increase the efficacy of GABA neurotransmission at pyramidal neuron axon initial segments. However, given the persistence of cognitive impairments in individuals with schizophrenia, these compensatory changes appear to be insufficient, and pharmacological augmentation of these responses might be of therapeutic value.170
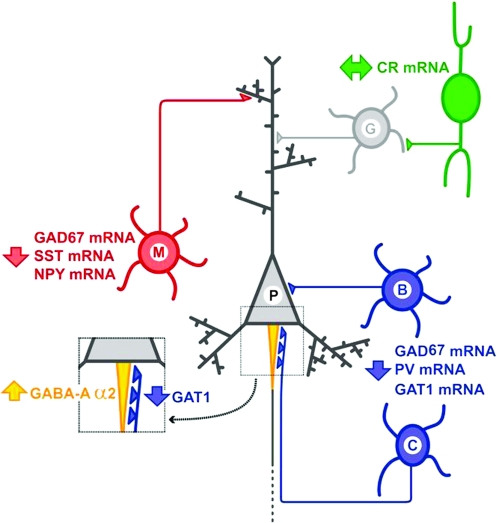
Fig. 3.
Alteration of markers of γ-aminobutyric acid (GABA) neurotransmission in the neocortex of subjects with schizophrenia. Highlighted in blue are the basket (B) and chandelier (C) neurons that contain the calcium-binding protein parvalbumin (PV), which synapse onto the soma and axon initial segment of pyramidal cells, respectively. In schizophrenia, PV-containing neurons have decreased levels of the mRNAs for PV, for the 67 kDa form of glutamic acid decarboxylase (GAD67) and for the GABA transporter 1 (GAT1). In addition, at synapses made by chandelier neurons, presynaptically there is a decrease in the concentration of GAT1 protein and postsynaptically an increase in the levels of the α2 subunits of GABA-A receptors. Schizophrenia is also associated with alterations in dendrite-targeting Martinotti neurons (M), indicated in red, in which mRNA levels are reduced for GAD67, somatostatin (SST), and neuropeptide Y (NPY). In contrast to the alterations in PV- and SST-containing neurons, GABA neurons that contain the calcium-binding protein calretinin (CR, in green), which mostly target other GABA neurons (G, in grey), do not seem to be altered in the cortex of subjects with schizophrenia. For additional details, see text.
The compensatory changes at chandelier cell synapses might be insufficient to produce normal patterns of network activity because additional interneuron subtypes are also functionally deficient in schizophrenia. For example, pre- and postsynaptic changes similar to those occurring at chandelier synapses might also occur in the perisomatic inputs from PV-expressing basket neurons. Indeed, the density of PV-immunoreactive puncta, possibly the axon terminals of PV-basket neurons,171 is reduced in the middle, but not the superficial, layers of the DLPFC of subjects with schizophrenia.172 These changes parallel the laminar pattern of decreased expression of PV mRNA in schizophrenia.173 Furthermore, an increase in GABA-A receptor density, as determined with ligand binding,174 was reported to be most prominent at pyramidal neuron cell bodies.174 If GABA-A receptor upregulation results from decreased GABA release, then these findings would suggest that GABA synthesis is decreased in both PV chandelier and PV basket cells. As noted above, PV basket cells produce IPSPs via α1-containing GABA-A receptors. Moreover, unlike PV chandelier cells that synapse exclusively onto pyramidal neurons, PV basket cells also synapse onto other PV neurons, a connection that is critical for oscillatory network synchrony by mutual inhibition. Because GAD67 deficits probably have an impact at all synapses made by a PV neuron, then a compensatory GABA-A receptor upregulation at PV basket cell synapses would require increased postsynaptic α1-containing GABA-A receptors in both pyramidal cells and in PV neurons.
However, we recently found that the cortical levels of the α1 subunit mRNA are decreased in the cortex of schizophrenia subjects,173 suggesting that the mechanisms of GABA-A receptor regulation at PV chandelier and PV basket cell synapses may differ in schizophrenia. Although both types of PV neurons share a number of physiological properties,125,162,175–178 a recent study suggested a fundamental functional difference. Specifically, activation of GABA-A receptors at the chandelier cell synapses was reported to produce chloride ion flow from inside neurons to the extracellular space.179 This unusual direction of chloride flow through GABA-A receptor channels is due to a chloride concentration gradient at the axon initial segment that is reversed compared to the cell body and proximal dendrites where FS basket cells synapse and produce hyperpolarizing IPSPs. As a consequence, chandelier cells could produce depolarizing/excitatory, instead of hyperpolarizing/inhibitory, synaptic potentials.179 The functional consequences of such surprising and interesting differences at the level of synchronized network oscillations remain to be determined.
Interestingly, at another type of synapse made by PV neurons, the synaptic potentials mediated by GABA-A receptors also differ from the classic hyperpolarizing IPSP. Specifically, the fast IPSPs reciprocally connecting FS/PV cells102 have a slight depolarizing but still inhibitory physiological effect called shunting.36,74 Computational modeling work suggests that the shunting effect of IPSPs at PV-to-PV connections is indeed critical for the role of such connections in the mechanisms of gamma oscillations.2,36,74 Whether or not shunting inhibition is important for oscillations in actual cortical networks awaits experimental demonstration; however these findings suggest diversity in the chloride concentration gradients in the compartments of different synapses made by PV neurons and that regulation of chloride ion distribution is a critical factor for normal GABA-A mediated signaling in cortical cell networks. The mechanisms regulating chloride ion gradients are therefore an important potential substrate for alterations of GABA transmission in schizophrenia. Specifically, the intracellular chloride concentration depends on the relative concentrations, in the adjacent plasma membrane, of the potassium/chloride cotransporter KCC2 that mediates chloride extrusion and the sodium/potassium/chloride transporter NKCC1 that mediates chloride uptake. At the axon initial segment of layer 3 pyramidal cells, where PV-positive chandelier cells make connections, the depolarizing effect of GABA is due both to the absence of KCC2179 and the presence of NKCC1.180
Conclusions
GABA neuron-mediated inhibition is crucial for the mechanisms underlying synchronization of neuronal activity in cortical microcircuits. Particularly important are the FS/PV subtypes of GABA neurons, which make synapses onto the perisomatic compartment of the pyramidal cell membrane. This location of the synaptic contacts endows FS/PV cells with a strong inhibitory effect because action potentials are typically initiated within this membrane compartment.181,182 It is likely that synchronization of pyramidal cell activity critically depends on such a strong inhibitory effect. Indeed, FS/PV neurons appear to participate in theta,86 gamma,89 and high ripple frequency oscillations.86
If synchronized oscillations critically depend on the efficacy4 of FS/PV-mediated signaling, then decreased inhibitory strength at FS/PV cell synapses due to reduced GABA synthesis is likely to contribute to the alterations of neural synchrony in schizophrenia. Specifically, if both the mutual inhibition between FS/PV neurons and the FS/PV inhibitory inputs onto pyramidal cells are altered, then both the mechanisms potentially setting oscillation frequency and distributing the synchronized activity to the pyramidal cell population may be impaired. Weaker FS/PV cell synapses in schizophrenia may decrease the efficacy of pyramidal cell synchronization by decreasing the synchrony of post-IPSP neuronal spikes and also by decreasing the number of pyramidal cells synchronized by individual GABA neurons (figure 1). Both effects would lead to decreased power of the population oscillation, as measured with EEG methods.
In addition to generating synchrony in local circuits, GABA neurons may play a role in the synchronization of rhythms across brain regions.2 For example, they could be targets of long distance projections between different cortical areas or may be the targets of a common input that provides a synchronization signal across cortical regions. Thus, alterations in GABA neurotransmission might also contribute to the reported deficits in synchronization of oscillation phase, or phase locking, in schizophrenia. Interestingly, recent data indicate that the GABA-related alterations found originally in schizophrenia studies of the DLPFC circuits are actually conserved across multiple cortical areas.183
It is important to note that although we have focused in this review on the alterations in FS/PV neurons, schizophrenia may be associated with alterations in other populations of GABA neurons as well. For example, significant alterations are found in the illness in transcripts that are expressed by other subtypes of GABA neurons, such as SST and NPY173,184 (figure 3), and which are commonly present in dendrite-targeting Martinotti cells. In contrast, other subpopulations, like the interneuron-targeting CR-containing neurons seem to be unaffected in schizophrenia (figure 3).
Because basic research studies continue to provide insight into the role of GABA neuron-mediated inhibition in cortical network oscillations, an important challenge for future studies is to clearly identify whether GABA transmission-related changes in schizophrenia represent a cause, consequence, compensation, or confound in the disease process.78 Therapeutic interventions aimed at diminishing consequences or boosting compensations at PV and SST cell synapses might provide a powerful way to improve oscillatory activity that depends on such synaptic inputs and therefore ameliorate the cognitive deficits of the illness.
Funding
National Institutes of Health (MH045156, MH051234, and MH043784) and NARSAD Young Investigator Award to the work cited in this article and published by the authors.
Acknowledgments
We thank Drs Bard Ermentrout and John Lisman for comments on a previous version of this manuscript. We thank Ms Mary Brady for excellent assistance creating the illustrations.
References
- 1.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mann EO, Paulsen O. Role of GABAergic inhibition in hippocampal network oscillations. Trends Neurosci. 2007;30:343–349. doi: 10.1016/j.tins.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 3.Howard MW, Rizzuto DS, Caplan JB, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 4.Tallon-Baudry C, Mandon S, Freiwald WA, Kreiter AK. Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cereb Cortex. 2004;14:713–720. doi: 10.1093/cercor/bhh031. [DOI] [PubMed] [Google Scholar]
- 5.Sejnowski TJ, Paulsen O. Network oscillations: emerging computational principles. J Neurosci. 2006;26:1673–1676. doi: 10.1523/JNEUROSCI.3737-05d.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lisman JE, Buzsaki G. A neural coding scheme formed by the combined function of gamma and theta oscillations. Schizoph Bull. doi: 10.1093/schbul/sbn060. June 16, 2008;doi:10.1093/schbul/sbn060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spencer KM, Nestor PG, Niznikiewicz MA, Salisbury DF, Shenton ME, McCarley RW. Abnormal neural synchrony in schizophrenia. J Neurosci. 2003;23:7407–7411. doi: 10.1523/JNEUROSCI.23-19-07407.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci USA. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uhlhaas PJ, Linden DE, Singer W, et al. Dysfunctional long-range coordination of neural activity during Gestalt perception in schizophrenia. J Neurosci. 2006;26:8168–8175. doi: 10.1523/JNEUROSCI.2002-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 11.Nunez PL, Srinivasan R. The Physics-EEG Interface. Electric Fields of the Brain—The Neurophysics of EEG. 2nd ed. New York: Oxford University Press; 2006. pp. 3–55. [Google Scholar]
- 12.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 13.Cobb SR, Buhl EH, Halasy K, Paulsen O, Somogyi P. Synchronization of neuronal activity in hippocampus by individual GABAergic interneurons. Nature. 1995;378:75–78. doi: 10.1038/378075a0. [DOI] [PubMed] [Google Scholar]
- 14.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 15.Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493(pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang XJ, Rinzel J. Spindle rhythmicity in the reticularis thalami nucleus: synchronization among mutually inhibitory neurons. Neuroscience. 1993;53:899–904. doi: 10.1016/0306-4522(93)90474-t. [DOI] [PubMed] [Google Scholar]
- 17.van Vreeswijk C, Abbott LF, Ermentrout GB. When inhibition not excitation synchronizes neural firing. J Comput Neurosci. 1994;1:313–321. doi: 10.1007/BF00961879. [DOI] [PubMed] [Google Scholar]
- 18.Wang XJ, Buzsaki G. Gamma oscillation by synaptic inhibition in a hippocampal interneuronal network model. J Neurosci. 1996;16:6402–6413. doi: 10.1523/JNEUROSCI.16-20-06402.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulyas AI, Hajos N, Freund TF. Interneurons containing calretinin are specialized to control other interneurons in the rat hippocampus. J Neurosci. 1996;16:3397–3411. doi: 10.1523/JNEUROSCI.16-10-03397.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gulyas AI, Megias M, Emri Z, Freund TF. Total number and ratio of excitatory and inhibitory synapses converging onto single interneurons of different types in the CA1 area of the rat hippocampus. J Neurosci. 1999;19:10082–10097. doi: 10.1523/JNEUROSCI.19-22-10082.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melchitzky DS, Eggan SM, Lewis DA. Synaptic targets of calretinin-containing axon terminals in macaque monkey prefrontal cortex. Neuroscience. 2005;130:185–195. doi: 10.1016/j.neuroscience.2004.08.046. [DOI] [PubMed] [Google Scholar]
- 22.Melchitzky DS, Lewis DA. Dendritic-targeting GABA neurons in monkey prefrontal cortex: comparison of somatostatin- and calretinin-immunoreactive axon terminals. Synapse. 2008;62:456–465. doi: 10.1002/syn.20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cobb SR, Halasy K, Vida I, et al. Synaptic effects of identified interneurons innervating both interneurons and pyramidal cells in the rat hippocampus. Neuroscience. 1997;79:629–648. doi: 10.1016/s0306-4522(97)00055-9. [DOI] [PubMed] [Google Scholar]
- 24.Tamas G, Somogyi P, Buhl EH. Differentially interconnected networks of GABAergic interneurons in the visual cortex of the cat. J Neurosci. 1998;18:4255–4270. doi: 10.1523/JNEUROSCI.18-11-04255.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamas G, Buhl EH, Somogyi P. Massive autaptic self-innervation of GABAergic neurons in cat visual cortex. J Neurosci. 1997;17:6352–6364. doi: 10.1523/JNEUROSCI.17-16-06352.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 27.Galarreta M, Hestrin S. Electrical and chemical synapses among parvalbumin fast-spiking GABAergic interneurons in adult mouse neocortex. Proc Natl Acad Sci USA. 2002;99:12438–12443. doi: 10.1073/pnas.192159599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurons and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- 29.Hestrin S, Galarreta M. Electrical synapses define networks of neocortical GABAergic neurons. Trends Neurosci. 2005;28:304–309. doi: 10.1016/j.tins.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Beierlein M, Gibson JR, Connors BW. A network of electrically coupled interneurons drives synchronized inhibition in neocortex. Nat Neurosci. 2000;3:904–910. doi: 10.1038/78809. [DOI] [PubMed] [Google Scholar]
- 31.Pawelzik H, Hughes DI, Thomson AM. Modulation of inhibitory autapses and synapses on rat CA1 interneurones by GABA(A) receptor ligands. J Physiol. 2003;546(pt 3):701–716. doi: 10.1113/jphysiol.2002.035121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bacci A, Huguenard JR, Prince DA. Functional autaptic neurotransmission in fast-spiking interneurons: a novel form of feedback inhibition in the neocortex. J Neurosci. 2003;23:859–866. doi: 10.1523/JNEUROSCI.23-03-00859.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bacci A, Huguenard JR. Enhancement of spike-timing precision by autaptic transmission in neocortical inhibitory interneurons. Neuron. 2006;49:119–130. doi: 10.1016/j.neuron.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda K, Bekkers JM. Autapses. Curr Biol. 2006;16:R308. doi: 10.1016/j.cub.2006.03.085. [DOI] [PubMed] [Google Scholar]
- 35.Kopell N, Ermentrout B. Chemical and electrical synapses perform complementary roles in the synchronization of interneuronal networks. Proc Natl Acad Sci USA. 2004;101:15482–15487. doi: 10.1073/pnas.0406343101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 37.Traub RD, Whittington MA, Colling SB, Buzsaki G, Jefferys JG. Analysis of gamma rhythms in the rat hippocampus in vitro and in vivo. J Physiol. 1996;493(pt 2):471–484. doi: 10.1113/jphysiol.1996.sp021397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Whittington MA, Traub RD, Kopell N, Ermentrout B, Buhl EH. Inhibition-based rhythms: experimental and mathematical observations on network dynamics. Int J Psychophysiol. 2000;38:315–336. doi: 10.1016/s0167-8760(00)00173-2. [DOI] [PubMed] [Google Scholar]
- 39.White JA, Banks MI, Pearce RA, Kopell NJ. Networks of interneurons with fast and slow gamma-aminobutyric acid type A (GABAA) kinetics provide substrate for mixed gamma-theta rhythm. Proc Natl Acad Sci USA. 2000;97:8128–8133. doi: 10.1073/pnas.100124097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- 41.Groc L, Gustafsson B, Hanse E. AMPA signalling in nascent glutamatergic synapses: there and not there! Trends Neurosci. 2006;29:132–139. doi: 10.1016/j.tins.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nicholson DA, Trana R, Katz Y, Kath WL, Spruston N, Geinisman Y. Distance-dependent differences in synapse number and AMPA receptor expression in hippocampal CA1 pyramidal neurons. Neuron. 2006;50:431–442. doi: 10.1016/j.neuron.2006.03.022. [DOI] [PubMed] [Google Scholar]
- 44.Angulo MC, Rossier J, Audinat E. Postsynaptic glutamate receptors and integrative properties of fast-spiking interneurons in the rat neocortex. J Neurophysiol. 1999;82:1295–1302. doi: 10.1152/jn.1999.82.3.1295. [DOI] [PubMed] [Google Scholar]
- 45.Geiger JR, Lubke J, Roth A, Frotscher M, Jonas P. Submillisecond AMPA receptor-mediated signaling at a principal neuron-interneuron synapse. Neuron. 1997;18:1009–1023. doi: 10.1016/s0896-6273(00)80339-6. [DOI] [PubMed] [Google Scholar]
- 46.Gonzalez-Burgos G, Kroener S, Seamans JK, Lewis DA, Barrionuevo G. Dopaminergic modulation of short-term synaptic plasticity in fast-spiking interneurons of primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;94:4168–4177. doi: 10.1152/jn.00698.2005. [DOI] [PubMed] [Google Scholar]
- 47.Wang H-W, Gao WJ. Cell type-specific development of NMDA receptors in the interneurons of rat prefrontal cortex. Soc Neurosci Abst. 2007 doi: 10.1038/npp.2009.20. 576.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fuchs EC, Zivkovic AR, Cunningham MO, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 49.Frerking M, Nicoll RA. Synaptic kainate receptors. Curr Opin Neurobiol. 2000;10:342–351. doi: 10.1016/s0959-4388(00)00094-5. [DOI] [PubMed] [Google Scholar]
- 50.Cossart R, Epsztein J, Tyzio R, et al. Quantal release of glutamate generates pure kainate and mixed AMPA/kainate EPSCs in hippocampal neurons. Neuron. 2002;35:147–159. doi: 10.1016/s0896-6273(02)00753-5. [DOI] [PubMed] [Google Scholar]
- 51.Goldin M, Epsztein J, Jorquera I, et al. Synaptic kainate receptors tune oriens-lacunosum moleculare interneurons to operate at theta frequency. J Neurosci. 2007;27:9560–9572. doi: 10.1523/JNEUROSCI.1237-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali AB. Involvement of post-synaptic kainate receptors during synaptic transmission between unitary connections in rat neocortex 13. Eur J Neurosci. 2003;17:2344–2350. doi: 10.1046/j.1460-9568.2003.02677.x. [DOI] [PubMed] [Google Scholar]
- 53.Nusser Z, Lujan R, Laube G, Roberts JDB, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- 54.Nusser Z. AMPA and NMDA receptors: similarities and differences in their synaptic distribution. Curr Opin Neurobiol. 2000;10:337–341. doi: 10.1016/s0959-4388(00)00086-6. [DOI] [PubMed] [Google Scholar]
- 55.Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gulyas AI, Miles R, Sik A, et al. Hippocampal pyramidal cells excite inhibitory neurons through a single release site. Nature. 1993;366:683–687. doi: 10.1038/366683a0. [DOI] [PubMed] [Google Scholar]
- 57.Beierlein M, Gibson JR, Connors BW. Two dynamically distinct inhibitory networks in layer 4 of the neocortex. J Neurophysiol. 2003;90:2987–3000. doi: 10.1152/jn.00283.2003. [DOI] [PubMed] [Google Scholar]
- 58.Holmgren C, Harkany T, Svennenfors B, Zilberter Y. Pyramidal cell communication within local networks in layer 2/3 of rat neocortex. J Physiol. 2003;551(pt 1):139–153. doi: 10.1113/jphysiol.2003.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonzalez-Burgos G, Krimer LS, Urban NN, Barrionuevo G, Lewis DA. Synaptic efficacy during repetitive activation of excitatory inputs in primate dorsolateral prefrontal cortex. Cereb Cortex. 2004;14:530–542. doi: 10.1093/cercor/bhh015. [DOI] [PubMed] [Google Scholar]
- 60.Povysheva NV, Gonzalez-Burgos G, Zaitsev AV, et al. Properties of excitatory synaptic responses in fast-spiking interneurons and pyramidal cells from monkey and rat prefrontal cortex. Cereb Cortex. 2006;16:541–552. doi: 10.1093/cercor/bhj002. [DOI] [PubMed] [Google Scholar]
- 61.van Hooft JA, Giuffrida R, Blatow M, Monyer H. Differential expression of group I metabotropic glutamate receptors in functionally distinct hippocampal interneurons. J Neurosci. 2000;20:3544–3551. doi: 10.1523/JNEUROSCI.20-10-03544.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez R, Kallenbach U, Singer W, Munk MH. Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci. 2004;24:10369–10378. doi: 10.1523/JNEUROSCI.1839-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hentschke H, Perkins MG, Pearce RA, Banks MI. Muscarinic blockade weakens interaction of gamma with theta rhythms in mouse hippocampus. Eur J Neurosci. 2007;26:1642–1656. doi: 10.1111/j.1460-9568.2007.05779.x. [DOI] [PubMed] [Google Scholar]
- 65.Kawaguchi Y. Selective cholinergic modulation of cortical GABAergic cell subtypes. J Neurophysiol. 1997;78:1743–1747. doi: 10.1152/jn.1997.78.3.1743. [DOI] [PubMed] [Google Scholar]
- 66.Gorelova N, Seamans JK, Yang CR. Mechanisms of dopamine activation of fast-spiking interneurons that exert inhibition in rat prefrontal cortex. J Neurophysiol. 2002;88:3150–3166. doi: 10.1152/jn.00335.2002. [DOI] [PubMed] [Google Scholar]
- 67.Kroner S, Krimer LS, Lewis DA, Barrionuevo G. Dopamine increases inhibition in the monkey dorsolateral prefrontal cortex through cell type-specific modulation of interneurons. Cereb Cortex. 2007;17:1020–1032. doi: 10.1093/cercor/bhl012. [DOI] [PubMed] [Google Scholar]
- 68.Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- 69.Ikegaya Y, Aaron G, Cossart R, et al. Synfire chains and cortical songs: temporal modules of cortical activity. Science. 2004;304:559–564. doi: 10.1126/science.1093173. [DOI] [PubMed] [Google Scholar]
- 70.Bandyopadhyay S, Gonzalez-Islas C, Hablitz JJ. Dopamine enhances spatiotemporal spread of activity in rat prefrontal cortex. J Neurophysiol. 2005;93:864–872. doi: 10.1152/jn.00922.2004. [DOI] [PubMed] [Google Scholar]
- 71.Bandyopadhyay S, Hablitz JJ. Dopaminergic modulation of local network activity in rat prefrontal cortex. J Neurophysiol. 2007;97:4120–4128. doi: 10.1152/jn.00898.2006. [DOI] [PubMed] [Google Scholar]
- 72.Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- 73.Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 74.Vida I, Bartos M, Jonas P. Shunting inhibition improves robustness of gamma oscillations in hippocampal interneuron networks by homogenizing firing rates. Neuron. 2006;49:107–117. doi: 10.1016/j.neuron.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 75.Banke TG, McBain CJ. GABAergic input onto CA3 hippocampal interneurons remains shunting throughout development. J Neurosci. 2006;26:11720–11725. doi: 10.1523/JNEUROSCI.2887-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marchionni I, Omrani A, Cherubini E. In the developing rat hippocampus a tonic GABAA-mediated conductance selectively enhances the glutamatergic drive of principal cells. J Physiol. 2007;581(pt 2):515–528. doi: 10.1113/jphysiol.2006.125609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562(pt 1):9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewis DA, Gonzalez-Burgos G. Neuroplasticity of neocortical circuits in schizophrenia. Neuropsychopharmacology. 2008;33:141–165. doi: 10.1038/sj.npp.1301563. [DOI] [PubMed] [Google Scholar]
- 79.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 80.Yuste R. Origin and classification of neocortical interneurons. Neuron. 2005;48:524–527. doi: 10.1016/j.neuron.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 81.Nelson SB, Sugino K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci. 2006;29:339–345. doi: 10.1016/j.tins.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 82.Soghomonian JJ, Martin DL. Two isoforms of glutamate decarboxylase: why? Trends Pharmacol Sci. 1998;19:500–505. doi: 10.1016/s0165-6147(98)01270-x. [DOI] [PubMed] [Google Scholar]
- 83.Conde F, Lund JS, Jacobowitz DM, Baimbridge KG, Lewis DA. Local circuit neurons immunoreactive for calretinin, calbindin D-28k or parvalbumin in monkey prefrontal cortex: distribution and morphology. J Comp Neurol. 1994;341:95–116. doi: 10.1002/cne.903410109. [DOI] [PubMed] [Google Scholar]
- 84.Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- 85.Wang Y, Toledo-Rodriguez M, Gupta A, et al. Anatomical, physiological and molecular properties of Martinotti cells in the somatosensory cortex of the juvenile rat. J Physiol. 2004;561(pt 1):65–90. doi: 10.1113/jphysiol.2004.073353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Klausberger T, Magill PJ, Marton LF, et al. Brain-state- and cell-type-specific firing of hippocampal interneurons in vivo. Nature. 2003;421:844–848. doi: 10.1038/nature01374. [DOI] [PubMed] [Google Scholar]
- 87.Klausberger T, Marton LF, Baude A, Roberts JD, Magill PJ, Somogyi P. Spike timing of dendrite-targeting bistratified cells during hippocampal network oscillations in vivo. Nat Neurosci. 2004;7:41–47. doi: 10.1038/nn1159. [DOI] [PubMed] [Google Scholar]
- 88.Klausberger T, Marton LF, O'Neill J, et al. Complementary roles of cholecystokinin- and parvalbumin-expressing GABAergic neurons in hippocampal network oscillations. J Neurosci. 2005;25:9782–9793. doi: 10.1523/JNEUROSCI.3269-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tukker JJ, Fuentealba P, Hartwich K, Somogyi P, Klausberger T. Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. J Neurosci. 2007;27:8184–8189. doi: 10.1523/JNEUROSCI.1685-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hajos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gloveli T, Dugladze T, Saha S, et al. Differential involvement of oriens/pyramidale interneurones in hippocampal network oscillations in vitro. J Physiol. 2005;562(pt 1):131–147. doi: 10.1113/jphysiol.2004.073007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 93.Oren I, Mann EO, Paulsen O, Hajos N. Synaptic currents in anatomically identified CA3 neurons during hippocampal gamma oscillations in vitro. J Neurosci. 2006;26:9923–9934. doi: 10.1523/JNEUROSCI.1580-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gillies MJ, Traub RD, LeBeau FE, et al. A model of atropine-resistant theta oscillations in rat hippocampal area CA1. J Physiol. 2002;543(pt 3):779–793. doi: 10.1113/jphysiol.2002.024588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fellous JM, Sejnowski TJ. Cholinergic induction of oscillations in the hippocampal slice in the slow (0.5-2 Hz), theta (5-12 Hz), and gamma (35-70 Hz) bands. Hippocampus. 2000;10(2):187–197. doi: 10.1002/(SICI)1098-1063(2000)10:2<187::AID-HIPO8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 96.Buzsaki G. Cycle 7: The brain's default state: self-organized oscillations in rest and sleep. In: Buzsaki G, editor. Rhythms of the Brain. New York: Oxford University Press; 2006. pp. 175–205. [Google Scholar]
- 97.Buzsaki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 98.Manseau F, Goutagny R, Danik M, Williams S. The Hippocamposeptal pathway generates rhythmic firing of GABAergic neurons in the medial septum and diagonal bands: an investigation using a complete septohippocampal preparation in vitro. J Neurosci. 2008;28:4096–4107. doi: 10.1523/JNEUROSCI.0247-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Garner HL, Whittington MA, Henderson Z. Induction by kainate of theta frequency rhythmic activity in the rat medial septum-diagonal band complex in vitro. J Physiol. 2005;564(pt 1):83–102. doi: 10.1113/jphysiol.2004.080622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mann EO, Radcliffe CA, Paulsen O. Hippocampal gamma-frequency oscillations: from interneurones to pyramidal cells, and back. J Physiol. 2005;562(pt 1):55–63. doi: 10.1113/jphysiol.2004.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;3:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 102.Bartos M, Vida I, Frotscher M, et al. Fast synaptic inhibition promotes synchronized gamma oscillations in hippocampal interneuron networks. Proc Natl Acad Sci USA. 2002;99:13222–13227. doi: 10.1073/pnas.192233099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rotstein HG, Pervouchine DD, Acker CD, et al. Slow and fast inhibition and an H-current interact to create a theta rhythm in a model of CA1 interneuron network. J Neurophysiol. 2005;94:1509–1518. doi: 10.1152/jn.00957.2004. [DOI] [PubMed] [Google Scholar]
- 104.Mohler H. GABA(A) receptor diversity and pharmacology. Cell Tissue Res. 2006;326:505–516. doi: 10.1007/s00441-006-0284-3. [DOI] [PubMed] [Google Scholar]
- 105.Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABA(A) receptor channels are dependent on alpha-subunit isoform. Biophys J. 1997;73:2518–2526. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Klausberger T, Roberts JD, Somogyi P. Cell type- and input-specific differences in the number and subtypes of synaptic GABA(A) receptors in the hippocampus. J Neurosci. 2002;22:2513–2521. doi: 10.1523/JNEUROSCI.22-07-02513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- 108.Thomson AM, Bannister AP, Hughes DI, Pawelzik H. Differential sensitivity to Zolpidem of IPSPs activated by morphologically identified CA1 interneurons in slices of rat hippocampus. Eur J Neurosci. 2000;12:425–436. doi: 10.1046/j.1460-9568.2000.00915.x. [DOI] [PubMed] [Google Scholar]
- 109.Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci USA. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bacci A, Rudolph U, Huguenard JR, Prince DA. Major differences in inhibitory synaptic transmission onto two neocortical interneuron subclasses. J Neurosci. 2003;23:9664–9674. doi: 10.1523/JNEUROSCI.23-29-09664.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ali AB, Thomson AM. Synaptic alpha 5 subunit containing GABAA receptors mediate IPSPs elicited by dendrite-preferring cells in rat neocortex. Cereb Cortex. 2007;18:1260–1271. doi: 10.1093/cercor/bhm160. [DOI] [PubMed] [Google Scholar]
- 112.Dunning DD, Hoover CL, Soltesz I, Smith MA, O'Dowd DK. GABA(A) receptor-mediated miniature postsynaptic currents and alpha-subunit expression in developing cortical neurons. J Neurophysiol. 1999;82:3286–3297. doi: 10.1152/jn.1999.82.6.3286. [DOI] [PubMed] [Google Scholar]
- 113.Fritschy JM, Paysan J, Enna A, Mohler H. Switch in the expression of rat GABAA-receptor subtypes during postnatal development: an immunohistochemical study. J Neurosci. 1994;14:5302–5324. doi: 10.1523/JNEUROSCI.14-09-05302.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nguyen QL, Hashimoto T, Lewis DA. Postnatal development of GABAA receptor a1 and a2 subunit mRNA expression in the monkey dorsolateral prefrontal cortex. Soc Neurosci Abst. 2006 190.7. [Google Scholar]
- 115.Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, de Blas AL. Synaptic and nonsynaptic localization of GABAA receptors containing the alpha5 subunit in the rat brain. J Comp Neurol. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 117.Spampanato J, Mody I. Spike timing of lacunosom-moleculare targeting interneurons and CA3 pyramidal cells during high frequency network oscillations in vitro. J Neurophysiol. 2007;98:96–104. doi: 10.1152/jn.00188.2007. [DOI] [PubMed] [Google Scholar]
- 118.Kamondi A, Acsady L, Wang XJ, Buzsaki G. Theta oscillations in somata and dendrites of hippocampal pyramidal cells in vivo: activity-dependent phase-precession of action potentials. Hippocampus. 1998;8(3):244–261. doi: 10.1002/(SICI)1098-1063(1998)8:3<244::AID-HIPO7>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 119.Penttonen M, Kamondi A, Acsady L, Buzsaki G. Gamma frequency oscillation in the hippocampus of the rat: intracellular analysis in vivo. Eur J Neurosci. 1998;10:718–728. doi: 10.1046/j.1460-9568.1998.00096.x. [DOI] [PubMed] [Google Scholar]
- 120.Brager DH, Johnston D. Plasticity of intrinsic excitability during long-term depression is mediated through mGluR-dependent changes in I(h) in hippocampal CA1 pyramidal neurons. J Neurosci. 2007;27:13926–13937. doi: 10.1523/JNEUROSCI.3520-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Ulrich D. Dendritic resonance in rat neocortical pyramidal cells. J Neurophysiol. 2002;87:2753–2759. doi: 10.1152/jn.2002.87.6.2753. [DOI] [PubMed] [Google Scholar]
- 122.Pike FG, Goddard RS, Suckling JM, Ganter P, Kasthuri N, Paulsen O. Distinct frequency preferences of different types of rat hippocampal neurones in response to oscillatory input currents. J Physiol. 2000;529(pt 1):205–213. doi: 10.1111/j.1469-7793.2000.00205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spruston N, McBain CJ. Structural and functional properties of hippocampal neurons. In: Andersen P, Morris R, Amaral DG, Bliss T, O'Keefe J, editors. The Hippocampus Book. New York: Oxford University Press; 2007. pp. 133–204. [Google Scholar]
- 124.Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 125.Gonzalez-Burgos G, Krimer LS, Povysheva NV, Barrionuevo G, Lewis DA. Functional properties of fast spiking interneurons and their synaptic connections with pyramidal cells in primate dorsolateral prefrontal cortex. J Neurophysiol. 2005;93:942–953. doi: 10.1152/jn.00787.2004. [DOI] [PubMed] [Google Scholar]
- 126.Galarreta M, Hestrin S. Spike transmission and synchrony detection in networks of GABAergic interneurons. Science. 2001;292:2295–2299. doi: 10.1126/science.1061395. [DOI] [PubMed] [Google Scholar]
- 127.Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32:1888–1902. doi: 10.1038/sj.npp.1301312. [DOI] [PubMed] [Google Scholar]
- 128.Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7:48–55. doi: 10.1016/j.coph.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 129.Woo TU, Shrestha K, Amstrong C, Minns MM, Walsh JP, Benes FM. Differential alterations of kainate receptor subunits in inhibitory interneurons in the anterior cingulate cortex in schizophrenia and bipolar disorder. Schizophr Res. 2007;96:46–61. doi: 10.1016/j.schres.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lewis DA, Gonzalez-Burgos G. Pathophysiologically based treatment interventions in schizophrenia. Nat Med. 2006;12:1016–1022. doi: 10.1038/nm1478. [DOI] [PubMed] [Google Scholar]
- 131.Nyiri G, Stephenson BM, Freund TF, Somogyi P. Large variability in synaptic N-methyl-D-aspartate receptor density on interneurons and a comparison with pyramidal-cell spines in the rat hippocampus. Neuroscience. 2003;119:347–363. doi: 10.1016/s0306-4522(03)00157-x. [DOI] [PubMed] [Google Scholar]
- 132.Goldberg JH, Tamas G, Aronov D, Yuste R. Calcium microdomains in aspiny dendrites. Neuron. 2003;40:807–821. doi: 10.1016/s0896-6273(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 133.Goldberg JH, Yuste R, Tamas G. Ca2+ imaging of mouse neocortical interneurone dendrites: contribution of Ca2+-permeable AMPA and NMDA receptors to subthreshold Ca2+dynamics. J Physiol. 2003;551(pt 1):67–78. doi: 10.1113/jphysiol.2003.042598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Lu JT, Li CY, Zhao JP, Poo MM, Zhang XH. Spike-timing-dependent plasticity of neocortical excitatory synapses on inhibitory interneurons depends on target cell type. J Neurosci. 2007;27:9711–9720. doi: 10.1523/JNEUROSCI.2513-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ermentrout GB, Kopell N. Fine structure of neural spiking and synchronization in the presence of conduction delays. Proc Natl Acad Sci USA. 1998;95:1259–1264. doi: 10.1073/pnas.95.3.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Kinney JW, Davis CN, Tabarean I, Conti B, Bartfai T, Behrens MM. A specific role for NR2A-containing NMDA receptors in the maintenance of parvalbumin and GAD67 immunoreactivity in cultured interneurons. J Neurosci. 2006;26:1604–1615. doi: 10.1523/JNEUROSCI.4722-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Behrens MM, Ali SS, Dao DN, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 138.Lisman JE, Fellous JM, Wang XJ. A role for NMDA-receptor channels in working memory. Nat Neurosci. 1998;1:273–275. doi: 10.1038/1086. [DOI] [PubMed] [Google Scholar]
- 139.Wang X-J. Synaptic reverberation underlying mnemonic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- 140.Durstewitz D, Gabriel T. Dynamical basis of irregular spiking in NMDA-driven prefrontal cortex neurons. Cereb Cortex. 2007;17:894–908. doi: 10.1093/cercor/bhk044. [DOI] [PubMed] [Google Scholar]
- 141.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 142.Romo R, Brody CD, Hernandez A, Lemus L. Neuronal correlates of parametric working memory in the prefrontal cortex. Nature. 1999;399:470–473. doi: 10.1038/20939. [DOI] [PubMed] [Google Scholar]
- 143.Pinto DJ, Hartings JA, Brumberg JC, Simons DJ. Cortical damping: analysis of thalamocortical response transformations in rodent barrel cortex. Cereb Cortex. 2003;13:33–44. doi: 10.1093/cercor/13.1.33. [DOI] [PubMed] [Google Scholar]
- 144.Okun M, Lampl I. Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci. 2008;11:535–537. doi: 10.1038/nn.2105. [DOI] [PubMed] [Google Scholar]
- 145.Jiao Y, Zhang C, Yanagawa Y, Sun QQ. Major effects of sensory experiences on the neocortical inhibitory circuits. J Neurosci. 2006;26:8691–8701. doi: 10.1523/JNEUROSCI.2478-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Cunningham MO, Hunt J, Middleton S, et al. Region-specific reduction in entorhinal gamma oscillations and parvalbumin-immunoreactive neurons in animal models of psychiatric illness. J Neurosci. 2006;26:2767–2776. doi: 10.1523/JNEUROSCI.5054-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schmitz D, Schuchmann S, Fisahn A, et al. Axo-axonal coupling: a novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 148.Mercer A, Bannister AP, Thomson AM. Electrical coupling between pyramidal cells in adult cortical regions. Brain Cell Biol. 2006;35(1):13–27. doi: 10.1007/s11068-006-9005-9. [DOI] [PubMed] [Google Scholar]
- 149.Traub RD, Bibbig A, Fisahn A, LeBeau FE, Whittington MA, Buhl EH. A model of gamma-frequency network oscillations induced in the rat CA3 region by carbachol in vitro. Eur J Neurosci. 2000;12:4093–4106. doi: 10.1046/j.1460-9568.2000.00300.x. [DOI] [PubMed] [Google Scholar]
- 150.Akbarian S, Huang HS. Molecular and cellular mechanisms of altered GAD1/GAD67 expression in schizophrenia and related disorders. Brain Res Rev. 2006;52(2):293–304. doi: 10.1016/j.brainresrev.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 151.Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–1069. doi: 10.1001/archpsyc.57.11.1061. [DOI] [PubMed] [Google Scholar]
- 152.Benes FM, Todtenkopf MS, Logiotatos P, Williams M. Glutamate decarboxylase(65)-immunoreactive terminals in cingulate and prefrontal cortices of schizophrenic and bipolar brain. J Chem Neuroanat. 2000;20:259–269. doi: 10.1016/s0891-0618(00)00105-8. [DOI] [PubMed] [Google Scholar]
- 153.Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 154.Volk DW, Austin MC, Pierri JN, Sampson AR, Lewis DA. Decreased glutamic acid decarboxylase67 messenger RNA expression in a subset of prefrontal cortical gamma-aminobutyric acid neurons in subjects with schizophrenia. Arch Gen Psychiatry. 2000;57:237–245. doi: 10.1001/archpsyc.57.3.237. [DOI] [PubMed] [Google Scholar]
- 155.Hashimoto T, Volk DW, Eggan SM, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Woo TU, Miller JL, Lewis DA. Schizophrenia and the parvalbumin-containing class of cortical local circuit neurons. Am J Psychiatry. 1997;154:1013–1015. doi: 10.1176/ajp.154.7.1013. [DOI] [PubMed] [Google Scholar]
- 157.Beasley CL, Zhang ZJ, Patten I, Reynolds GP. Selective deficits in prefrontal cortical GABAergic neurons in schizophrenia defined by the presence of calcium-binding proteins. Biol Psychiatry. 2002;5:708–715. doi: 10.1016/s0006-3223(02)01360-4. [DOI] [PubMed] [Google Scholar]
- 158.Pierri JN, Volk CL, Auh S, Sampson A, Lewis DA. Decreased somal size of deep layer 3 pyramidal neurons in the prefrontal cortex of subjects with schizophrenia. Arch Gen Psychiatry. 2001;58:466–473. doi: 10.1001/archpsyc.58.5.466. [DOI] [PubMed] [Google Scholar]
- 159.Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 160.Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- 161.Huang HS, Matevossian A, Whittle C, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–11262. doi: 10.1523/JNEUROSCI.3272-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Overstreet LS, Westbrook GL. Synapse density regulates independence at unitary inhibitory synapses. J Neurosci. 2003;23:2618–2626. doi: 10.1523/JNEUROSCI.23-07-02618.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Keros S, Hablitz JJ. Subtype-specific GABA transporter antagonists synergistically modulate phasic and tonic GABAA conductances in rat neocortex. J Neurophysiol. 2005;94:2073–2085. doi: 10.1152/jn.00520.2005. [DOI] [PubMed] [Google Scholar]
- 164.Gonzalez-Burgos G, Rotaru DC, Zaitsev AV, Povysheva NV, Krimer LS, Lewis DA. Modulation of inhibitory transmission by GABA transporter 1 in primate dorsolateral prefrontal cortex. Soc Neurosci Abst. 2006 728.13. [Google Scholar]
- 165.Collin T, Chat M, Lucas MG, et al. Developmental changes in parvalbumin regulate presynaptic Ca2+ signaling. J Neurosci. 2005;25:96–107. doi: 10.1523/JNEUROSCI.3748-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Muller M, Felmy F, Schwaller B, Schneggenburger R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of held that accelerates the decay of Ca2+ and short-term facilitation. J Neurosci. 2007;27:2261–2271. doi: 10.1523/JNEUROSCI.5582-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Vreugdenhil M, Jefferys JG, Celio MR, Schwaller B. Parvalbumin-deficiency facilitates repetitive IPSCs and gamma oscillations in the hippocampus. J Neurophysiol. 2003;89:1414–1422. doi: 10.1152/jn.00576.2002. [DOI] [PubMed] [Google Scholar]
- 168.Hefft S, Jonas P. Asynchronous GABA release generates long-lasting inhibition at a hippocampal interneuron-principal neuron synapse. Nat Neurosci. 2005;8:1319–1328. doi: 10.1038/nn1542. [DOI] [PubMed] [Google Scholar]
- 169.Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci USA. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lewis DA, Volk DW, Hashimoto T. Selective alterations in prefrontal cortical GABA neurotransmission in schizophrenia: a novel target for the treatment of working memory dysfunction. Psychopharmacology (Berl) 2004;174:143–150. doi: 10.1007/s00213-003-1673-x. [DOI] [PubMed] [Google Scholar]
- 171.Erickson SL, Lewis DA. Postnatal development of parvalbumin- and GABA transporter-immunoreactive axon terminals in monkey prefrontal cortex. J Comp Neurol. 2002;448:186–202. doi: 10.1002/cne.10249. [DOI] [PubMed] [Google Scholar]
- 172.Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- 173.Hashimoto T, Arion D, Unger T, et al. Alterations in GABA-related transcriptome in the dorsolateral prefrontal cortex of subjects with schizophrenia. Mol Psychiatry. 2008;13:147–161. doi: 10.1038/sj.mp.4002011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Benes FM, Vincent SL, Marie A, Khan Y. Up-regulation of GABAA receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- 175.Maccaferri G, Roberts JD, Szucs P, Cottingham CA, Somogyi P. Cell surface domain specific postsynaptic currents evoked by identified GABAergic neurones in rat hippocampus in vitro. J Physiol. 2000;524:91–116. doi: 10.1111/j.1469-7793.2000.t01-3-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Krimer LS, Zaitsev AV, Czanner G, et al. Cluster analysis-based physiological classification and morphological properties of inhibitory neurons in layers 2-3 of monkey dorsolateral prefrontal cortex. J Neurophysiol. 2005;94:3009–3022. doi: 10.1152/jn.00156.2005. [DOI] [PubMed] [Google Scholar]
- 177.Zaitsev AV, Gonzalez-Burgos G, Povysheva NV, Kroner S, Lewis DA, Krimer LS. Localization of calcium-binding proteins in physiologically and morphologically characterized interneurons of monkey dorsolateral prefrontal cortex. Cereb Cortex. 2005;15:1178–1186. doi: 10.1093/cercor/bhh218. [DOI] [PubMed] [Google Scholar]
- 178.Buhl EH, Halasy K, Somogyi P. Diverse sources of hippocampal unitary inhibitory postsynaptic potentials and the number of synaptic release sites. Nature. 1994;368:823–828. doi: 10.1038/368823a0. [DOI] [PubMed] [Google Scholar]
- 179.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science. 2006;311:233–235. doi: 10.1126/science.1121325. [DOI] [PubMed] [Google Scholar]
- 180.Khirug S, Yamada J, Afzalov R, Voipio J, Khirough R, Kaila K. GABAergic depolarization of the axon initial segment in cortical principal neurons is caused by the Na-K-2Cl- contransporter NKCC1. J Neurosci. 2008;28:4635–4639. doi: 10.1523/JNEUROSCI.0908-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Palmer LM, Stuart GJ. Site of action potential initiation in layer 5 pyramidal neurons. J Neurosci. 2006;26:1854–1863. doi: 10.1523/JNEUROSCI.4812-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kole MH, Ilschner SU, Kampa BM, Williams SR, Ruben PC, Stuart GJ. Action potential generation requires a high sodium channel density in the axon initial segment. Nat Neurosci. 2008;11:178–186. doi: 10.1038/nn2040. [DOI] [PubMed] [Google Scholar]
- 183.Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, Lewis DA. Conserved regional patterns of GABA-related transcript expression in the neocortex of subjects with schizophrenia. Am J Psychiatry. 2008;162:479–489. doi: 10.1176/appi.ajp.2007.07081223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Morris HM, Hashimoto T, Lewis DA. Alterations in somatostatin mRNA expression in the dorsolateral prefrontal cortex of subjects with schizophrenia or schizoaffective disorder. Cereb Cortex. 2008;18:1575–1587. doi: 10.1093/cercor/bhm186. [DOI] [PMC free article] [PubMed] [Google Scholar]