Abstract
Cell differentiation requires the ability to detect and respond appropriately to a variety of extracellular signals. Here we investigate a differentiation switch induced by changes in the concentration of a single stimulus. Yeast cells exposed to high doses of mating pheromone undergo cell division arrest. Cells at intermediate doses become elongated and divide in the direction of a pheromone gradient (chemotropic-growth). Either of the pheromone-responsive MAP kinases, Fus3 and Kss1, promotes cell elongation, but only Fus3 promotes chemotropic growth. Whereas Kss1 is activated rapidly and with a graded dose-response profile, Fus3 is activated slowly and exhibits a steeper dose-response relationship (ultrasensitivity). Fus3 activity requires the scaffold protein Ste5; when binding to Ste5 is abrogated Fus3 behaves like Kss1, and the cells no longer respond to a gradient or mate efficiently with distant partners. We propose that scaffold proteins serve to modulate the temporal and dose-response behavior of the MAP kinase.
INTRODUCTION
Different environmental stimuli often employ the same set of signaling proteins to achieve very different developmental outcomes. A prototypical example occurs in the yeast Saccharomyces cerevisiae. Haploid cells exposed to mating pheromones will undergo cell division arrest and polarized cell expansion leading to shmoo formation. These events prepare haploid a-and α-type cells to mate, resulting in the formation of an a/α diploid (Wang and Dohlman, 2004). Alternatively, cells in nutrient-poor media exhibit an altered budding pattern, long branching filaments, as well as increased adherence and invasion of the substratum. This behavior is variously known as invasive-growth, pseudohyphal-growth, or filamentous-growth (Fig. 1A). Despite striking differences in cell morphology and behavior, the pheromone-and nutrient-response pathways employ the same protein kinase cascade comprised of Ste20, Ste11, Ste7 and either of two mitogen-activated protein (MAP) kinases, Kss1 and Fus3 (Liu et al., 1993; Roberts and Fink, 1994). Kss1 is needed for invasive-growth, while Fus3 is required for growth-arrest (Cook et al., 1997; Madhani and Fink, 1997; Roberts et al., 2000; Roberts and Fink, 1994; Sabbagh et al., 2001) (Fig. 1A).
Fig. 1. Yeast cell differentiation induced by different concentrations of pheromone.
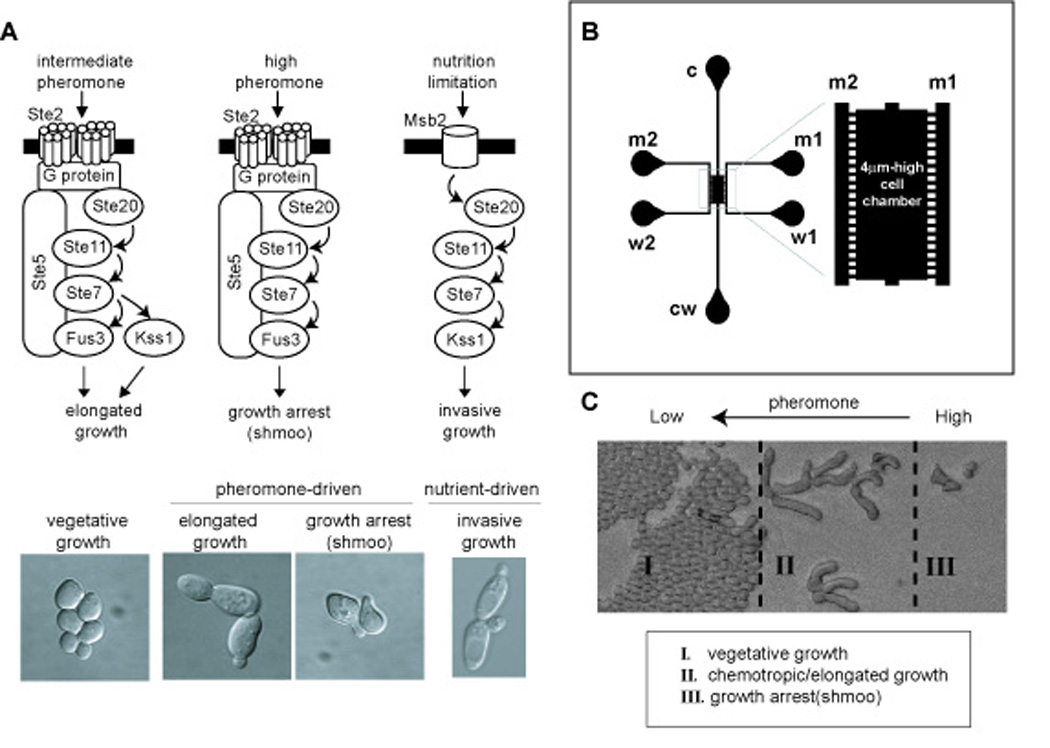
(A) Common signaling components are used for nutrient-driven invasive growth, as well as for the pheromone-driven switch from vegetative-growth to elongated-or chemotropic-growth and growth-arrest. (B) Schematic of the microfluidic gradient device. Ports c and cw correspond to cell loading and cell waste, respectively. Ports m1 and m2 are for media supply, w1 and w2 are media waste ports. Gradient within the chamber is established by diffusion between m1 and m2 via the microchannels that connect the media channels to the cell chamber. (C) Yeast cells undergo three distinct developmental fates in a pheromone gradient. Cells are loaded evenly and at a very low density, but rapidly dividing cells eventually fill the chamber at the lowest concentrations of pheromone (zone I).
Another lesser-known example of component sharing is the differentiation switch triggered by alterations in pheromone concentration. Whereas yeast cells exposed to a high dose of pheromone undergo cell division arrest and shmoo formation, at lower doses they transiently arrest, become elongated and then divide, presumably in the direction of a pheromone gradient (Dorer et al., 1995; Erdman and Snyder, 2001; Paliwal et al., 2007; Segall, 1993) (Fig. 1A). The growth-arrest and elongated-growth pathways employ the same cell surface receptor, G protein transducer, and downstream protein kinases (Fig. 1A). Whereas either Fus3 or Kss1 will sustain the elongated cell morphology, Fus3 alone is required for growth-arrest and shmoo formation (Erdman and Snyder, 2001). It is not known if Fus3 or Kss1 contribute to a chemotropic-growth response.
In the past, stimulus-specific responses have been ascribed to the scaffolded-association of specific protein kinases. The term “scaffold” is commonly used to describe a protein that can assemble multiple distinct binding partners and promote their mutual interactions. In yeast the scaffold protein Ste5 has binding partners that include Ste11, Ste7, and Fus3. Whereas Ste5 is absolutely required for activation of Fus3 (Andersson et al., 2004; Breitkreutz et al., 2001; Flatauer et al., 2005; Kusari et al., 2004; Maleri et al., 2004), Ste5 binds poorly to Kss1 and is not required for the Kss1-mediated invasive-growth response (Choi et al., 1994; Kusari et al., 2004; Printen and Sprague, 1994). Based on these differences, it has been widely assumed that Ste5 insulates Fus3 from other MAP kinases that use the same upstream components, and thus prevents activation by irrelevant signals. While clearly required for Fus3 activity, however, Ste5 nevertheless allows activation of Kss1 by pheromone (Andersson et al., 2004; Breitkreutz et al., 2001; Choi et al., 1994; Flatauer et al., 2005; Maleri et al., 2004; Printen and Sprague, 1994). These observations have prompted a search for additional functions for Ste5.
More recently, Ste5 was reported to undergo a stimulus-induced conformational change and to allosterically regulate Fus3 kinase activity (Bhattacharyya et al., 2006; Sette et al., 2000). Ste5 stimulates Fus3 auto-phosphorylation at Tyr-182, one of two sites normally phosphorylated upon full activation of the kinase (Gartner et al., 1992). This mono-phosphorylated form of Fus3 is partially active. Moreover the interaction of Ste5 with Fus3 results in diminished overall signaling at the level of new gene transcription (Bhattacharyya et al., 2006; Sette et al., 2000). Thus Ste5 is essential for Fus3 signaling but also limits the overall output of the Fus3 signaling pathway.
Here we establish a role for Ste5 in mediating chemotropic-growth behavior in yeast. We show that Ste5 acts as a dynamic modulator of Fus3, conferring slow kinase activation and a sigmoidal (ultrasensitive) pheromone dose-response profile. By comparison, Kss1 activation is rapid and exhibits a graded dose-response behavior. We propose that Ste5 allows cells to discriminate between pheromone doses appropriate for chemotropic-growth versus growth-arrest and mating, and does so by altering the time-and dose-dependent behavior of Fus3.
RESULTS
Yeast cells are well-known to undergo a developmental switch from nutrient-driven invasive-growth to pheromone-driven growth-arrest and mating. Previous investigations have highlighted the fact that these pathways share many, but not all, component proteins. However it remains unclear which pathway-specific components are required for signal fidelity as opposed to pathway-specific behaviors such as altered morphology or invasion. As an alternative approach, we investigated a distinct differentiation switch triggered by a common stimulus (Fig. 1A). At high doses of pheromone, cells arrest in G1 and exhibit the characteristic shmoo morphology. At intermediate doses they grow slowly and appear elongated (Fig. 1A). Given that these events require the same receptor and G protein, as well as many of the same effector kinases, this system provides an excellent opportunity to determine the function of pathway-specific components. Here we investigate the role of Ste5.
Initially we investigated dose-dependent responses to pheromone, using a variety of functional assays. Pheromone-induced transcription has been shown to exhibit a graded dose-response profile (Poritz et al., 2001), whereas downstream processes such as G1 arrest and mating are switch-like. Switch-like behavior is an extreme example of ultrasensitivity, defined as any response with a Hill coefficient greater than one (Ferrell and Machleder, 1998; Goldbeter and Koshland, 1981). To determine if other aspects of the pheromone response exhibit ultrasensitivity, we monitored differentiation of cells exposed to a range of pheromone concentrations. Cells were classified into one of three categories: vegetative-growth, elongated-growth, or shmoo. Vegetative-growth occurs in the absence of pheromone. In this case cells divide rapidly and bud axially (new buds emerge from the daughter cell in the direction of the mother cell). At intermediate doses of pheromone the cells appear elongated, proliferate slowly, and divide in a bipolar fashion, opposite the mother cell (Dorer et al., 1995; Erdman and Snyder, 2001; Madden and Snyder, 1992). At high doses of pheromone the cells arrest and form a shmoo. Each of these morphologies is illustrated in Fig. 1. As shown in Fig. 2A, >85% of wild-type cells grow vegetatively at pheromone concentrations of 0.1 µM or less. At concentrations of 0.3 – 3 µM the population transitions from vegetative-growth to elongated-growth, even in the absence of a pheromone gradient. At 10 µM nearly all cells undergo growth-arrest and shmoo formation. The first transition occurs over a broader range of pheromone concentrations, as compared with the second transition. Thus the differentiation switch from elongated-growth to growth-arrest may exhibit ultrasensitivity.
Fig. 2. Experimental measurements of yeast cell differentiation.
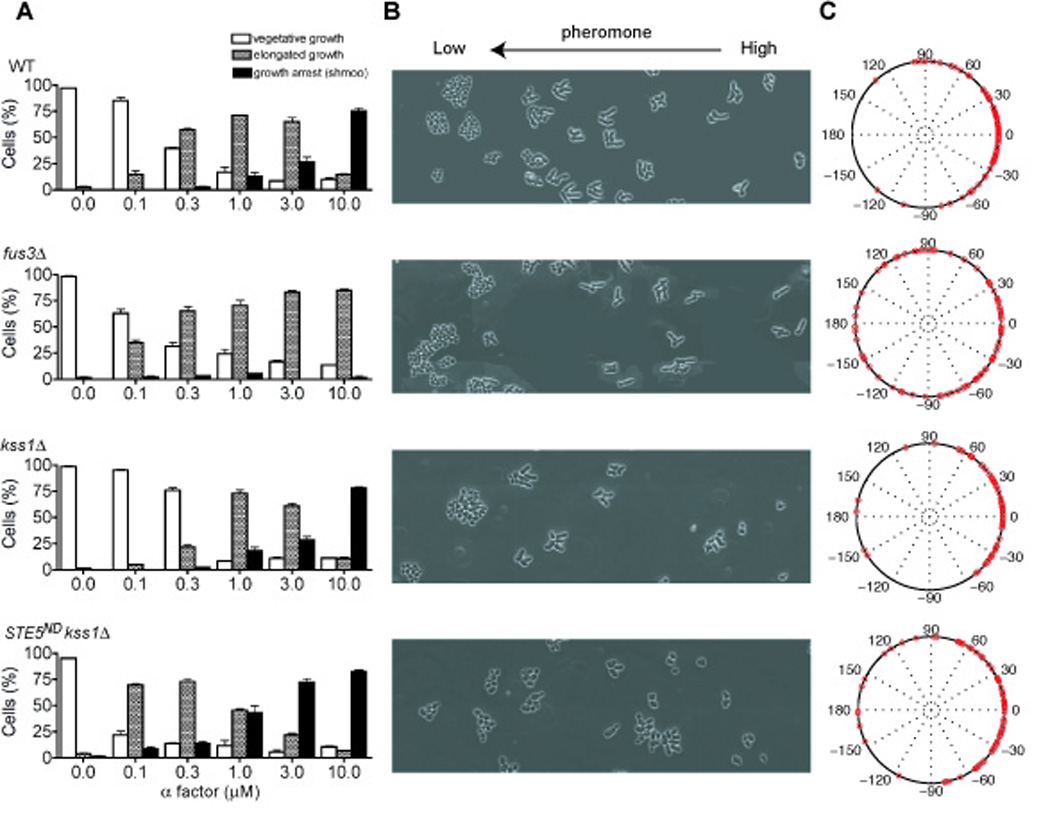
(A) Cell morphologies classified as: vegetative-growth, elongated-growth (length:width ≥1.6), or shmoo. Wild-type and mutant cells exposed to a uniform concentration of pheromone exhibit each of the three morphologies. (B) Cells exposed to a linear gradient of pheromone exhibit the three morphologies depending on their position within the chamber. Cell differentiation was recorded at 600 min for 3 independent experiments. (C) Chemotropic-growth quantified as the angle of cell projections and the direction of the gradient, represented with polar plots. Error bars, +/− standard error of the mean.
We then investigated how the MAP kinases contribute to the two developmental transitions. It is well-established that mutants lacking Fus3 are unable to shmoo, but maintain the ability to transition from vegetative-growth to elongated-growth (Fig. 2A, second row). Conversely, cells lacking Kss1 still undergo both transitions (Fig. 2A, third row), but the dose-response profile for the transition from vegetative-growth to elongated-growth is significantly steeper than that of cells containing both MAP kinases or Kss1 alone. Signaling exclusively through Fus3 increases the pheromone concentration required for elongated-growth, thereby reducing the range of pheromone levels for which this behavior is observed. Kss1 broadens this range allowing cells to become elongated at lower doses of pheromone, as compared with cells that express both MAP kinases. Thus Kss1 and Fus3 confer distinct dose-response characteristics on cell growth: Kss1-mediated responses are graded while Fus3 responses appear ultrasensitive.
We next examined whether elongated-growth corresponds to chemotropic-growth, defined here as cell expansion and division in the direction of a stimulus gradient. It has been suggested that chemotropic-growth could allow yeast, which are otherwise non-motile, to orient new bud formation in the direction of a weak pheromone stimulus and thus towards a distant mating partner (Dorer et al., 1995; Erdman and Snyder, 2001; Madden and Snyder, 1992). To this end we constructed a microfluidic chamber capable of exposing cells to a precisely-controlled linear concentration gradient. The gradient is achieved by passive diffusion between two parallel microchannels containing either no pheromone or a dose of pheromone sufficient to induce cell division arrest (Fig. 1B and Supplementary Materials). There is no active flow within the growth chamber, thereby allowing the non-adherent cells to remain stationary during the course of the experiment. In this method the cells are at low density and the pheromone is constantly replenished, resulting in a lower dose-activity profile (see below).
As shown in Fig. 1C, Fig 2B, as well as in Supplementary Fig. S1 and the accompanying movies (Supplementary Information), cells within the growth chamber exhibit all three morphologies, depending on the local concentration of pheromone. Cells exposed to minimal doses of pheromone grow vegetatively and divide axially (Dorer et al., 1995; Madden and Snyder, 1992). Cells exposed to high doses of pheromone undergo cell division arrest and form a shmoo. At intermediate doses the cells appear elongated but continue to divide slowly and in the direction of increasing pheromone concentrations (Fig. 2B). This chemotropic-growth response generally entails just one round of elongated-growth, after which the daughter cell detaches from the mother cell and forms a shmoo. This is in contrast to invasive-growth, where cells undergo several rounds of polarized budding to form a long filament. In wild-type cells the vast majority of new buds and cell projections emerge within +/− 60° of the gradient, and very few are outside +/− 90° (Fig. 2C). Mutants lacking Fus3 or Kss1 retain the ability to undergo the elongated-growth response. Likewise, mutants lacking Kss1 are able to divide in the direction of the gradient, although Kss1 may contribute at very low doses of pheromone or in mutants that are supersensitive to pheromone (Fig. 2B and 2C) (Dorer et al., 1995; Erdman and Snyder, 2001; Paliwal et al., 2007; Segall, 1993). In cells lacking Fus3 however, newly formed buds emerge randomly with respect to the gradient and the cells no longer undergo growth-arrest and shmoo formation (Fig. 2B and 2C). Thus Fus3 is specifically required for chemotropic-growth but is dispensable for elongated-growth.
Cellular responses mediated by Fus3 appear to be ultrasensitive, while responses mediated by Kss1 appear graded (Fig. 2). Measurements of cell morphology are somewhat qualitative, however, so we also measured changes that occur at a molecular level. Specifically, we asked if there is ultrasensitivity at the level of protein kinase activity. To this end we monitored Fus3 and Kss1 activation in cells treated with a range of pheromone concentrations, using antibodies that recognize the dually-phosphorylated (fully activated) form of each protein. As shown in Fig. 3A and Fig. S2 the temporal-and dose-response profiles for Fus3 and Kss1 phosphorylation are distinct. At 1 µM pheromone (a dose sufficient to trigger chemotropic-growth but not shmoo formation) Fus3 is phosphorylated to 22% of maximum while Kss1 is phosphorylated to 48% of maximum. At 10 µM pheromone (a dose sufficient to trigger cell division arrest and shmoo formation) both kinases are maximally activated. Remarkably, we found that maximum kinase activity occurs at different times depending on the pheromone concentration; for example Fus3 activity peaks at 60 min in response to 10 µM pheromone whereas it peaks at 15 min in 1 µM pheromone (Fig. 3A). Thus two different doses may produce identical kinase activity at a single (early) time point, but nevertheless exhibit dramatic differences in the duration and final maximum level of kinase activation. Accordingly, we quantified peak kinase activity (Fig. 3D) and area under the curve (Fig. 3F) for each dose of pheromone. The latter incorporates the elements of duration and activity into a single parameter. Both of these methods accurately account for the observed differences in activity, and yielded similar results. As shown in Fig.3F, the effective Hill coefficient for activation of Fus3 is substantially higher than for activation of Kss1 (nH = 2.2 and 1.3, respectively). This difference mirrors the dose-response profiles for Fus3-and Kss1-mediated cell differentiation responses, as reported in Fig. 2. Note that we measured the MAP kinase activation of a population of cells. The response might be even more switch-like in individual cells.
Fig. 3. Experimental and computational analysis of MAP kinase activation with or without Ste5.
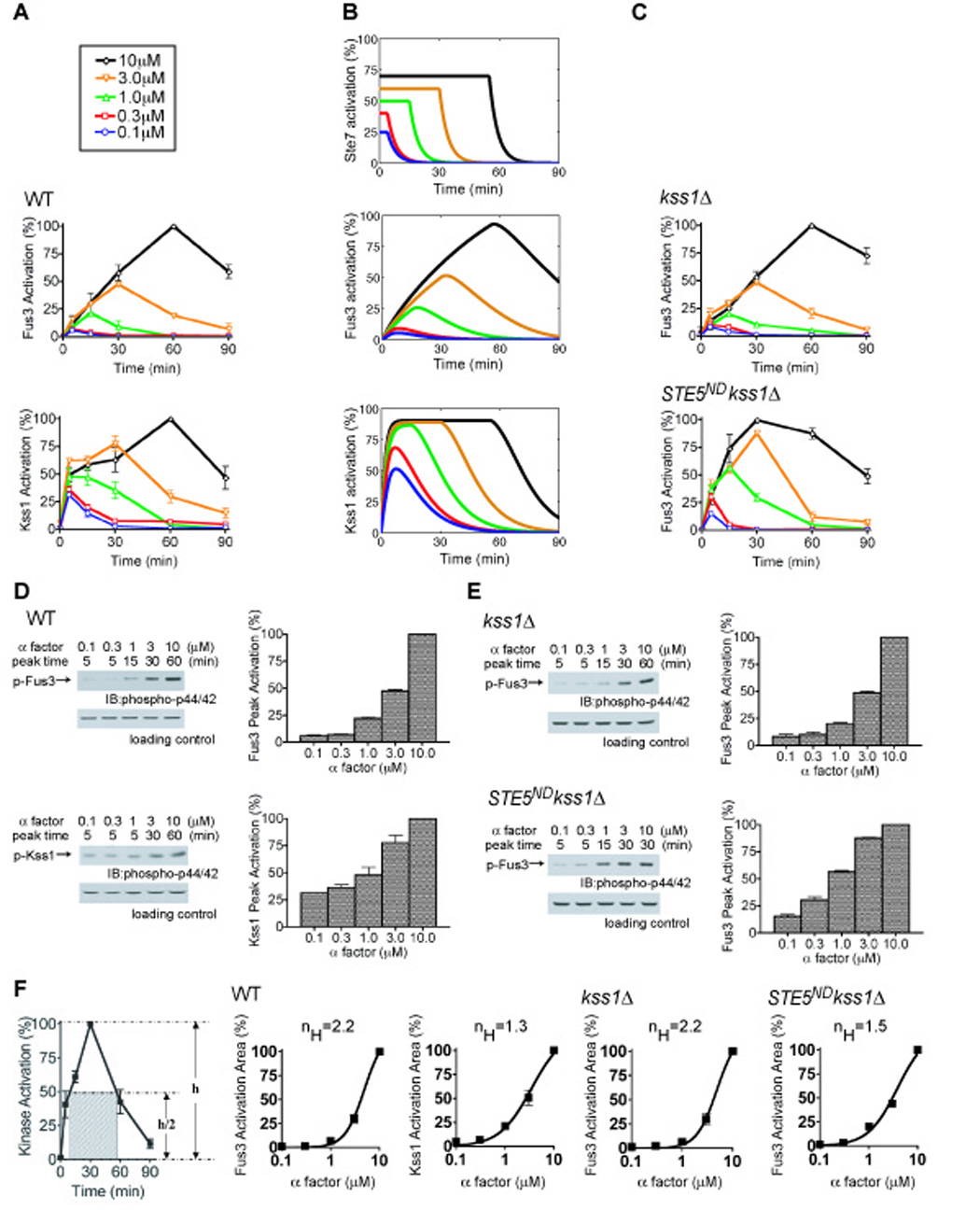
Time course of MAP kinase activation monitored in wild-type (A, D) and mutant (C, E) strains treated with the indicated pheromone concentrations, using phospho-p42/44 antibodies that recognize the dually-phosphorylated (fully activated) form of each protein. (B) Simple mathematical models qualitatively reproduce the experimental data for Fus3 and Kss1 activation. Each model assumes that the upstream signal (e.g. Ste7) is saturated at early times and at low pheromone concentrations, and any further increases in pheromone concentration prolong the duration of the upstream signal. (D, E) Kinase activation quantified as the percentage of maximum activation obtained at each dose (Peak Activation). (F) Kinase activation at different concentrations of pheromone quantified as the area of the rectangle fit to half-maximum activity (Activation Area) and fit by the Hill equation (nH, Hill coefficient). Band intensity from 3 or more experiments was quantified by scanning densitometry presented as percentage of maximum intensity for each experiment. Error bars, +/− standard error of the mean.
Fus3 and Kss1 are activated by the same upstream protein kinases, most immediately by the MAP kinase kinase Ste7. Nevertheless we have shown above that Fus3 and Kss1 exhibit very different temporal-and dose-dependent behaviors. Whereas Kss1 activity peaks quickly, Fus3 activity increases slowly at a constant rate (slope) that is independent of the pheromone level. In both cases, increasing the pheromone level delays the time at which MAP kinase activity begins to decline. One way to account for this unusual behavior is to assume that the predominant effect of increases in pheromone concentration is to prolong the duration of Ste7 activity (Fig. 3B, top panel). To address this possibility we constructed simple mathematical models of Fus3 and Kss1 activation and investigated each model’s response to varying durations of Ste7 activation. Both models assume that the phosphorylation and dephosphorylation reactions follow Michaelis-Menten kinetics. Each model further assumes that the νmax for phosphorylation of the MAP kinase is proportional to the active Ste7 concentration. The difference between the models is that the rate constants describing phosphorylation and dephosphorylation of Kss1 are larger than those of Fus3 (Supplementary Materials). Fig. 3B shows the Ste7 activation profile used as the input signal for both MAP kinases (top panel), as well as the activity for Fus3 (middle panel) and Kss1 (bottom panel) predicted by the corresponding models. There is a very good qualitative agreement between these simulations (Fig. 3B) and the experimental data (Fig. 3A). The models also demonstrate how a purely kinetic mechanism (slow activation) can be used to convert a graded response to one that is more switch-like (see Discussion).
We then considered why Fus3 is activated more slowly than Kss1. This difference could be an inherent property of each kinase. Alternatively, slow activation of Fus3 could be due to binding of Ste5. Indeed, Ste5 was reported previously to diminish Fus3-mediated transcription responses (Bhattacharyya et al., 2006). To investigate the contribution of Ste5 to Fus3 dynamics, we monitored kinase activation in the absence of Ste5-Fus3 interaction. Deletion of STE5 blocks signaling altogether, so as an alternative we used a mutant form of Ste5 in which Fus3 docking is disrupted (“non-docking” allele, Ste5ND). This mutant binds poorly to Fus3 (Maeder et al., 2007), yet produces an enhanced transcription-induction response (Bhattacharyya et al., 2006). Thus we investigated how Ste5ND affects dose-and time-dependent signaling events including cell morphogenesis, gradient-sensing and kinase-activation. To eliminate any possibility that Kss1 can substitute for Fus3 or otherwise modulate Fus3 activity in the absence of Ste5, these experiments were conducted in a Kss1-deficient strain. As shown in Fig. 2A, cells that express Ste5ND are able to undergo the transitions from vegetative-growth to elongated-growth and then to growth-arrest. However, the transitions occur at lower pheromone concentrations and the dose-response profile is more graded than that seen in wild-type cells. Indeed we observed considerable overlap in the distribution of all three categories of cells within the gradient chamber. Moreover, cells that express Ste5ND exhibit diminished elongation and orientation towards the source of pheromone (Fig. 2B and 2C). Likewise, Fus3 becomes fully activated in the Ste5ND strain, but activation occurs with faster kinetics (Fig. 3C). Additionally, the Hill coefficient for activation of Fus3 is reduced from nH=2.2 in wild-type cells to nH=1.5 in Ste5ND mutant cells, a value similar to that normally observed for Kss1 (nH= 1.3) (Fig.3F). Thus under conditions where it is no longer modulated by Ste5, the dynamic and dose-dependent behavior of Fus3 resembles that of Kss1.
We also considered the possibility that Ste5ND might affect the activity of other signaling components upstream of Fus3, such as Ste11 or Ste7. To test this we examined whether Ste5ND alters the activity of Kss1, which likewise requires Ste11 and Ste7. In accordance with the model, Ste5ND (in the absence of Fus3 expression) had no effect on Kss1 activation (Fig. S3). Another upstream mechanism of regulation is pheromone degradation. The pheromone protease Bar1 is up-regulated upon prolonged pathway activation, and different degrees of induction by Fus3 and Kss1 might somehow underlie the differences observed for the two kinases. To address this concern, and as a further test of our computational model, we measured Fus3 and Kss1 activation in a bar1Δ mutant strain (Fig. S4A). As anticipated, differences in the dynamic and dose-dependent behaviors of Fus3 and Kss1 remain unchanged. Strikingly, by altering only the input signal profile the equations and parameters that govern kinase activation in wild-type cells could also be used to accurately describe kinase activation in the bar1Δ mutant cells (Fig. S4B).
Finally, we examined the physiological importance of the elongated-and chemotropic-growth behaviors in mating. Previous investigations employed the “pheromone-confusion” assay, which compares overall mating efficiency in the presence or absence of added pheromone. The underlying assumption is that exogenous pheromone will obscure natural pheromone gradients, and thereby diminish the ability of cells to detect a potential mating partner (Dorer et al., 1995; Nern and Arkowitz, 1998; Strickfaden and Pryciak, 2007; Valtz et al., 1995). One drawback to this method is that addition of excess exogenous pheromone is also likely to obscure or otherwise interfere with pheromone clearance by Bar1 protease, receptor occupancy, as well as adaptation/desensitization responses that further impinge on mating efficiency. As an alternative approach, we measured mating efficiency under conditions where potential mating partners are rare (10:1 ratio) and spatially segregated (plated on solid growth medium). As shown in Fig. 4, the Ste5ND mutants mate substantially better than wild-type cells, presumably because they can better detect and respond to a distant mating partner. Conversely, Ste5ND mutants mate more poorly under conditions where potential mating partners are more abundant (1:10 ratio) but still dispersed, presumably because they can no longer orient towards the closest of multiple potential mating partners. This reduction in mating efficiency occurs despite the increase in pheromone sensitivity.
Fig. 4. Physiological analysis of the role of Ste5 in mating.
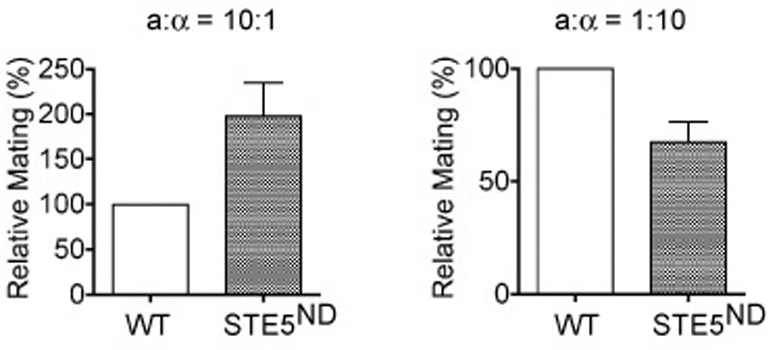
Wild-type or STE5ND a-type cells and α-type tester cells were spread separately and evenly onto plates containing selective medium. Colonies of a/□diploids were counted after 2 days. Mating efficiencies averaged from 3 independent experiments are relative to wild-type (WT). Error bars, +/− standard error of the mean.
From these data we conclude that Ste5 confers the slow and ultrasensitive responses characteristic of Fus3. If Fus3 cannot bind to Ste5 the cells no longer undergo chemotropic growth and exhibit an altered ability to mate efficiently with partners at a distance.
DISCUSSION
The pheromone response pathway is perhaps the best-characterized signal transduction system in any eukaryote, and it has long served as a prototype for hormone, neurotransmitter, and sensory-response systems in humans (Wang and Dohlman, 2004). Genetic and biochemical studies revealed that pheromone-induced growth-arrest requires a protein kinase cascade comprised of Ste20, Ste11, Ste7 and Fus3. These same signaling components are required for pheromone-induced elongation and gradient sensing behavior. Thus an important question is how the same pathway elicits different developmental responses. Here we demonstrate that Ste5 selectively alters the temporal and dose-dependent behavior of Fus3, and thereby allows cells to perceive a stimulus gradient. The cells are subsequently able to undergo chemotropic-growth, growth-arrest, and effective fusion with a distant mating partner.
Experiments conducted in a variety of other systems have revealed that MAP kinases can produce distinct cellular responses depending on the temporal behavior of the pathway. In one oft-cited example, epidermal growth factor causes transient activation of the ERK MAP kinase leading to cell proliferation, while nerve growth factor causes sustained ERK activation and cell differentiation (Marshall, 1995). Similarly in yeast, temporal differences in MAP kinase activation can dictate whether cells follow a mating or nutrient-driven invasive-growth program. Specifically, when upstream kinases Ste11 or Ste7 are mutated so as to be permanently active, Kss1 is activated in a sustained manner, in preference to Fus3, and this condition leads to invasive-growth but not mating (Andersson et al., 2004; Flatauer et al., 2005; Maleri et al., 2004).
Another way MAP kinases can produce distinct cellular responses is through changes in the dose-dependent behavior of the pathway. MAP kinase signaling pathways have long been known to exhibit ultrasensitivity (Koshland et al., 1982). For example, MAP kinase cascades in Xenopus oocytes use a variety of mechanisms to produce an all-or-none response leading to cell maturation (Ferrell and Machleder, 1998). On the other hand the pheromone-induced transcription response in yeast exhibits a more graded dose-response profile (Poritz et al., 2001), even when downstream processes such as G1 arrest and mating are switch-like. Our data reveal that the two pheromone-responsive MAP kinases exhibit distinct dose-dependent behaviors. Whereas activation of Kss1 is graded, activation of Fus3 is ultrasensitive. The ultrasensitivity exhibited by Fus3 is not as dramatic as that demonstrated in Xenopus, but is comparable to the benchmark example hemoglobin, which exhibits a Hill coefficient of 2.8.
Our data show that Ste5 is required for the unique time-and dose-dependent characteristics exhibited by Fus3. Ste5 was originally considered to function as a passive tether for protein kinases. More recently, Ste5 was shown to be required for Fus3 activation, but also impedes full activation of the signaling pathway (Bhattacharyya et al., 2006). That loss of signaling was demonstrated using a reporter transcription assay. To determine the contribution of Ste5 to MAP kinase function, we evaluated signaling in the absence of Fus3 binding (Ste5ND). Signaling was monitored by cell differentiation, gradient sensing, and MAP kinase activation assays. By all of these measures it appears that Ste5 is necessary for the slow and ultrasensitive responses exhibited by Fus3. When Ste5 binding is abrogated, Fus3 behaves like Kss1.
A distinction between our analysis and prior investigations is that we considered the role of pheromone gradient sensing. Most studies of the yeast pheromone response have been done with uniform and saturating concentrations of pheromone. In a physiological setting however, yeast cells are likely to be exposed to a pheromone gradient emanating from a potential mating partner. When that partner is too distant for mating to occur, the gradient will be weak and some mechanism must exist to bridge the divide. Here we demonstrate that Ste5 promotes cell elongation in the direction of a pheromone gradient, thereby increasing the probability of mating with a distant partner.
Finally, our computational analysis reveals how a kinetic mechanism (specifically, slow activation) can potentially be used to convert a graded response to one that is more switch-like. We postulate that the main effect of increasing pheromone concentration is to increase the time the signal is on. In this scenario the fast activation kinetics of Kss1 allows it to faithfully track the input signal. Conversely, the slow activation kinetics of Fus3 means that it does not saturate while the incoming signal is present. Because Fus3 activity increases linearly with time, the response increases with time and thus with pheromone concentration.
In summary, the findings presented here reveal one way that scaffolds contribute to protein kinase activity. In this example Ste5 alters the dynamic behavior of Fus3, and these changes promote gradient sensing and orientation towards a distant mating partner. Given the prevalence of scaffold proteins in signaling cascades, the mechanisms elucidated here for Ste5 will likely prove applicable to cell differentiation programs in other cellular systems.
EXPERIMENTAL PROCEDURES
Strains and Plasmids
Standard methods for cell growth and maintenance were used throughout. Strain BY4741 (MATa leu2Δ met15Δ his3Δ ura3Δ) and BY4741-derived mutants were used unless otherwise indicated. Details of plasmid construction are provided in the Supplementary Materials
Immunoblot Analysis
Cell extracts (20 µg/lane) were resolved by 12% SDS-polyacrylamide gel electrophoresis and immunoblotting as described (Sabbagh et al., 2001). Band intensity was quantified by scanning densitometry using ImageJ (NIH).
Microfluidics Chamber and Quantification of Gradient Sensing
A microfluidic device was constructed similar to one described previously (Paliwal et al., 2007), and as detailed in Supplementary Materials. Image acquisition was done with a Nikon Diaphot TMD epifluorescence or Olympus IX81 inverted microscope. Image processing and analysis was done using Slide Book 4.1 Advanced Imaging Software and ImageJ. Polar plots are presented using MATLAB (Mathworks).
Quantitative Mating Efficiency Assay
DC17 (MATα his1) and BY4741 cells were grown to A600nm ~1.0, diluted with water and spread separately and evenly onto plates containing synthetic growth medium. Diploid colonies were counted after 2 days. Mating was conducted with 5×104 αcells and 5×105 a cells (1:10), or 5×105 αcells and 5×104 a cells (10:1).
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Andersson J, Simpson DM, Qi M, Wang Y, Elion EA. Differential input by Ste5 scaffold and Msg5 phosphatase route a MAPK cascade to multiple outcomes. Embo J. 2004;23:2564–2576. doi: 10.1038/sj.emboj.7600250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya RP, Remenyi A, Good MC, Bashor CJ, Falick AM, Lim WA. The Ste5 scaffold allosterically modulates signaling output of the yeast mating pathway. Science. 2006;311:822–826. doi: 10.1126/science.1120941. [DOI] [PubMed] [Google Scholar]
- Breitkreutz A, Boucher L, Tyers M. MAPK specificity in the yeast pheromone response independent of transcriptional activation. Curr Biol. 2001;11:1266–1271. doi: 10.1016/s0960-9822(01)00370-0. [DOI] [PubMed] [Google Scholar]
- Choi KY, Satterberg B, Lyons DM, Elion EA. Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell. 1994;78:499–512. doi: 10.1016/0092-8674(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Cook JG, Bardwell L, Thorner J. Inhibitory and activating functions for MAPK Kss1 in the S. cerevisiae filamentous-growth signalling pathway. Nature. 1997;390:85–88. doi: 10.1038/36355. [DOI] [PubMed] [Google Scholar]
- Dorer R, Pryciak PM, Hartwell LH. Saccharomyces cerevisiae cells execute a default pathway to select a mate in the absence of pheromone gradients. J Cell Biol. 1995;131:845–861. doi: 10.1083/jcb.131.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman S, Snyder M. A filamentous growth response mediated by the yeast mating pathway. Genetics. 2001;159:919–928. doi: 10.1093/genetics/159.3.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrell JEJ, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science. 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- Flatauer LJ, Zadeh SF, Bardwell L. Mitogen-activated protein kinases with distinct requirements for Ste5 scaffolding influence signaling specificity in Saccharomyces cerevisiae. Mol Cell Biol. 2005;25:1793–1803. doi: 10.1128/MCB.25.5.1793-1803.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner A, Nasmyth K, Ammerer G. Signal transduction in Saccharomyces cerevisiae requires tyrosine and threonine phosphorylation of FUS3 and KSS1. Genes Dev. 1992;6:1280–1292. doi: 10.1101/gad.6.7.1280. [DOI] [PubMed] [Google Scholar]
- Goldbeter A, Koshland DE., Jr An amplified sensitivity arising from covalent modification in biological systems. Proc Natl Acad Sci U S A. 1981;78:6840–6844. doi: 10.1073/pnas.78.11.6840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland DE, Jr, Goldbeter A, Stock JB. Amplification and adaptation in regulatory and sensory systems. Science. 1982;217:220–225. doi: 10.1126/science.7089556. [DOI] [PubMed] [Google Scholar]
- Kusari AB, Molina DM, Sabbagh W, Jr, Lau CS, Bardwell L. A conserved protein interaction network involving the yeast MAP kinases Fus3 and Kss1. J Cell Biol. 2004;164:267–277. doi: 10.1083/jcb.200310021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Styles CA, Fink GR. Elements of the yeast pheromone response pathway required for filamentous growth of diploids. Science. 1993;262:1741–1744. doi: 10.1126/science.8259520. [DOI] [PubMed] [Google Scholar]
- Madden K, Snyder M. Specification of sites for polarized growth in Saccharomyces cerevisiae and the influence of external factors on site selection. Mol Biol Cell. 1992;3:1025–1035. doi: 10.1091/mbc.3.9.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhani HD, Fink GR. Combinatorial control required for the specificity of yeast MAPK signaling. Science. 1997;275:1314–1317. doi: 10.1126/science.275.5304.1314. [DOI] [PubMed] [Google Scholar]
- Maeder CI, Hink MA, Kinkhabwala A, Mayr R, Bastiaens PI, Knop M. Spatial regulation of Fus3 MAP kinase activity through a reaction-diffusion mechanism in yeast pheromone signalling. Nat Cell Biol. 2007;9:1319–1326. doi: 10.1038/ncb1652. [DOI] [PubMed] [Google Scholar]
- Maleri S, Ge Q, Hackett EA, Wang Y, Dohlman HG, Errede B. Persistent activation by constitutive Ste7 promotes Kss1-mediated invasive growth but fails to support Fus3-dependent mating in yeast. Mol Cell Biol. 2004;24:9221–9238. doi: 10.1128/MCB.24.20.9221-9238.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Nern A, Arkowitz RA. A GTP-exchange factor required for cell orientation. Nature. 1998;391:195–198. doi: 10.1038/34458. [DOI] [PubMed] [Google Scholar]
- Paliwal S, Iglesias PA, Campbell K, Hilioti Z, Groisman A, Levchenko A. MAPK-mediated bimodal gene expression and adaptive gradient sensing in yeast. Nature. 2007;446:46–51. doi: 10.1038/nature05561. [DOI] [PubMed] [Google Scholar]
- Poritz MA, Malmstrom S, Kim MK, Rossmeissl PJ, Kamb A. Graded mode of transcriptional induction in yeast pheromone signalling revealed by single-cell analysis. Yeast. 2001;18:1331–1338. doi: 10.1002/yea.777. [DOI] [PubMed] [Google Scholar]
- Printen JA, Sprague GF., Jr Protein-protein interactions in the yeast pheromone response pathway: Ste5p interacts with all members of the MAP kinase cascade. Genetics. 1994;138:609–619. doi: 10.1093/genetics/138.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts CJ, Nelson B, Marton MJ, Stoughton R, Meyer MR, Bennett HA, He YD, Dai H, Walker WL, Hughes TR, et al. Signaling and circuitry of multiple MAPK pathways revealed by a matrix of global gene expression profiles. Science. 2000;287:873–880. doi: 10.1126/science.287.5454.873. [DOI] [PubMed] [Google Scholar]
- Roberts RL, Fink GR. Elements of a single MAP kinase cascade in Saccharomyces cerevisiae mediate two developmental programs in the same cell type: mating and invasive growth. Genes Dev. 1994;8:2974–2985. doi: 10.1101/gad.8.24.2974. [DOI] [PubMed] [Google Scholar]
- Sabbagh W, Jr, Flatauer LJ, Bardwell AJ, Bardwell L. Specificity of MAP kinase signaling in yeast differentiation involves transient versus sustained MAPK activation. Mol Cell. 2001;8:683–691. doi: 10.1016/s1097-2765(01)00322-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segall JE. Polarization of yeast cells in spatial gradients of alpha mating factor. Proc Natl Acad Sci U S A. 1993;90:8332–8336. doi: 10.1073/pnas.90.18.8332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sette C, Inouye CJ, Stroschein SL, Iaquinta PJ, Thorner J. Mutational analysis suggests that activation of the yeast pheromone response mitogen-activated protein kinase pathway involves conformational changes in the ste5 scaffold protein. Mol Biol Cell. 2000;11:4033–4049. doi: 10.1091/mbc.11.11.4033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickfaden SC, Pryciak PM. Distinct Roles for Two G{alpha}-G{beta} Interfaces in Cell Polarity Control by a Yeast Heterotrimeric G Protein. Mol Biol Cell. 2007 doi: 10.1091/mbc.E07-04-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valtz N, Peter M, Herskowitz I. FAR1 is required for oriented polarization of yeast cells in response to mating pheromones. J Cell Biol. 1995;131:863–873. doi: 10.1083/jcb.131.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Dohlman HG. Pheromone signaling mechanisms in yeast: a prototypical sex machine. Science. 2004;306:1508–1509. doi: 10.1126/science.1104568. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


