Abstract
We investigated the mechanism of synaptic suppression by P2Y receptors in mixed hippocampal cultures wherein networked neurons exhibit synchronized Ca2+ oscillations (SCO) due to spontaneous glutamatergic synaptic transmission. Pharmacological studies suggested that SCO suppression was mediated by P2Y2/P2Y4 receptors. Immunostaining studies and characterization of ATP/UTP-stimulated Ca2+ responses in solitary neurons and astrocytes revealed that the SCO attenuation was effectuated by astrocytes. We demonstrate that nitric oxide released from activated astrocytes causes synaptic suppression by inhibiting neurotransmitter release. Physiological concentrations of ATP and UTP evoked NO production in astrocytes. SCO suppression was considerably diminished by removal of extracellular NO by membrane-impermeable scavenger c-PTIO or by pretreatment of cells with nitric oxide synthase inhibitor L-NAME. The nitric oxide donor DETA/NO effectively suppressed the SCO. ATP/UTP inhibited KCl-induced exocytosis at presynaptic terminals in an NO-dependent manner. In the absence of exogenously added ATP/UTP, both the NO scavenger and NOS inhibitor enhanced the frequency of SCO, implying that astrocytes release NO during spontaneous synaptic activity and exert a suppressive effect. We report for the first time that under physiological conditions astrocytes use NO as a messenger molecule to modulate the synaptic strength in the networked neurons.
INTRODUCTION
Increasing evidence suggests that astrocytes actively participate in dynamic control of synaptic transmission. Glutamatergic and purinergic receptor-mediated Ca2+ signaling plays the key role in crosstalk between neurons and astrocytes (Fields and Burnstock, 2006). Glutamate and ATP are coreleased during neuronal activity. These neurotransmitters are sensed by astrocytic receptors capable of generating and propagating Ca2+ waves. Activated astrocytes release gliotransmitters including glutamate and ATP that in turn can modulate the activity of neighboring neurons (Haydon, 2001; Newman, 2003; Fellin et al., 2004; Fiacco and McCarthy, 2004; Halassa et al., 2007). Several studies have demonstrated the modulatory role of ATP on synaptic transmission. Endogenously released ATP caused homo and heterosynaptic suppression in cultured hippocampal neurons (Zhang et al., 2003). The tonic suppression of glutamatergic synapses was dependent on the presence of astrocytes in the culture. ATP released upon mechanical stimulation of astrocytes and exogenously applied ATP decreased the glutamatergic synaptic transmission in hippocampal neurons (Koizumi et al., 2003; Koizumi and Inoue, 1997). Synaptic inhibition by exogenous or endogenously released ATP has also been shown in mouse hippocampal slices (Bowser and Khakh, 2004; Kawamura et al., 2004). It has been shown that ATP modulates neurotransmission by facilitating IPSCs in interneurons. Another study has suggested that ATP can act presynaptically to facilitate or inhibit glutamate release from hippocampal neurons (Rodrigues et al., 2005). The mechanisms suggested so far consider direct action of ATP on neuronal P2 receptors and do not fully explain the dynamic regulation of synaptic transmission by astrocytes. In the present study we tested the hypothesis that an astrocyte-derived diffusible messenger molecule is causal in ATP-induced synaptic modulation. Nitric oxide is a highly probable candidate because (a) P2 receptor activation induces nitric oxide synthase in many cell types (Ohtani et al., 2000; Auld and Robitaille, 2003; Silva et al., 2006); (b) astrocytes are endowed with nitric oxide synthase (Murphy, 2000; Kozuka et al., 2007) and they can produce nitric oxide in response to activation by various agents including ATP (Li et al., 2003; Murakami et al., 2003); (c) NO is a membrane-permeable molecule that can diffuse from astrocytes to neurons; and (e), lastly but most importantly, NO is the prevailing mediator of plastic changes in synaptic transmission implicated in long term potentiation (Wang et al., 2004) and long term depression (Stanton et al., 2003). In the present paper we show that the inhibitory effect of exogenously applied nucleotides and endogenously released ATP on synaptic transmission is mediated by nitric oxide.
Hippocampal neurons in culture form synaptically connected network and exhibit spontaneous synaptic activity. The networked neurons exhibit synchronized Ca2+ oscillations that correlate with periodic burst firing of action potentials (Liu et al., 2003; Dravid and Murray, 2004; Tanaka et al., 2007). The synchronized Ca2+ oscillations are the faithful indicator of synaptic events and they have been used to study the synaptic modulation. We have used this experimental system to gain insight of dynamic control of synaptic plasticity imposed by astrocytes. Our study describes a novel mechanism of neuronal plasticity caused by nitric oxide–mediated neuron–glia crosstalk.
MATERIALS AND METHODS
Chemicals
Fluorescence probes Fura-2 acetoxymethyl ester (AM), FM1-43, diaminofluorescein diacetate (DAF-DA), Alexa Fluor 594–conjugated cholera toxin B subunit, pluronic and Slowfade antifade reagent were from Molecular Probes, Invitrogen. 2-Amino-5-phosphonopentanoic acid (APV), 6-cyano-7-nitroquinozaline-2,3-dione (CNQX), (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) amino] diazen-1-ium-1,2-diolate (DETA/NO), (2-(4-carboxyphenyl)-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide potassium salt), carboxy-PTIO (c-PTIO), G-nitro-l-arginine methyl ester (L-NAME), and Eagle's minimal essential medium with Earle's salt (EMEM) were purchased from Sigma-Aldrich. Horse serum and FBS were obtained from Invitrogen. All other chemicals were of analytical grade obtained from commercial sources.
Antibodies
Primary polyclonal antibodies for P2Y1, P2Y2, and P2Y4 receptors were from Alomone Laboratories. Monoclonal anti MAP-2 antibody and anti-synaptophysin were from BD Biosciences. Secondary antibodies Alexa Fluor 488 goat anti-rabbit IgG1 and Alexa Fluor 633 goat anti-mouse IgG1 were from Molecular Probes, Invitrogen.
Hippocampal Neuron–Astrocyte Mixed Culture
Primary mixed cultures of hippocampal neurons and astrocytes were obtained from 0–1-d-old Sprague Dawley rats as previously described (Vergun et al., 1999). In brief, the pups were anesthetized by hypothermia and then decapitated. The brain was rapidly removed and immersed in ice-cold HBSS buffer. Hippocampi were collected, stripped of blood vessels, treated with 0.05% trypsin for 25 min at 37°C, and minced. Cells were then dissociated by trituration in EMEM supplemented with 2 mM l-glutamine, 10% FBS, 10% horse serum, 100 IU/ml penicillin, and 0.10 mg/ml streptomycin, pH 7.4. Cells were plated onto poly-L-lysine–coated 22-mm coverslips placed in 33-mm Petri dish. The plating density was 4 million cells/coverslip. Cells were incubated at 37°C in a 5% CO2 and 95% humidity atmosphere. On the third day, 10 μM cytosine arabinoside (AraC) was added to the culture medium, and after 24 h the medium was replaced with fresh culture medium without AraC. Subsequently, the medium was changed every third day by replacing half of the medium with fresh EMEM supplemented with 10% horse serum. Cells grown for 10–14 d were used for the experiments.
Loading of Cells with Fluorescent Probes
Relative changes in intracellular Ca2+ and nitric oxide in single cells and exocytosis at synaptic terminals were measured by fluorescence imaging. Cells were loaded with specific probes as described below and maintained in HEPES buffer containing (in mM) NaCl 140, KCl 5, MgCl2 1, CaCl2 2, HEPES 10, and glucose 10, pH 7.4.
Intracellular calcium was monitored using the fluorescent indicator fura-2 as described earlier (Sen et al., 2008). Cultures grown on coverslips were incubated with 5 μM Fura-2AM in buffer containing 0.08% pluronic acid for 45 min at 37°C, washed three times with buffer, and kept in dark for an additional 20 min to allow for complete de-esterification of dye.
Cellular nitric oxide was measured with DAF-DA, a nonfluorescent compound that is irreversibly nitrosylated by NO to a fluorescent triazole. Cultures were incubated with 10 μM DAF-DA for 1 h at 37°C in HEPES buffer. Cells were then washed with buffer and further incubated at room temperature for 20 min.
Exocytosis at synaptic terminals was monitored using FM1-43. Cells were exposed to FM1-43 (10 μM) in HEPES buffer containing high K+ (50 mM KCl) for 2 min and washed with HEPES buffer. Subsequently, cells were further incubated with 10 μM FM1-43 in normal HEPES buffer (containing 5 mM KCl) for 20 min at room temperature. Cells were then washed with buffer for 30 min to remove nonspecific binding of FM1-43 to extrasynaptic sites.
Immunocytochemistry
The immunofluorescence experiments were performed to detect the expression of P2Y receptors in hippocampal neurons and astrocytes. Cells grown on polylysine-coated coverslips for 10–14 d were fixed with 4% paraformaldehyde in PBS for 30 min followed by permeabilization with 0.2% Triton X-100 in PBS for 10 min. Nonspecific binding sites were blocked by incubating the cells with 10% heat-inactivated goat serum in PBS for 30 min. Cells were incubated simultaneously with polyclonal antibody against a specific P2Y receptor and monoclonal antibody against neuron marker protein MAP-2 overnight at 4°C. The antibody dilutions were P2Y1 (1:100), P2Y4 (1:100), P2Y2 (1:100), P2Y11 (1:100), and MAP-2 (1:500). Subsequently, the cultures were washed three times with PBS and incubated with Alexafluor 488–conjugated goat anti-rabbit and Alexafluor 633–conjugated goat anti-mouse secondary antibodies for 1 h. Coverslips were mounted with Slowfade antifade reagent in 50% glycerol buffer. In control experiments, primary antibodies were omitted from the staining protocol. For quantification, the average fluorescence intensities at a given region of interest (ROI) in control samples were measured using Olympus Fluoview software. Test samples showing intensity values higher than average intensity +3 SD in controls were considered as positive immunoreactivity.
Neuronal network in the mixed culture was visualized by staining the cultures with Alexa Fluor 594–conjugated cholera toxin B subunit, which binds to ganglioside GM1 on neuronal membranes.
Fluorescence Imaging
Fluorescence imaging of intracellular Ca2+ and NO was performed with epifluorescence imaging system (TILL Photonics) coupled to an Olympus IX71 microscope as described earlier (Sen et al., 2008). Cells grown on the coverslips and loaded with appropriate fluorescent probes were mounted on a perfusion chamber on the microscope stage. The bath volume of the chamber was 0.5 ml. For fura-2 imaging, cells were alternately excited at 340 and 380 nm. Change in [Ca2+]i in single neurons and astrocytes was quantified by selecting relevant ROI and represented as change in 340 nm/380 nm fluorescence intensity ratio. The baseline ratio (340 nm/380 nm) was 0.46 ± 0.87 for neurons (n = 50) and 0.41 ± 0.09 for astrocytes (n = 50). Any peak value that was three SD above the baseline mean value was considered as the positive response to the stimulus. The same criterion was used for considering the spontaneous [Ca2+]i fluctuations as spontaneous Ca2+ oscillations.
DAF fluorescence was imaged with 485-nm excitation. Change in NO concentration is represented as percentage increase in DAF fluorescence (F − F0) × 100/F0 where F0 and F are the fluorescence intensities at the beginning of recording and at a given time point, respectively.
Exocytosis at single synapses was visualized and quantified by confocal imaging with Olympus FV1000 microscope. FM1-43–labeled cells were mounted on perfusion chamber, and fluorescence images were captured by excitation with 488-nm laser. Baseline fluorescence in HEPES buffer (low K+) was recorded for 2–3 min followed by perfusion with high K+ buffer with and without given drugs. To quantify exocytosis, ROIs were selected at brightly stained synapses and percent decrease in fluorescence was measured.
Fluorescence images of immunostained cultures were obtained with confocal microscope using 488, 591, and 633-nm lasers.
Drug Treatment
During recording ATP, UTP, and other pharmacological agents CNQX, APV, nimodipine, suramin, and, picrotoxin were added to the static bath and were washed with perfusate after given durations. For exocytosis experiments, ATP, UTP, or other drugs were applied through exchange of perfusate. In all the experiments the ATP and UTP concentrations used were 100 and 30 μM, respectively, unless otherwise stated.
Statistical Analysis
The difference in test and control groups was analyzed by Student's t test using Sigma Plot 10.0 Software.
RESULTS
Spontaneous Synaptic Transmission in Hippocampal Neuronal Network in Culture
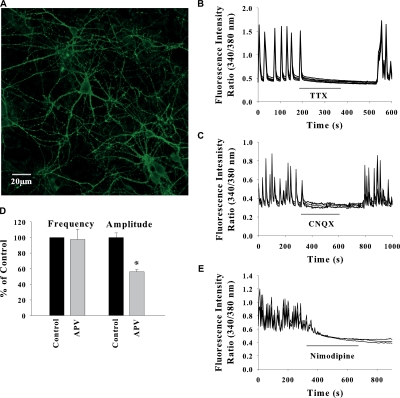
Hippocampal neurons in culture formed extensive network (Fig. 1 A) and exhibited spontaneous synaptic activity that is manifested as synchronized oscillations in intracellular calcium in a group of neurons. The SCO were abolished in the presence of the sodium channel blocker tetrodotoxin (Fig. 1 B) and in Ca2+-free medium (not depicted), suggesting that they originate from the spontaneous synaptic transmission in the neuronal network. Addition of AMPA/kainate receptor antagonist CNQX to the perfusate completely abolished the SCO and this effect was reverted after washout of CNQX (Fig. 1 C). The SCO were partially inhibited by NMDA receptor antagonist APV (Fig. 1 D). A maximum inhibition of 52 ± 5% in the amplitude was observed with 10 μM APV without any significant change in the frequency. SCO were also eliminated by L-type voltage-gated Ca2+ channel blocker nimodipine (Fig. 1 E). Thus the SCO represent spontaneous synaptic transmission in the neuronal network mediated by postsynaptic AMPA, NMDA receptors, and L-type voltage-gated Ca2+ channels. The frequency and amplitude of SCO was highly variable and dependent on the density of neurons in the network. In different cultures, the peak [Ca2+]i measured as 340/380-nm fluorescence ratio varied from 0.5 to 1.8 and the frequency from one to five oscillations per minute. The amount of spontaneously released neurotransmitter in sparsely distributed neurons may not be sufficient to maintain the synchronized Ca2+ oscillations.
Figure 1.
Synchronized Ca2+ oscillations in networked hippocampal neurons represent spontaneous glutamatergic synaptic transmission. (A) Network of neurons in mixed hippocampal cultures visualized by Alexa Fluor 594–conjugated Cholera toxin B subunit that binds to ganglioside GM1 on neuronal membrane. (B) TTX (10 nM) sensitive Ca2+ oscillations in a group of neurons. Bath application of 10 μM CNQX (C), 10 μM APV (D), or 1 μM nimodipine inhibited the SCO. For clarity, the data shown in B–D are for five neurons in a field from a typical experiment repeated three to five times. Data in D are mean ± SEM for 30 neurons from three experiments (*, P < 0.005). The controls were taken as SCO in each experiment before drug treatment.
P2Y Receptors Modulate Spontaneous Synaptic Activity
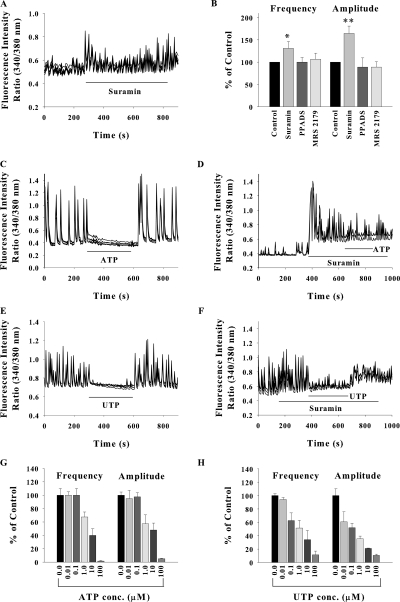
ATP and its analogues have been shown to inhibit the spontaneous Ca2+ oscillations in hippocampal neurons (Koizumi and Inoue, 1997). We observed that nonspecific P2 receptor antagonist suramin facilitated the SCO (Fig. 2 A). In a neuronal network exhibiting few oscillations of very low amplitude, blockade of P2 receptors with suramin caused marked enhancement in the frequency and amplitude of SCO. Another nonspecific P2 receptor antagonist PPADS and specific inhibitor of P2Y1 receptor MRS2179 did not alter either the frequency or the amplitude of SCO (Fig. 2 B).
Figure 2.
Modulation of SCO by P2 receptor agonists and antagonists. (A and B) Bath application of suramin (50 μM) facilitated the SCO but PPADS (10 μM) and MRS2179 (10 μM) were ineffective. Data shown in B are mean ± SEM from 20–40 cells from at least three experiments for each drug. SCO before drug addition were treated as control. Bath application of 100 μM ATP (C) or 30 μM UTP (E) blocked the SCO. UTP inhibited SCO more potently than ATP (G and H). The SCO persisted upon application of 100 μM ATP or 30 μM UTP along with 50 μM suramin (D and F). Data shown in G and H are mean ± SEM for 20–30 neurons from three experiments. (*, P < 0.01; **, P < 0.001).
Exogenously added ATP attenuated the neuronal Ca2+ oscillations in a reversible manner (Fig. 2 C). The suppression of SCO was significantly less when ATP was added in presence of suramin (Fig. 2 D). It is noteworthy that in the absence of suramin, 100 μM ATP completely abolished the SCO, whereas in presence of 50 μM suramin, the SCO persisted though with lower amplitude and frequency, confirming the role of P2 receptors in synaptic modulation. Similar results were obtained with UTP (Fig. 2, E and F), which was slightly more potent in suppressing SCO as compared with ATP (Fig. 2, G and H).
These data suggest that spontaneous synaptic activity is modulated by endogenously released or exogenously added ATP/UTP through suramin-sensitive and PPADS-insensitive P2 receptors. This pharmacological profile points toward P2Y2/P2Y4 receptors. UTP specifically activates P2Y2 and P2Y4 receptors, which are also activated by ATP and these receptors are suramin sensitive but insensitive to PPADS (Fields and Burnstock, 2006).
Inhibitory Effect of ATP and UTP on Neuronal SCO Is Effectuated by Astrocytes
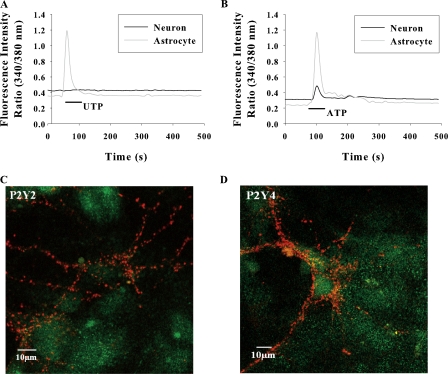
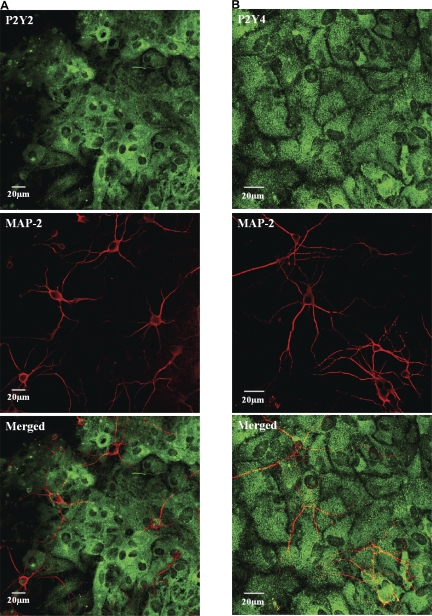
ATP/UTP can inhibit SCO by directly acting on neuronal P2 receptors or via astrocytic receptors. To determine the site of action, the nucleotide-induced Ca2+ signals were monitored in solitary neurons and astrocytes. In low density co-cultures, where neurons are unable to elicit network activity, UTP elevated the [Ca2+]i exclusively in astrocytes (Fig. 3 A), suggesting the absence of functional P2Y2/P2Y4 receptors in neurons. This was confirmed by immunostaining of hippocampal cultures with specific antibodies. P2Y2 and P2Y4 receptors were profusely expressed in astrocytes but no immunofluorescence was seen in neurons (Fig. 4, A and B). The synaptic terminals identified by synaptophysin staining were also completely devoid of anti-P2Y2 or P2Y4 immunofluorescence (Fig. 3, C and D). The lack of P2Y2 and P2Y4 immunoreactivity in neurons was confirmed by quantification of fluorescence images using the criterion that the average intensity of a positively stained cell should be greater than average intensity +3 SD of cells that were incubated with secondary antibody alone, as described in the Materials and methods. ATP induced a robust [Ca2+]i signal in astrocytes but marginally elevated [Ca2+]i in neurons (Fig. 3 B), possibly through ionotropic P2X receptors (Khakh et al., 2001; Pankratov et al., 2002).
Figure 3.
Representative traces of [Ca2+]i response in solitary neurons and astrocytes in low density cultures stimulated with 30 μM UTP (A); 100 μM ATP (B). The traces shown are from a single neuron and astrocyte representing 15 neurons and 40 astrocytes from eight experiments. (C and D) Confocal images of mixed hippocampal cultures immunostained with anti-P2Y receptors (green) and synaptophysin (red). P2Y2 and P2Y4 receptors are expressed only in astrocytes. P2Y2 and P2Y4 receptor immunofluorescence was not colocalized with synaptophysin fluorescence at synaptic terminals.
Figure 4.
Confocal images of mixed hippocampal cultures immunostained with anti-P2Y receptors (green) and neuronal markers anti-MAP-2 (red). Both P2Y2 and P2Y4 receptor immunostaining is not seen in neuronal cell bodies or processes but astrocytes are profusely stained. In merged images, some pixels appear yellow because of the neurons (red) overlying the brightly stained (green) astrocytes.
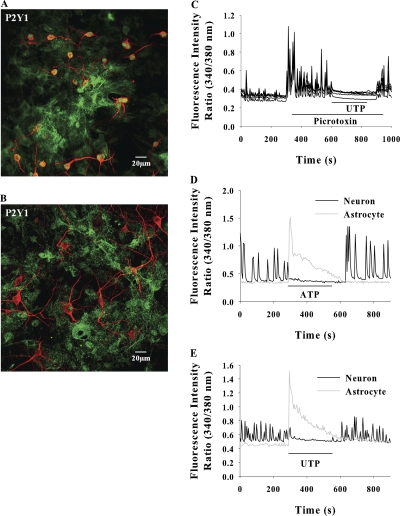
P2Y1 receptor immunofluorescence was observed in smaller neurons (somal diameters ≤ 10 μm, possibly the GABAergic neurons) but was not seen in larger neurons (Fig. 5, A and B). In all our experiments, the SCO were monitored in larger neurons only (somal diameter ≥ 15 μm). In hippocampal slices, activation of P2Y1 receptors in interneurons has been shown to facilitate the GABAergic IPSCs causing synaptic inhibition (Bowser and Khakh, 2004). We tested this possibility in our experimental system. Inhibition of GABA receptors by picrotoxin dramatically enhanced the SCO, however, addition of ATP or UTP still suppressed the SCO (Fig. 5 C), implying that under our experimental conditions the inhibitory effect of ATP/UTP is not mediated by GABAergic neurons.
Figure 5.
P2Y1 receptors are expressed in smaller neurons with somal diameter ≤ 10 μm (A) but are absent in larger neurons (B). (C) Inhibition of GABA receptors with 1 μM picrotoxin enhanced the SCO, and application of 100 μM ATP efficiently inhibited the SCO. Data shown are for five neurons in a typical experiment repeated three times. 100 μM ATP (D) or 30 μM UTP (E) induced large [Ca2+]i response in astrocytes that was coincident with SCO inhibition in neurons. The data shown are from neurons and astrocytes in the same field in a typical experiment.
In the absence of functional UTP receptors (P2Y2 and P2Y4) in postsynaptic neurons and presynaptic terminals, the suppression of SCO by UTP/ATP most likely occurs via astrocytes. Analysis of [Ca2+]i changes in networked neurons and the astrocytes in the same field revealed that ATP/UTP induced a robust but transient [Ca2+]i signal in astrocytes that coincided with depression of neuronal SCO (Fig. 5, D and E).
Nitric Oxide Mediates UTP/ATP-induced Synaptic Inhibition
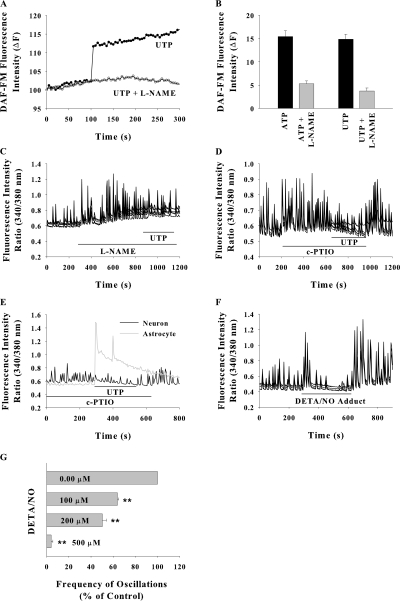
We hypothesized nitric oxide (NO) as the putative diffusible messenger from astrocyte to neurons. In hippocampal mixed cultures loaded with nitric oxide probe DAF, exogenously added UTP and ATP considerably increased the fluorescence intensity in astrocytes and it was inhibited by pretreatment of cells with nitric oxide synthase inhibitor L-NAME (Fig. 6, A and B). The possible role of NO in ATP/UTP-induced SCO suppression was tested. A significant enhancement in SCO amplitude was observed after treatment with nitric oxide synthase inhibitor L-NAME, and subsequent addition of UTP did not abolish the SCO (Fig. 6 C). When UTP was added in presence of membrane-impermeable NO scavenger c-PTIO, the [Ca2+]i signal in astrocytes was comparable to that observed in the absence of scavenger but the neuronal SCO persisted (Fig. 6 E), indicating the involvement of diffusible NO in SCO suppression. c-PTIO significantly enhanced the SCO amplitude in control (nucleotide untreated) cultures (Fig. 6 D). The nitric oxide donor DETA/NO (200 μM) considerably decreased the frequency as well as amplitude of SCO after a delay of ∼50 s (Fig. 6, F and G). These data suggest the involvement of diffusible NO in SCO suppression by ATP.
Figure 6.
ATP and UTP-induced nitric oxide formation in astrocytes and its effect on neuronal SCO. (A) Increase in DAF fluorescence stimulated with 30 μM UTP in astrocytes in a mixed culture with and without pretreatment with 100 μM L-NAME. The traces are from single astrocytes. (B) Mean ± SEM. DAF fluorescence in 10–15 astrocytes from three experiments for each treatment. (C) SCO inhibition with UTP was prevented by pretreatment of cells with L-NAME or by 100 μM c-PTIO in the perfusate (D). (E) UTP induced [Ca2+]i rise in astrocytes in presence of c-PTIO. (F and G) Effect of nitric oxide donor DETA/NO on SCO frequency. Data in G are for 10–15 neurons for each data concentration from three experiments (**, P < 0.001).
NO-mediated Inhibition Does Not Occur through Postsynaptic Receptors
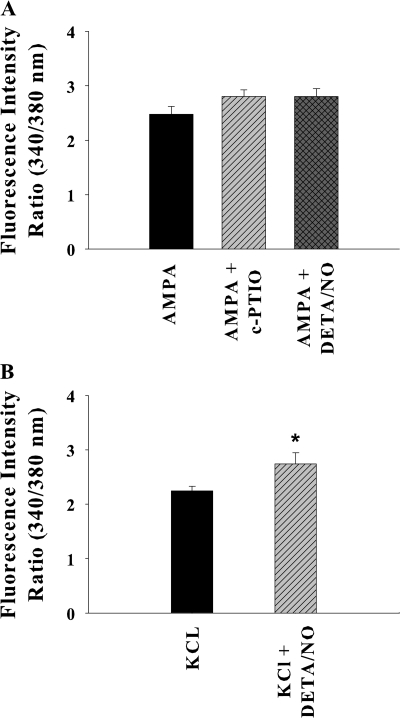
As shown above, the SCO are generated due to Ca2+ entry through voltage-gated Ca2+ channels consequent to AMPA receptor activation during spontaneous activity in the neuronal network. The effect of NO on neuronal AMPA receptors and VGCC was examined. There was no significant effect of c-PTIO and DETA/NO on AMPA-evoked [Ca2+]i (Fig. 7 A). Ca2+ influx through VGCC activated by KCl depolarization was slightly enhanced in the presence of NO donor DETA/NO (Fig. 7 B). These data suggest that the suppression of SCO is not due to modulation of post synaptic receptors involved in SCO, as previously reported (Jian et al., 2007).
Figure 7.
Effect of nitric oxide on postsynaptic receptors. (A) [Ca2+]i rise induced by 50 μM AMPA was not significantly affected by presence of 100 μM c-PTIO or 500 μM DETA/NO. (B) DETA/NO modestly enhanced the KCl (60 mM) induced [Ca2+]i rise. The data shown are mean ± SEM for 10–35 neurons from at least three experiments for each treatment (*, P < 0.01).
NO Released Consequent to P2Y Receptor Stimulation Inhibits Neurotransmitter Release
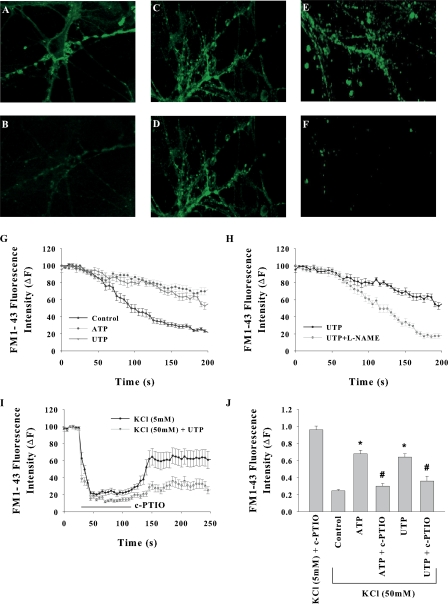
Effect of ATP/UTP on neurotransmitter release and the involvement of NO therein were determined by monitoring the exocytotic events. Neurons were loaded with membrane probe FM1-43 that is released into the medium during exocytosis. Exocytosis was induced by depolarization with 50 mM KCl in the perfusate, and change in fluorescence intensity at single synapses was measured by confocal imaging. Other agents described below were included in the perfusate. Depolarization of neurons drastically diminished the fluorescence intensity at brightly stained synaptic terminals within 100 s (Fig. 8, A, B, and G). In the presence of ATP, the reduction in fluorescence upon KCl depolarization was markedly less, suggesting the attenuation of neurotransmitter release (Fig. 8, C, D, and G). The ATP-induced attenuation was not observed in cells pretreated with 100 μM L-NAME (Fig. 8 H). Addition of c-PTIO in to the perfusate caused strong quenching of FM1-43 fluorescence. This quenching was observed even in perfusate containing low potassium (5 mM KCl), however it recovered almost to the original level after washout of c-PTIO (Fig. 8 I). We attributed this quenching to fluorescence energy transfer as the fluorescence spectrum of FM1-43 strongly overlaps with the absorption spectrum of c-PTIO; nevertheless the quenching was relieved after removal of c-PTIO. In the experiments wherein c-PTIO was used, the fluorescence levels were compared at 60 s after c-PTIO washout, i.e., when the fluorescence would have fully recovered from c-PTIO quenching in the absence of depolarization. As shown in Fig. 8 (E, F, and J), the neurotransmitter release in presence of ATP and c-PTIO was similar to that observed in the absence of ATP. Overall the data shown in Fig. 8 provide the evidence for attenuation of neurotransmitter release by NO produced as a result of P2Y receptor activation.
Figure 8.
Role of nitric oxide in ATP/UTP-induced exocytosis. (A–F) Confocal images of FM1-43–loaded cultures (A, C, and E) before and (B, D, and F) 150 s after depolarization. 40 s after the start of imaging, cells were depolarized by perfusing high K+ (60 mM) HEPES buffer. (A and B) control, (C and D) in presence of 30 μM UTP, (E and F) in presence of 30 μM UTP and 100 μM c-PTIO. (G) KCl-induced reduction in FM1-43 fluorescence was inhibited by ATP and UTP. (H) Inhibitory effect of UTP was prevented by pretreatment of cells with L-NAME. (I) Inhibitory effect of UTP was prevented by bath-applied c-PTIO. Application of 100 μM quenched the FM1-43 fluorescence even in the absence of depolarization but it was fully recovered after removal of c-PTIO. (J) Relative fluorescence intensities at 150 s after KCl depolarization along with given treatments. Fluorescence in 5 mM KCl buffer is also included for comparison. The data shown are mean ± SEM for 10–20 synapses from at least three experiments for each treatment (*, P < 0.0001; #, P > 0.1).
DISCUSSION
Our data provide evidence for control of neuronal activity induced by P2Y receptor activation in astrocytes, concomitant release of nitric oxide, and coupling with presynaptic neurotransmitter release. The spontaneous synaptic activity is manifested as synchronized Ca2+ oscillations in networked neurons. SCO are triggered by action potential in the neuronal network. The SCO were blocked by nimodipine and CNQX completely abolished the SCO, suggesting that the SCO arise due to Ca2+ entry through voltage-gated Ca2+ channels for which AMPA receptor activation is mandatory. Glutamate released during spontaneous synaptic activity can activate the AMPA receptor in postsynaptic neuron, causing membrane depolarization and activation of VGCC. Ca2+ influx through NMDA receptor also partially contributes to SCO as antagonist APV diminished the amplitude of SCO without causing a significant change in the frequency.
Exogenously added ATP and UTP suppressed the SCO and their effect was prevented by P2 receptor antagonist suramin (Fig. 2, C–H). ATP and UTP can activate several P2X and P2Y receptors (Fields and Burnstock, 2006). UTP activates three P2Y receptors: P2Y2 receptor that is activated equipotently by ATP and UTP, P2Y4 receptor most potently activated by UTP, and the P2Y6 receptor that can be selectively activated by UDP. In contrast with ATP and UTP, bath application of UDP facilitated the SCO (unpublished data); thus the involvement of P2Y6 receptors is ruled out. We think that the SCO suppression is mediated by P2Y2 and P2Y4 receptors because UTP mimicked ATP in suppressing the SCO with almost the same potency, and the suppression was sensitive to suramin but not PPADS, which are the characteristics of these receptors (Fields and Burnstock, 2006). However, we cannot conclusively affirm their role because no specific antagonists are available for these receptors.
By immunostaining of synaptosomes, a subpopulation of glutamatergic nerve terminals was shown to possess the P2Y2 and P2Y4 receptors (Rodrigues et al., 2005). However in the hippocampal cultures we did not find P2Y2 or P2Y4 immunostaining either in neuronal soma or the synaptic release sites identified by synaptophysin labeling (Figs. 3 and 4). Astrocytes in the same cultures showed abundant immunofluorescence for P2Y2 as well as P2Y4 receptors (Figs. 3 and 4). Imaging the [Ca2+]i changes simultaneously in solitary neurons and astrocytes in low density hippocampal cultures showed that neurons were absolutely silent upon UTP stimulation and elicited a very small [Ca2+]i elevation in response to ATP. In contrast, astrocytes in the same field elicited a robust [Ca2+]i elevation. These data along with the immunocytochemistry results suggest the absence of functional P2Y2/P2Y4 receptors in neurons but abundant expression in astrocytes in hippocampal cultures. The ATP-induced small signal in neurons can be attributed to Ca2+ entry through ionotropic P2X receptors (Khakh et al., 2001; Pankratov et al., 2002). We observed that a pulse of extracellular ATP/UTP suppressed the SCO in a reversible manner and a robust Ca2+ signal in astrocytes was concomitant with SCO inhibition. Taken together these data imply that activation of P2Y2/P2Y4 receptors on astrocytes causes inhibition of SCO.
We hypothesized that a messenger molecule other than ATP released from astrocytes acts on neurons to suppress the SCO. NO was considered as the putative messenger molecule. Astrocytes produced appreciable levels of nitric oxide in response to ATP and UTP stimulation (Fig. 6, A and B). The inhibitory effect of ATP/UTP was markedly diminished by nitric oxide synthase inhibitor L-NAME and membrane-impermeant NO scavenger c-PTIO. These results established the involvement of NO in nucleotide-induced suppression of SCO. NO can also be generated in neurons by activation of nNOS and the role of NO as a diffusible retrograde messenger from postsynaptic to presynaptic neuron and its involvement in long term potentiation is well known. In our experimental system, induction of nNOS by UTP/ATP in postsynaptic neurons is unlikely because of the lack of UTP-inducible receptors in neurons. Moreover the membrane-impermeable NO scavenger c-PTIO eliminated the inhibitory effect of ATP and UTP. We therefore infer that NO produced in astrocytes diffuses into the neurons and thus attenuates SCO. Effect of NO on postsynaptic AMPA receptor and voltage-gated ion channels was also tested. NO donor DETA/NO did not affect the Ca2+ signal induced by AMPA. On the other hand, a modest increase in Ca2+ influx through VGCC was observed. ATP and UTP attenuated the exocytosis at synaptic release sites and this attenuation was prevented by L-NAME and c-PTIO (Fig. 8). Our data demonstrate that NO released from activated astrocytes acts presynaptically to suppress the SCO. This is consistent with the earlier reports demonstrating NO-mediated inhibition of synaptic glutamate release in hippocampal CA1 neurons and cerebellar granule cells (Tsutsuki et al., 2007). NO causes long term depression by the cGMP pathway (Gage et al., 1997).
All the earlier studies on NO signaling in astrocytes have focused on its role in neurodegeneration under pathological conditions. In this paper, we have demonstrated for the first time that under physiological conditions astrocytes use NO as a messenger molecule to modulate the synaptic strength in the networked neurons. In the absence of exogenously added ATP/UTP, both the NO scavenger and NOS inhibitor enhanced the frequency of SCO, implying that NO is released even during spontaneous synaptic transmission and exerts a suppressive effect on synaptic activity. Similarly P2 receptor antagonist suramin and PPADS heightened the SCO, suggesting negative regulation of synaptic strength by endogenously released ATP/UTP during spontaneous synaptic activity. A large body of evidence exists, demonstrating the release of ATP from stimulated astrocytes. Constitutive and regulated release of UTP has been shown in many cell types, including rat brain astrocytes (Lazarowski et al., 1997, 2003).
The spontaneous synaptic activity and synchronized Ca2+ oscillations in neuronal network are commonly observed in developing neurons and have been implicated in neuronal migration, axonal and dendritic growth, refinement of neural connections and synapses, gene regulations, neurotransmitter receptor expression, and channel maturation (Aguado et al., 2002; Katz and Shatz, 1996), etc. Large scale oscillatory Ca2+ waves regulate long distance wiring in immature cortex. Local and global spontaneous Ca2+ oscillations are key regulators for onset of neuronal phenotype during neural precursor differentiation and cell specification in the developing nervous system (Ciccolini et al., 2003). Early spontaneous synchronous network activity is able to convert silent synapses to active ones, which in turn prevents later arriving axons from making afferent connections and thus limit the range of neocortical connections (Voigt et al., 2005). The proposed nitric oxide–mediated intercellular signaling loop between neurons and astrocytes could be an important mechanism for dynamic control of synaptic plasticity in developing nervous system and differentiation of neural stem cells.
Acknowledgments
This work was supported by Department of Biotechnology, India, and Fellowship to B. Mehta by Council of Scientific and Industrial Research, India.
Lawrence G. Palmer served as editor.
Abbreviations used in this paper: APV, 2-amino-5-phosphonopentanoic acid; CNQX, 6-cyano-7-nitroquinozaline-2,3-dione; DAF-DA, diaminofluorescein diacetate; DETA-NO, (Z)-1-[N-(2-aminoethyl)-N-(2-ammonioethyl) amino] diazen-1-ium-1,2-diolate; ROI, region of interest.
References
- Aguado, F., J.F. Espinosa-Parrilla, M.A. Carmona, and E. Soriano. 2002. Neuronal activity regulates correlated network properties of spontaneous calcium transients in astrocytes in situ. J. Neurosci. 22:9430–9444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auld, D.S., and R. Robitaille. 2003. Glial cells and neurotransmission: an inclusive view of synaptic function. Neuron. 40:389–400. [DOI] [PubMed] [Google Scholar]
- Bowser, D.N., and B.S. Khakh. 2004. ATP excites interneurons and astrocytes to increase synaptic inhibition in neuronal networks. J. Neurosci. 24:8606–8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciccolini, F., T.J. Collins, J. Sudhoelter, P. Lipp, M.J. Berridge, and M.D. Bootman. 2003. Local and global spontaneous calcium events regulate neurite outgrowth and onset of GABAergic phenotype during neural precursor differentiation. J. Neurosci. 23:103–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dravid, S.M., and T.F. Murray. 2004. Spontaneous synchronized calcium oscillations in neocortical neurons in the presence of physiological [Mg2+]: involvement of AMPA/kainate and metabotropic glutamate receptors. Brain Res. 1006:8–17. [DOI] [PubMed] [Google Scholar]
- Fellin, T., O. Pascual, S. Gobbo, T. Pozzan, P.G. Haydon, and G. Carmignoto. 2004. Neuronal synchrony mediated by astrocytic glutamate through activation of extrasynaptic NMDA receptors. Neuron. 43:729–743. [DOI] [PubMed] [Google Scholar]
- Fiacco, T.A., and K.D. McCarthy. 2004. Intracellular astrocyte calcium waves in situ increase the frequency of spontaneous AMPA receptor currents in CA1 pyramidal neurons. J. Neurosci. 24:722–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields, R.D., and G. Burnstock. 2006. Purinergic signalling in neuron-glia interactions. Nat. Rev. Neurosci. 7:423–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage, A.T., M. Reyes, and P.K. Stanton. 1997. Nitric-oxide-guanylyl-cyclase-dependent and -independent components of multiple forms of long-term synaptic depression. Hippocampus. 7:286–295. [DOI] [PubMed] [Google Scholar]
- Halassa, M.M., T. Fellin, and P.G. Haydon. 2007. The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol. Med. 13:54–63. [DOI] [PubMed] [Google Scholar]
- Haydon, P.G. 2001. GLIA: listening and talking to the synapse. Nat. Rev. Neurosci. 2:185–193. [DOI] [PubMed] [Google Scholar]
- Jian, K., M. Chen, X. Cao, X.H. Zhu, M.L. Fung, and T.M. Gao. 2007. Nitric oxide modulation of voltage-gated calcium current by S-nitrosylation and cGMP pathway in cultured rat hippocampal neurons. Biochem. Biophys. Res. Commun. 359:481–485. [DOI] [PubMed] [Google Scholar]
- Katz, L.C., and C.J. Shatz. 1996. Synaptic activity and the construction of cortical circuits. Science. 274:1133–1138. [DOI] [PubMed] [Google Scholar]
- Kawamura, M., C. Gachet, K. Inoue, and F. Kato. 2004. Direct excitation of inhibitory interneurons by extracellular ATP mediated by P2Y1 receptors in the hippocampal slice. J. Neurosci. 24:10835–10845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh, B.S., W.B. Smith, C.S. Chiu, D. Ju, N. Davidson, and H.A. Lester. 2001. Activation-dependent changes in receptor distribution and dendritic morphology in hippocampal neurons expressing P2X2-green fluorescent protein receptors. Proc. Natl. Acad. Sci. USA. 98:5288–5293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi, S., K. Fujishita, M. Tsuda, Y. Shigemoto-Mogami, and K. Inoue. 2003. Dynamic inhibition of excitatory synaptic transmission by astrocyte-derived ATP in hippocampal cultures. Proc. Natl. Acad. Sci. USA. 100:11023–11028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi, S., and K. Inoue. 1997. Inhibition by ATP of calcium oscillations in rat cultured hippocampal neurones. Br. J. Pharmacol. 122:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka, N., Y. Kudo, and M. Morita. 2007. Multiple inhibitory pathways for lipopolysaccharide- and pro-inflammatory cytokine-induced nitric oxide production in cultured astrocytes. Neuroscience. 144:911–919. [DOI] [PubMed] [Google Scholar]
- Lazarowski, E.R., L. Homolya, R.C. Boucher, and T.K. Harden. 1997. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J. Biol. Chem. 272:24348–24354. [DOI] [PubMed] [Google Scholar]
- Lazarowski, E.R., R.C. Boucher, and T.K. Harden. 2003. Mechanisms of release of nucleotides and integration of their action as P2X- and P2Y-receptor activating molecules. Mol. Pharmacol. 64:785–795. [DOI] [PubMed] [Google Scholar]
- Li, N., J.Y. Sul, and P.G. Haydon. 2003. A calcium-induced calcium influx factor, nitric oxide, modulates the refilling of calcium stores in astrocytes. J. Neurosci. 23:10302–10310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Z., L. Geng, R. Li, X. He, J.Q. Zheng, and Z. Xie. 2003. Frequency modulation of synchronized Ca2+ spikes in cultured hippocampal networks through G-protein-coupled receptors. J. Neurosci. 23:4156–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami, K., Y. Nakamura, and Y. Yoneda. 2003. Potentiation by ATP of lipopolysaccharide-stimulated nitric oxide production in cultured astrocytes. Neuroscience. 117:37–42. [DOI] [PubMed] [Google Scholar]
- Murphy, S. 2000. Production of nitric oxide by glial cells: regulation and potential roles in the CNS. Glia. 29:1–13. [DOI] [PubMed] [Google Scholar]
- Newman, E.A. 2003. Glial cell inhibition of neurons by release of ATP. J. Neurosci. 23:1659–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani, Y., M. Minami, and M. Satoh. 2000. Expression of inducible nitric oxide synthase mRNA and production of nitric oxide are induced by adenosine triphosphate in cultured rat microglia. Neurosci. Lett. 293:72–74. [DOI] [PubMed] [Google Scholar]
- Pankratov, Y.V., U.V. Lalo, and O.A. Krishtal. 2002. Role for P2X receptors in long-term potentiation. J. Neurosci. 22:8363–8369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues, R.J., T. Almeida, P.J. Richardson, C.R. Oliveira, and R.A. Cunha. 2005. Dual presynaptic control by ATP of glutamate release via facilitatory P2X1, P2X2/3, and P2X3 and inhibitory P2Y1, P2Y2, and/or P2Y4 receptors in the rat hippocampus. J. Neurosci. 25:6286–6295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen, I., D.C. Joshi, P.G. Joshi, and N.B. Joshi. 2008. NMDA and non-NMDA receptor-mediated differential Ca2+ load and greater vulnerability of motor neurons in spinal cord cultures. Neurochem. Int. 52:247–255. [DOI] [PubMed] [Google Scholar]
- Silva, G., W.H. Beierwaltes, and J.L. Garvin. 2006. Extracellular ATP stimulates NO production in rat thick ascending limb. Hypertension. 47:563–567. [DOI] [PubMed] [Google Scholar]
- Stanton, P.K., J. Winterer, C.P. Bailey, A. Kyrozis, I. Raginov, G. Laube, R.W. Veh, C.Q. Nguyen, and W. Muller. 2003. Long-term depression of presynaptic release from the readily releasable vesicle pool induced by NMDA receptor-dependent retrograde nitric oxide. J. Neurosci. 23:5936–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka, M., K. Kawahara, T. Kosugi, T. Yamada, and T. Mioka. 2007. Changes in the spontaneous calcium oscillations for the development of the preconditioning-induced ischemic tolerance in neuron/astrocyte co-culture. Neurochem. Res. 32:988–1001. [DOI] [PubMed] [Google Scholar]
- Tsutsuki, H., T. Kohda, M. Hara, S. Kozaki, and H. Ihara. 2007. Nitric oxide inhibits depolarization-evoked glutamate release from rat cerebellar granule cells. Nitric Oxide. 16:217–227. [DOI] [PubMed] [Google Scholar]
- Vergun, O., J. Keelan, B.I. Khodorov, and M.R. Duchen. 1999. Glutamate-induced mitochondrial depolarisation and perturbation of calcium homeostasis in cultured rat hippocampal neurones. J. Physiol. 519(Pt 2):451–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voigt, T., T. Opitz, and A.D. de Lima. 2005. Activation of early silent synapses by spontaneous synchronous network activity limits the range of neocortical connections. J. Neurosci. 25:4605–4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y., N.J. Haughey, M.P. Mattson, and K. Furukawa. 2004. Dual effects of ATP on rat hippocampal synaptic plasticity. Neuroreport. 15:633–636. [DOI] [PubMed] [Google Scholar]
- Zhang, J.M., H.K. Wang, C.Q. Ye, W. Ge, Y. Chen, Z.L. Jiang, C.P. Wu, M.M. Poo, and S. Duan. 2003. ATP released by astrocytes mediates glutamatergic activity-dependent heterosynaptic suppression. Neuron. 40:971–982. [DOI] [PubMed] [Google Scholar]