Abstract
Aldehyde modified proteins have been associated with the development and/or progression of alcoholic liver disease (ALD). These protein adducts are capable of initiating many immunological responses that are harmful to the normal homeostasis of organism function. Previous studies have shown that malondialdehyde (MDA) and acetaldehyde (AA) synergistically form a unique adduct (MAA) with soluble proteins, which are capable of inducing cytokine release, T-cell proliferation, and antibody production. The purpose of this study was to determine whether MAA-adduction can elicit similar responses to cells using a well-defined tumor model. The mouse mastocytoma P815 tumor cell line was modified with MAA (P815-MAA) or left unmodified (P815) and 106 irradiated cells were injected into DBA/2 mice once a week for 5 weeks. Serum was collected and tested for antibody responses to P815 cells and the MAA epitope. Immunization of MAA-adducted P815 cells into syngeneic DBA/2 mice induced a strong antibody response to the MAA epitope as determined by ELISA on Alb and MAA-Alb (508 µg/ml and 1092 µg/ml, respectively). In addition, antibody to unmodified P815 cells was detected by fluorescent technique. Mice immunized with P815 cells or PBS showed little or no reactivity to the MAA epitope or P815 cells. Studies to assess IL-12 stimulation showed that peritoneal macrophages from P815 and PBS immunized animals produced modest amounts of IL-12 (20 and 35 pg/ml) when stimulated with Alb or MAA-Alb. However, macrophage from P815-MAA immunized mice responded to soluble MAA-adduct (142 pg/ml). Finally, in tumor survival studies the mean survival was 14.25 days in PBS treated mice; 15.75 days with P815 immunized mice and 18.25 days with P815-MAA immunized mice. Therefore, these data strongly suggest that antibody responses are induced by P815 cells modified with MAA-adducts. This may be a possible tool to begin looking at how alcohol metabolites potentially modify cells and/or cellular components making them recognizable to the immune system as foreign. It is thought that these studies define a model system that will be useful in assessing antibody and potentially T cell responses to cells that are modified by MAA.
Keywords: P815 mastocytoma, aldehyde adducts, DBA/2 mice, tumor immunology
Introduction
A relationship between the immune system and alcoholic liver disease (ALD) has been reported by many investigators (1–5). For example, there is an influx of immune cells found in liver biopsies from alcoholic liver disease patients (1). Additionally, it has been shown that substantial abnormalities occur in the humoral and cellular immune responses of patients with liver disease associated with alcohol (6–10). While a number of different antigens have been involved in these immune responses, no one epitope has been identified as the true mediator of ALD.
Support for immune involvement in ALD is found in the detection of autoantibodies to various antigens found in the serum of chronic ethanol-fed rats or patients with alcohol induced liver injury. These autoantibodies include; CYP2E1 (11), alcohol dehydrogenase (ADH) (12) hydroxyl ethyl free radicals (13), acetaldehyde/malondialdehyde modified protein adducts (14–18), liver specific protein (LSP) (19, 20), and liver membrane protein (LMA) (21, 22), all of which have been shown to play a role in alcohol liver disease. The ability of these chemicals or proteins to modify cell surface membranes or cellular material under the right conditions could help break tolerance and initiate an autoimmune response. Circulating autoantibodies specific for liver proteins or antibodies to adducts on proteins could trigger antibody-dependent cell medicated cytotoxcity, killing or severely damaging hepatocytes, and causing increased inflammation and eventually liver failure. This has been demonstrated using hydroxyl ethyl radical-CYP2E1 adducts on the surface of isolated hepatocytes exposed to ethanol in the presence of normal human peripheral blood mononuclear cells (23–26). Clearly, the sustained ingestion of alcohol in most people appears result in the development of an array of auto-antibodies to cellular proteins potentially initiating a T-cytotoxic response to hepatocytes (23, 27, 28).
The finding of circulating antibodies specific for alcohol metabolites indicates there maybe a cellular component involved. A report by Terabayashi; et al. supports the concept that cytotoxic T lymphocytes can be generated that recognize syngeneic cells modified with acetaldehyde (28). In studies consistent with findings reported in this manuscript, malondialdehyde-acetaldehyde modified proteins have been shown to elicit T-cell proliferative responses through antigen processing and presentation by macrophages and dendritic cells (29). Therefore, if these cellular proteins are available for aldehyde binding and the right conditions are met to produce these adducts, the potential for modification of self proteins or cells exists. These self protein adducts could potentially induce an autoimmune disease mediated by antibodies, CD4+ T-cells, antigen presenting cells, and/or by antibody-dependent cell mediated cytotoxicity.
Current work in this laboratory has shown that the in vivo metabolism of alcohol generates the reaction of malondialdehyde (MDA) and acetaldehyde (AA) to form a unique adduct, that has been designated as MAA (18). These MAA-adducts have been shown to elicit antibody and T-cell response in the absence of adjuvants (29, 30). However, these studies have all utilized soluble proteins and no data is available with regard to the immune response to MAA-modified cells. Therefore, the demonstration that MAA-modified cells can induce T-cell responses would suggest a possible mechanism for alcohol-induced liver damage. Therefore, it was the purpose of this study to determine if MAA-adduction of tumor cells can elicit an immune response that may serve as a model system of MHC class I restricted immune response in ALD.
Materials and Methods
Mice
Syngeneic DBA/2 mice were obtained from the National Cancer Institute via an interagency agreement with the Department of Veterans Affairs and maintained on water and laboratory chow ad libitum. The mice were monitored and determined to be pathogen-free. All procedures were approved by the animal subcommittee of the Omaha VA Medical Center (accredited by American Association for the Accreditation of Laboratory Animal AAALAC) and were in accordance with guidelines of the National Institutes of Health (1985).
Cell line
The DBA/2 P815 mastocytoma cell line was purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in RPMI 1640 medium supplemented with 10% fetal calf, 25 mM HEPES, 2 mM L-glutamine, gentamycin (100 U/ml), and incubated at 37°C in the presences of 5% CO2.
Chemicals and Proteins
Bovine serum albumin (Alb) was purchased from Cal Biochem (La Jolla, CA). Acetaldehyde (AA) was obtained from Aldrich Chemical Co. (Milwaukee, WI). Malondialdehyde (MDA) was obtained as the sodium salt (MDA~Na) by treatment of tetramethoxypropane (Aldrich Chemical Co.) with NaOH, according to the method of Kikugawa and Ido (31). Phytic acid (PA), and diethylenetriaminepentaacetic acid (DTPA) were obtained from Sigma Chemical Co. (St. Louis, MO).
Preparation and Labeling of Ligands and P815 Cells
Bovine serum albumin-MAA (MAA-Alb) was prepared by reacting 1.0 mM AA and 2.0 mM MDA with 2 mg of bovine serum albumin (Alb) in 0.1 M phosphate buffer pH 7.2, containing 2 mM DTPA and 2mM PA at 37°C for 3 days, followed by dialysis against 3 changes of 0.1 M sodium phosphate buffer for 24 hours at 4°C (18). P815 cells were modified by adjusting to 1 × 106 cells/ml and reacting with 1.0 mM AA and 1.0 mM MDA in 0.1 M phosphate buffer pH 7.2 containing 2 mM DTPA and 2 mM PA and incubated for 3 days at 37°C. Cells were checked for modification by the amount of fluorescent MAA adduct present (excitation 398 nm and emission 460 nm) using a Perkin-Elmer (Norwalk, CT) LS-5B spectrofluorometer as previously described by Tuma et. al. (18).
Immunization of DBA/2 mice
Prior to injection P815 or P815 AA and MDA adducted (P815-MAA) cells at a concentration of 1 × 106 cells/ml were irradiated with 2000 Rads to guarantee that tumor cells would not proliferate and kill the animal prior to challenging with lethal cells. Mice were immunized intra peritoneal once a week for 5 weeks with 1 × 106 P815, P815-MAA, or phosphate buffered saline (PBS) control irradiated cells. At the end of 6 weeks the mice were bled and infused via the tail vein with a lethal dose of P815 cells which had been passaged through the ascites of a normal DBA/2 mouse.
Lethal Dose Survival Curve
In order to determine the appropriate amount of lethal P815 cells to inject into DBA/2 mice that would elicit tumor growth, a dose response of varying concentrations of cells was done. To make the P815 cell line virulent, cells had to be injected into the peritoneal cavity of a DBA/2 mouse and the ascites fluid drawn out after approximately a week of growth. These cells were counted and doses ranging from 102 to 106 were injected into animals, which were monitored for days of survival. A liner dose curve was generated and 105 cells were used as this would give us killing at around 14–15 days.
Assay for detection of antibody to the MAA-adduct
Sera from mice injected with PBS, irradiated P815 cells, or irradiated P815-MAA adducted cells were screened by a direct ELISA for the presence of the MAA epitope. For these experiments, 96 well Immulon IV (Nunc, Fisher Scientific, St. Louis, MO) microtiter plates were coated with 20 µg/well Alb or MAA-Alb in the presence of bicarbonate buffer (pH 9.6) as previously described (32). After an overnight incubation at 37°C, the plates were washed three times with phosphate buffer saline containing 0.05% Tween 20 (PBST), the serum added at a 1:50 dilution, and incubated at 37°C for 45 minutes. Plates were washed in PBST and a secondary antibody (alkaline phosphatase rabbit anti-mouse IgG (H&L); Zymed Laboratories, San Francisco, CA), was added and incubated at 37°C for 45 minutes. Previous work has demonstrated that antibodies to MAA-modified proteins are IgG, therefore no other isotypes were assayed (30). The plates were then washed and the substrate p-nitrophenyl phosphate (Sigma Chemical Co) was added. Color changes were monitored by a Dynatech MicroELISA Reader MR 7000 (Dynatech, Chantilly, VA) at 410 nm. Standard curves were established using known concentrations of mouse IgG (Sigma Chemical Co.), and the concentrations of unknown samples were extrapolated by using the BioLinx computer program (Dynatech, Chantilly, VA). The means +/− SE of the relative concentrations of antibody from individual mice assayed in duplicated are reported in micrograms per milliliter.
Assay for detection of antibody to the P815 Cells
P815 cells were plated into a 24 well plate and allowed to grow confluent for 3 days. Serum from mice injected with PBS, P815 cells, or P815-MAA were incubated with the cells at a 1:50 dilution, and incubated at 4°C overnight. The plate was washed 3 times with Hanks Balanced Salt Solution (HBSS) containing 2% BSA 100 µl of FITC Rb × Ms IgG antibody (Zymed Laboratories, San Francisco, CA) was added and the plate incubated for 45 minutes at 4°C. The plate was washed 3 times with HBSS and fluorescent binding to the cells evaluated using a Flourolite spectrophotometer (Dynatech, Chantilly, VA). The values are expressed as relative fluorescence following subtraction from the secondary antibody. As a positive control, to determine assay function, a FITC Rb × Ms Class I H2kd antibody (BD Biosciences, San Jose, CA) was incubated with the P815 cells as these cells constitutively express MHC Class I.
IL-12 Production by Peritoneal Macrophages
DBA/2 mice immunized with PBS, P815 cells, or P815-MAA were injected with one milliliter of 3% Brewer’s thioglycollate medium intra peritoneal (33). Mice were killed 7 days later by cervical dislocation, 10 ml of DMEM-10 was injected intra peritoneal, and peritoneal fluid was withdrawn and placed in sterile 15 ml siliconized tubes (Becton Dickinson Vacutainer System, Franklin Lakes, NJ). Cells were placed in 24 well culture plates and exposed to 25 µg/ml of Alb or MAA-Alb at 37°C for 2, 4, 6, and 24 hours. A commercially available mouse IL-12 cytokine kit form (BD Biosciences, San Jose, CA) was used to look for IL-12 production in the supernatant.
Lethal P815 Dose
In order to determine the minimum concentration of viable P815 cells that resulted in 100% mortality in DBA/2 mice, a dose response assay was done. P815 cells were first passaged through the peritoneal cavity of a DBA/2 mouse. The mouse was watched for the presence of ascites. After a week of incubation the cells were removed, counted, and normal DBA/2 mice were injected via the tail vein with various doses of the viable (non-irradiated) P815 cells. Mice were watched daily and the time of death recorded. A survival curve was used to determine the proper dose with which to challenge immunized mice.
P815 Challenge after MAA Injections
After 6 weeks of PBS, P815, or P815-MAA injections the mice were challenged with a lethal dose via the tail vein of P815 cells at a concentration of 1 × 105 cells. Mice were watched daily and checked for survival. A survival curve was generated by plotting the number of days following the injection with the tumor by the percent of mouse survival after the challenge.
Statistical analysis
Results were expressed as means +/− SEM. Statistical significance was achieved if P values were less than 0.05. All statistical analysis was performed using the Students T-test in SigmaStat (Jandel Scientific, 2002).
Results
Antibody to the MAA Epitope
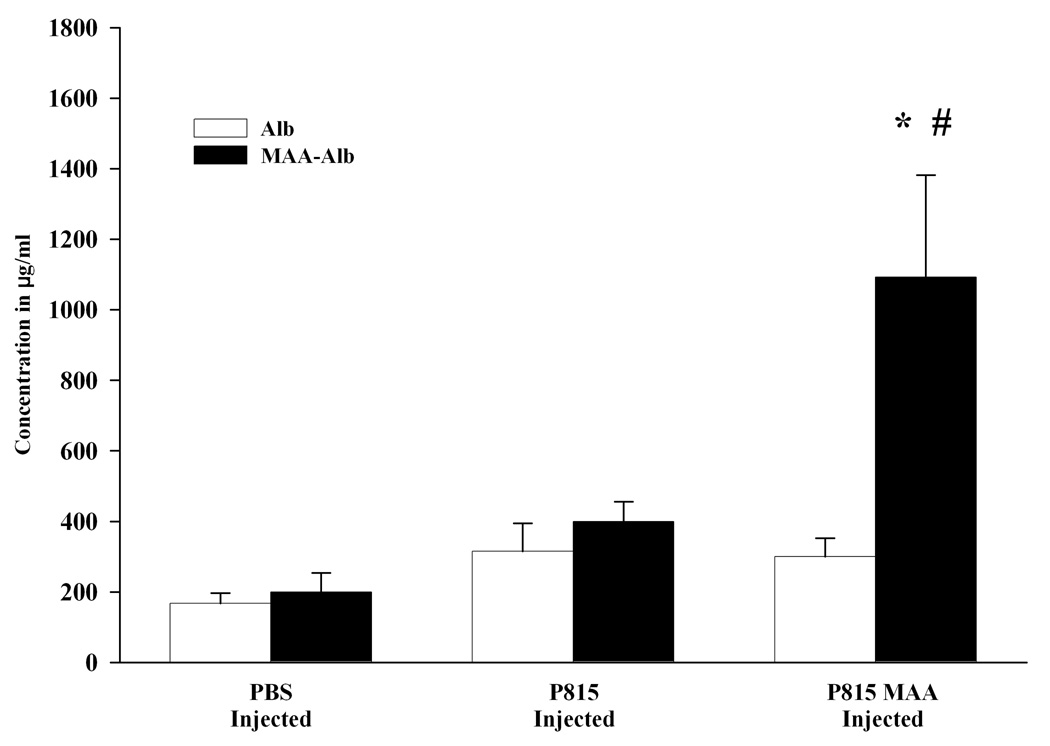
To begin assessing the antibody response to the MAA epitope, sera from mice injected with PBS, P815 or P815-MAA were screened for antibody to MAA or the carrier cell proteins. As shown in Figure 1, PBS injected animals had background levels of antibody to the Alb or MAA-Alb adduct (167 and 200 µg/ml respectively). These numbers increased (315 Alb and 508 µg/ml MAA-Alb) when serum from P815 irradiated cells were screened on Alb or MAA-Alb. However, P815-MAA injection increased the antibody to the MAA-Alb epitope 2 fold over P815 irradiated cells (300 Alb and 1092 MAA-Alb µg/ml). Immunizations of 1mM AA-modified or 1mM MDA-modified cells in the absence of adjuvant were looked and demonstrated background levels of antibodies to the cells or the AA or MDA adduct (data not shown). These data demonstrate that antibody to the MAA adduct is present in these animals.
Figure 1.
Antibody activity to the MAA epitope in DBA/2 mice immunized with PBS, P815 or P815-MAA modified cells as assayed on Alb or MAA-Alb. Serum from DBA/2 mice injected with P815-MAA modified cells contained a 2 fold increase in MAA antibody when compared to the DBA/2 mice injected with P815 cells. Mice injected with PBS had only background antibody production to Alb or MAA-Alb. Each bar is the mean SE of 5 mice. * P< 0.05, significantly different from PBS and P815 injected animals when sera was screened on MAA-Alb. # P < 0.05 significantly different from PBS and P815 injected animals when screened on Alb.
Antibody to P815 Cells
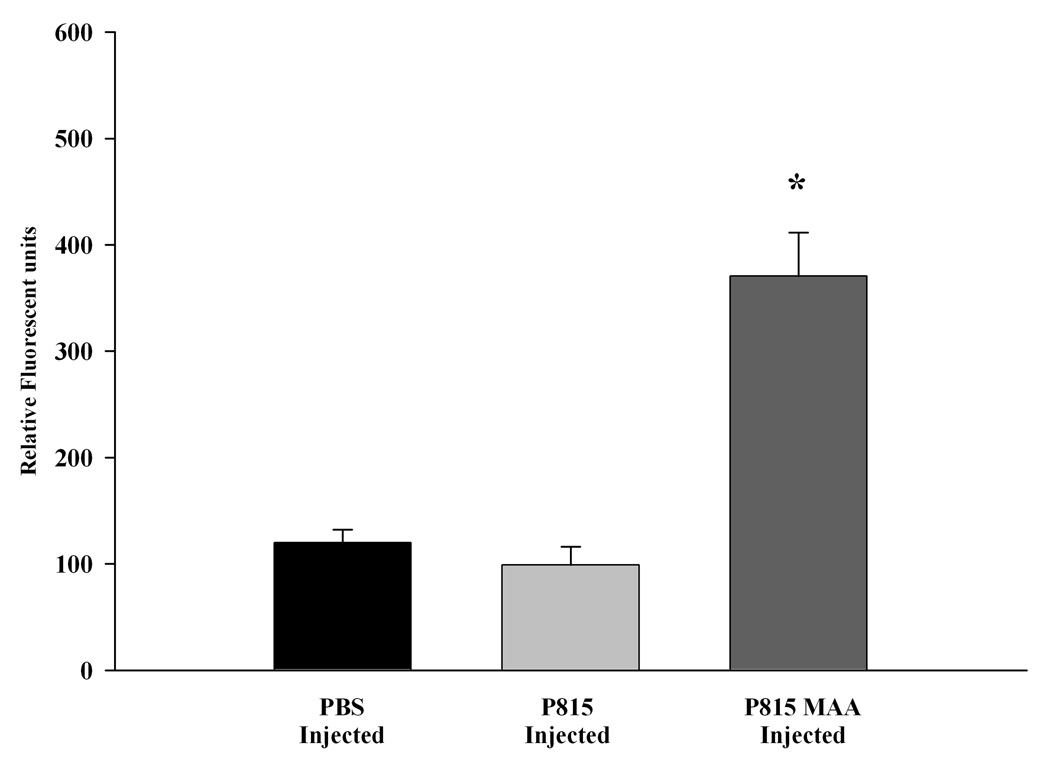
To determine if P815-MAA injection induced antibodies against the P815 cells, a fluorescent assay was developed as described in materials and methods. For these studies, serum from mice immunized with PBS, P815 irradiated or P815-MAA irradiated cells were incubated with P815 cells and detected by immunofluorescence. Figure 2 shows only modest increases in antibody to P815 cells in serum from PBS or P815 irradiated and injected animals (120 and 99 relative fluorescent units respectfully). Serum from animals immunized with P815-MAA irradiated cells showed an increased activity to the P815 cells 3–4 fold (371 relative fluorescent units) as compared to the controls.
Figure 2.
Antibody activity to P815 cells in DBA/2 mice immunized with PBS, P815 or P815-MAA modified cells. Serum from DBA/2 mice was incubated with P815 cells and the antibody activity determined. Mice injected with P815-MAA had a 3 fold increase in antibody activity to the P815 cells, while the mice injected with P815 cells or PBS had background levels of approximately 100 relative fluorescent units. Each bar is the mean SE of 5 mice. * P < 0.001, significantly different from PBS and P815 injected animals.
Peritoneal Macrophage IL-12 Production
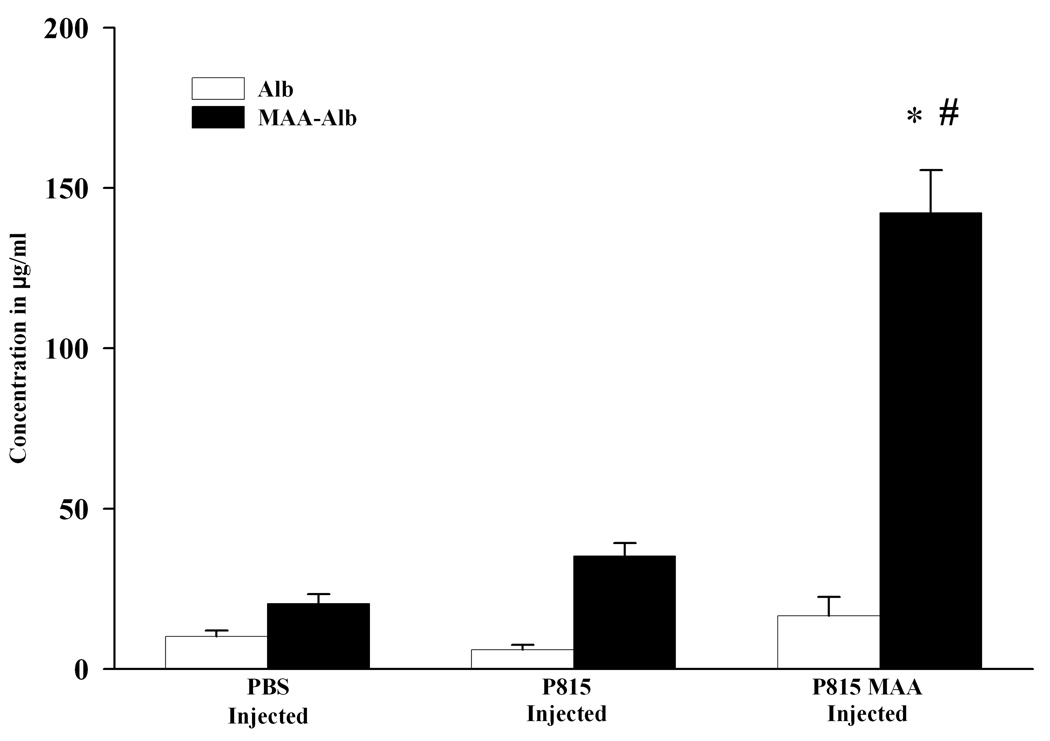
To determine whether macrophage sensitization, by the MAA adduct occurs, peritoneal macrophage were isolated from PBS, P815 irradiated or P815-MAA irradiated and immunized animals, stimulated with Alb or MAA-Alb (in vitro), and assayed for IL-12 release. As shown in Figure 3 animals immunized with PBS or P815 irradiated cells showed small amounts of IL-12 production (20 and 35 ng/ml respectfully). P815-MAA injected animals increase IL-12 release 3 fold over the controls (142 ng/ml), and stimulation by Alb had only minimal effects on the production of IL-12. These data demonstrate that macrophages from P815-MAA injected mice are sensitized to the MAA adduct.
Figure 3.
IL-12 release by peritoneal macrophages presensitized with P815-MAA cells. Peritoneal macrophage from DBA/2 mice injected with PBS, P815 or P815-MAA modified cells were incubated in the presence of (25 µg/ml) Alb or MAA-Alb for 3 hours. Supernatant was assayed for IL-12 production. P815-MAA injected animals showed a 3 fold increase in IL-12 over the PBS or P815 injected animals. Each bar is the mean SE of 5 mice. * P < 0.001, significantly different from PBS and P815 injected animals when MAA-Alb was used as the antigen. # P < 0.001 significantly different from PBS or P815 injected animals when Alb was used as the antigen.
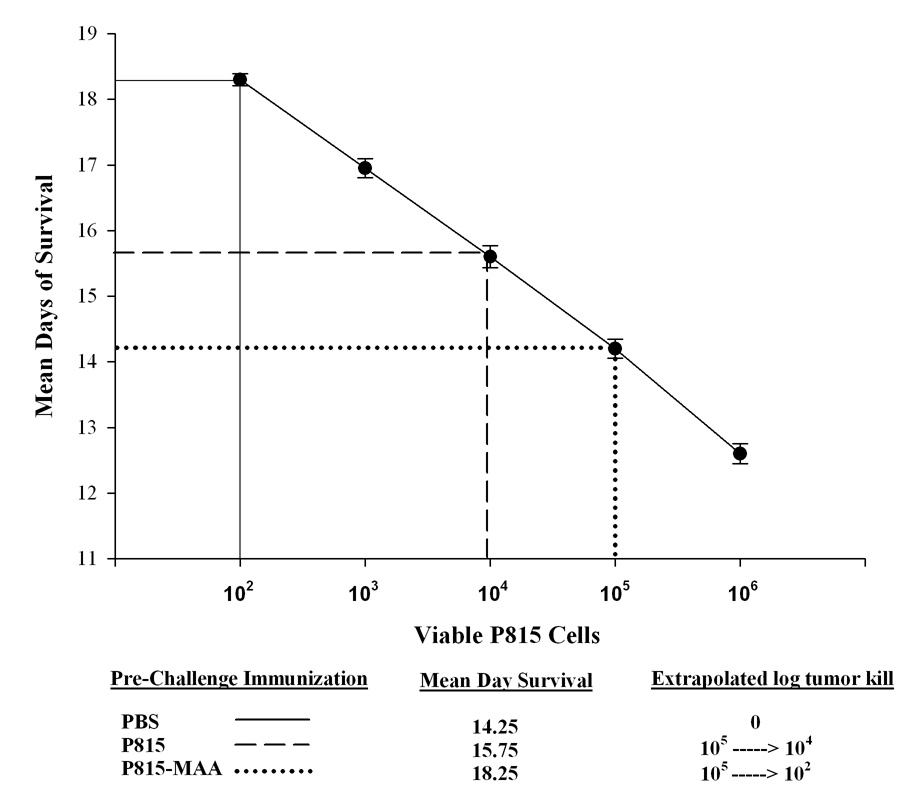
Survival of DBA/2 mice after P815-MAA Injection
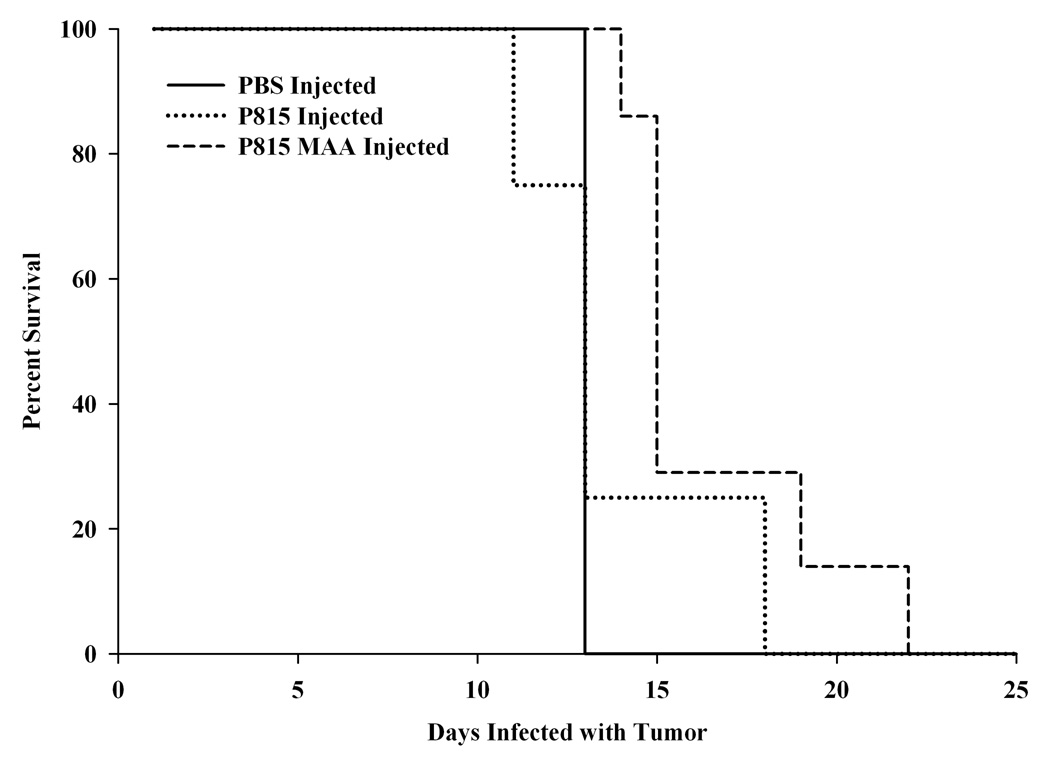
After 5 weeks of injection with PBS and P815 or P815-MAA irradiated cells, the mice were injected via the tail vein with 105 lethal P815 cells. The mice were monitored and counted daily for survival. As shown in Figure 4, 100% of the mice preimmunized with PBS survived only 14 days post tumor injection. Mice preimmunized with P815 irradiated cells after 14 days showed only a 20% survival and at 18 days all had died. However, P815-MAA preimmunized animal survival was increased by 4 days. Figure 5 demonstrates the tumor log kill of P815 cells following a challenge with 105 P815 viable cells. These data show that after a lethal dose of P815 cells the PBS preimmunized animals had a mean survival of 14.25 days and the immune system only killed 102 tumor cells prior to death. Mice preimmunized with P815 irradiated cells had a mean survival of 15.75 days and approximately 104 tumor cells were killed prior to death. DBA/2 mice that were preimmunized with P815-MAA had a mean survival of 18.25 days and the immune system eradicated 105 tumor cells prior to death. These data demonstrate that P815-MAA immunized animals survive longer and are capable of clearing a larger number of tumor cells prior to death of the animal.
Figure 4.
Survival of mice immunized with PBS, P815 or P815-MAA cells modified following the administration of a lethal dose of P815 cells. Mice immunized with PBS lived 14 days, while P815 injected animals lived 14–18 days. However, mice immunized with P815-MAA modified cells lived 4 days longer than the PBS of P815 injected animals. Each point is the mean SE of 6 mice.
Figure 5.
Survival of DBA/2 mice challenged with P815 cells. Data were extrapolated and expressed to show the concentration of cells needed to kill mice. Briefly, mice immunized with PBS and P815 cells require 102 and 104 cells respectfully in order to induce tumor and death. However, mice pre-immunized with P815-MAA cells required 105 cells to induce tumor and death. Each point is the mean SE of 6 mice.
Discussion
It has been suggested that immune components are associated with the development and/or progression of ALD. Previous studies performed by our laboratory have demonstrated that Malondialdehyde-Acetaldehyde (MAA) modified proteins are formed under physiological conditions (16) and are immunogenic in the absence of adjuvant (30). However, the cellular mechanism(s) by which tissue damage is accomplished is unclear. In an effort to begin examining these cellular mechanisms, which are most likely initiated during the killing of hepatocytes modified with MAA (34, 35), experiments were designed using the P815 model of tumor rejection. Therefore, it was the purpose of these experiments to determine if MAA-modified P815 tumor cells could be used as a model system for examining the cytotoxicity of hepatocytes that has been observed in ALD.
P815 cells modified with MAA and injected into DBA/2 mice, generated serum antibody titers to the MAA epitope that was 2 fold higher over mice injected with only irradiated cells. These experiments demonstrated that antibody against MAA epitopes were produced in these animals. Importantly, it was also possible to show that antibody to P815 cells was present in the mouse sera, and was increased 4 fold in serum from P815-MAA immunized mice as compared to sera from P815 or the PBS immunized control mice. Thus, the antibody response was not only to the adduct, but to the cellular material. Should antibody be involved through complement activation or antibody dependent cellular cytotoxicity, then the response to the cell and not just the adduct would lend greater specificity to the tissue damage.
The cytokine IL-12 has long been known to play a role in both the innate and adaptive immune response to tumor cells by inducing macrophages, CD8+ T cells, and secondary cytokines that destroy tumor cells (36, 37). Previous studies performed by our laboratory have shown that MAA-adducted proteins bind to scavenger receptors on peritoneal macrophages, sinusoidal liver endothelial cells, and Kupffer cells (29, 38, 39). It was also shown that macrophages could release pro-inflammatory cytokines in response to MAA-modified proteins (39). In this present study peritoneal macrophages were isolated from DBA/2 mice that had been immunized with PBS, P815 irradiated cells or P815-MAA adducted and irradiated cells. The cells were then incubated with Alb or MAA-Alb and the supernatant was assayed for the release of IL-12, and showed there was a 3 fold increase in IL-12 production over the Alb control. It is tempting to speculate that a Th1 type of immune response has been elicited to activate macrophages and Th1 cytokine responses.
The ability of these mice to generate antibody to both the MAA adduct and macromolecules on the P815 cells, in conjunction with the production of IL-12 by peritoneal macrophages, provides a possible mechanism by which immune responses to cells of the liver (hepatocytes) may be initiated in ALD. For these studies P815 cells modified with MAA were injected into DBA/2 mice as described, and a lethal dose of P815 cells was intravenously injected. Mice injected with PBS or P815 irradiated cells only, lived for 14 and 15 days respectfully after infusion of lethal doses of P815 cells. However, mice immunized with P815 MAA modified cells and infused with P815 cells lived for 18 days, increasing their survival by 3 to 4 days. When mean log tumor kill was assessed, P815-MAA injected animals killed more tumor cells than either the PBS or P815 irradiated cells. These data strongly suggest that an immune response to the tumor cell proteins was generated in response to the MAA-modified P815 cells. If these were MAA modified liver cells, then the target organ would be the liver, and cell death could occur in a similar fashion as the P815 cells.
Data generated from these studies show that modification of tumor cells with malondialdehyde and acetaldehyde (MAA) causes significant immune responses in the absence of adjuvant. These MAA adducts have already been detected in both human (16) and rat livers (40) chronically consuming ethanol. It has been suggested that if these adducts are formed during chronic inflammation (alcohol and oxidative stress) and modify cells of the liver, harmful results may occur. If these modified self proteins from the liver are processed by dendritic cells with the right co-stimulatory molecules (B7-1/B7-2) (41), it may be possible to generate T helper, T-cytotoxic, and antibody to the self proteins, potentially initiating damage to the liver cells similar to that observed using the P815 model used in this study. Importantly, this may serve as a model system to investigate NK and T-cytotoxic cell death in the context of ALD due to the metabolites of chronic ethanol consumption.
In summary, when DBA/2 mice are immunized with P815 MAA-modified cell lysates, an immune response to both the P815 cells and the MAA epitope are observed. Peritoneal macrophages from these animals are pre-sensitized to MAA and release IL-12 in response to MAA stimulations. When DBA/2 mice, pre-immunized with P815 MAA modified cells are challenged with lethal tumor cells, an increased tumor kill in vivo is observed, which serves as a model of tissue specific cellular cytotoxicity. More work is needed to be performed to find the underlying mechanisms by which MAA induces these responses.
Acknowledgments
Supported by: National Institutes of Health Grants: R01 AA10435 and R37 AA07818. Also supported by a Department of Veterans Affairs Merit Review and The Department of Veterans Affairs Alcohol Research Center at the Omaha VA Medical Center.
Abbreviations used in this paper
- MDA
Malondialdehyde
- AA
Acetaldehyde
- IL-12
Interleukin Twelve
- Alb
Bovine Serum Albumin
- PBS
Phosphate Buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All work was performed at the Veterans Administration Alcohol Research Center, Omaha Veterans Administration Medical Center, 4101 Woolworth Avenue, Omaha, NE 68105.
References
- 1.Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22(9):1927–1942. [PubMed] [Google Scholar]
- 2.Deviere J. Immune mechanisms in alcoholic liver disease. Acta Gastroenterol Belg. 1992;55(5–6):450–456. [PubMed] [Google Scholar]
- 3.Duryee MJ, Willis MS, Freeman TL, Kuszynski CA, Tuma DJ, Klassen LW, et al. Mechanisms of alcohol liver damage: aldehydes, scavenger receptors, and autoimmunity. Front Biosci. 2004;9:3145–3155. doi: 10.2741/1467. [DOI] [PubMed] [Google Scholar]
- 4.Paronetto F. Immunologic reactions in alcoholic liver disease. Semin Liver Dis. 1993;13(2):183–195. doi: 10.1055/s-2007-1007348. [DOI] [PubMed] [Google Scholar]
- 5.Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis. 2004;24(3):273–287. doi: 10.1055/s-2004-832940. [DOI] [PubMed] [Google Scholar]
- 6.Si L, Whiteside TL, Schade RR, Van Thiel D. Lymphocyte subsets studied with monoclonal antibodies in liver tissues of patients with alcoholic liver disease. Alcohol Clin Exp Res. 1983;7(4):431–435. doi: 10.1111/j.1530-0277.1983.tb05501.x. [DOI] [PubMed] [Google Scholar]
- 7.Si L, Whiteside TL, Van Thiel DH, Rabin BS. Lymphocyte subpopulations at the site of "piecemeal" necrosis in end stage chronic liver diseases and rejecting liver allografts in cyclosporine-treated patients. Lab Invest. 1984;50(3):341–347. [PubMed] [Google Scholar]
- 8.Poralla T, Hutteroth TH, Meyer zum, Buschenfelde KH. Cellular cytotoxicity against autologous hepatocytes in alcoholic liver disease. Liver. 1984;4(2):117–121. doi: 10.1111/j.1600-0676.1984.tb00915.x. [DOI] [PubMed] [Google Scholar]
- 9.Izumi N, Hasumura Y, Takeuchi J. Lymphocyte cytotoxicity for autologous human hepatocytes in alcoholic liver disease. Clin Exp Immunol. 1983;54(1):219–224. [PMC free article] [PubMed] [Google Scholar]
- 10.Cochrane AM, Moussouros A, Portmann B, McFarlane IG, Thomson AD, Eddleston, et al. Lymphocyte cytotoxicity for isolated hepatocytes in alcoholic liver disease. Gastroenterology. 1977;72(5 Pt 1):918–923. [PubMed] [Google Scholar]
- 11.Lytton SD, Helander A, Zhang-Gouillon ZQ, Stokkeland K, Bordone R, Arico S, et al. Autoantibodies against cytochromes P-4502E1 and P-4503A in alcoholics. Mol Pharmacol. 1999;55(2):223–233. doi: 10.1124/mol.55.2.223. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Meregalli M, Hodges S, Davies N, Bogdanos DP, Fargion S, et al. Alcohol dehydrogenase: an autoantibody target in patients with alcoholic liver disease. Int J Immunopathol Pharmacol. 2005;18(1):173–182. doi: 10.1177/039463200501800118. [DOI] [PubMed] [Google Scholar]
- 13.Niemela O. Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radic Biol Med. 2001;31(12):1533–1538. doi: 10.1016/s0891-5849(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 14.Niemela O, Parkkila S, Pasanen M, Iimuro Y, Bradford B, Thurman RG. Early alcoholic liver injury: formation of protein adducts with acetaldehyde and lipid peroxidation products, and expression of CYP2E1 and CYP3A. Alcohol Clin Exp Res. 1998;22(9):2118–2124. doi: 10.1111/j.1530-0277.1998.tb05925.x. [DOI] [PubMed] [Google Scholar]
- 15.Paradis V, Scoazec JY, Kollinger M, Holstege A, Moreau A, Feldmann G, et al. Cellular and subcellular localization of acetaldehyde-protein adducts in liver biopsies from alcoholic patients. J Histochem Cytochem. 1996;44(9):1051–1057. doi: 10.1177/44.9.8773571. [DOI] [PubMed] [Google Scholar]
- 16.Rolla R, Vay D, Mottaran E, Parodi M, Traverso N, Arico S, et al. Detection of circulating antibodies against malondialdehyde-acetaldehyde adducts in patients with alcohol-induced liver disease. Hepatology. 2000;31(4):878–884. doi: 10.1053/he.2000.5373. [DOI] [PubMed] [Google Scholar]
- 17.Viitala K, Makkonen K, Israel Y, Lehtimaki T, Jaakkola O, Koivula T, et al. Autoimmune responses against oxidant stress and acetaldehyde-derived epitopes in human alcohol consumers. Alcohol Clin Exp Res. 2000;24(7):1103–1109. [PubMed] [Google Scholar]
- 18.Tuma DJ, Thiele GM, Xu D, Klassen LW, Sorrell MF. Acetaldehyde and malondialdehyde react together to generate distinct protein adducts in the liver during long-term ethanol administration. Hepatology. 1996;23(4):872–880. doi: 10.1002/hep.510230431. [DOI] [PubMed] [Google Scholar]
- 19.McFarlane IG, Wojcicka BM, Williams R. Antigens of the human liver. Clin Exp Immunol. 1980;40(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- 20.Behrens UJ, Vernace S, Paronetto F. Studies on "liver-specific" antigens. II. Detection of serum antibodies to liver and kidney cell membrane antigens in patients with chronic liver disease. Gastroenterology. 1979;77(5):1053–1061. [PubMed] [Google Scholar]
- 21.Manns M, Meyer zum, Buschenfelde KH, Hess G. Autoantibodies against liver-specific membrane lipoprotein in acute and chronic liver diseases: studies on organ-, species-, and disease-specificity. Gut. 1980;21(11):955–961. doi: 10.1136/gut.21.11.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perperas A, Tsantoulas D, Portmann B, Eddleston AL, Williams R. Autoimmunity to a liver membrane lipoprotein and liver damage in alcoholic liver disease. Gut. 1981;22(2):149–152. doi: 10.1136/gut.22.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clot P, Parola M, Bellomo G, Dianzani U, Carini R, Tabone M, et al. Plasma membrane hydroxyethyl radical adducts cause antibody-dependent cytotoxicity in rat hepatocytes exposed to alcohol. Gastroenterology. 1997;113(1):265–276. doi: 10.1016/s0016-5085(97)70104-5. [DOI] [PubMed] [Google Scholar]
- 24.Loeper J, Descatoire V, Maurice M, Beaune P, Feldmann G, Larrey D, et al. Presence of functional cytochrome P-450 on isolated rat hepatocyte plasma membrane. Hepatology. 1990;11(5):850–858. doi: 10.1002/hep.1840110521. [DOI] [PubMed] [Google Scholar]
- 25.Loeper J, Descatoire V, Maurice M, Beaune P, Belghiti J, Houssin D, et al. Cytochromes P-450 in human hepatocyte plasma membrane: recognition by several autoantibodies. Gastroenterology. 1993;104(1):203–216. doi: 10.1016/0016-5085(93)90853-5. [DOI] [PubMed] [Google Scholar]
- 26.Wu D, Cederbaum AI. Presence of functionally active cytochrome P-450IIE1 in the plasma membrane of rat hepatocytes. Hepatology. 1992;15(3):515–524. doi: 10.1002/hep.1840150326. [DOI] [PubMed] [Google Scholar]
- 27.Clot P, Bellomo G, Tabone M, Arico S, Albano E. Detection of antibodies against proteins modified by hydroxyethyl free radicals in patients with alcoholic cirrhosis. Gastroenterology. 1995;108(1):201–207. doi: 10.1016/0016-5085(95)90025-x. [DOI] [PubMed] [Google Scholar]
- 28.Terabayashi H, Kolber MA. The generation of cytotoxic T lymphocytes against acetaldehyde-modified syngeneic cells. Alcohol Clin Exp Res. 1990;14(6):893–899. doi: 10.1111/j.1530-0277.1990.tb01833.x. [DOI] [PubMed] [Google Scholar]
- 29.Willis MS, Thiele GM, Tuma DJ, Klassen LW. T cell proliferative responses to malondialdehyde-acetaldehyde haptenated protein are scavenger receptor mediated. Int Immunopharmacol. 2003;3(10–11):1381–1399. doi: 10.1016/S1567-5769(03)00136-X. [DOI] [PubMed] [Google Scholar]
- 30.Thiele GM, Tuma DJ, Willis MS, Miller JA, McDonald TL, Sorrell MF, et al. Soluble proteins modified with acetaldehyde and malondialdehyde are immunogenic in the absence of adjuvant. Alcohol Clin Exp Res. 1998;22(8):1731–1739. [PubMed] [Google Scholar]
- 31.Kikugawa K, Kosugi H, Asakura T. Effect of malondialdehyde, a product of lipid peroxidation, on the function and stability of hemoglobin. Arch Biochem Biophys. 1984;229(1):7–14. doi: 10.1016/0003-9861(84)90124-3. [DOI] [PubMed] [Google Scholar]
- 32.Xu D, Thiele GM, Kearley ML, Haugen MD, Klassen LW, Sorrell MF, et al. Epitope characterization of malondialdehyde-acetaldehyde adducts using an enzyme-linked immunosorbent assay. Chem Res Toxicol. 1997;10(9):978–986. doi: 10.1021/tx970069t. [DOI] [PubMed] [Google Scholar]
- 33.Edelson PJ, Zwiebel R, Cohn ZA. The pinocytic rate of activated macrophages. J Exp Med. 1975;142(5):1150–1164. doi: 10.1084/jem.142.5.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamate C, Baloul S, Grootenboer S, Pessis E, Chevrot A, Tulliez M, et al. Inflammation and cancer, the mastocytoma P815 tumor model revisited: triggering of macrophage activation in vivo with pro-tumorigenic consequences. Int J Cancer. 2002;100(5):571–579. doi: 10.1002/ijc.10519. [DOI] [PubMed] [Google Scholar]
- 35.Knisely TL, Luckenbach MW, Fischer BJ, Niederkorn JY. Destructive and nondestructive patterns of immune rejection of syngeneic intraocular tumors. J Immunol. 1987;138(12):4515–4523. [PubMed] [Google Scholar]
- 36.Langrish CL, McKenzie BS, Wilson NJ, de Waal Malefyt R, Kastelein RA, Cua DJ. IL-12 and IL-23: master regulators of innate and adaptive immunity. Immunol Rev. 2004;202:96–105. doi: 10.1111/j.0105-2896.2004.00214.x. [DOI] [PubMed] [Google Scholar]
- 37.Colombo MP, Trinchieri G. Interleukin-12 in anti-tumor immunity and immunotherapy. Cytokine Growth Factor Rev. 2002;13(2):155–168. doi: 10.1016/s1359-6101(01)00032-6. [DOI] [PubMed] [Google Scholar]
- 38.Duryee MJ, Freeman TL, Willis MS, Hunter CD, Hamilton BC, 3rd, Suzuki H, et al. Scavenger receptors on sinusoidal liver endothelial cells are involved in the uptake of aldehyde-modified proteins. Mol Pharmacol. 2005;68(5):1423–1430. doi: 10.1124/mol.105.016121. [DOI] [PubMed] [Google Scholar]
- 39.Duryee MJ, Klassen LW, Freeman TL, Willis MS, Tuma DJ, Thiele GM. Lipopolysaccharide is a cofactor for malondialdehyde-acetaldehyde adduct-mediated cytokine/chemokine release by rat sinusoidal liver endothelial and Kupffer cells. Alcohol Clin Exp Res. 2004;28(12):1931–1938. doi: 10.1097/01.alc.0000148115.90045.c5. [DOI] [PubMed] [Google Scholar]
- 40.Xu D, Thiele GM, Beckenhauer JL, Klassen LW, Sorrell MF, Tuma DJ. Detection of circulating antibodies to malondialdehyde-acetaldehyde adducts in ethanol-fed rats. Gastroenterology. 1998;115(3):686–692. doi: 10.1016/s0016-5085(98)70148-9. [DOI] [PubMed] [Google Scholar]
- 41.Thiele GM, Klassen LW, Tuma DJ, Willis MS. The increased binding of MAA (malondialdehyde-acetaldehyde) adducted protein to scavenger receptors and the up-regulation of B7 may play a role in immunogenicity. Hepatology. 1999;30(4):328A–328A. [Google Scholar]