Abstract
Brain oscillations are important in controlling the timing of neuronal firing. This process has been extensively analyzed in connection with gamma frequency oscillations and more recently with respect to theta frequency oscillations. Here we review evidence that theta and gamma oscillations work together to form a neural code. This coding scheme provides a way for multiple neural ensembles to represent an ordered sequence of items. In the hippocampus, this coding scheme is utilized during the phase precession, a phenomenon that can be interpreted as the recall of sequences of items (places) from long-term memory. The same coding scheme may be used in certain cortical regions to encode multi-item short-term memory. The possibility that abnormalities in theta/gamma could underlie symptoms of schizophrenia is discussed.
Keywords: phase precession, synchronization, hippocampus, memory
Although brain oscillations have been known for a long time, it is only recently that attempts have been made to understand how brain rhythms support cognitive operations.1 There is now considerable interest in the role of gamma oscillations (40–100 Hz) in cognitive processes and the possibility that abnormalities in these oscillations might underlie symptoms of schizophrenia (see reviews in this issue2,3). However, prominent oscillations at lower frequency, notably in the theta range (4–10 Hz), also occur during cognitive processes. In this brief review, we will describe studies indicating that theta and gamma oscillations work together to create a neural code, the function of which is to allow representation of multiple items in a defined order. The possibility that this coding scheme is also utilized in other brain regions will be discussed.
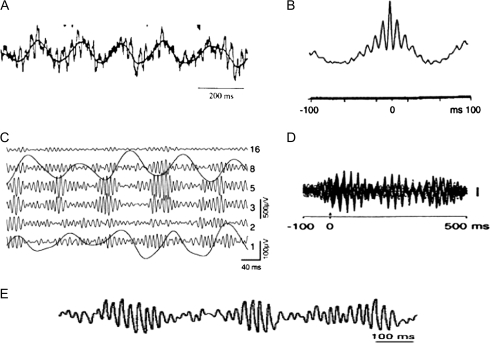
Figure 1 shows field recordings from various brain regions that demonstrate the existence of both theta and gamma oscillations. These regions include the hippocampus, cortical regions that interact with the hippocampus (entorhinal cortex) and primary sensory cortex (olfactory cortex). Two important conclusions can be reached by simple inspection. First, both oscillations occur simultaneously. Second, the 2 oscillations are not independent, but show clear signs of interactions: this is evident from the fact that the amplitude of gamma oscillations is modulated at theta frequency.
Fig. 1.
Recordings of Dual Theta/Gamma Oscillations in the Hippocampus and Various Brain Regions. In some cases, what is clearest in these figures is the theta modulation of gamma amplitude. This modulation is an indication of the interaction of the 2 oscillations within the same network, but it remains unclear whether there is any functional significance of these amplitude changes. (A) Intracellular recording from hippocampal neuron.30 (B) Field recordings from hippocampus; average triggered on peak of the gamma frequency field potential oscillation.31 (C) Field recordings from entorhinal cortex. Highly filtered records show the slow theta component in isolation.32 (D) magnetoencepholography from human midline cortex.33 (E) Field recording from olfactory cortex.34 Reproduced with permission from Lisman.20
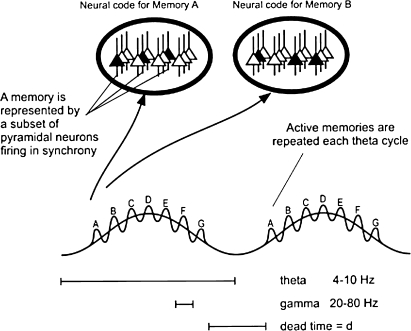
The way in which these dual oscillations could form a coding scheme is illustrated by the model shown in figure 2. This model4 builds on the idea of a neural ensemble, a group of active cells whose spatial pattern represents a particular percept, memory, or thought (henceforth termed an “item,” eg, the representation of a place, face, or event). Networks in the hippocampus, such as the dentate, CA3, or CA1, have a few hundred thousand to a few million cells depending on the species (reviewed in Johnston and Amaral5), only a small subset of which are firing under a particular condition. The subset that fires in a given temporal window is considered the ensemble that represents an item. It has been estimated that the properties of CA3 are such that it can store about 20 000 items using partially overlapping ensembles of 300 active neurons.6 These items can be selectively activated (recalled) because they are encoded in a distributed fashion by the synaptic weights in the network. But what if multiple items are to be recalled? One reason this might be needed is if the memory is actually a sequence of events. The hypothesized coding scheme for the representation of multiple items is acheived by the model of figure 2 in the following way. Each item is represented by the cells that fire during a given gamma cycle. The first item is represented by the cells that fire in the first gamma cycle; subsequent items in the sequence are stored in successive gamma cycles. In the sections that follow, we will review the now substantial evidence that such a coding scheme is utilized in the hippocampus.
Fig. 2.
Scheme of Theta-Gamma Discrete Phase Code. Two theta cycles are shown, each of which contains 7 gamma cycles. Different items (A–G) are represented by activity in different gamma cycles. The ovals above show the network activity that represents memory A during the first gamma cycle; note that a spatial code of active cells (black) is used to encode the memory. During the next gamma cycle, different cells in the same network are active, thereby encoding memory B. This entire pattern can repeat on the next theta cycle. Note that in this schematic, the theta modulation of gamma amplitude is not shown.
Relationship of cell firing to theta phase
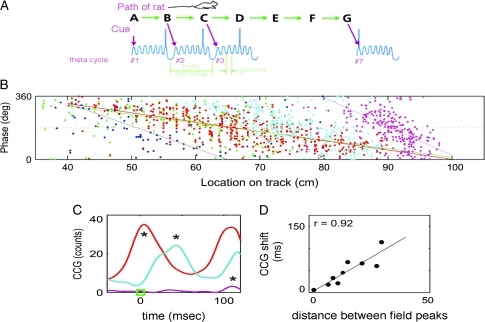
According to the scheme of figure 2, cells representing different items should fire within different gamma cycles of a theta cycle. Because the period (cycle) of theta is slower than the period of gamma, this is equivalent to stating that these ensembles fire at different phases of the theta cycle. The experiments of O'Keefe were the first to demonstrate that the hippocampus uses a coding scheme in which theta phase is an important variable.7 Single cells were monitored in awake, behaving rats. Particular cells in the hippocampus respond to particular regions of the environment termed the place field. As the rat traverses the place field (∼1 s), about 7 theta oscillations occur. What O'Keefe observed was that as the rat moved through the place field of a cell, the theta phase of spikes from that cell underwent a systematic change of phase (schematized in figure 3A). A cell tended to fire at late theta phase as the animal entered the place field, but on each successive theta cycle firing occurred with earlier phase. This remarkable finding (figure 3B) has been replicated in subsequent studies.8,9
Fig. 3.
Ensembles During the Phase Precession. (A) Schematic showing that firing occurs at earlier theta phase as the rat moves along a track and through the place field. (B) Different CA1 cells (in different colors) respond to different positions along the track. Dots represent spikes as a function of position (x-axis) and theta phase (y-axis); as the rat moves, each neuron systematically changes its preferred phase of spiking within the theta cycle (although at different rates). (C) Cross-correlogram (CCG) between different cell pairs made from data obtained on track. The CCG between the green and red cells peak at zero (red curve), indicating that the cells are part of the same ensemble (always both active at the same theta phase, despite the change in theta phase). In contrast, the green and light blue cells have a 45-ms phase shift (blue circle) and are thus part of different ensembles. (D) The millisecond shift in the CCG is directly proportional to the difference of place field centers. Thus, cells with the same place field fire in a correlated way with no temporal shift; cells with slightly different place fields fire in different ensembles that fire with a temporal shift within a theta cycle. Modified with permission from Dragoi and Buzsaki.9
Relationship of ensemble firing to theta phase
The development of closely spaced multielectrode systems and software to identify firing of individual cells (spike sorting algorithms) has made it possible to obtain simultaneous information about over 100 cells in a hippocampal region. It has therefore been possible to actually identify ensembles and to ask whether ensembles change their phase in a systematic way. Figure 3C and D provides evidence that this is the case.9 This work supports 3 conclusions:
There are cells that systematically fire within a few milliseconds of each other, even though they fire at varying theta phase as the rat traverses the place field; we consider such cells to be part of the same ensemble (figure 3B, red).
Other cell pairs fire systematically at a temporal separation that is significant fraction of the theta cycle, eg at 45 ms separation; these we consider to represent different ensembles (figure 3B blue)
The temporal separation of different ensembles is directly related to the spatial separation of their place fields (figure 3D).
Taken together, these results provide direct evidence for ensembles (cells that fire closely together in time) and show that theta phase coding is not just a single-cell phenomenon.
A second way of testing the importance of ensemble activity in theta phase coding is derived from more formal theories of neural coding. In this strategy, hypothetical codes are defined (eg, using theta phase or not) based on the correlation of cell firing with the rat's position. This is done for all the recorded cells using data obtained during the first half of an experiment. Then, the information content (ability to predict the rat's position) of the cell is tested using the second half of the experiment: specifically, the position of the rat is decoded from the spike trains of the cell population. The accuracy of the prediction can be determined by comparison to the actual position. This provides an objective way of comparing different coding strategies. It was found10,11 that a code which takes theta phase into consideration produces a dramatic increase in the accuracy with which the position of a rat can be predicted.
Relationship of cell firing to both theta phase and gamma phase
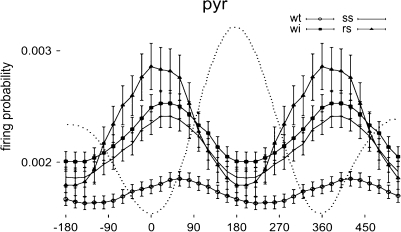
The fact that gamma and theta oscillations occur simultaneously in the field potential does not necessarily mean that the same cells are influenced by both oscillations. Recent experiments12–14 show, however, that this is the case. In the experiments by Senior and colleagues,13 place cells were recorded from the hippocampus, and the theta phase precession was verified. Field recordings were used to record the simultaneous gamma oscillations, and firing of the place cell was analyzed during each gamma cycle (figure 4). The results showed that on each gamma cycle, firing tended to occur at a preferred phase. As the animal progressed through the place field, the same cells showed theta phase precession. These findings demonstrate that firing of a cell is dependent on both theta and gamma and that the theta phase code is therefore discrete.
Fig. 4.
Gamma-modulated Firing of Rat Pyramidal Cell During Different States (wt, Waking Theta; wi, Waking Immobility; ss, Slow Wave Sleep; rs, REM Sleep). Dashed line is gamma waveform in field potential. The “waking” data are taken at a time that the theta phase precession was occurring, thus demonstrating that both theta and gamma oscillations influence the timing of firing. Modified from Senior, Huxter, Allen, et al.13
Function of theta/gamma in the recall of sequences from hippocampal long-term memory
There is significant experimental support for the idea that the phase precession reflects the cued recall of upcoming places in a known environment.10,15 According to this interpretation, in a given theta cycle, the current position is the cue. This cue is input to the hippocampus at the beginning of the theta cycle. Then, through asymmetric connections between ensembles, the ensemble that represents the forthcoming place becomes active. This occurs in the second gamma cycle (∼20 ms later). This process of one ensemble stimulating the next is called “chaining” and it continues throughout the subsequent gamma cycles of the theta cycle. Thus, what is being played out is a whole sequence of upcoming places in their actual order. Let us say that the ensemble active in the last gamma cycle represents a hole in the wall through which the rat can escape. Now let us consider what happens on the next theta cycle. Because the rat has been running and time has passed, the cue is now a subsequent position along its path. This cue will initiate a chaining process in the next theta cycle that is different than it was on the first; specifically, the ensemble representing the hole in the wall will now fire with earlier theta phase (on the second to last gamma cycle and thus with earlier theta phase). One can see from this example how a cued chaining process explains why the phase precession occurs as the rat runs through the environment. Importantly, in temporal sequences, not only neighboring items (first order chain) but also higher order (non-neighbor) information is represented,9 supporting the idea that such a mechanism could be involved in the storage of episodic memories which often involve complex sequences of events.
Several experiments have further tested this cued sequence recall model. If a rat runs on a running wheel, the environmental and body cues do not change over time.12 If the cue is constant, sequence recall should be the same on each theta cycle and the phase precession should therefore not be observed. Consistent with this prediction, recordings show that theta oscillations occur on the running wheel, but there is no phase precession. This is found under conditions where the animal has no immediate expectation of getting off the running wheel, but there are other conditions where phase precession can occur.
According to the cued sequence recall model, the firing on each theta cycle is dependent on the cue that arrives at the beginning of each theta cycle. An alternative model of the phase precession is that it depends only on a single cue (at the beginning of the place field) and that the firing on all subsequent theta cycles is dependent on intrahippocampal processes stimulated by that cue. According to this latter model, the phase precession should be irreversibly interrupted by briefly inhibiting the hippocampus during the period of the phase precession. It was found, however, that the phase precession resumes after brief inhibition.16 These results support a model in which the phase precession is due to an internal mechanism that is initiated at the beginning of each theta cycle by an external cue.
Some progress has been made at defining which synapses in the hippocampus store the different types of information required to produce the phase precession. One type of information is autoassociative, ie involves linkages between different cells in the same network that represent an item. The function of such linkages is to make the network robust against noise or partial information; if only a subset of cells representing are stimulated by the chaining process (see above), the cells that do fire perform a “pattern completion process” in which they trigger the remaining cells that represent the item. This process is thought to occur in the CA3 region because this region has the numerous recurrent connections needed to perform such pattern completion (for a review see Witter17). Furthermore, disruption of associative synaptic modification at recurrent synapses interferes with pattern completion as measured behaviorally.18 The localization of heterosynaptic linkages that underlie the chaining process (ie, where cells representing item n selectively synapse onto cells representing the n + 1 item) is less clear. Theoretical arguments suggest that these synapses are in feedback connections from CA3 and mossy cells onto granule cells.6
Recent work19 provides a fascinating view of different type of memory readout process that also appears to depend on theta and gamma. As the rat approaches a decision point in a T-maze, the rat initiates a process of remembering what is down the left or right arm. This is evident during particular theta cycles (with associated gamma) as the sequential activation of ensembles representing sequential positions along the left or right arm. Different theta cycles involve the readout of information about separate arms. This kind of look-ahead process provides a way of organizing intelligent behavior based on the recall of stored sequences.
Function of theta/gamma in multi-item short-term memory (7 ± 2)
As illustrated in figure 1, linked theta and gamma oscillations are not found only in the hippocampus and indeed can be recorded in sensory cortex (olfactory) (reviewed in Lisman20). This suggests that the theta-gamma coding scheme may subserve other types of information processes in addition to the recall from long-term memory described above. Indeed, the original formulation of the theta-gamma coding hypothesis4 was based on tantalizing similarities of theta-gamma organization to properties of short-term memory. Classic studies of short-term memory in humans have established the capacity limit of short-term memory as 7 ± 2. Subsequent work by Sternberg21 suggested that during recall from short-term memory, items are serially scanned at a rate of about 30 ms per item. These findings could be explained if short-term memory involved the representation of different memory items in sequential gamma cycles of a theta cycle. The capacity limit would arise because of the approximately 7 gamma cycles within a theta cycle. The scan rate of 30 ms would arise because this is approximately the temporal separation of gamma cycles.22
Several experimental tests support aspects of this model. Intracranial recordings were conducted during the Sternberg memory task and showed cortical regions where the theta oscillations were strongly linked to the requirements of short-term memory; the power strongly increased at the onset of working memory requirements and strongly decreased when memory was no longer required by the task.23 This work provides the clearest evidence to date for the involvement of theta oscillations in short-term memory. Single unit studies24 in cortical area V4 in monkeys provide important additional evidence. It was found that while the monkey held information in short-term memory, cells tended to fire at a particular phase of theta as measured by the local field potential. A critical additional prediction of the model of figure 2, that different short-term memories fire at different theta phase, is yet to be tested.
Comparison to other models of the role of gamma
Important initial work on the role of gamma oscillations in sensory cortex came from Wolf Singer's laboratory and has led to models somewhat different from the one described above. A key difference is the definition of an ensemble. As described above, an ensemble is the group of cells that fire during a gamma cycle. This implies that the exact timing of firing during a gamma cycle is not important. In contrast, models developed by Singer and Hopfield25,26 ascribe importance to small differences in gamma phase (ie, a few milliseconds). Thus, different postulated ensembles may fire at different phases of a gamma cycle.
Experiments in the hippocampus provide evidence that small differences in gamma phase may not be important in this structure. These experiments involved recordings from hippocampal place cells that have nearly the same position. It could then be analyzed whether the spiking of one cell could be best predicted from others using a very short temporal window (in the millisecond range) or using a longer window (in the 10–25 ms range).27 The longer window proved better, suggesting that ensemble lifetime is a substantial fraction of a gamma cycle. The physiological advantage of this temporal organization is that the ensemble lifetime is comparable to the membrane time constant (this defines the integration time of synaptic currents) of target neurons downstream to the ensemble. Consequently, neuronal coalitions brought about within this time scale are synergistic in discharging their postsynaptic target. A corollary to this definition of an ensemble is that the exact timing of cell firing during the gamma cycle is not important.
It remains to be determined how these different ideas about neural coding can be reconciled. The best evidence for the importance of timing within a gamma cycle comes from work on visual cortex, whereas the work against the importance of such precise timing comes from hippocampus. It is therefore possible that both types of coding occur, but in different brain regions.
Implications for mental disease
A deficit in gamma oscillations would be expected to lead to a deficit in the synchrony that is necessary to distinguish between sequential items. Indeed, an important recent article28 shows that relatively small changes in the excitatory inputs to fast-spiking interneurons reduces the amplitude of gamma oscillations and leads to a large reduction in the synchrony of pyramidal cell firing. Such a reduction could hinder the triggering of downstream neurons by individual items, an ability that presumably depends on coincidence detection mechanisms. The result could be that downstream cells might respond to the superposition of 2 or more items; this could be as confusing as the superposition of 2 conversations on a bad phone connection. Consistent with this suggestion, the mice with reduced gamma oscillations had deficits in memory performance. Along similar lines, it was recently demonstrated that CB1 (cannabinoid) receptor activation, which reduces transmitter release, leads to a reduction of theta and gamma power and to a robust loss of the synchronization that defines ensembles.29 Importantly, the magnitude of synchrony impairment was correlated with the degree of the deterioration of memory performance.
Deficits in theta would be expected to have a very different effect on brain processing. Gamma oscillations typically involve local mechanisms and the slower theta oscillation can temporally coordinate these local assemblies over a large volume of neuronal substrate. By the mechanisms of theta-gamma coupling, different phases of theta waves order gamma cycles, and the corresponding ensembles, into temporal sequences (which would repeat during working memory). Without theta, it would become impossible to assign order in such repeating sequences. Perhaps the best analogy is that absence of punctuation that marks would make it impossible to determine where sentences end and begin. Thus, abnormalities in theta might be expected to produce confusion in the order of thoughts or percepts, as can occur in schizophrenia.
These speculative ideas point out the fundamental importance of understanding the neural codes. The way in which information is organized and transmitted in the brain must depend on the formats provided by such coding mechanisms. The theta-gamma code discussed here now has considerable experimental support in the hippocampal region and may well be of more general importance. Abnormalities in such a fundamental aspect of neural processing are likely to produce deficits in cognitive processes.
Funding
National Institutes of Health Conte Center (P50 MH060450) to J.L.
References
- 1.Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004;304(5679):1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 2.Gonzalez-Burgos G, Lewis D. GABA neurons and the mechanisms of network oscillations: implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bull. 2008 doi: 10.1093/schbul/sbn070. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhlhaas P, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophrenia Bull. 2008 doi: 10.1093/schbul/sbn062. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lisman JE, Idiart MA. Storage of 7 +/- 2 short-term memories in oscillatory subcycles. Science. 1995;267(5203):1512–1515. doi: 10.1126/science.7878473. [DOI] [PubMed] [Google Scholar]
- 5.Johnston D, Amaral DG. Hippocampus. In: Shepherd GM, editor. The Synaptic Organization of the Brain. 4th ed. New York, NY: Oxford University Press, Inc.; 1998. pp. 417–458. [Google Scholar]
- 6.de Almeida L, Idiart M, Lisman JE. Memory retrieval time and memory capacity of the CA3 network: role of gamma frequency oscillations. Learn Mem. 2007;14(11):795–806. doi: 10.1101/lm.730207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Keefe J, Recce ML. Phase relationship between hippocampal place units and the EEG theta rhythm. Hippocampus. 1993;3(3):317–330. doi: 10.1002/hipo.450030307. [DOI] [PubMed] [Google Scholar]
- 8.Skaggs WE, McNaughton BL, Wilson MA, Barnes CA. Theta phase precession in hippocampal neuronal populations and the compression of temporal sequences. Hippocampus. 1996;6(2):149–172. doi: 10.1002/(SICI)1098-1063(1996)6:2<149::AID-HIPO6>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 9.Dragoi G, Buzsaki G. Temporal encoding of place sequences by hippocampal cell assemblies. Neuron. 2006;50(1):145–157. doi: 10.1016/j.neuron.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 10.Jensen O, Lisman JE. Hippocampal CA3 region predicts memory sequences: accounting for the phase precession of place cells. Learn Mem. 1996;3(2–3):279–287. doi: 10.1101/lm.3.2-3.279. [DOI] [PubMed] [Google Scholar]
- 11.Jensen O, Lisman JE. Position reconstruction from an ensemble of hippocampal place cells: contribution of theta phase coding. J Neurophysiol. 2000;83(5):2602–2609. doi: 10.1152/jn.2000.83.5.2602. [DOI] [PubMed] [Google Scholar]
- 12.Csicsvari J, Hirase H, Czurko A, Mamiya A, Buzsaki G. Fast network oscillations in the hippocampal CA1 region of the behaving rat. J Neurosci. 1999;19(16):RC20. doi: 10.1523/JNEUROSCI.19-16-j0001.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Senior TJ, Huxter JR, Allen K, O'Neill J, Csicsvari J. Gamma oscillatory firing reveals distinct populations of pyramidal cells in the CA1 region of the hippocampus. J Neurosci. 2008;28(9):2274–2286. doi: 10.1523/JNEUROSCI.4669-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hirase H, Czurko A, Csicsvari J, Buzsaki G. Firing rate and theta-phase coding by hippocampal pyramidal neurons during ‘space clamping’. Eur J Neurosci. 1999;11(12):4373–4380. doi: 10.1046/j.1460-9568.1999.00853.x. [DOI] [PubMed] [Google Scholar]
- 15.Tsodyks MV, Skaggs WE, Sejnowski TJ, McNaughton BL. Population dynamics and theta rhythm phase precession of hippocampal place cell firing: a spiking neuron model. Hippocampus. 1996;6(3):271–280. doi: 10.1002/(SICI)1098-1063(1996)6:3<271::AID-HIPO5>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.Zugaro MB, Monconduit L, Buzsaki G. Spike phase precession persists after transient intrahippocampal perturbation. Nat Neurosci. 2005;8(1):67–71. doi: 10.1038/nn1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Witter MP. Intrinsic and extrinsic wiring of CA3: indications for connectional heterogeneity. Learn Mem. 2007;14(11):705–713. doi: 10.1101/lm.725207. [DOI] [PubMed] [Google Scholar]
- 18.Nakazawa K, Quirk MC, Chitwood RA, et al. Requirement for hippocampal CA3 NMDA receptors in associative memory recall. Science. 2002;297(5579):211–218. doi: 10.1126/science.1071795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson A, Redish AD. Neural ensembles in CA3 transiently encode paths forward of the animal at a decision point. J Neurosci. 2007;27(45):12176–12189. doi: 10.1523/JNEUROSCI.3761-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lisman J. The theta/gamma discrete phase code occurring during the hippocampal phase precession may be a more general brain coding scheme. Hippocampus. 2005;15(7):913–922. doi: 10.1002/hipo.20121. [DOI] [PubMed] [Google Scholar]
- 21.Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- 22.Jensen O, Lisman JE. An oscillatory short-term memory buffer model can account for data on the Sternberg task. J Neurosci. 1998;18(24):10688–10699. doi: 10.1523/JNEUROSCI.18-24-10688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raghavachari S, Kahana MJ, Rizzuto DS, et al. Gating of human theta oscillations by a working memory task. J Neurosci. 2001;21(9):3175–3183. doi: 10.1523/JNEUROSCI.21-09-03175.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45(1):147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 25.Hopfield JJ. Pattern recognition computation using action potential timing for stimulus representation. Nature. 1995;376(6535):33–36. doi: 10.1038/376033a0. [DOI] [PubMed] [Google Scholar]
- 26.Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30(7):309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 27.Harris KD, Csicsvari J, Hirase H, Dragoi G, Buzsaki G. Organization of cell assemblies in the hippocampus. Nature. 2003;424(6948):552–556. doi: 10.1038/nature01834. [DOI] [PubMed] [Google Scholar]
- 28.Fuchs EC, Zivkovic AR, Cunningham MO, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53(4):591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 29.Robbe D, Montgomery SM, Thome A, Rueda-Orozco PE, McNaughton BL, Buzsaki G. Cannabinoids reveal importance of spike timing coordination in hippocampal function. Nat Neurosci. 2006;9(12):1526–1533. doi: 10.1038/nn1801. [DOI] [PubMed] [Google Scholar]
- 30.Soltesz I, Deschenes M. Low- and high-frequency membrane potential oscillations during theta activity in CA1 and CA3 pyramidal neurons of the rat hippocampus under ketamine-xylazine anesthesia. J Neurophysiol. 1993;70(1):97–116. doi: 10.1152/jn.1993.70.1.97. [DOI] [PubMed] [Google Scholar]
- 31.Bragin A, Jando G, Nadasdy Z, Hetke J, Wise K, Buzsaki G. Gamma (40–100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci. 1995;15(1 Pt 1):47–60. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chrobak JJ, Buzsaki G. Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci. 1998;18(1):388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Llinas R, Ribary U. Coherent 40-Hz oscillation characterizes dream state in humans. Proc Natl Acad Sci U S A. 1993;90(5):2078–2081. doi: 10.1073/pnas.90.5.2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolley DE, Timiras PS. Prepyriform electrical activity in the rat during high altitude exposure. Electroencephalogr Clin Neurophysiol. 1965;18:680–690. doi: 10.1016/0013-4694(65)90112-4. [DOI] [PubMed] [Google Scholar]