Abstract
The scatter factor/hepatocyte growth factor regulates scattering and morphogenesis of epithelial cells through activation of the MET tyrosine kinase receptor. In particular, the noncatalytic C-terminal tail of MET contains two autophosphorylation tyrosine residues, which form a multisubstrate-binding site for several cytoplasmic effectors and are thought to be essential for signal transduction. We show here that a MET receptor mutated on the four C-terminal tyrosine residues, Y1311F, Y1347F, Y1354F, and Y1363F, can induce efficiently a transcriptional response and cell scattering, whereas it cannot induce cell morphogenesis. Although the mutated receptor had lost its ability to recruit and/or activate known signaling molecules, such as GRB2, SHC, GAB1, and PI3K, by using a sensitive association–kinase assay we found that the mutated receptor can still associate and phosphorylate a ∼250-kDa protein. By further examining signal transduction mediated by the mutated MET receptor, we established that it can transmit efficient RAS signaling and that cell scattering by the mutated MET receptor could be inhibited by a pharmacological inhibitor of the MEK-ERK (MAP kinase kinase–extracellular signal-regulated kinase) pathway. We propose that signal transduction by autophosphorylation of the C-terminal tyrosine residues is not the sole mechanism by which the activated MET receptor can transmit RAS signaling and cell scattering.
INTRODUCTION
The scatter factor/hepatocyte growth factor (SF/HGF) is a mesenchymal cytokine that acts predominantly on cells of epithelial origin. According to its original identifications, SF/HGF promotes the scattering and/or growth of various epithelial cells (Nakamura et al., 1984, 1989; Stoker et al., 1987; Naldini et al., 1991). The scattering response comprises initial cell dissociation that involves cytoskeletal reorganization and loss of intercellular junctions, followed by active migration. In addition, SF/HGF has been shown to induce formation of branches and tubules, suggesting that it plays a role during epithelial morphogenesis (Montesano et al., 1991; Tsafarty et al., 1992). The expression patterns in combination with the pleiotropic properties of SF/HGF support the idea that this ligand is an important mediator of the interaction between the epithelial and stromal compartments of various tissues during normal and pathological development (reviewed in Birchmeier and Birchmeier, 1993; Matsumoto and Nakamura, 1996).
A high-affinity receptor for SF/HGF has been identified as the MET tyrosine kinase receptor (Park et al., 1987; Giordano et al., 1989; Bottaro et al., 1991; Naldini et al., 1991). It is a 190-kDa transmembrane protein, composed of an α subunit of 45 kDa disulfide linked to a β subunit of 145 kDa, which contains the catalytic domain. Upon ligand binding and subsequent dimerization of MET, several tyrosine residues in the intracellular region of the β subunit become phosphorylated (Ponzetto et al., 1993; Zhu et al., 1994): Y1232, Y1233, Y1347, and Y1354, or Y8, Y9, Y14, and Y15 when intracellular tyrosine residues of mouse MET were numbered 1–16 (Weidner et al., 1995). Within the tyrosine kinase domain, Y8 and Y9 are the major autophosphorylation sites, and their mutations abolish the biological activity (Longati et al., 1994). Outside the kinase domain, the two autophosphorylation sites in the C-terminal region, Y14 and Y15, are responsible for the binding of various proteins known to interact with MET, which include GRB2, SRC, PI3K, PLCγ, SHC, SHP-2, GAB1, and most likely STAT3 (Ponzetto et al., 1994; Pelicci et al., 1995; Fournier et al., 1996; Weidner et al., 1996; Nguyen et al., 1997; Boccaccio et al., 1998). More precisely, it was found that GRB2 is only recruited by Y15, whereas seven distinct proteins (SRC, PI3K, PLCγ, SHC, SHP-2, GAB1, and STAT3) are recruited by both Y14 and Y15. All these signaling molecules bind phosphorylated tyrosine residues via their respective Src homology 2 domains, except for GAB1, which binds via a novel MET-binding domain (Weidner et al., 1996). Taken together, this points to an interesting characteristic of MET: a short sequence located in the C-terminal tail and containing two tyrosine residues (Y14 and Y15), is alone responsible for the recognition of several cytoplasmic effectors.
This multiplicity of substrates for the same region of a receptor questions the sequential recognition and relative contribution of these substrates to the pleiotropic activities of MET in epithelial cells, which include cell motility and morphogenesis. To unravel this issue, several groups used a chimeric receptor strategy. They established Madin–Darby canine kidney (MDCK) cell lines expressing mutated MET chimeric receptors in which tyrosine residues have been substituted to phenylalanine to prevent their phosphorylation. Using these cell lines, the ability of mutated receptors to promote cell scattering (motile response) and branching morphogenesis in collagen gels (morphogenesis response) was determined. Single mutations of Y15 led to controversial results on both cell motility and morphogenesis (Ponzetto et al., 1994; Zhu et al., 1994; Weidner et al., 1995). For example, mutation of this tyrosine residue abolished cell motility in one study (Zhu et al., 1994) but had no effect in another (Weidner et al., 1995). Double mutations of both Y14 and Y15 consistently had an effect on cell motility, resulting in either abolition (Ponzetto et al., 1994) or reduction of cell motility (Weidner et al., 1995). In addition, other tyrosine residues were found to contribute to these biological activities. For example, Y16, like Y15, could reduce cell morphogenesis, and mutation of all C-terminal tyrosine residues, Y13–16, could reduce but not abolish cell motility (Weidner et al., 1995).
Using transactivation assays, we recently demonstrated that SF/HGF can induce a transcriptional response in MDCK epithelial cells; this effect involves RAS and ETS1 and results in the activation of specific promoters containing ETS/AP1-binding sites (Fafeur et al., 1997). To establish the relative contribution of the C-terminal tyrosine residues of MET in transmitting transcriptional activation, we cotransfected different reporter genes containing ETS/AP1-binding sites with TRK–MET hybrid receptors, mutated or not on various tyrosine residues. This led us to characterize further in stable cell lines the biological effects mediated by a TRK–MET receptor in which the four C-terminal tyrosine residues (Y13–16) of MET were mutated. Our results suggest that all signaling and biological activities of the activated MET receptor are not mediated by the C-terminal tyrosine residues.
MATERIALS AND METHODS
Cytokines, Drug, and Cell Cultures
Human recombinant forms of SF/HGF and β-nerve growth factor (β-NGF) were purchased from R & D Systems (Minneapolis, MN). PD98059, a specific inhibitor of MEK1, was purchased from Calbiochem (La Jolla, CA). MDCK epithelial cells (kindly provided by Dr. J. Jouanneau, Ecole Normale Supérieure, Paris, France) and NIH-3T3 fibroblasts were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Grand Island, NY) supplemented with 10% FCS and antibiotics at 37°C.
Plasmids
The various expression vectors of TRK–MET were previously described (Weidner et al., 1995). Briefly, the cDNA encoding the extracellular domain of the human high-affinity NGF receptor (TRK) was fused to the sequence encoding the transmembrane and cytoplasmic region of mouse MET. Tyrosine-to-phenylalanine substitutions were introduced into the TRK–MET cDNA by site-directed mutagenesis using mismatched oligonucleotide primers.
The vector expressing the dominant negative form of RAS (pRSV-RASN17) was provided by Dr. S. Rayter (Sugen, Redwood City, CA). The expression vector expressing the dominant negative form of CDC42 (pMT90-CDC42N17) was provided by Dr. P. Chavrier (Center d’Immunologie, Marseille, France).
The reporter vectors used were as follows: Py-Luc contains three tandem copies of a polyoma virus enhancer–derived sequence with ETS/AP1-binding sites linked to the thymidine kinase promoter and drives the luciferase reporter gene (Wasylyk et al., 1990); urokinase plasminogen activator (uPA)-Luc contains a 90-bp HaeIII fragment of the mouse uPA promoter (−2446,−2356) linked to the uPA proximal promoter (−114,+398) and drives the luciferase reporter gene (Stacey et al., 1995); collagenase-Luc contains the (−517,+63) region of the human collagenase 1 promoter sequence linked to the luciferase gene (Schneikert et al., 1996).
Antibodies
Rabbit polyclonal antibodies anti-ERK2 and anti-MET SP260 (directed against the C-terminal tail of mouse c-MET) were purchased from Santa-Cruz Biotechnology (Santa Cruz, CA). Rabbit polyclonal antibodies anti-phospho-specific-MAPK (ERK), anti-SHC, and anti-GAB1 were purchased from Biolabs (Northbrook, IL), Transduction Laboratories (Lexington, KY), and Upstate Biotechnology (Lake Placid, NY), respectively. Mouse monoclonal antibodies 4G10 anti-phosphotyrosine and anti-GRB2 were purchased from Upstate Biotechnology and Transduction Laboratories, respectively. Alkaline phosphatase conjugates of antibodies directed against rabbit and mouse immunoglobulin G were purchased from Sigma (St. Louis, MO).
Transactivation Assays
The transactivation assays were performed as described previously with minor modifications (Fafeur et al., 1997). Briefly, 15 × 103 MDCK cells were cultured in 12-well plates and were transiently transfected using a lipofection method. Cells were incubated with a mixture of DNA (2.5 μg/ml) and LipofectAMINE (Life Technologies; 20 μg/ml). In each experiment, plates were incubated with the same total amount of plasmid DNA, completed as necessary with the corresponding empty expression vector. The following day, cells were stimulated with 10 ng/ml SF/HGF or 100 ng/ml NGF. Controls consisted of unstimulated cells. Twenty-four hours later, cells were disrupted in reporter lysis buffer (Promega, Madison, WI). The supernatant was assayed for protein content and processed for luciferase assays. The value obtained was normalized to the protein content of the cell extract. Fold activation is the ratio from each luciferase value, relative to the luciferase activity from the reporter gene with empty expression vector. Triplicate samples were performed in each experiment, and SDs are shown. Each experiment was repeated at least twice with independent plasmid preparations to assess reproducibility.
Stable Transfections
MDCK cells (6 × 105/100-mm dish) were cultured for 24 h and then transfected by lipofection with 10 μg of expression vectors, either empty (pBAT), wild-type (WT) TRK–MET, or Y13–16F TRK–MET, and 1 μg of pSV2-neo. For each vector, 20 clones from two independent transfections were selected after culture in neomycin-selective medium (0.8 mg/ml G418/DMEM and 10% FCS). The clones were analyzed by Western blotting for expression of TRK–MET hybrid receptors.
Metabolic Labeling and Immunoprecipitation
For metabolic labeling, MDCK cells (1.5 × 105 cells/100-mm dish) were cultured for 2–3 d in DMEM and 10% FCS. The media were replaced for 1 h by methionine- and cysteine-free modified Eagle’s medium (MEM, Met−, Cys−; Life Technologies). During the last 2 h of incubation cells were cultured in fresh MEM, Met−, Cys−, with 250 μCi/3 ml of l-[35S]methionine and cysteine (Tran35S-Label, 1066 Ci/mmol; ICN, Costa Mesa, CA). At the end of the experiments, cells were lysed and processed for immunoprecipitation as described (Gilles et al., 1996). Briefly, identical trichloroacetic acid-precipitable counts of proteins were immunoprecipitated and separated onto 10% SDS-polyacrylamide gels. Gels were fixed and treated with DMSO containing 20% 2,5-diphenyloxazole for autoradiographic enhancement and exposed for autoradiography using Hyperfilm-MP (Amersham International, Little Chalfont, England).
Immunoprecipitation and Western Blotting
MDCK cells (106 cells/100-mm dish) were cultured 1 d in DMEM and 10% FCS and grown for a further 24 h in serum-free MEM. Cells were lysed in lysis buffer (20 mM Tris-HCl, pH 7.8, 50 mM NaCl, 5 mM EGTA, and 1% vol/vol Triton X-100) containing freshly added protease and phosphatase inhibitors (1 mM phenylmethyl sulfonyl fluoride, 1 μM leupeptin, 2 μM aprotinin, 50 mM sodium fluoride, and 4 mM sodium orthovanadate). Lysates were clarified by centrifugation at 4°C, and protein concentration was determined by Bio-Rad (Hercules, CA) protein assay. Appropriate antibodies were incubated with total cell lysates overnight at 4°C, and antibodies were then adsorbed on protein A-Sepharose for 1 h at 4°C. Immune complexes were washed four times with ice-cold lysis buffer, eluted, and denatured by heating for 3 min at 95°C in reducing Laemmli buffer. Immunoprecipitates (or cell lysates for direct analysis by Western blotting) were separated on SDS-polyacrylamide gels. For immunoblot analysis, proteins were transferred onto polyvinylidene difluoride filters (Millipore, Bedford, MA). Filters were incubated 1 h at 22°C in blocking buffer (0.2% wt/vol caseine and 0.1% vol/vol Tween 20 dissolved in PBS) and probed 1 h at 22°C with specific antibodies diluted in blocking buffer. After extensive washing, immune complexes were detected with species-specific secondary antiserum conjugated with alkaline phosphatase followed by an enhanced chemiluminescence detection system (Aurora, ICN). For membrane reprobing, filters were incubated in stripping buffer (0.2 M glycine, pH 2.2, 0.1% SDS, and 1% Tween 20); after extensive washing, filters were incubated in blocking buffer and reprobed with specific antibodies.
Association–Kinase Assay
This assay was performed either on stable transfectants or on transiently transfected cells. MDCK cells (106 cells/100-mm dish) were cultured for 24 h in DMEM and 10% FCS and transiently transfected or not using the lipofection method. The cells were then grown for 24 h in DMEM and 0.5% FCS. The cells were then stimulated for 10 min with 100 ng/ml NGF or 10 ng/ml SF/HGF. After growth factor treatment, cells were disrupted and immunoprecipitated with the anti-MET antibody in lysis buffer, as described above. Immune complexes and associated proteins were washed four times with ice-cold lysis buffer and once in kinase buffer (50 mM HEPES, pH 7.5, 150 mM NaCl, and 12.5 mM MgCl2). In vitro kinase assays were performed at 37°C in 50 μl of kinase buffer containing 10 μCi [γ-33P]ATP (4000 Ci/mmol) and 10 μM cold ATP for 15 min. Phosphorylated proteins were then resolved on SDS-polyacrylamide gels and visualized by autoradiography.
Assays for Cell Scattering
Scattering from cell islets was performed as follows. MDCK cells (6 × 104 cells per well) were seeded onto six-well plates and cultured for 24 h in DMEM and 10% FCS, with or without 10 ng/ml SF/HGF or 100 ng/ml NGF. The cell scattering from circular wounds was performed essentially as described previously (Klein-Soyer et al., 1986). Briefly, MDCK cells were seeded onto 12-well plates and grown to confluence in DMEM and 10% FCS. A circular wound was made in the center of the well using a calibrated Whatman (Clifton, NJ) 3 MM paper disk (5 mm in diameter). MDCK cells were cultured 4 d in DMEM and 10% FCS with or without 10 ng/ml SF/HGF or 10 ng/ml NGF. At the end of the experiments, cells were fixed and stained (Diff-Quik; Dade AG, Düdingen, Switzerland), and their morphologies were examined by light microscopy.
Assays for Cell Morphogenesis
Formation of cellular networks on Matrigel gels was performed as follows. MDCK cells (4 × 104 cells per well) were plated on a layer of 300 μl of Matrigel (Becton Dickinson, San Jose, CA) in 24-well plates in DMEM and 10% FCS. The next day, cultures were stimulated with or without 10 ng/ml SF/HGF or 100 ng/ml NGF for 24 h. At the end of the experiments, cells were stained 20 min at 37°C with neutral red (0.5% wt/vol), and fixed with 4% paraformaldehyde, and their morphologies were examined by light microscopy.
RESULTS
The Y13–16 C-Terminal Tyrosine Residues of MET Are Not Required for Efficient Transcriptional Activation
We transiently cotransfected MDCK epithelial cells with both the TRK–MET hybrid receptor mutated on specific tyrosine residues and the Py-Luc reporter, which contains an ETS/AP1-responsive element. The TRK–MET hybrid receptor consists of the ligand-binding domain of the NGF receptor (TRK) fused to the kinase domain of MET. This hybrid receptor has been shown to mediate MET-specific signals in epithelial cells in response to NGF (Weidner et al., 1993, 1995).
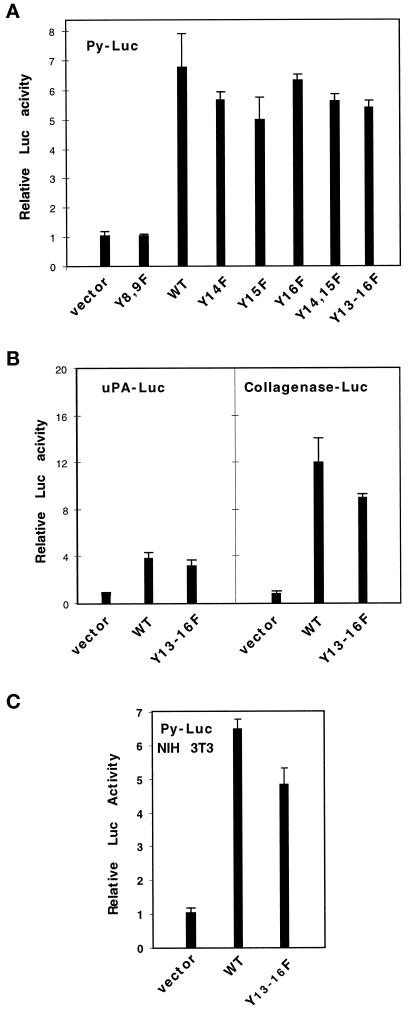
We found that TRK–MET mutated on tyrosine residues 8 and 9 (Y8,9F) of the tyrosine kinase region was unable to induce transactivation of the Py-Luc reporter (Figure 1A). These results showed that the tyrosine kinase activity of the MET receptor is essential for inducing this transcriptional response.
Figure 1.
Mutation of the C-terminal tyrosine residues of MET does not abolish its ability to transmit a transcriptional response, involving ETS/AP1-binding sites. (A) MDCK cells were transiently cotransfected with 0.5 μg/ml Py-Luc reporter and 1 μg/ml vectors, either empty (vector), or expressing WT TRK–MET receptor (WT) or TRK–MET receptor, mutated on various tyrosine residues (Y–F). The following day, MDCK cells were stimulated with 100 ng/ml NGF, and the luciferase activity was measured 24 h later. (B) MDCK cells were transiently cotransfected with 1 μg/ml vectors, either empty or expressing WT or Y13–16F TRK–MET receptor, and 0.5 μg/ml uPA- or collagenase-Luc reporters. Treatment with NGF and measurement of the relative luciferase activity were as described above. (C) NIH-3T3 cells were transiently cotransfected with 1 μg/ml vectors, either empty or expressing WT or Y13–16F TRK–MET receptor, and 0.5 μg/ml Py-Luc reporter. Treatment with NGF and measurement of the relative luciferase activity were as described above.
By contrast, mutations of tyrosine residues of the C-terminal tail of MET did not abrogate its ability to transmit transactivation. As shown in Figure 1A, single (Y13, Y14, Y15, or Y16), double (Y14–15), or complete Y to F (Y13–16) mutations of C-terminal tyrosine residues of the receptor mediated a transcriptional response, like the WT receptor. Similar results were obtained using uPA- or collagenase-Luc reporters (Figure 1B), in which functional ETS/AP1 have been identified (Gutman and Wasylyk, 1990; Rorth et al., 1990; Fafeur et al., 1997). In all of these transactivation assays, a decrease in transcriptional activation in cells transfected by Y13–16F TRK–MET versus WT TRK–MET receptors was never >30% and was not systematically observed. In addition, dose–response experiments were performed using increasing concentrations of expression vectors for WT and Y13–16F TRK–MET. Each concentration was found to be effective: optimal induction was obtained with 200 ng/ml expression vector for WT or Y13–16F TRK–MET, and higher concentrations (up to 1 μg/ml) did not decrease transactivation (our unpublished results).
The WT and Y13–16F TRK–MET receptors were also able to induce a transcriptional response in NIH-3T3 cells (Figure 1C). Unlike MDCK cells, NIH-3T3 cells do not respond to SF/HGF in these transactivation assays (our unpublished results). These results demonstrated that the Y13–16F TRK–MET receptor can also transduce a signal leading to the transcriptional activation of the Py-Luc reporter independently of endogenous functional MET receptors.
Taken together these results demonstrated that a TRK–MET receptor mutated on Y13–16 residues can transmit a transcriptional response, involving activation of promoter elements containing ETS/AP1-binding sites.
The Y13–16F TRK–MET Receptor Can Promote Cell Motility but Fails to Promote Cell Morphogenesis
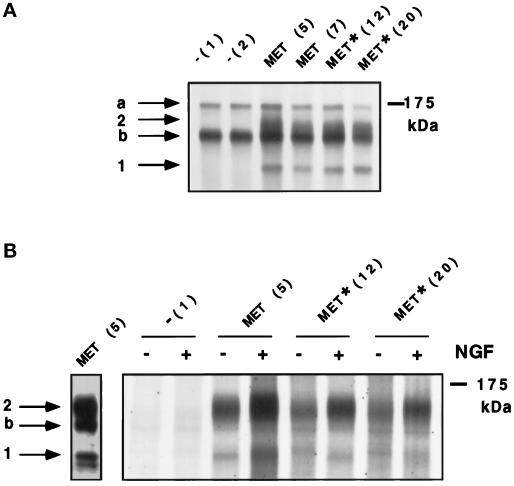
To investigate whether these sites are required for cell motility and morphogenesis, we have generated stable MDCK cell lines expressing WT and Y13–16F TRK–MET receptor. Stable transfectants of MDCK cells were selected for the expression of the TRK–MET hybrid receptor, and clones, obtained from independent transfections, were further analyzed. Immunoprecipitations were performed using an anti-MET antibody, which recognizes both hybrid TRK–MET and endogenous MET receptors. In nontransfected MDCK cells, immunoprecipitation analysis revealed the presence of the 170-kDa precursor and the 145-kDa β-subunit of the endogenous MET receptor (Figure 2A). In MDCK cells transfected with the TRK–MET receptors, two new bands were detected; an upper band at ∼150 kDa and a lower band at ∼125 kDa, which correspond to the fully glycosylated and nonglycosylated hybrid receptors, respectively (Ponzetto et al., 1996) (Figure 2A). After kinase assay, phosphorylation of TRK–MET, but not of endogenous MET, was induced by NGF in all TRK–MET cell lines (Figure 2B).
Figure 2.
Expression and NGF-dependent autophosphorylation of WT and Y13–16F TRK–MET receptors in stably transfected MDCK cells. (A) Proteins from stable transfectants of MDCK cells were metabolically labeled with [35S]methionine and [35S]cysteine, and both endogenous and hybrid receptors were immunoprecipitated using the anti-MET antibody. The proteins were resolved by 7% SDS-PAGE and visualized by autoradiography. On the top of each lane, the name of the cell line is indicated, corresponding to stable transfection by empty vector (−), WT TRK–MET receptor (MET), or Y13–16F TRK–MET receptor (MET*), and the clone number is indicated in brackets. (B) Stable transfectants of MDCK cells were stimulated (+) or not (−) with 100 ng/ml NGF for 10 min. Endogenous and hybrid receptors were immunoprecipitated using the anti-MET antibody, and immunoprecipitates were subjected to an in vitro kinase assay, using [γ-33P]ATP. The proteins were resolved by 5% SDS-PAGE and visualized by autoradiography. Left panel, sample from the MET (5) cell line, immunoprecipitated and revealed by immunoblot using the anti-MET antibody. Arrows a, b, 1, and 2 on the left indicate positions of MET precursor, MET β-subunit, nonglycosylated TRK–MET, and fully glycosylated TRK–MET, respectively. Right, molecular mass markers.
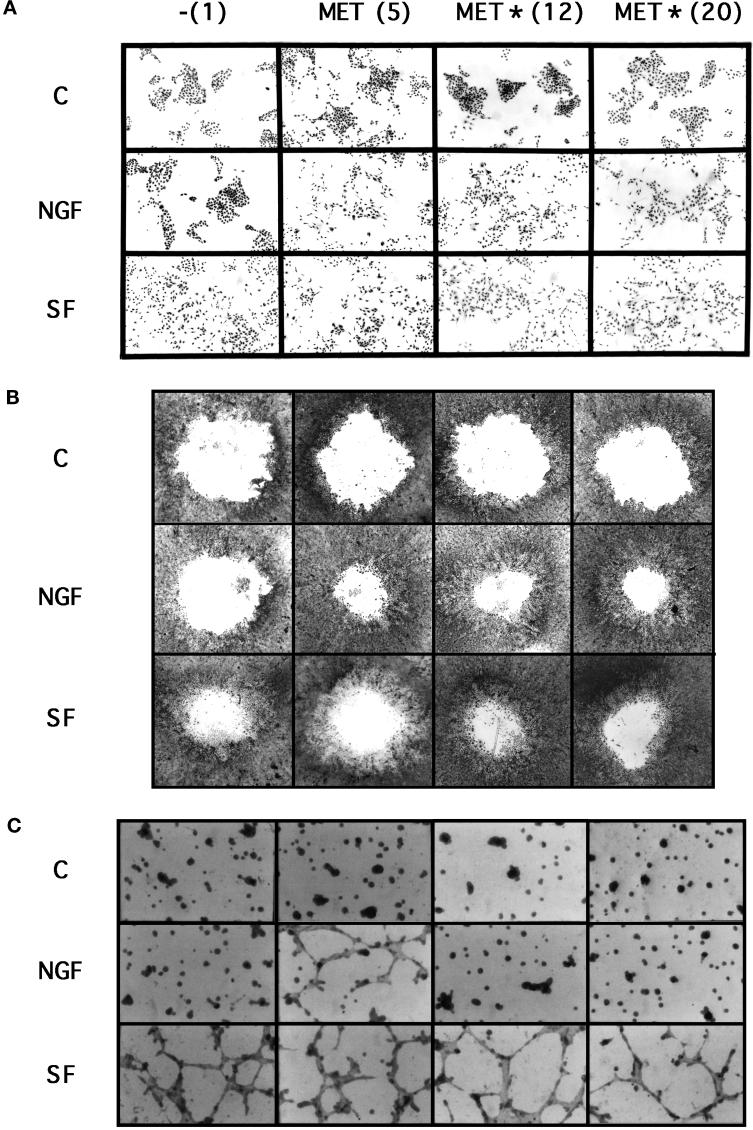
The motile responses of the stable cell lines were examined in two different assays. First, the cells were seeded at low density, allowing the cells to grow as islets. Addition of SF/HGF for 24 h caused scattering of all cell lines (Figure 3A), whereas addition of NGF caused cell scattering only in TRK–MET cell lines (Figure 3A). Similar results were observed in a wound assay, in which scattering is measured from confluent monolayers. Stable transfectants were grown up to confluence, and circular wounds were performed with a calibrated disk. From this wound, the cells can dissociate, migrate, and proliferate. In the presence of serum, the size of the hole was smaller in SF/HGF-treated cells compared with untreated cells (Figure 3B). In parallel experiments, we established that SF/HGF was unable to induce proliferation of MDCK cells in culture medium containing either low (0.5%) or regular (10%) serum concentration (our unpublished results). In this wound assay, we propose that we primarily demonstrated the scattering effect of SF/HGF from the wound, allowing the loss of cell contact inhibition, leading, as a secondary effect, to an increase in cell proliferation and to the reconstitution of the cell layer. This wound assay also demonstrated that NGF caused scattering in all TRK–MET cell lines (Figure 3B).
Figure 3.
Effect of SF/HGF and NGF on the induction of cell scattering and morphogenesis in stably transfected MDCK cells. (A) Scattering from cell islets. Stable transfectant MDCK cells were seeded at low density. The next day cells were cultured for 24 h in the absence (C) or presence of 10 ng/ml SF/HGF (SF) or 100 ng/ml NGF. Magnification, 20×. (B) Scatttering from confluent cells. Stable transfectant MDCK cells were grown to confluence. Circular wounds were performed with a calibrated disk, and cultures were stimulated or not with 10 ng/ml SF/HGF or 100 ng/ml NGF for 4 d. Magnification, 8×. (C) Morphogenesis in Matrigel gel. Stable transfectants of MDCK cells were cultured on Matrigel gels. The following day, cells were incubated with or without (C) 10 ng/ml SF/HGF or 100 ng/ml NGF for 24 h. Magnification, 25×.
The response of TRK–MET cell lines to NGF was different in morphogenesis assays. After seeding on Matrigel gels, small aggregates of cells were obtained within 24 h. The next day, SF/HGF was added for 24 h, and most of the cells became fusiform and propelled extensions, resulting in the formation of a spider web–like network in all stable transfectant MDCK cells (Figure 3C). A similar network formation was obtained with NGF but only in the stable cell line expressing WT TRK–MET (Figure 3C). The absence of morphogenetic effect of NGF on mutated TRK–MET cell lines was confirmed in a collagen gel assay. Stable transfectants were cultured at low density within the collagen gel until spherical cyst formation occurred. SF/HGF was then added for 3 d, resulting in the formation of branching cords extending from the cysts into the surrounding collagen gel in all stable cell lines. Consistent with previous studies (Weidner et al., 1995), NGF induced a similar morphological effect in stable cell lines expressing WT TRK–MET and was unable to induce this effect in stable cell lines expressing Y13–16F TRK–MET receptor (our unpublished results).
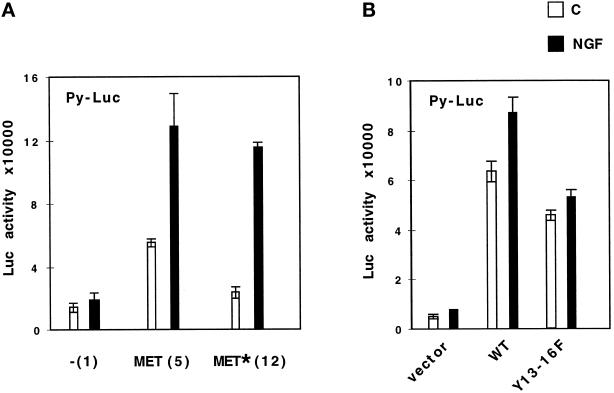
The Y13–16F TRK–MET Receptor Retains the Ability to Induce Transcriptional Activation in Stable Transfectants
The ability of the Y13–16F TRK–MET receptor to transmit a transcriptional response (see Figure 1) was confirmed using transfection of the Py-Luc reporters in stable MDCK clones expressing TRK–MET receptors. Both cell lines expressing WT or mutated TRK–MET receptor, stimulated by NGF, could transmit a transcriptional response (Figure 4A) at a similar level than after stimulation by SF/HGF (also see Figure 7A). It should be noted that in the absence of NGF, the cell line expressing WT TRK–MET showed a higher basal transcriptional activation compared with the control and mutated receptor cell line (Figure 4A). This was consistent with a higher basal scattering and phosphorylation of this WT TRK–MET receptor cell line (Figures 2B and 3A).
Figure 4.
The Y13–16F TRK–MET receptor retains the ability to transactivate the Py-Luc reporter in stable transfectants. (A) Stable transfectants of MDCK cells were transiently transfected with 0.5 μg/ml Py-Luc reporter. The following day, cells were stimulated for 24 h with 100 ng/ml NGF (black bars) or without (white bars), and luciferase activity was measured. (B) MDCK cells were transiently cotransfected with 1 μg/ml vectors, either empty or expressing WT or Y13–16F TRK–MET receptor, and 0.5 μg/ml Py-Luc reporter. The following day, MDCK cells were stimulated with 100 ng/ml NGF, and the luciferase activity was measured 24 h later.
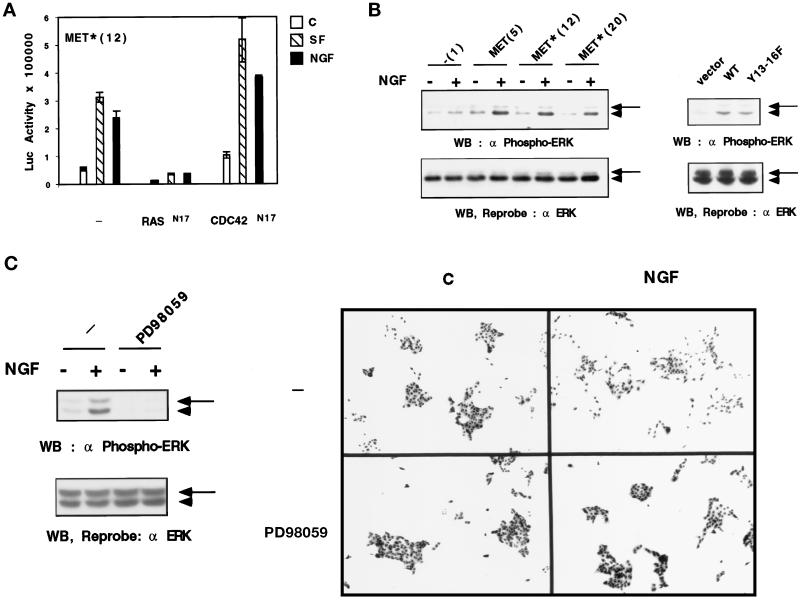
Figure 7.
Mutated MET receptor induces RAS signaling, which is necessary for cell scattering. (A) Effect of RAS N17 and CDC42 N17 on transactivation of the Py-Luc reporter vector in a mutated receptor cell line. The stable transfectant of the mutated receptor (MET*, clone 12) was transiently transfected with 0.5 μg/ml Py-Luc reporter and 0.5 μg/ml expression vectors of RAS N17 or CDC42 N17 dominant negative mutants. Cells were incubated the next day with 10 ng/ml SF/HGF (dashed bars) or 100 ng/ml NGF (black bars) or without (white bars) for 24 h, and luciferase activity was measured. (B) Effect of the mutated MET receptor on ERK phosphorylation. Left panel, stable transfectants were stimulated (+) or not (−) with 100 ng/ml NGF for 10 min. Right panel, MDCK cells were transiently transfected with 1 μg/ml empty, WT, or Y13–16F TRK–MET expression vectors and were stimulated the next day by NGF (100 ng/ml). Cellular extracts (50 μg) were resolved by 10% SDS-PAGE. The proteins were analyzed by Western blotting using an antibody directed against phosphorylated ERK1,2 (top). The filter was dehybridized and reprobed using the anti-ERK2 antibody, which also recognizes ERK1 (bottom). Arrowhead, position of ERK1; arrow, position of ERK2. (C) Effect of an inhibitor of MEK1 on cell scattering in a mutated receptor cell line. Left panel, stable transfectants expressing the mutated receptor (clone 12) were treated for 45 min with 50 μM PD98059 and then stimulated (+) or not (−) with 100 ng/ml NGF for 10 min. Fifty micrograms of cellular extract were resolved by 10% SDS-PAGE. The proteins were analyzed by Western blotting using an antibody directed against phosphorylated ERK1,2 (top). The filter was dehybridized and reprobed using the anti-ERK2 antibody, which also recognizes ERK1 (bottom). Arrowhead, position of ERK1; arrow, position of ERK2. Right panel, stable transfectants expressing the mutated receptor (clone 12) were seeded at low density. The following day cells were treated for 45 min with 50 μM PD98059 and cultured for 24 h in the presence or absence of 100 ng/ml NGF. Magnification, 20×.
The principal difference between stable cell lines and transiently transfected cells was a stronger response to NGF (Figure 4, compare A and B). Transient transfection can result in overexpression of the transfected tyrosine kinase receptors leading to autonomous dimerization and autophosphorylation in the absence of the ligand (Taylor et al., 1995). By measuring tyrosine phosphorylation of the immunoprecipitated MET receptor in transiently transfected cells, we verified that the TRK–MET receptors were phosphorylated without NGF and that this effect was only slightly enhanced by NGF (our unpublished results). In addition, the activation of the TRK–MET receptor in the absence of its ligand appeared to be related to the transient transfection, because in stably transfected cells a lower basal transcriptional effect was found (Figure 4, compare A and B).
The Y13–16F TRK–MET Receptor Fails to Bind or Activate GRB2, SHC, PI3K, and GAB1
The transcriptional and the motile responses mediated by the mutated TRK–MET receptor suggested that MET can activate signaling pathways independently of the phosphorylation of its C-terminal tyrosine residues. Nonetheless, previous studies demonstrated that these tyrosine residues are required for recruitment and activation of multiple proteins known to be involved in signal transduction by MET (Ponzetto et al., 1994; Pelicci et al., 1995; Fournier et al., 1996; Weidner et al., 1996; Nguyen et al., 1997). To check the ability of the WT and mutated TRK–MET receptor to recruit these proteins in vivo, we performed coimmunoprecipitation experiments in MDCK cells.
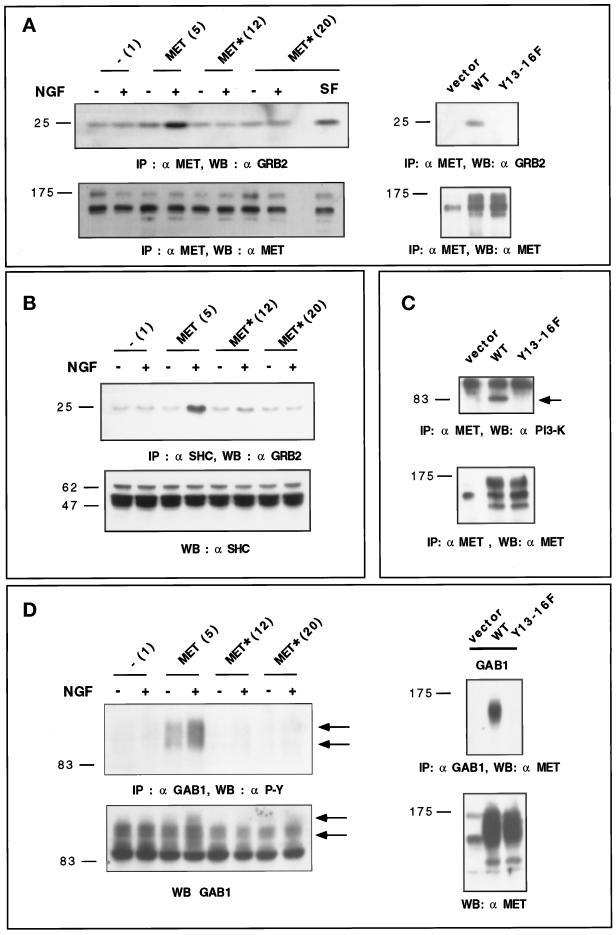
After autophosphorylation, the MET receptor can recruit GRB2 directly (Ponzetto et al., 1994; Fournier et al., 1996). Immunoprecipitations were performed using the anti-MET antibody, and immunoprecipitates were analyzed by Western blotting using the anti-GRB2 antibody. As expected, NGF stimulation enhanced the recruitment of GRB2 in WT TRK–MET cell lines, whereas the amount of GRB2 was similar with or without NGF stimulation in stable cell lines expressing Y13–16F TRK–MET (Figure 5A, left panel). The fact that in the Y13–16F TRK–MET cell line (Met* 20) SF/HGF, but not NGF, enhanced GRB2 recruitment demonstrated that signaling mediated by endogenous and chimeric receptors are distinguishable. Similarly, in normal MDCK cells, NGF could induce GRB2 recruitment, after transient transfection of WT, but not of mutated Y13–16F TRK–MET receptors (Figure 5A, right panel).
Figure 5.
Y13–16F TRK–MET receptor fails to bind or activate known signaling proteins. (A) Left panel, stable transfectants were stimulated (+) or not (−) with 100 ng/ml NGF or 10 ng/ml SF/HGF (SF) for 10 min. Endogenous and hybrid receptors were immunoprecipitated using the anti-MET antibody. Proteins were resolved by 12% SDS-PAGE and analyzed by Western blotting. Part of the filter was incubated with the anti-GRB2 antibody (top), whereas the other part was incubated with the anti-MET antibody (bottom). Right panel, MDCK cells were transiently transfected with 2 μg/ml empty, WT, or Y13–16F expression vectors. The following day, cells were stimulated with 100 ng/ml NGF for 10 min. Endogenous and hybrid receptors were then immunoprecipitated using the anti-MET antibody. The proteins were resolved by 10% SDS-PAGE and analyzed by Western blotting. Part of the filter was incubated with the anti-GRB2 antibody (top), whereas the other was incubated with the anti-MET antibody (bottom). (B) Stable transfectants were stimulated (+) or not (−) with 100 ng/ml NGF for 10 min. Cellular extracts were immunoprecipitated using the anti-SHC antibody. Immunoprecipitates were resolved in 10% SDS-PAGE and analyzed by Western blotting using the anti-GRB2 antibody (top). The amount of SHC in each cellular extract (30 μg of protein) was determined by Western blotting using the anti-SHC antibody (bottom). (C) MDCK cells were transiently transfected with 2 μg/ml empty, WT, or Y13–16F expression vectors. The following day, cells were stimulated with 100 ng/ml NGF for 10 min. Endogenous and hybrid receptors were immunoprecipitated using the anti-MET antibody. The proteins were resolved by 7% SDS-PAGE and analyzed by Western blotting. Part of the filter was incubated with the anti-PI3K antibody (top), whereas the other was incubated with the anti-MET antibody (bottom). (D) Left panel, stable transfectants were stimulated (+) or not (−) with 100 ng/ml NGF for 10 min. Cellular extracts were immunoprecipitated using the an-GAB1 antibody. Immunoprecipitates were resolved in 7% SDS-PAGE and analyzed by Western blotting using the anti-phosphotyrosine antibody (top). The amount of GAB1 in each cellular extract (30 μg of protein) was determined by Western blotting using the anti-GAB1 antibody (bottom), and corresponding positions of GAB1 are indicated by arrows. Right panel, MDCK cells were transiently cotransfected with 1 μg/ml expression vectors of Flag-GAB1 and 1 μg/ml empty, WT, or Y13–16F expression vectors. The following day, cells were stimulated with 100 ng/ml NGF for 10 min. GAB1 was immunoprecipitated using the anti-Flag antibody, and the proteins were resolved by 7% SDS-PAGE and analyzed by Western blotting using the anti-MET antibody (top). The amount of endogenous and transfected receptor in each cellular extract (50 μg of protein) was determined by Western blotting using the anti-MET antibody (bottom). Direct Western blot in transient transfection does not allow a clear separation of the ∼150-kDa TRK–MET and 145-kDa β-subunit of MET. Left, molecular mass markers.
The MET receptor can also recruit GRB2 indirectly, after SHC adaptor recruitment and activation by MET (Pelicci et al., 1995). After immunoprecipitation of SHC, proteins were analyzed by Western blotting using an anti-GRB2 antibody. In stable cell lines expressing WT TRK–MET receptor, NGF stimulation enhanced recruitment of GRB2 by SHC, whereas in stable cell lines expressing Y13–16F TRK–MET receptor, the quantities of GRB2 were similar with or without NGF stimulation (Figure 5B).
The phosphorylated MET receptor can associate with PI3K (Ponzetto et al., 1993; Rahimi et al., 1996). The association of p85 PI3K with the WT TRK–MET receptor was not detected in stable MDCK cell lines. Because transient transfection allows high expression of the chimeric receptor (see Figure 5A), the recruitment of p85 PI3K was examined in MDCK cells transiently transfected by TRK–MET receptors. Although p85 PI3K was recruited by the WT TRK–MET receptor, it was not recruited by the mutated Y13–16F TRK–MET receptor (Figure 5C). The same result was obtained after cotransfection of TRK–MET receptors and p85 PI3K (our unpublished results).
Finally, we investigated the ability of the WT and mutated TRK–MET receptor to activate or to associate GAB1 (Weidner et al., 1996; Nguyen et al., 1997). After immunoprecipitation of GAB1, proteins were analyzed by Western blotting using an anti-phosphotyrosine antibody. In stable cell lines expressing WT TRK–MET receptors, phosphorylation of GAB1 was detected, and stimulation by NGF enhanced its phosphorylation. In contrast, in stable cell lines expressing Y13–16F TRK–MET receptor, phosphorylation of GAB1 was not detected either with or without NGF (Figure 5D, left panel). Similar results were obtained with MDCK cells transiently cotransfected by TRK–MET receptors and an expression vector for GAB1 tagged with a Flag epitope. In contrast to the WT TRK–MET receptor, no detectable binding of mutated TRK–MET receptor was found after immunoprecipitation of GAB1, indicating that the WT but not the mutated TRK–MET receptors can associate with GAB1 (Figure 5D, right panel).
The Mutated TRK–MET Receptor Retains the Ability to Associate and Phosphorylate a ∼250-kDa Protein
To determine the ability of the MET receptor to recruit and phosphorylate proteins in MDCK cells, we used a sensitive association–kinase assay, in which immunoprecipitated MET receptors are subjected to an in vitro kinase assay using radiolabeled [γ-33P]ATP (Figure 6A). The immunoprecipitated MET receptors can phosphorylate both themselves and cytoplasmic ligands (Bardelli et al., 1997; Nguyen et al., 1997). In preliminary experiments, we established that this assay was more sensitive than simple association experiments, in which the phosphorylated proteins are revealed by immunoblot using an anti-phosphotyrosine antibody after immunoprecipitation of MET. The sensitivity of the test was also enhanced by performing transient transfections of the TRK–MET receptors in MDCK cells.
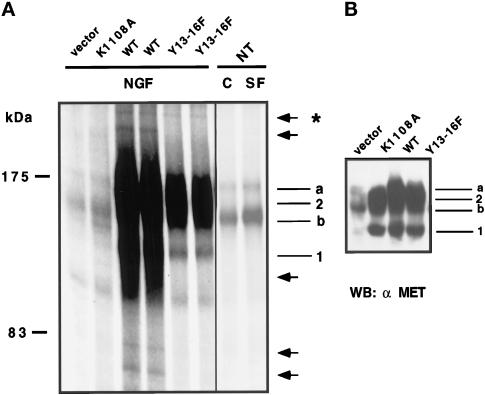
Figure 6.
Detection of phosphorylated MET-associated proteins. (A) MDCK cells were transiently transfected with 2 μg/ml empty (−), kinase-inactive (K1108A), WT, or Y13–16F TRK–MET expression vectors. The following day, cells were stimulated with 100 ng/ml NGF for 10 min. Last two columns, nontransfected MDCK cells were stimulated or not with 10 ng/ml SF/HGF for 10 min. Endogenous and hybrid receptors were then immunoprecipitated using the anti-MET antibody. Immunoprecipitates were subjected to an in vitro kinase assay using [γ-33P]ATP. Phosphorylated proteins were resolved on the same 7% SDS-polyacrylamide gel and visualized by autoradiography. Left, molecular mass markers. Right, MET precursor (a), MET β-subunit (b), nonglycosylated TRK–MET (1), and fully glycosylated TRK–MET (2). Arrows indicate phosphorylated bands detected in WT transfected cells, and the asterisk indicates the phosphorylated band detected both in WT and Y13–16F-transfected cells. (B) Cellular extracts (50 μg of protein) of A were resolved by 7% SDS-PAGE and analyzed by Western blotting using the anti-MET antibody.
As shown in Figure 6, phosphorylation of the endogenous MET receptor was similarly detected in cells nontransfected (NT) or transfected with the empty vector (vector) or a kinase inactive receptor (K1108A, which fails to bind ATP). In nontransfected cells, stimulation by SF/HGF enhanced the amount of phosphorylated MET, but none of the other phosphorylated bands was clearly detected.
The WT and Y13–16F TRK–MET receptor samples were assayed in duplicate to ensure reproducibility (Figure 6A), and the level of expression of the transfected receptors was verified by Western blotting (Figure 6B). In WT TRK–MET-transfected cells, a number of phosphorylated bands were detected, in particular at molecular masses ranging from 180 to 140 kDa. A lower exposure of the gel allowed identification of the MET and TRK–MET receptors. Outside of this region of the gel, five less-phosphorylated bands were detected at molecular masses of ∼250, 230, 110, 80, and 70 kDa (Figure 6B, arrows). In Y13–16F TRK–MET-transfected cells, the TRK–MET receptor was less phosphorylated than in WT TRK–MET-transfected cells; a result consistent with the loss of the C-terminal autophosphorylation sites. In agreement with the demonstration that C-terminal tyrosine residues are necessary for recruitment of intracellular proteins (Ponzetto et al., 1994; Pelicci et al., 1995; Fournier et al., 1996; Weidner et al., 1996; Nguyen et al., 1997), four of the phosphorylated MET-associated bands (molecular masses of ∼230, 110, 80, and 70 kDa) were lost in the Y13–16F TRK–MET receptor versus the WT TRK–MET receptor. Most importantly, the ∼250-kDa phosphorylated band was still detected in the Y13–16F receptor, suggesting that independently of the phosphorylation of the C-terminal tyrosine residues, the MET receptor can still bind and phosphorylate an intracellular protein. This phosphorylated band of ∼250 kDa did not correspond to a known ligand and did not comigrate (our unpublished results) in SDS-PAGE with the ∼220- and ∼185-kDa tyrosine-phosphorylated proteins previously identified in MDCK1 cells, an MDCK cell line constitutively dissociated (Webb et al., 1996).
Y13–16F TRK–MET Retains the Ability to Activate RAS Signaling, Which Is Involved in the Scattering Response
As previously reported, SF/HGF and RAS have a similar ability to induce a transcriptional response involving ETS/AP1-binding sites (Fafeur et al., 1997). To investigate whether the RAS pathway is involved in mediating this transcriptional response, stable cell lines were transiently transfected with both the Py-Luc reporter and dominant negative mutants of small GTP-binding proteins, RAS N17 and CDC42 N17. We found that in MET*(12) cells, RASN17 was able to inhibit basal and SF/HGF- or NGF-stimulated transactivation of the Py-Luc reporter gene. In contrast, CDC42N17 was unable to inhibit transactivation; rather, it enhanced these transcriptional responses (Figure 7A). It is worth noting that both stimulated and basal activities could be mediated by the same factors. Similar results were obtained in stable cell lines expressing WT TRK–MET (our unpublished results).
We also investigated in MDCK cells expressing the mutated receptor whether the phosphorylation of ERK1 and ERK2, two target kinases of RAS, were induced by NGF. Cellular extracts of MDCK cells from stable (Figure 7B, left panel) or transient (Figure 7B, right panel) transfections were Western blotted using an antibody directed against phosphorylated ERK1,2. After NGF stimulation, both WT and Y13–16F TRK–MET receptors were able to mediate the phosphorylation of ERK1,2 in a similar manner (Figure 7B). These phosphorylations of ERK1,2, induced by NGF in the mutated receptor cell lines, were determined on the same samples that allowed the demonstration of an absence of GRB2 recruitment through SHC activation (see Figure 5B).
Finally, we used PD98059, a pharmacological inhibitor of MEK1, which belongs to the RAS signal transduction cascade leading to the phosphorylation of ERK1,2. In stable transfectants expressing the mutated Y13–16F receptor, the scattering induced by NGF was abolished by addition of 50 μM PD98059, demonstrating that the motile response induced by the mutated TRK–MET receptor is transmitted by this downstream pathway of RAS (Figure 7D). In the same experiment, cell extracts were obtained in parallel, and this concentration of PD98059 was found to be effective in inhibiting phosphorylation of ERK1,2 (Figure 7D).
These results demonstrated that the motile response induced by the mutated TRK–MET receptor correlated with its ability to induce ERK1,2 phosphorylation and RAS-dependent transcriptional activation and was abolished after treatment of the cells by an inhibitor of the MEK–ERK1,2 pathway.
DISCUSSION
The recruitment via phosphorylated tyrosine residues of proteins responsible for activation of a signaling pathway is a well-established mechanism used by tyrosine kinase receptors to mediate signal transduction (reviewed in Schlessinger, 1994; Van der Geer et al., 1994). After ligand binding, receptor dimerization, and autophosphorylation, various proteins are recruited to phosphorylated tyrosine residues on the receptor. The MET receptor fits into this scenario, because the two autophosphorylation sites in the C-terminal region of MET, Y14 and Y15, are responsible for the binding of several proteins known to interact with the receptor; these include GRB2, SHC, GAB1, PI3K, PLCγ, SHP2, SRC, and most likely STAT3 (Ponzetto et al., 1994; Pelicci et al., 1995; Fournier et al., 1996; Weidner et al., 1996; Nguyen et al., 1997; Boccaccio et al., 1998). Most of these proteins are capable of activating RAS signaling. For example, the Y15 of MET can specifically recruit GRB2, an adaptor protein that couples activated receptor tyrosine kinases to SOS, promoting activation of RAS (Buday and Downward, 1993; Gale et al., 1993; Li et al., 1993). Both Y14 and Y15 form a multisubstrate-binding site for all other cytoplasmic effectors. When SHC is recruited and phosphorylated by the receptor, it can then associate with GRB2, which activates the RAS pathway (Pelicci et al., 1995). GAB1 is a multifunctional protein that can interact with MET both directly and indirectly via GRB2 (Holgado et al., 1996; Weidner et al., 1996; Nguyen et al., 1997). Its overexpression in epithelial cells leads to phosphorylation of downstream targets of the RAS pathway, the MAP kinases ERK1,2 (Weidner et al., 1996). PI3K consists of two subunits, p85, which contains Src homology 2 domains involved in MET receptor interaction, and p110, the catalytic subunit. The RAS protein has been shown to bind to the p110 subunit (Rodriguez-Viciana et al., 1994), and a constitutively activated mutant form of PI3K can activate the RAS pathway in fibroblasts (Hu et al., 1995). Taken together, these data suggest that mutations of both Y14 and Y15 of the MET receptor could impair activation of the RAS cascade.
The Y13–16F MET Transmits Efficient RAS Signaling
By establishing the relative contribution of these tyrosine residues in transmitting transcriptional activation by the MET receptor, we found that neither single, double, nor complete mutations of the four C-terminal tyrosine residues impaired the transcriptional response. This led us to demonstrate in vivo that the Y13–16F MET receptor can transmit efficient RAS signaling, including ERK phosphorylation and RAS-dependent transcriptional activation, despite the fact that this mutated MET receptor, as expected, was unable to recruit GRB2, SHC, GAB1, and PI3K.
Within the family of tyrosine kinase receptors, similar approaches have been undertaken to examine the relative contribution of tyrosine residues in transmitting signal transduction. Mutations of tyrosine residues of the platelet-derived growth factor and NGF receptors abolished the recruitment of signaling proteins and consequently abolished the induction of signaling pathways, including the RAS cascade (Valius and Kazlauskas, 1993; Stephens et al., 1994). Interestingly, the mutations of the tyrosine residues of the EGF and fibroblast growth factor (FGF) receptors abolished the recruitment of all previously identified proteins, including GRB2, but did not impair induction of the RAS cascade (Li et al., 1994; Mohammadi et al., 1996). It is clear that the MET receptor behaves differently than the EGF and basic FGF receptors. Indeed, it was demonstrated that both EGF and FGF receptors mutated on autophosphorylation tyrosine residues can recruit GRB2 indirectly, via SHC activation (Li et al., 1994; Mohammadi et al., 1996), whereas in our study SHC is not activated by the mutated MET receptor.
According to other studies, signal transduction by the mutated tyrosine kinase receptor can be explained by heterodimerization with related endogenous receptors (Wright et al., 1995). In the present study, we provide evidence that the TRK–MET receptor does not function through endogenous MET. In MDCK cell lines expressing TRK–MET receptors, we found that stimulation with NGF induced TRK–MET phosphorylation without affecting phosphorylation of the endogenous MET receptor (Figure 2B). Furthermore, we found that the mutated TRK–MET receptor cannot transmit signal transduction through the endogenous MET receptor. First, using the anti-MET antibody, both the endogenous and transfected receptors were immunoprecipitated from MDCK cells. In cells expressing the mutated TRK–MET receptor, NGF did not induce either recruitment or activation of endogenous MET substrates, such as GRB2, PI3K, and GAB1, whereas SF/HGF was able to induce recruitment of GRB2 (Figure 5). Second, we did not observe a RAS-dependent transcriptional response using kinase defective MET receptors (Y8,9F, which is mutated on tyrosine residues of the kinase region, or K1108A, unable to bind ATP; Figure 1 and our unpublished results). This is in contrast to the demonstration that a kinase-defective EGF receptor can heterodimerize with an ERB2/NEU receptor and that this heterodimer causes activation of RAS signaling (Wright et al., 1995). Finally, by performing transactivation assays in NIH 3T3 fibroblasts, we found that TRK–MET receptors induced a transcriptional response, whereas these cells did not respond to SF/HGF, suggesting that this response is independent of the presence of functional endogenous MET receptors (Figure 1C). It is therefore unlikely that TRK–MET functions through indirect recruitment of proteins via endogenous MET.
A most likely interpretation of our data is that the mutated MET receptor can still recruit and phosphorylate original proteins. To detect the MET-associated and -phosphorylated proteins, a sensitive association–kinase assay was performed from MDCK cells transiently transfected by the TRK–MET receptors. In cells transfected with the WT receptor, at least five MET-associated phosphorylated bands (molecular masses of ∼250, 230, 110, 80, and 70 kDa) were detected, which were not observed in nontransfected cells. The phosphorylated bands at ∼110, 80, and 70 kDa could correspond to known signaling proteins, i.e., GAB1 (110 kDa), PI3K (85 kDa), SHP2 (80 kDa), and the high-molecular-weight form of SHC (66 kDa). In contrast, the phosphorylated bands of ∼250 and 230 kDa did not correspond to known ligands and did not comigrate (our unpublished results) in SDS-PAGE with the ∼220- and 185-kDa tyrosine-phosphorylated proteins previously identified in MDCK1 cells, an MDCK cell line constitutively dissociated (Webb et al., 1996). In cells transfected with the mutated receptor, the ∼220-, 110-, 80-, and 70-kDa MET-associated bands were lost, whereas the ∼250-kDa band was not. This result suggests that the MET receptor is able to bind and phosphorylate a protein independently of the phosphorylation of the C-terminal residues. Further experiments will be aimed to identify this protein and its possible implication in biological effects transmitted by the MET receptor.
RAS Signaling Transmitted by Y13–16F MET Is Sufficient to Promote Cell Motility but Not Cell Morphogenesis
We investigated cell morphogenesis and scattering transmitted by the chimeric TRK–MET receptors. We found that the mutated receptor was unable to promote branching morphogenesis of MDCK cells. This lost capacity is in agreement with the conclusion of previous studies performed in three-dimensional cultures in collagen gels (Zhu et al., 1994; Weidner et al., 1995; Fournier et al., 1996; Sachs et al., 1996). In the present study, we also demonstrated that the morphogenetic capacity of MDCK cells can be investigated on Matrigel gels; this assay allows the formation of a cellular network by branching morphogenesis over 24 h, a time scale comparable to the one used in a classical scattering assay (Zhu et al., 1994; Weidner et al., 1995; present study), whereas branching morphogenesis in collagen gels requires 1–2 wk.
In contrast to cell morphogenesis, the measurement of cell scattering has given contradictory results as reported by Ponzetto et al. (1994), Zhu et al. (1994), Weidner et al. (1995), and Sachs et al. (1996). For example, the mutation of the sole Y15 residue resulted in the abolition of cell scattering in one study (Zhu et al., 1994), whereas mutations of all four Y13–16 residues reduced, but did not abolish, cell scattering in others (Weidner et al., 1995; Sachs et al., 1996). Our present data are in agreement with these last results; we found that the mutations of the four C-terminal tyrosine residues did not impair the ability of the mutated receptor to transmit cell scattering. In contrast, our results contradict those of Zhu et al. (1994) and Ponzetto et al. (1994), which led to the demonstration that single (Y15) or double mutations (Y14 and Y15) abolished MET receptor-mediated cell scattering. A possible explanation of these contradictory results is suggested by the difficulty in quantifying this response. We circumvented the problem by confirming the cell scattering activity of the mutated MET receptor on representative cell populations and in two different assays (Figure 3, A and B).
The scattering response obtained with WT and Y13–16F TRK–MET receptors correlated with RAS-dependent transcriptional activation of promoters containing ETS/AP1-binding sites. The fact that a pharmacological inhibitor of MEK abolished cell scattering in the mutated cell line demonstrated the implication of this downstream pathway of RAS in the biological response. We propose that in these cell lines, RAS activation contributes to cell scattering by its ability to induce transcriptional activation of cellular gene promoters. These include the promoters of the uPA and collagenase genes. These proteases belong to a complex enzymatic cascade that degrades the extracellular matrix and can contribute to the motility of the cells.
It is still a matter of debate whether cell scattering and morphogenesis are distinct biological activities, occurring through different cytoplasmic effectors and signaling pathways. Nonetheless, when measurements of cell scattering and morphogenesis were established in parallel, it was found that any reduction in cell scattering correlated with a loss in cell morphogenesis, whereas the inverse relationship was not found (Weidner et al., 1995; Sachs et al., 1996; present study). Furthermore, morphogenetic activity of MET can be transferred onto a scattering receptor, TRK-A, by fusing to it the C-terminal tail of MET containing the Y14-Y15 tyrosine docking site (Sachs et al., 1996). These results indicate that the signaling pathways leading to cell scattering are necessary for cell morphogenesis and/or that morphogenesis requires stronger signaling than scattering. Accordingly, a possible interpretation of our data is that RAS signaling mediated by mutated TRK–MET receptor was sufficient to induce cell scattering, but not cell morphogenesis.
The current model for signal transduction by the MET receptor postulates that the C-terminal tyrosine residues of MET is a unique multifunctional docking site. After phosphorylation of its C-terminal tyrosine residues, various signaling proteins are recruited and activated, which in turn activate signaling pathways and biological effects of SF/HGF, including cell scattering and morphogenesis. We propose that the MET receptor can promote signaling both by recruitment of proteins via the C-terminal docking site and by an additional mechanism, which leads to efficient activation of the RAS pathway and cell scattering.
ACKNOWLEDGMENTS
We thank Dr. Sydonia Rayter for the dominant negative form of RAS, Dr. Philippe Chavrier for the dominant negative form of CDC42, Dr. Michael Ostrowski for the uPA-luciferase plasmid, and Dr. Andrew Cato for the collagenase-luciferase plasmid. We also thank Dr. Jean Coll, Dr. Raymond Pierce, and Dr. Catherine Duggan for critical reading of the manuscript. This work was supported by the Institut Pasteur de Lille and the Centre National de la Recherche Scientifique and by grants from the Ligue Nationale contre le Cancer and the Association pour la recherche contre le Cancer.
REFERENCES
- Bardelli A, Longati P, Gramaglia D, Stella MC, Comoglio PM. Gab1 coupling to the HGF/Met receptor multifunctional docking site requires binding of Grb2 and correlates with the transforming potential. Oncogene. 1997;15:3103–3111. doi: 10.1038/sj.onc.1201561. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W. Molecular aspects of mesenchymal-epithelial interactions. Annu Rev Cell Biol. 1993;9:511–540. doi: 10.1146/annurev.cb.09.110193.002455. [DOI] [PubMed] [Google Scholar]
- Boccaccio C, Ando M, Tamagnone L, Bardelli A, Michieli P, Battistini C, Comoglio PM. Induction of epithelial tubules by growth factor HGF depends on the STAT pathway. Nature. 1998;391:285–288. doi: 10.1038/34657. [DOI] [PubMed] [Google Scholar]
- Bottaro DP, Rubin JS, Faletto DL, Chan AM, Kmiecik TE, Vande Woude G, Aaronson SA. Identification of the hepatocyte growth factor receptor as the c-met proto-oncogene product. Science. 1991;251:802–804. doi: 10.1126/science.1846706. [DOI] [PubMed] [Google Scholar]
- Buday L, Downward J. Epidermal growth factor regulates p21ras through the formation of a complex of receptor, Grb2 adapter protein, and Sos nucleotide exchange factor. Cell. 1993;73:611–620. doi: 10.1016/0092-8674(93)90146-h. [DOI] [PubMed] [Google Scholar]
- Fafeur V, Tulasne D, Quéva C, Vercamer C, Dimster V, Mattot V, Stéhelin D, Desbiens X, Vandenbunder B. The Ets1 transcription factor is expressed during epithelial-mesenchymal transitions in the chick embryo and is activated in scatter factor-stimulated MDCK epithelial cells. Cell Growth & Differ. 1997;8:655–665. [PubMed] [Google Scholar]
- Fournier TM, Kamikura D, Teng K, Park M. Branching tubulogenesis but not scatter of Madin-Darby canine kidney cells requires a functional grb2 binding site in the met receptor tyrosine kinase. J Biol Chem. 1996;271:22211–22217. doi: 10.1074/jbc.271.36.22211. [DOI] [PubMed] [Google Scholar]
- Gale NW, Kaplan S, Lowenstein EJ, Schlessinger J, Bar SD. Grb2 mediates the EGF-dependent activation of guanine nucleotide exchange on Ras. Nature. 1993;363:88–92. doi: 10.1038/363088a0. [DOI] [PubMed] [Google Scholar]
- Gilles F, Raes MB, Stéhelin D, Vandenbunder B, Fafeur V. The c-ets-1 proto-oncogene is a new early-response gene differentially regulated by cytokines and growth factors in human fibroblasts. Exp Cell Res. 1996;222:370–378. doi: 10.1006/excr.1996.0046. [DOI] [PubMed] [Google Scholar]
- Giordano S, Ponzetto C, Di RM, Cooper CS, Comoglio PM. Tyrosine kinase receptor indistinguishable from the c-met protein. Nature. 1989;339:155–156. doi: 10.1038/339155a0. [DOI] [PubMed] [Google Scholar]
- Gutman A, Wasylyk B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990;9:2241–2246. doi: 10.1002/j.1460-2075.1990.tb07394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holgado MM, Emlet DR, Moscatello DK, Godwin AK, Wong AJ. A Grb2-associated docking protein in EGF- and insulin-receptor signaling. Nature. 1996;379:560–564. doi: 10.1038/379560a0. [DOI] [PubMed] [Google Scholar]
- Hu Q, Klippel A, Muslin AJ, Fantl WJ, Williams LT. Ras-dependent induction of cellular responses by constitutively active phosphatidylinositol-3 kinase. Science. 1995;268:100–102. doi: 10.1126/science.7701328. [DOI] [PubMed] [Google Scholar]
- Klein-Soyer C, Bereetz A, Millon-Collard R, Abecassis J, Cazenave JP. A simple in vitro model of mechanical injury of confluent cultured endothelial cells to study quantitatively the repair process. Thromb Hemost. 1986;56:232–235. [PubMed] [Google Scholar]
- Li N, Batzer A, Daly R, Yajnik V, Skolnik E, Chardin P, Bar SD, Margolis B, Schlessinger J. Guanine-nucleotide-releasing factor hSos1 binds to Grb2 and links receptor tyrosine kinases to Ras signaling. Nature. 1993;363:85–88. doi: 10.1038/363085a0. [DOI] [PubMed] [Google Scholar]
- Li N, Schlessinger J, Margolis B. Autophosphorylation mutants of the EGF-receptor signal through auxiliary mechanisms involving SH2 domain proteins. Oncogene. 1994;9:3457–3465. [PubMed] [Google Scholar]
- Longati P, Bardelli A, Ponzetto C, Naldini L, Comoglio PM. Tyrosines1234–1235 are critical for activation of the tyrosine kinase encoded by the MET proto-oncogene (HGF receptor) Oncogene. 1994;9:49–57. [PubMed] [Google Scholar]
- Matsumoto K, Nakamura T. Emerging multipotent aspects of hepatocyte growth factor. J Biochem. 1996;119:591–600. doi: 10.1093/oxfordjournals.jbchem.a021283. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Dikic I, Sorokin A, Burgess WH, Jaye M, Schlessinger J. Identification of six novel autophosphorylation sites on fibroblast growth factor receptor 1 and elucidation of their importance in receptor activation and signal transduction. Mol Cell Biol. 1996;16:977–989. doi: 10.1128/mcb.16.3.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Matsumoto K, Nakamura T, Orci L. Identification of a fibroblast-derived epithelial morphogen as hepatocyte growth factor. Cell. 1991;67:901–908. doi: 10.1016/0092-8674(91)90363-4. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nawa K, Ichihara A. Partial purification and characterization of hepatocyte growth factor from serum of hepatectomized rats. Biochem Biophys Res Commun. 1984;122:1450–1459. doi: 10.1016/0006-291x(84)91253-1. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Nishizawa T, Hagiya M, Seki T, Shimonishi M, Sugimura A, Tashiro K, Shimizu S. Molecular cloning and expression of human hepatocyte growth factor. Nature. 1989;342:440–443. doi: 10.1038/342440a0. [DOI] [PubMed] [Google Scholar]
- Naldini L, et al. Scatter factor and hepatocyte growth factor are indistinguishable ligands for the MET receptor. EMBO J. 1991;10:2867–2878. doi: 10.1002/j.1460-2075.1991.tb07836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L, Holgado MM, Maroun C, Fixman ED, Kamikura D, Fournier T, Charest A, Tremblay ML, Wong AJ, Park M. Association of the multisubstrate docking protein Gab1 with the hepatocyte growth factor receptor requires a functional Grb2 binding site involving tyrosine 1356. J Biol Chem. 1997;272:20811–20819. doi: 10.1074/jbc.272.33.20811. [DOI] [PubMed] [Google Scholar]
- Park M, Dean M, Kaul K, Braun MJ, Gonda MA, Vande Woude G. Sequence of MET proto-oncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc Natl Acad Sci USA. 1987;84:6379–6383. doi: 10.1073/pnas.84.18.6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci G, et al. The motogenic and mitogenic responses to HGF are amplified by the SHC adaptor protein. Oncogene. 1995;10:1631–1638. [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Maina F, Longati P, Panayotou G, Dhand R, Waterfield MD, Comoglio PM. A novel recognition motif for phosphatidylinositol 3-kinase binding mediates its association with the hepatocyte growth factor/scatter factor receptor. Mol Cell Biol. 1993;13:4600–4608. doi: 10.1128/mcb.13.8.4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzetto C, Bardelli A, Zhen Z, Maina F, Zonca PD, Giordano S, Graziani A, Panayotou G, Comoglio PM. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell. 1994;77:261–271. doi: 10.1016/0092-8674(94)90318-2. [DOI] [PubMed] [Google Scholar]
- Ponzetto C, Zhen Z, Audero E, Maina F, Bardelli A, Basile ML, Giordano S, Narsimhan R, Comoglio P. Specific uncoupling of GRB2 from the met receptor—differential effects on transformation and motility. J Biol Chem. 1996;271:14119–14123. doi: 10.1074/jbc.271.24.14119. [DOI] [PubMed] [Google Scholar]
- Rahimi N, Tremblay E, Elliott B. Phosphatidylinositol 3-kinase activity is required for hepatocyte growth factor-induced mitogenic signals in epithelial cells. J Biol Chem. 1996;271:24850–24855. doi: 10.1074/jbc.271.40.24850. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Rorth P, Nerlov C, Blasi F, Johnsen M. Transcription factor PEA3 participates in the induction of urokinase plasminogen activator transcription in murine keratinocytes stimulated with epidermal growth factor or phorbol-ester. Nucleic Acids Res. 1990;18:5009–5017. doi: 10.1093/nar/18.17.5009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs M, Weidner KM, Brinkmann V, Walther I, Obermeier A, Ullrich A, Birchmeier W. Motogenic and morphogenic activity of epithelial receptor tyrosine kinases. J Cell Biol. 1996;133:1095–1107. doi: 10.1083/jcb.133.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J. SH2/SH3 signaling proteins. Curr Opin Genet Dev. 1994;4:25–30. doi: 10.1016/0959-437x(94)90087-6. [DOI] [PubMed] [Google Scholar]
- Schneikert J, Peterziel H, Defossez PA, Klocker H, de Launoit Y, Bato AC. Androgen receptor-Ets protein interaction: a novel mechanism for steroid hormone mediated down-modulation of matrix-metalloproteinase expression. J Biol Chem. 1996;271:23907–23913. doi: 10.1074/jbc.271.39.23907. [DOI] [PubMed] [Google Scholar]
- Stacey KJ, Fowles LF, Colman MS, Ostrowski MC, Hume DA. Regulation of urokinase-type plasminogen activator gene transcription by macrophage colony-stimulating factor. Mol Cell Biol. 1995;15:3430–3441. doi: 10.1128/mcb.15.6.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens RM, Loeb DM, Copeland TD, Pawson T, Greene LA, Kaplan DR. Trk receptors use redundant signal transduction pathways involving SHC and PLC-gamma 1 to mediate NGF responses. Neuron. 1994;12:691–705. doi: 10.1016/0896-6273(94)90223-2. [DOI] [PubMed] [Google Scholar]
- Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Taylor IC, Roy S, Varmus HE. Overexpression of Sky receptor tyrosine kinase at the cell surface or in the cytoplasm results in ligand-independent activation. Oncogene. 1995;11:2619–2626. [PubMed] [Google Scholar]
- Tsafarty I, Resau JH, Rulong I, Keydar D, Faletto DL, Vande Woude G. The met proto-oncogene receptor and lumen formation. Science. 1992;257:1258–1261. doi: 10.1126/science.1387731. [DOI] [PubMed] [Google Scholar]
- Valius M, Kazlauskas A. Phospholipase C-γ and phosphatidylinositol 3 kinase are the downstream mediators of the PDGF receptor’s mitogenic signal. Cell. 1993;73:321–334. doi: 10.1016/0092-8674(93)90232-f. [DOI] [PubMed] [Google Scholar]
- Van der Geer P, Hunter T, Lindberg RA. Receptor protein-tyrosine kinases and their signal transduction pathways. Annu Rev Cell Biol. 1994;10:251–337. doi: 10.1146/annurev.cb.10.110194.001343. [DOI] [PubMed] [Google Scholar]
- Wasylyk B, Wasylyk C, Flores P, Begue A, Leprince D, Stéhelin D. The c-ets proto-oncogenes encode transcription factors that cooperate with c-Fos and c-Jun for transcriptional activation. Nature. 1990;346:191–193. doi: 10.1038/346191a0. [DOI] [PubMed] [Google Scholar]
- Webb CP, Lane K, Dawson AP, Vandewoude GF, Warn RM. C-Met signaling in an HGF/SF-insensitive variant MDCK cell line with constitutive motile/invasive behavior. J Cell Sci. 1996;109:2371–2381. doi: 10.1242/jcs.109.9.2371. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Dicesare S, Sachs M, Brinkmann V, Behrens J, Birchmeier W. Interaction between gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature. 1996;384:173–176. doi: 10.1038/384173a0. [DOI] [PubMed] [Google Scholar]
- Weidner KM, Sachs M, Birchmeier W. The Met receptor tyrosine kinase transduces mobility, proliferation, and morphogenic signals of scatter factor/hepatocyte growth factor in epithelial cells. J Cell Biol. 1993;121:145–154. doi: 10.1083/jcb.121.1.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner KM, Sachs M, Birchmeier W. Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the met receptor in epithelial cells. Proc Natl Acad Sci USA. 1995;92:2597–2601. doi: 10.1073/pnas.92.7.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright JD, Reuter CW, Weber MJ. An incomplete program of cellular tyrosine phosphorylations induced by kinase-defective epidermal growth factor receptors. J Biol Chem. 1995;270:12085–12093. doi: 10.1074/jbc.270.20.12085. [DOI] [PubMed] [Google Scholar]
- Zhu H, Naujokas MA, Fixman ED, Torossian K, Park M. Tyrosine 1356 in the carboxy-terminal tail of the HGF/SF receptor is essential for the transduction of signals for cell motility and morphogenesis. J Biol Chem. 1994;269:29943–29948. [PubMed] [Google Scholar]