Abstract
Signal transduction by transforming growth factor β (TGFβ) coordinates physiological responses in diverse cell types. TGFβ signals via type I and type II receptor serine/threonine kinases and intracellular Smad proteins that regulate transcription. Strength and duration of TGFβ signaling is largely dependent on a negative-feedback program initiated during signal progression. We have identified an inducible gene target of TGFβ/Smad signaling, the salt-inducible kinase (SIK), which negatively regulates signaling together with Smad7. SIK and Smad7 form a complex and cooperate to down-regulate the activated type I receptor ALK5. We further show that both the kinase and ubiquitin-associated domain of SIK are required for proper ALK5 degradation, with ubiquitin functioning to enhance SIK-mediated receptor degradation. Loss of endogenous SIK results in enhanced gene responses of the fibrotic and cytostatic programs of TGFβ. We thus identify in SIK a negative regulator that controls TGFβ receptor turnover and physiological signaling.
Introduction
The profound roles that TGFβ plays during embryogenesis, adult tissue homeostasis, and disease pathogenesis necessitate deeper understanding of mechanisms that regulate this signaling pathway. Signaling occurs via the TGFβ type II receptor (TβRII) that trans-phosphorylates TβRI, also known as activin receptor-like kinase (ALK) 5. Activated ALK5 phosphorylates receptor-regulated Smads (Smad2 and Smad3), promotes their association with Smad4, and leads to regulation of transcription (Feng and Derynck, 2005).
Smad7, a negative regulator of TGFβ/Smad signaling, is an immediate early gene target of the pathway (for review see Itoh and ten Dijke, 2007). Smad7 binds to ALK5, competing with Smad2/3 phosphorylation and mediating receptor ubiquitination, mechanisms which link to the process of TGFβ receptor internalization and lysosomal degradation (Di Guglielmo et al., 2003).
We have identified gene targets of Smad signaling (Kowanetz et al., 2004). A highly regulated gene, SNF1LK (sucrose nonfermented 1-like kinase) or salt-inducible kinase 1 (SIK1, hereby abbreviated as SIK), encodes a serine/threonine kinase. SIK is one of 14 AMP-activated protein kinases that can be phosphorylated by the tumor suppressor LKB1 (Alessi et al., 2006). SIK expression is induced during cardiogenesis and skeletal muscle differentiation (Ruiz et al., 1994) or in adrenal glands, leading to steroidogenesis (Okamoto et al., 2004).
We uncover a new functional role of SIK in the TGFβ pathway. SIK induction is necessary for physiological TGFβ signaling. Ubiquitin promotes association of SIK to Smad7, which down-regulates ALK5. Thus, SIK defines a new negative-feedback loop initiated by incoming Smad signals.
Results and discussion
TGFβ induces SIK in a Smad-dependent manner
We have reported Smad4-dependant gene profiles in response to TGFβ1 or bone morphogenetic protein (BMP) 7 using human breast carcinoma MDA-MB-468 cells that lack both Smad4 gene copies (Kowanetz et al., 2004). After reconstitution with Smad4, TGFβ1 or BMP7 rapidly induced SIK mRNA, followed by slow decrease (Fig. 1, A and B). In human HaCaT keratinocytes, TGFβ1 induced and sustained SIK mRNA and protein levels (Fig. 1, C and D; and Fig. S1 A, available at http://www.jcb.org/cgi/content/full/jcb.200804107/DC1). Endogenous TGFβ1-induced SIK protein showed punctate nuclear, cytoplasmic, and peripheral localization (Fig. 1 E). SIK represents a new gene target of TGFβ/BMP7 Smad signaling. Interestingly, the Caenorhabditis elegans orthologue of SIK, Kin-29, regulates chemosensory neuronal signaling and body size, a process which is dependent on TGFβ/Smad (Lanjuin and Sengupta, 2002; Maduzia et al., 2005), which is in agreement with our data.
Figure 1.
Smad signaling induces endogenous SIK. (A) cDNA microarray analysis of SIK mRNA in Smad4-deficient MDA-MB-468 cells after infection with adenovirus expressing LacZ or Smad4 and stimulation with 2 ng/ml TGFβ1 or 300 ng/ml BMP7. Normalized mean expression values with error bars from triplicate microarray expressions are shown (arbitrary units). (B and C) Semiquantitative RT-PCR analysis of SIK and GAPDH mRNA in MDA-MB-468 cells as in A (B) or in response to 2 ng/ml TGFβ1 in HaCaT cells (C). Amplified cDNA sizes are in bp. (D) SIK and α-tubulin loading control protein profiles in response to 5 ng/ml TGFβ1 in HaCaT cells. Protein sizes are in kilodaltons. (E) Induction of nuclear and cytoplasmic levels of endogenous SIK by 5 ng/ml TGFβ1 in HaCaT cells (bar, 10 μm).
SIK down-regulates activated ALK5
Gain-of-function experiments with transfected SIK showed specific down-regulation of ALK5 after TGFβ1 stimulation, whereas minor effects were scored without stimulation (Fig. 2 A). SIK did not down-regulate Smad7 or GFP (Fig. S1 B), excluding translational inhibition or induction of proteolysis.
Figure 2.
SIK down-regulates activated ALK5. (A) Immunoblot of ALK5 expressed in COS1 cells together with TβRII and stimulation or not with 2 ng/ml TGF-β1 for 12 h in the absence or presence of increasing wild-type GFP-SIK amounts. (B and C) Pulse-chase analysis of CA-ALK5, KR-ALK5, and CIN85 in the absence (−) or presence (+) of cotransfected SIK (S). A 30-min pulse was followed by chase for the indicated times. Treatment with proteasomal (LLnL 200 μM; S + L) or lysosomal (chloroquine 400 μM; S + Q) inhibitor during the chase is shown in C. Radioactive quantitation of ALK5 or CIN85 protein is plotted relative to the pulse signal. Representative data from three independent repeats are shown. Protein autoradiograms are shown below each graph. (D) Immunoblot of CA-ALK5 in COS1 cells cotransfected with wild-type and mutant SIK constructs. (E, top) quantitative real-time RT-PCR analysis of SIK mRNA in HaCaT cells after transfection with control (siLuc) and specific (siSIK) siRNAs and 8 h of stimulation with 5 ng/ml TGFβ1. Mean fold induction of TGFβ-stimulated SIK (black bars) relative to unstimulated levels (gray bars) are plotted, with standard errors determined from triplicate samples. (E, bottom) Immunoblot of endogenous SIK, ALK5, and α-tubulin loading control in HaCaT total cell lysates (TCL) prepared as per mRNA analysis. (F) Mv1Lu cells transfected with control (siLuc) or specific (siSIK) siRNA were incubated with radio-TGFβ1 before cross-linking and incubation at 37°C for the indicated time periods. Cell surface receptor autoradiograms show the three TGF-β receptors (TβR) marked as type I, II, and III, and the unbound ligand (TGFβ) migrating at the bottom. Protein sizes are in kilodaltons. Black lines indicate that intervening lanes have been spliced out.
SIK affected ALK5 turnover as it reduced the half-life of a constitutively active (CA) ALK5 from 5.5 to 2.8 h without affecting a KR- (kinase-dead) ALK5 or an unrelated adaptor protein CIN85 (Fig. 2 B). Proteasomal (LLnL) and lysosomal (chloroquine) inhibitors stabilized the ALK5 levels in the presence of SIK (Fig. 2 C), suggesting both proteasomal and lysosomal mechanisms in ALK5 down-regulation by SIK.
SIK has an N-terminal kinase domain with lysine 56 binding to ATP and a central ubiquitin-associated (UBA) domain, which regulates conformation and kinase activity (Jaleel et al., 2006). A catalytically inactive (K56R) SIK or a deletion mutant lacking the UBA domain (ΔUBA) failed to down-regulate CA-ALK5 (Fig. 2 D). This suggests that both catalytic activity and UBA domain of SIK affect ALK5 turnover.
RNAi against SIK significantly enhanced endogenous ALK5 levels (Fig. 2 E). Increased presence of endogenous cell surface receptors measured with chemically cross-linked radioligand upon depletion of SIK further strengthened this evidence (Fig. 2 F, 0 h). Thus, endogenous SIK must regulate total ALK5 levels, which also affects cell surface receptor numbers, available for signaling. Receptor down-regulation was also slower after SIK RNAi. Significantly higher ligand-bound receptor levels were observed for up to 1 h of internalization compared with control (Fig. 2 F).
Because proteasomal and lysosomal inhibitors block ALK5 degradation (Fig. 2 C), we suggest that ALK5 turnover takes place in lysosomes. Proteasomes might promote trafficking to the lysosome, as has already been established for the EGF receptor (Longva et al., 2002; Alwan et al., 2003). How proteasomes regulate TGF-β receptor internalization and degradation remains unclear.
SIK associates and cooperates with Smad7 for ALK5 down-regulation
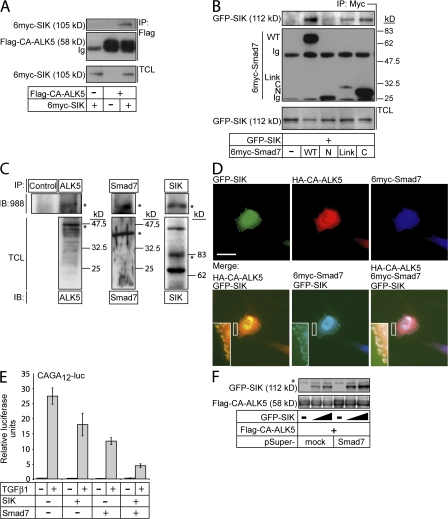
Down-regulation of ALK5 is mediated by Smad7 (for review see Itoh and ten Dijke, 2007). We reasoned that SIK could associate directly with ALK5 or Smad7. Coimmunoprecipitation assays showed that SIK formed complexes with both ALK5 and Smad7 (Fig. 3, A and B). To detect SIK–ALK5 complexes, we had to achieve low SIK expression and high CA-ALK5 levels, which resulted in active ALK5 signaling with small effects on receptor down-regulation. SIK bound stronger to the Smad7 linker and C-terminal (Mad homology 2) domains than to its N-terminal domain (Fig. 3 B). When endogenous human ALK5, Smad7, and SIK were immunoprecipitated from HaCaT lysates, SIK coprecipitated primarily with Smad7 and weakly with ALK5 (Fig. 3 C), confirming the evidence with transfected proteins. Thus, protein complexes between endogenous SIK, Smad7, and ALK5 occur in vivo.
Figure 3.
SIK associates and cooperates with Smad7 to down-regulate ALK5. (A) Coimmunoprecipitation of CA-ALK5 with wild-type (WT) SIK and TCL controls for SIK. Low levels of SIK coexpressed with high levels of CA-ALK5 allowed detection of SIK–ALK5 complexes with only small effects on receptor down-regulation. (B) Coimmunoprecipitation of wild-type SIK with wild type and various Smad7 domains (C, C-terminal; Link, linker; N, N-terminal). TCL controls for SIK and immunoglobulin chains (Ig) are shown. (C) Coimmunoprecipitation of endogenous SIK with endogenous ALK5 and Smad7. HaCaT cell lysates, pretreated with 50 μM MG132 and 5 ng/ml TGFβ1 overnight, were immunoprecipitated with anti-ALK5, anti-Smad7, and anti-SIK (988) antibodies, followed by immunoblotting with anti-SIK (988) antibody. TCL immunoblots with the three antibodies demonstrate specificity of each antiserum. Asterisks indicate specific protein bands and sizes are in kilodaltons. The top left panel (Mock and ALK5) was exposed four times longer than the top right panel (Smad7 and SIK). (D) Triple confocal immunofluorescence analysis of wild-type SIK, CA-ALK5, and wild-type Smad7 in transfected Mv1Lu cells. Insets show high magnifications of peripheral clusters just below the plasma membrane (bar, 10 μm). (E) SIK represses synergistically with Smad7 CAGA12 promoter induction by 0.5 ng/ml TGFβ1 for 6 h in HepG2 cells transfected with empty vector or Flag-SIK and Flag-Smad7. Normalized promoter activity is plotted as mean values from triplicate determinations with standard error bars. (F) Depletion of endogenous Smad7 stabilizes the CA-ALK5 receptor and wild-type SIK. Immunoblot of HEK293T cell extracts after transient transfection with shRNA vector pSuper-Smad7 or its empty (mock) version together with increasing amounts (triangles) of GFP-SIK and constant amount of CA-ALK5. The asterisks indicate nonspecific protein bands. Protein sizes are shown in kilodaltons.
Smad7 resides in the nucleus and moves to the cytoplasm in response to TGF-β (for review see Itoh and ten Dijke, 2007). SIK also resides in both nucleus and cytoplasm, and its localization is regulated by hormones in adrenal cells or by the 14–3-3 adaptor (Okamoto et al., 2004; Al-Hakim et al., 2005). In transfected TGFβ-sensitive Mv1Lu cells, SIK localized in nuclei and cytoplasm with pronounced punctate distribution proximal to the plasma membrane (Fig. 3 D), which is similar to endogenous SIK (Fig. 1 E). Colocalization of SIK, CA-ALK5, and Smad7 was primarily observed in peripheral clusters (Fig. 3 D, insets). Thus, SIK, Smad7, and ALK5 must form complexes in cytoplasmic regions proximal to the plasma membrane.
To verify the cooperation of Smad7 and SIK in negatively regulating TGFβ signaling, we measured the effects of SIK and Smad7 on the transcriptional induction of a Smad3/Smad4-specific promoter-reporter, CAGA12, by TGFβ1 (Fig. 3 E). SIK enhanced the inhibitory effect of Smad7 by more than twofold. Knockdown of endogenous Smad7 stabilized and rescued CA-ALK5 down-regulation by SIK, even though Smad7 depletion led to increased SIK levels (Fig. 3 F). Thus, SIK mediates down-regulation of signaling ALK5 receptor in a Smad7-dependent manner.
Ubiquitin and the UBA domain modulate ALK5 regulation by SIK
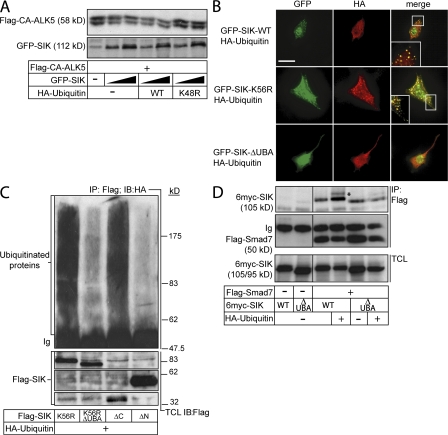
We examined whether ubiquitin affected the ability of SIK to facilitate ALK5 degradation (Fig. 4 A). Ubiquitin significantly enhanced the potency of CA-ALK5 down-regulation by SIK. A K48R ubiquitin mutant completely blocked this down-regulation. This implies that polyubiquitin chain formation via lysine 48 affects ALK5 degradation, which is compatible with a proteasome-dependent mechanism and inhibition of ALK5 degradation by LLnL (Fig. 2 D).
Figure 4.
Role of ubiquitin and the UBA domain of SIK in ALK5 down-regulation. (A) Immunoblot of CA-ALK5 in the presence of increasing doses of WT GFP-SIK and coexpression of WT or K48R mutant ubiquitin. (B) Confocal immunofluorescence analysis of wild-type or mutant SIK proteins cotransfected with HA-ubiquitin in Mv1Lu cells. Insets show higher magnifications of peripheral clusters below the plasma membrane (bar, 10 μm). (C) SIK lysate was mixed briefly with lysate for ubiquitinated proteins before immunoprecipitation. SIK mutant proteins were immunoprecipitated (anti-Flag) and ubiquitinated proteins are detected (anti-HA immunoblot). Total SIK levels (bottom) and immunoglobulin heavy chain (Ig) are shown. (D) Coimmunoprecipitation of wild-type Smad7 with wild-type myc-SIK or UBA deletion mutant in the absence or presence of ubiquitin. The asterisk indicates a slow migrating SIK species, possibly representing ubiquitinated SIK. TCL controls of SIK variants, the immunoglobulin heavy chain (Ig), and protein sizes in kilodaltons are shown. Black lines indicate that intervening lanes have been spliced out.
We examined SIK localization in relation to ubiquitin in Mv1Lu cells (Fig. 4 B). SIK distributed in nuclei, cytoplasm, and prominent peripheral structures, SIK-K56R showed more cytoplasmic enrichment, and both colocalized with cytoplasmic ubiquitin clusters. SIK lacking its UBA domain showed no colocalization, as it distributed diffusely in the cytoplasm, suggesting that SIK-ubiquitin clusters depend on the integrity of the UBA domain. These punctate structures were also enriched in 19S and 20S proteasomes (unpublished data).
We then expressed SIK and ubiquitin in different pools of cells before immunoprecipitation to measure association of SIK with ubiquitinated proteins but not SIK ubiquitination, per se (Fig. 4 C and Fig. S2 A, available at http://www.jcb.org/cgi/content/full/jcb.200804107/DC1). SIK readily coprecipitated ubiquitinated proteins of diverse sizes, confirming that they were distinct from ubiquitinated SIK. Wild-type and SIK-K56R bound to ubiquitinated proteins, whereas SIK-ΔUBA or a mutant lacking both kinase and UBA domains (ΔN) did not, suggesting that the SIK UBA domain is needed for binding to polyubiquitinated proteins (Fig. 4 C). C-terminal deletion of SIK, after its UBA domain, had no effect on binding ubiquitinated proteins (Fig. 4 C). Thus, SIK can associate with ubiquitinated proteins in cells.
Obvious ubiquitinated protein candidates to which SIK could bind were ALK5 and Smad7. Accordingly, the SIK–Smad7 complex was enhanced by coexpressing ubiquitin, whereas deletion of the UBA domain of SIK prohibited such enhancement (Fig. 4 D). A slow migrating SIK form was obvious in complex with Smad7 (Fig. 4 D, asterisk), which may represent ubiquitinated SIK. In contrast, neither ubiquitin nor UBA domain deletion had any effect on SIK–ALK5 complexes (Fig. S2 B). Ubiquitin therefore enhances association between SIK and Smad7, and the SIK UBA domain mediates formation of this ternary complex.
The SIK UBA domain was recently shown to not bind ubiquitin but to modulate SIK kinase activity in vitro (Jaleel et al., 2006). We also failed in demonstrating in vitro binding of recombinant mono- or oligoubiquitin chains to SIK (unpublished data). SIK immunopurified from mammalian cells clearly bound to ubiquitinated proteins via its UBA domain (Fig. 4 C and Fig. S2 A). This could reflect an in vivo modification or the requirement for another binding protein. Further studies will reveal the mechanism by which ubiquitin and the SIK UBA domain regulate its own function.
SIK negatively regulates TGFβ signaling
Stimulation of cells after endogenous SIK depletion enhanced the potency of TGF-β signaling (Fig. 5, A and B). Signaling was assessed by measuring mRNA levels of well characterized TGFβ gene targets, representing three distinct physiological programs: the fibrotic program (plasminogen activator inhibitor 1 [PAI-1], TGFβ inducible), the cytostatic program (cell cycle inhibitors p21 and p15, TGFβ inducible; transcriptional regulators c-myc and Id2, TGFβ repressible), and the negative-feedback program (Smad7, TGFβ-inducible; Fig. 5 B and Fig. S3, available at http://www.jcb.org/cgi/content/full/jcb.200804107/DC1). We also confirmed that SIK knockdown enhanced the magnitude of endogenous PAI-1 and p21 protein accumulation in response to TGFβ1 (Fig. 5 C). Finally, protein levels and pericellular localization of fibronectin, another TGFβ gene target from the fibrotic program, augmented significantly after depleting SIK (Fig. 5, D and E). Thus, induction of SIK by TGFβ represents a central step in negative regulation of TGFβ signaling.
Figure 5.
Endogenous SIK negatively regulates endogenous TGFβ signaling. (A) Immunoblot of endogenous SIK and α-tubulin loading control in HaCaT TCL after transfection with control (siLuc) and specific (siSIK) siRNAs and 12 h of stimulation with 5 ng/ml TGFβ1. The asterisk marks a nonspecific protein band. (B) Quantitative real-time RT-PCR analysis in HaCaT cells treated with siRNAs as in A and stimulated with 5 ng/ml TGFβ1 for 6 h (PAI-1 and p21) or 8 h (Smad7 and p15). Mean fold induction of TGFβ-stimulated mRNA levels (black bars) relative to unstimulated levels (gray bars) are plotted, with standard errors determined from triplicate or quadruplicate samples. Asterisks indicate significant difference (t test: P <0.05) in mRNA levels in cells transfected with siLuc versus siSIK siRNA and stimulated with TGFβ1. (C) Immunoblot of endogenous PAI-1, p21, and α-tubulin loading control in HaCaT cells treated as in A and stimulated with 5 ng/ml TGFβ1 for the indicated times. Black lines indicate that intervening lanes have been spliced out. (D) Immunofluorescence analysis of endogenous fibronectin in HaCaT cells treated as in A and stimulated with 5 ng/ml TGFβ1 for 12 h (bar, 10 μm). (E) Immunoblot of endogenous fibronectin and α-tubulin loading control in the same experimental setup as in D. Protein sizes are shown in kilodaltons. (F) Model of SIK as a negative regulator of TGFβ signaling. TGFβ receptors activate nuclear Smad complexes with transcription factors (TF) to mediate gene responses (green circles). Genes of the cytostatic (proliferation), differentiation, ECM, and negative-feedback programs are listed. Negative-feedback loop protein products assemble the machinery that inhibits signaling. SIK associates with the TβRI and Smad7, promoting internalization and termination of receptor signaling.
The identification of SIK as an immediate early gene induced by the Smad pathway led to important new insights into how TGFβ receptor signaling is negatively regulated (Fig. 5 F). SIK joins Smad7 in the negative-feedback loop of TGFβ signaling (for review see Itoh and ten Dijke, 2007). SIK and Smad7 interact, and ubiquitin enhances their complex. SIK also binds to ALK5 and down-regulates the receptor in a Smad7-dependent manner. This model applies to various epithelial cell models that are highly sensitive to TGFβ including keratinocytes and lung cells. Our study emphasizes the role of ubiquitin and proteasomes in regulation of TGFβ signaling by SIK. Our data also support a role for the kinase activity of SIK (Fig. 2 D). This offers the first evidence for a kinase/ubiquitin binding protein being involved in the down-regulation of a signaling receptor. Identifying possible SIK substrates within the ALK5–Smad7 complex and explaining their functional role in regulation of TGFβ signaling will be important. We provide evidence for lysosomal degradation of TGFβ receptors (Fig. 2 C). This is a relatively unexplored step during TGFβ receptor internalization. TGFβ receptor ubiquitination and proteasomal degradation is often reported (for review see Itoh and ten Dijke, 2007). A lysosomal pathway of receptor-ligand degradation would better fit to the classical mechanisms of receptor internalization and degradation. During this process, ubiquitin and proteasomes might act as intermediate processors of TGFβ receptor trafficking from the plasma membrane to the lysosome.
Evolutionary conservation of SIK from humans to C. elegans, and functional conservation of SIK as a regulator of homeostatic (this study) and developmental (Maduzia et al., 2005) pathways guided by TGFβ signaling opens new territories for exploration of such pathways across species. Characterizing the role of the Drosophila melanogaster SIK orthologue will greatly benefit this cause.
Because SIK is activated by the tumor suppressor kinase LKB1 (Alessi et al., 2006), our study suggests a role of LKB1 in processes of TGFβ signaling and receptor down-regulation. Thus, SIK being a component of the TβR–Smad7 complex provides a nodal point for crosstalk of the TGFβ pathway with LKB1 or AMP-activated protein kinase pathways. SIK therefore opens a new window for the elucidation of the process of down-regulation of serine/threonine kinase receptors.
Materials and methods
Reagents
HaCaT keratinocytes, MDA-MB-468, HEK293T and HepG2 carcinoma, COS1 and Mv1Lu cells, and culture conditions were as previously described (Kowanetz et al., 2004; Morén et al., 2005). Recombinant TGF-β1 was obtained from PeproTech. Anti–β-tubulin (T8535) and anti-Flag (M5) antibodies were obtained from Sigma-Aldrich; anti-GFP (A11122) from Invitrogen; anti–PAI-1 and anti-p21 (Cip1/WAF1) from BD Biosciences; anti-HA (Y-11), anti-TβRI (V-22), and anti–α-tubulin (TU-02) from Santa Cruz Biotechnology, Inc.; rabbit polyclonal anti–human SIK (988, raised against peptide CEEQDTQESLPSSTGRR mapping C-terminally from the UBA domain), anti-Smad7, and mouse monoclonal anti-myc (9E10) were made in house; rabbit polyclonal anti-CIN85(CT) was from I. Dikic (Goethe University, Frankfurt, Germany); secondary antibodies coupled to HRP from GE Healthcare; secondary antibodies coupled to FITC and TRITC from Dako; Alexa Fluor 546 from Invitrogen; and AMCA7 (7-amino-4-methylcoumarin-3-acetic acid) from Jackson ImmunoResearch Laboratories.
Human SIK-specific siRNA, ON-TARGET plus SMARTpool reagent L-003959-00, and control siRNA against the luciferase reporter vector pGL2 (X65324) were from Thermo Fisher Scientific. An EST clone BG719047 of human SIK complete cDNA was obtained from H. Takemori and M. Okamoto (National Institute of Biomedical Innovation, Osaka, Japan). The cDNA was fused to a Flag, 6myc, and GFP creating pcDNA3-Flag-hSIK, pcDNA3-6myc-hSIK, and pEGFP-hSIK. The SIK ATP binding site mutant K56R was created by the Quick-Change mutagenesis kit (Stratagene). Deletion mutants SIK-ΔUBA, SIK (K56R)-ΔUBA, SIK-ΔC, and SIK-ΔN were created using a PCR-based strategy and were subcloned in pcDNA3-Flag and pEGFP. DNA constructs were sequence verified.
Vectors pcDNA3-ALK5(wt)-HA (wild-type receptor with C-terminal hemagglutinin tag), pcDNA3-ALK5(CA)-HA (CA T204D mutant receptor), pcDNA3-ALK5(KR)-HA (kinase dead ATP-binding site mutant receptor), pcDNA3-Flag-Smad7, pcDNA3-6myc-Smad7, pcDNA3-6myc-Smad7 deletion mutants (N-, Linker, C-), pcDNA3-HA-ubiquitin, and pcDNA3-HA-ubiquitin(K48R) and gene reporters pGL3-CAGA12-luc and pCMV-βgal were previously described (Morén et al., 2005); pcDNA3-CIN85 was from I. Dikic (Goethe University, Frankfurt, Germany); pcDNA3-TβRI(wt)-Flag and pcDNA3-TβRII(wt)-Flag were from E.B. Leof (Mayo Clinic, Rochester, MN); pSuper-Smad7 expressing a short hairpin RNA against mouse and human Smad7 mRNAs was made by ligating the Smad7-specific hairpin (5′-GATCCCCGAGGCTGTGTTGCTGTGAATT CAAGAGATTCACAGCAACACAGCCTCTTTTTGGAAA-3′; underlined sequences show the hairpin stem) to pSuper (a gift from R. Agami, Netherlands Cancer Institute, Amsterdam, Netherlands; Brummelkamp et al., 2002).
Transient transfections
HaCaT cells were transfected with siRNAs via Lipofectamine 2000 (Invitrogen) or SiLentFect (Bio-Rad Laboratories), COS1 and Mv1Lu cells via Lipofectamine, and HEK293T and HepG2 cells via calcium phosphate as previously described (Kowanetz et al., 2004). 24 h after transfection, cells were stimulated with TGF-β1 as indicated in the figures.
RT-PCR
Total HaCaT or MDA-MB-468 RNA was analyzed by quantitative or semiquantitative RT-PCR as previously described (Niimi et al., 2007). PCR primers are listed in Tables I and II, and glyceraldehyde-3′-phosphate dehydrogenase (GAPDH) was the reference gene. A two-tailed paired Student's t test (significance at P < 0.05) was used to compare TGFβ1-inducible levels after control or SIK-specific knockdown in triplicate (PAI-1, p21, and p15) or quadruplicate (Smad7) determinations.
Table I. Human oligonucleotide primers used for RT-PCR.
| Gene | Primer Sequence | Strand | Product size | Temperature | PCR cycle | Reference |
|---|---|---|---|---|---|---|
| bp | °C | |||||
| SIK | 5′-GTCTGGGCGCACCTTTAGCA-3′ | + | 387 | 62 | 27 | NM_173354 |
| 5′-CAGCCTTTCGCCCAGTCTTT-3′ | − | |||||
| IDB2 | 5′-ACGACCCGATGAGCCTGCTA-3′ | + | 213 | 55 | 24 | Kowanetz et al. (2004) |
| 5′-TCCTGGAGCGCTGGTTCTG-3′ | − | |||||
| CDKN1A (p21) | 5′-CTGCCCAAGCTCTACCTTCC-3′ | + | 123 | 57 | 28 | Kowanetz et al. (2004) |
| 5′-CAGGTCCACATGGTCTTCCT-3′ | − | |||||
| c-MYC | 5′-ACCCGGACGACGAGACCTTCATCA-3′ | + | 683 | 63 | 23 | Kowanetz et al. (2004) |
| 5′-GGGGCTGGTGCATTTTCGGTTGTT-3′ | − | |||||
| MADH7 (Smad7) | 5′-CAGATTCCCAACTTCTTCTG-3′ | + | 533 | 53 | 27 | Kowanetz et al. (2004) |
| 5′-GTTGAAGATGACCTCCAGCC-3′ | − | |||||
| GAPDH | 5′-ATCACTGCCACCCAGAAGAC-3′ | + | 443 | 57 | 22 | Kowanetz et al. (2004) |
| 5′-ATGAGGTCCACCACCCTGTT-3′ | − |
A previously published reference or the NCBI database accession number is indicated.
Table II. Human oligonucleotide primers used for quantitative real-time RT-PCR.
| Gene | Primer Sequence | Strand | Product size | Temperature | Reference |
|---|---|---|---|---|---|
| bp | °C | ||||
| SIK | 5′-CAACCTGGGCGACTACGATGAGCA-3′ | + | 167 | 60 | NM_173354 |
| 5′-GGGCGCACTGGGCATTCCGATACT-3′ | − | ||||
| CDKN1B (p15) | 5′-TGGACCTGGTGGCTACGAAT-3′(+) | + | 150 | 60 | Niimi et al. (2007) |
| 5′-AGGGCCTAAGTTGTGGGTTCA-3′(−) | − | ||||
| CDKN1A (p21) | 5′-CTGCCCAAGCTCTACCTTCC-3′(+) | + | 123 | 60 | Niimi et al. (2007) |
| 5′-CAGGTCCACATGGTCTTCCT-3′(−) | − | ||||
| PAI-1 | 5′-GAGACAGGCAGCTCGGATTC-3′ | + | 101 | 60 | ENST00000223095 |
| 5′-GGCCTCCCAAAGTGCATTAC-3′ | − | ||||
| MADH7 (Smad7) | 5′-ACCCGATGGATTTTCTCAAACC-3′ | + | 85 | 60 | ENST00000262158 |
| 5′-GCCAGATAATTCGTTCCCCCT-3′ | − | ||||
| GAPDH | 5′-GGAGTCAACGGATTTGGTCGTA-3′(+) | + | 78 | 60 | Niimi et al. (2007) |
| 5′-GGCAACAATATCCACTTTACCAGAGT-3′(−) | − |
A previously published reference, the NCBI database accession number, or the Ensembl database transcript (ENST) number is indicated.
Promoter-reporter assays
HepG2 cells transfected with the Smad3-responsive reporter pGL3-CAGA12-luc for 36 h before stimulation with TGF-β1 for 6 h were analyzed with the enhanced luciferase assay kit (BD Biosciences). Normalized promoter activity is plotted as mean values from triplicate determinations with standard deviations.
Immunoblotting, immunoprecipitation, and ubiquitination assays
SDS-PAGE, immunoblot, coimmunoprecipitation, pulldown, and ubiquitination analysis was as previously described (Kowanetz et al., 2004; Morén et al., 2005). For efficient detection of endogenous SIK, HaCaT cells were pretreated with 50 μM MG132 (EMD) for 6 h at 37°C. For pulse-chase analysis, a 30 min pulse with 35S-methionine/cysteine was followed by a chase, as indicated in the Figures and as previously described (Morén et al., 2005). Cells treated with proteasomal inhibitor, calpain inhibitor I (N-Acetyl-Leu-Leu-norleucinal, LLnL; Roche) or lysosomal inhibitor chloroquine (Sigma-Aldrich) were also analyzed. To measure association between SIK and ubiquitinated proteins, HEK293T cells were transfected with Flag-SIK in one plate and HA-ubiquitin in another and treated with proteasomal inhibitors, MG132, or LLnL. Individual cell lysates were combined before immunoprecipitation.
Confocal microscopy
Approximately 70% confluent transfected Mv1Lu monolayers were analyzed by immunofluorescence 24 h after transfection as previously described (Kowanetz et al., 2004). Nuclei were counterstained with DAPI or propidium iodide. A confocal microscope (Axiovert 200 M; Carl Zeiss, Inc.) equipped with LSM 510 laser was used with the 63×/0.75 NA objective lens (Carl Zeiss, Inc.) and photographing at ambient temperature in the presence of immersion oil. Images acquired with a charge-coupled device camera (C4742-95; Hamamatsu Photonics) and the acquisition software QED Camera Plug-in v.1.1.6 (QED Imaging Inc.) were reduced in memory content using Photoshop 6.0 (Adobe), without additional processing.
Receptor affinity cross-linking
Mv1Lu cells incubated on ice with iodinated TGF-β1 were cross-linked, as previously described (Moustakas et al., 1993), and shifted to 37°C, as indicated in the figure, before SDS-PAGE and autoradiography.
Online supplemental material
Fig. S1 shows endogenous SIK protein levels in response to TGFβ1 and the lack of effect of SIK expression on GFP or Smad7 levels. Fig. S2 shows interaction of SIK with ubiquitinated proteins and interaction between SIK and ALK5 in presence of ubiquitin. Fig. S3 shows mRNA profiles of Smad7, p21, c-myc, and Id2 during a time course in response to TGFβ1 in control cells or after SIK knockdown. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.200804107/DC1.
Supplementary Material
Acknowledgments
We thank R. Agami, I. Dikic, E.B. Leof, M. Okamoto and H. Takemori for valuable reagents and A. Morén and U. Valcourt for assistance and suggestions.
The work was supported by the Ludwig Institute for Cancer Research, the Swedish Cancer Society (project number 4855-B03-01XAC), the Natural Sciences Foundation of Sweden (project number K2004-32XD-14936-01A), and the Strategic Japanese-Swedish Cooperative Program “Multidisciplinary BIO” (project number 26575-1) supported by the Swedish Agency for Innovation Systems and the Swedish Foundation for Strategic Research.
The authors declare no competing interests.
M. and K. Kowanetz's current address is Genentech, Inc., South San Francisco, CA 94080.
Abbreviations used in this paper: ALK, activin receptor-like kinase; BMP, bone morphogenetic protein; CA, constitutively active; GAPDH, glyceraldehyde-3′-phosphate dehydrogenase; PAI-1, plasminogen activator inhibitor 1; SIK, salt-inducible kinase; TβRII, TGFβ type II receptor; TCL, total cell lysate; UBA, ubiquitin associated.
References
- Alessi, D.R., K. Sakamoto, and J.R. Bayascas. 2006. LKB1-dependent signaling pathways. Annu. Rev. Biochem. 75:137–163. [DOI] [PubMed] [Google Scholar]
- Al-Hakim, A.K., O. Goransson, M. Deak, R. Toth, D.G. Campbell, N.A. Morrice, A.R. Prescott, and D.R. Alessi. 2005. 14-3-3 cooperates with LKB1 to regulate the activity and localization of QSK and SIK. J. Cell Sci. 118:5661–5673. [DOI] [PubMed] [Google Scholar]
- Alwan, H.A., E.J. van Zoelen, and J.E. van Leeuwen. 2003. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J. Biol. Chem. 278:35781–35790. [DOI] [PubMed] [Google Scholar]
- Brummelkamp, T.R., R. Bernards, and R. Agami. 2002. A system for stable expression of short interfering RNAs in mammalian cells. Science. 296:550–553. [DOI] [PubMed] [Google Scholar]
- Di Guglielmo, G.M., C. Le Roy, A.F. Goodfellow, and J.L. Wrana. 2003. Distinct endocytic pathways regulate TGF-β receptor signalling and turnover. Nat. Cell Biol. 5:410–421. [DOI] [PubMed] [Google Scholar]
- Feng, X.-H., and R. Derynck. 2005. Specificity and versatility in TGF-β signaling through Smads. Annu. Rev. Cell Dev. Biol. 21:659–693. [DOI] [PubMed] [Google Scholar]
- Itoh, S., and P. ten Dijke. 2007. Negative regulation of TGF-β receptor/Smad signal transduction. Curr. Opin. Cell Biol. 19:176–184. [DOI] [PubMed] [Google Scholar]
- Jaleel, M., F. Villa, M. Deak, R. Toth, A.R. Prescott, D.M. Van Aalten, and D.R. Alessi. 2006. The ubiquitin-associated domain of AMPK-related kinases regulates conformation and LKB1-mediated phosphorylation and activation. Biochem. J. 394:545–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowanetz, M., U. Valcourt, R. Bergström, C.-H. Heldin, and A. Moustakas. 2004. Id2 and Id3 define the potency of cell proliferation and differentiation responses to transforming growth factor β and bone morphogenetic protein. Mol. Cell. Biol. 24:4241–4254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanjuin, A., and P. Sengupta. 2002. Regulation of chemosensory receptor expression and sensory signaling by the KIN-29 Ser/Thr kinase. Neuron. 33:369–381. [DOI] [PubMed] [Google Scholar]
- Longva, K.E., F.D. Blystad, E. Stang, A.M. Larsen, L.E. Johannessen, and I.H. Madshus. 2002. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J. Cell Biol. 156:843–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maduzia, L.L., A.F. Roberts, H. Wang, X. Lin, L.J. Chin, C.M. Zimmerman, S. Cohen, X.-H. Feng, and R.W. Padgett. 2005. C. elegans serine-threonine kinase KIN-29 modulates TGFβ signaling and regulates body size formation. BMC Dev. Biol. 5:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morén, A., T. Imamura, K. Miyazono, C.-H. Heldin, and A. Moustakas. 2005. Degradation of the tumor suppressor Smad4 by WW and HECT domain ubiquitin ligases. J. Biol. Chem. 280:22115–22123. [DOI] [PubMed] [Google Scholar]
- Moustakas, A., H.Y. Lin, Y.I. Henis, J. Plamondon, M.D. O'Connor-McCourt, and H.F. Lodish. 1993. The transforming growth factor β receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J. Biol. Chem. 268:22215–22218. [PubMed] [Google Scholar]
- Niimi, H., K. Pardali, M. Vanlandewijck, C.-H. Heldin, and A. Moustakas. 2007. Notch signaling is necessary for epithelial growth arrest by TGF-β. J. Cell Biol. 176:695–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto, M., H. Takemori, and Y. Katoh. 2004. Salt-inducible kinase in steroidogenesis and adipogenesis. Trends Endocrinol. Metab. 15:21–26. [DOI] [PubMed] [Google Scholar]
- Ruiz, J.C., F.L. Conlon, and E.J. Robertson. 1994. Identification of novel protein kinases expressed in the myocardium of the developing mouse heart. Mech. Dev. 48:153–164. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.