Abstract
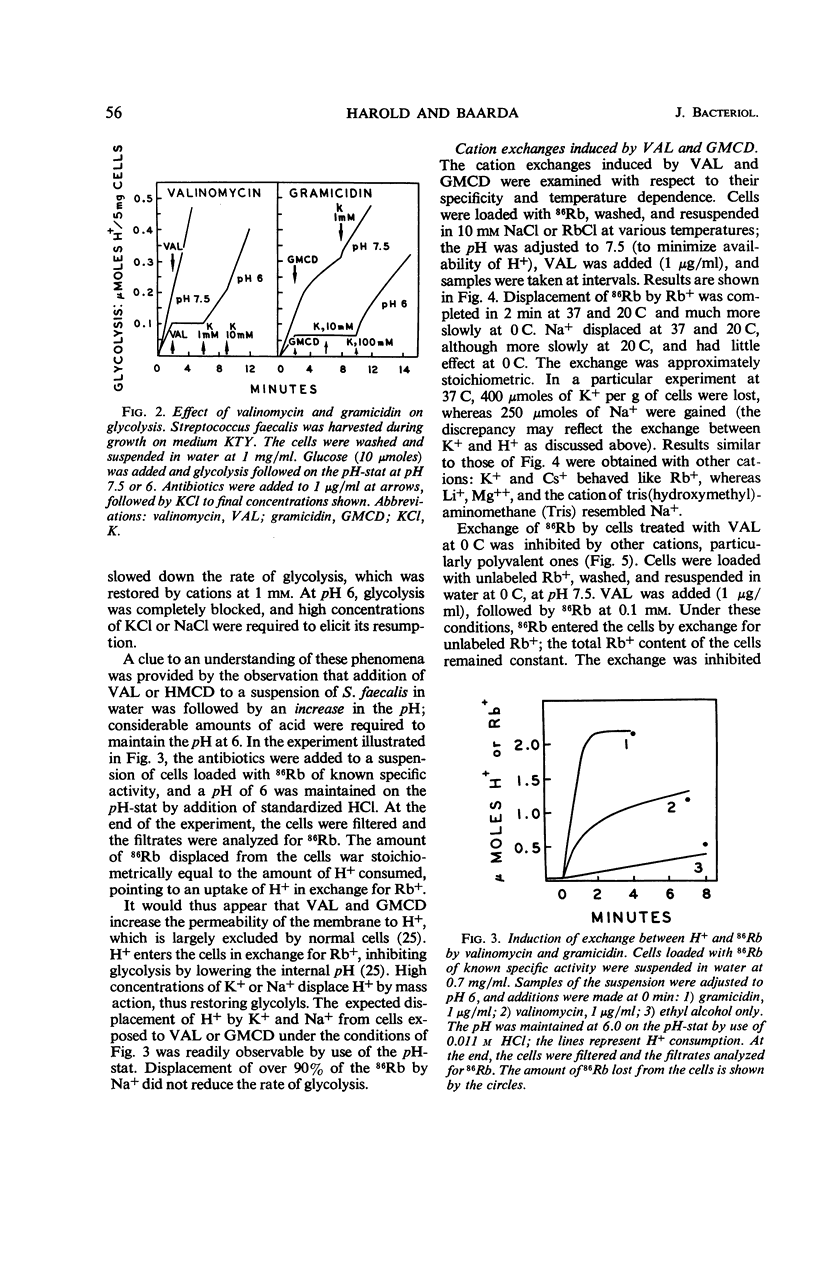
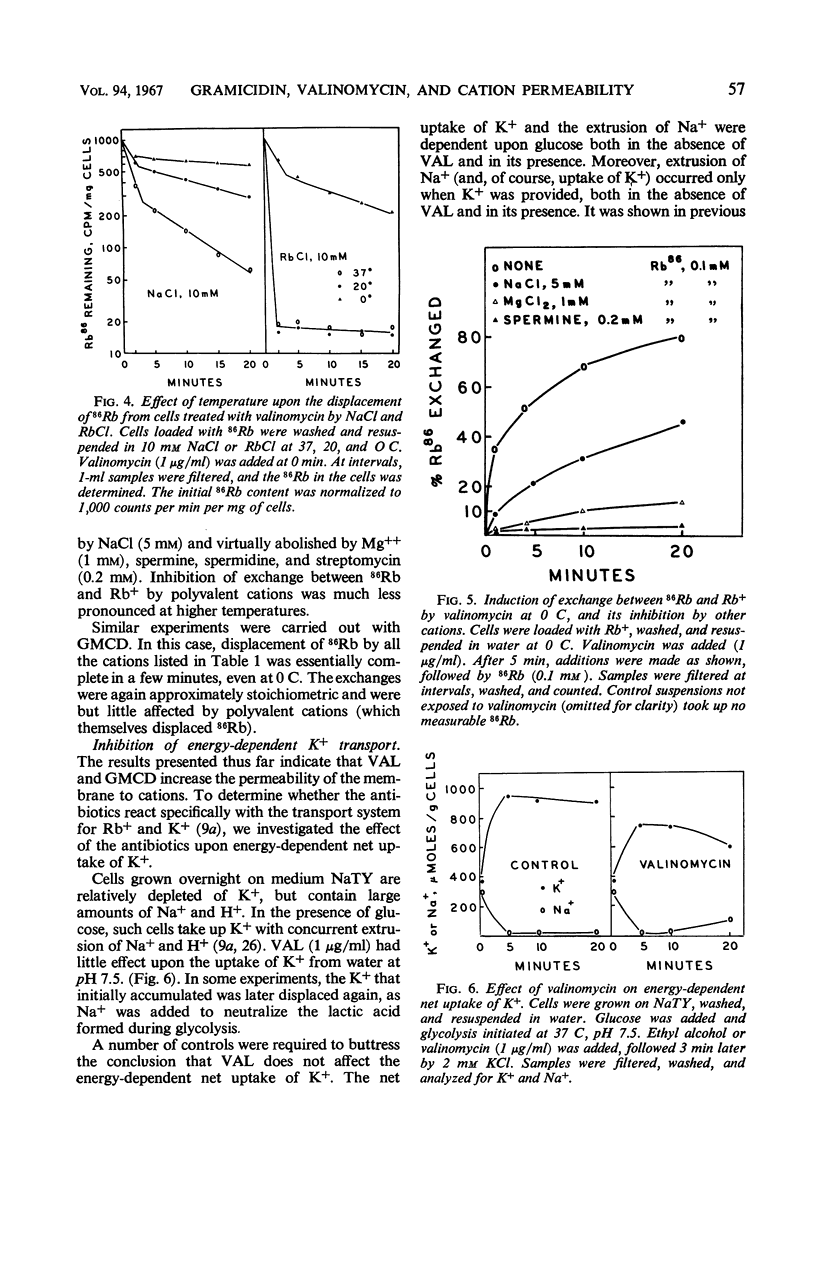
Gramicidin and valinomycin in concentrations of 10−7 and 10−6m, respectively, inhibited the growth of Streptococcus faecalis. Inhibition of growth was associated with loss of Rb+ and K+ from the cells, and could be reversed by addition of excess K+. Cells treated with these antibiotics exhibited greatly increased permeability to certain cations; no effect was observed on the penetration of other small molecules. Unlike normal cells, cells treated with gramicidin rapidly lost internal Rb+ by passive exchange with external cations, including H+, all monovalent alkali metals, NH4+, Mg++, and tris(hydroxymethyl)aminomethane. Exchange was rapid even at 0 C and was independent of energy metabolism. The effect of valinomycin was more selective. Cellular Rb+ was rapidly displaced by external H+, K+, Rb+, and Cs+; other cations were less effective. The exchange was independent of metabolism but strongly affected by temperature. Under certain conditions, polyvalent cations inhibited exchange between 86Rb and Rb+ induced by valinomycin. The antibiotic apparently neither stimulates nor inhibits the energy-dependent K+ pump of S. faecalis, but exerts its effect on the passive permeability of the membrane to cations. The increased permeability to specific cations induced by gramicidin and valinomycin is a sufficient explanation for the inhibition of growth, glycolysis, and other processes.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. Metabolically dependent penetration of oligosaccharides into bacterial cells and protoplasts. J Biol Chem. 1960 May;235:1281–1285. [PubMed] [Google Scholar]
- ABRAMS A. Reversible metabolic swelling of bacterial protoplasts. J Biol Chem. 1959 Feb;234(2):383–388. [PubMed] [Google Scholar]
- CHAPPELL J. B., CROFTS A. R. GRAMICIDIN AND ION TRANSPORT IN ISOLATED LIVER MITOCHONDRIA. Biochem J. 1965 May;95:393–402. doi: 10.1042/bj0950393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CIRILLO V. P., HARSCH M., LAMPEN J. O. ACTION OF THE POLYENE ANTIBIOTICS FILIPIN, NYSTATIN AND N-ACETYLCANDIDIN ON THE YEAST CELL MEMBRANE. J Gen Microbiol. 1964 May;35:249–259. doi: 10.1099/00221287-35-2-249. [DOI] [PubMed] [Google Scholar]
- GALE E. F. Assimilation of amino acids by Gram-positive bacteria and some actions of antibiotics thereon. Adv Protein Chem. 1953;8:285–391. doi: 10.1016/s0065-3233(08)60094-7. [DOI] [PubMed] [Google Scholar]
- GALE E. F. MECHANISMS OF ANTIBIOTIC ACTION. Pharmacol Rev. 1963 Sep;15:481–530. [PubMed] [Google Scholar]
- HAROLD F. M., HAROLD R. L., ABRAMS A. A MUTANT OF STREPTOCOCCUS FAECALIS DEFECTIVE IN PHOSPHATE UPTAKE. J Biol Chem. 1965 Jul;240:3145–3153. [PubMed] [Google Scholar]
- HAROLD F. M. STABILIZATION OF STREPTOCOCCUS FAECALIS PROTOPLASTS BY SPERMINE. J Bacteriol. 1964 Nov;88:1416–1420. doi: 10.1128/jb.88.5.1416-1420.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. J., Cockrell R., Pressman B. C. Induced and spontaneous movements of potassium ions into mitochondria. Biochem J. 1966 Apr;99(1):200–213. doi: 10.1042/bj0990200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUBIN M. INTRACELLULAR POTASSIUM AND CONTROL OF PROTEIN SYNTHESIS. Fed Proc. 1964 Sep-Oct;23:994–1001. [PubMed] [Google Scholar]
- Mach B., Slayman C. W. Mode of action of tyrocidine on Neupospora. Biochim Biophys Acta. 1966 Aug 24;124(2):351–361. doi: 10.1016/0304-4165(66)90198-x. [DOI] [PubMed] [Google Scholar]
- Newton B. A. Mechanisms of antibiotic action. Annu Rev Microbiol. 1965;19:209–240. doi: 10.1146/annurev.mi.19.100165.001233. [DOI] [PubMed] [Google Scholar]
- Ogata E., Rasmussen H. Valinomycin and mitochondrial ion transport. Biochemistry. 1966 Jan;5(1):57–66. doi: 10.1021/bi00865a009. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Induced active transport of ions in mitochondria. Proc Natl Acad Sci U S A. 1965 May;53(5):1076–1083. doi: 10.1073/pnas.53.5.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor C. W. STABILIZATION OF PROTOPLASTS AND SPHEROPLASTS BY SPERMINE AND OTHER POLYAMINES. J Bacteriol. 1962 May;83(5):1101–1111. doi: 10.1128/jb.83.5.1101-1111.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZARLENGO M., ABRAMS A. Selective penetration of ammonia and alkylamines into Streptococcus fecalis and their effect on glycolysis. Biochim Biophys Acta. 1963 Apr 2;71:65–77. doi: 10.1016/0006-3002(63)90986-7. [DOI] [PubMed] [Google Scholar]
- Zarlengo M. H., Schultz S. G. Cation transport and metabolism in Streptococcus fecalis. Biochim Biophys Acta. 1966 Oct 10;126(2):308–320. doi: 10.1016/0926-6585(66)90068-9. [DOI] [PubMed] [Google Scholar]



