Abstract
This study was undertaken to analyze the functional outcome of surgically treated spinal meningiomas and to determine factors for surgical morbidity. Between January 1990 and December 2006 a total of 131 patients underwent surgical resection of a spinal menigioma. There were 114 (87%) female and 17 (13%) male patients. Age ranged from 17 to 88 years (mean 69 years). The mean follow-up period was 61 months (range 1–116 months) including a complete neurological examination and postoperative MRI studies. The pre- and postoperative neurological state was graded according to the Frankel Scale. Surgery was performed under standard microsurgical conditions with neurophysiological monitoring. In 73% the lesion was located in the thoracic region, in 16% in the cervical region, in 5% at the cervico-thoracic junction, in 4.5% at the thoraco-lumbar junction and in 1.5% in the lumbar region. Surgical resection was complete in 127 patients (97%) and incomplete in 4 patients (3%). At the last follow-up the neurological state was improved or unchanged in 126 patients (96.2%) and worse in 4 patients (3%). Permanent operative morbidity and mortality rates were 3 and 0.8%, respectively. Extensive tumour calcification proved to be a significant factor for surgical morbidity (P < 0.0001). Radical resection of spinal meningiomas can be performed with good functional results. Extensive tumor calcification, especially in elderly patients proved to harbor an increased risk for surgical morbidity.
Keywords: Spinal meningioma, Outcome, Spinal tumours, Surgery, Meningioma
Introduction
Meningiomas involving the spinal compartment are relatively rare in comparison to the intracranial compartment accounting approximately 1.2% of all meningiomas of the central nervous system. Mostly, they are located in the intradural compartment, generally respecting the pial layer of the spinal cord. Isolated extradural spinal meningiomas are rare [4, 13, 18, 19]. By the introduction of modern neuroimaging techniques and standard microneurosurgical procedures, these tumours nowadays can be removed with a low morbidity and mortality.
Spinal menigiomas are more common in elderly patients, thus, the occurrence in younger patients should raise the suspicion of a genetically determined disorder as neurofibromatosis 2 or an association with aggressive histological subtypes [2]. They are slow growing tumours and therefore, they lead to symptoms only after reaching a distinct size due to significant spinal cord compression. Initially, local pain is one of the leading symptoms, however, in a considerable number of patients diagnosis is confirmed not until neurological deficits or gait disturbances are present. These extensive tumours demand challenge to neurosurgical skills, in particular lesions, which are located ventrally to the spinal cord.
In this study, we report on our experience of 131 patients operated on a spinal meningioma at our institution. Special consideration was focused on patients with postoperative functional deterioration in order to identify factors with impact on surgical outcome.
Patients and methods
Between January 1990 and December 2006 a total of 131 patients with a spinal meningioma were referred to our institution and underwent microneurosurgical resection. The female gender was clearly predominant with 114 female patients and 17 male patients (female/male ratio 5:1). The patients’ age ranged from 17 to 88 years with a mean age of 69 years.
All patients were followed-up clinically and by spinal MRI. The mean follow-up period was 61 months (range 1–116 months). Histological examination of the specimen confirmed a grade I meningioma according to the WHO classification in 129 patients and an atypical meningioma grade II in 2 patients.
The patient’s pre- and postoperative neurological state was classified according to the Frankel scale in order to achieve a grading of functional disturbance of daily life activities and gait disturbances [3]. According to the Frankel grade outcome was classified as poor (A + B), fair (C), and good (D + E).
Diagnostics and classification
Contrast enhanced spinal magnetic resonance imaging (MRI) was the diagnostic tool of choice and performed routinely for preoperative evaluation in all patients. Both, T1 and T2 weighted images in sagittal and axial planes were done to determine the spinal level, size and the dural attachment of the meningioma and its relation to the spinal cord. The latter was additionally assessed based on intraoperative observations. Pre- and postoperative plain radiographs were obtained routinely in all cases either to demonstrate the extent of bone resection in patients undergoing laminectomy as surgical approach or to document the spinal reconstruction in patients undergoing osteoplastic laminotomy.
Surgical approach
During the first years of this study, mono- or multisegmental laminectomy or hemilaminectomy was performed to access the spinal meningioma, however, this strategy later was changed and replaced by osteoplastic laminotomy with following reconstruction of the posterior spinal column with microplates as our standard surgical approach [21].
Operative removal of the spinal meningioma was performed under standard microsurgical conditions with intraoperative monitoring of somatosensory evoked potentials (SSEP).
Although generally the grading system according to Simpson (1957) was used to determine the extent of resection in meningiomas [17], in our opinion this grading system can not be utilised unrestricted for meningiomas in this area, such as for meningiomas of the convexity, where the dural attachment can be resected more radically. Therefore, in the subtype of meningiomas of this study resection was defined as “complete” according to intraoperative observations and postoperative MRI corresponding to Simpson’s grade I or II.
Calcification of the spinal meningioma was classified as complete, partial or absent.
Recurrence free survival was evaluated using the Kaplan-Meier analysis. Recurrence free period was defined as the interval between surgery and the last follow-up without clinical and radiological evidence of tumor regrowth.
The functional outcome was analyzed depending on histological features, tumour localization and extension of the tumour.
Statistical analyses were performed using SPSS (SPSS Inc., version 11.0). Crosstables were calculated and Chi-quadrate test was performed to assess the impact of different variables on outcome. Functional outcome was dichotomized into categories ‘good’ versus ‘fair and poor’.
Results
Leading symptoms were sensory and motor deficits in 84% of the patients. Gait disturbances were observed in 83%. In 39% the patients were unable to walk due to significant neurological deficits (Frankel grade A–C). Almost the half of the patients (47%) complained about local pain.
The most frequently involved localization was the thoracic region followed by the cervical region. An overview is given in Table 1.
Table 1.
Localization of the spinal meningioma
| Localization | Number of patients (%) |
|---|---|
| Cervical | 21 (16) |
| Cervicothoracic | 7 (5) |
| Thoracic | 95 (73) |
| Thoracolumbar | 6 (4.5) |
| Lumbar | 2 (1.5) |
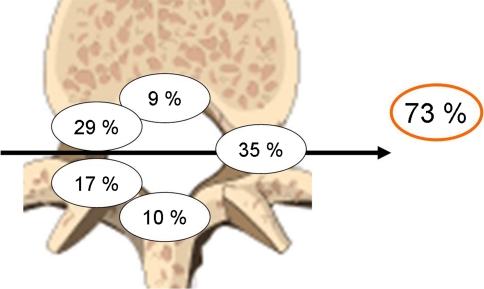
The dural attachment of the spinal meningioma was predominantly localized laterally or ventrolaterally in 35 and 29%, respectively, as determined by preoperative MRI and intraoperative observation. In addition to 9% of patients with a ventrally located spinal meningioma, the dural attachment was ventrally to the dentate ligament of the spinal canal in 73% and only in 27% dorsally (Fig. 1).
Fig. 1.
Localization of the dural attachment of the spinal meningioma
Surgery
Surgery was mostly performed in a semisitting position if the spinal meningioma was located in the cervical or upper thoracic region and in a prone position for tumours located below the upper thoracic region or the lumbar region.
Laminectomy or hemilaminectomy was performed to access the tumour, but this approach was more recently abandoned and replaced by osteoplastic laminotomy with reconstruction of the posterior spinal column [21].
In 127 patients (97%) the spinal meningioma was removed completely (Simpson’s grade I or II) and in 4 patients (3%) incompletely (Simpson’s grade III). Partial tumour calcification was observed in 15 patients (11.5%) and complete calcification in 6 cases (4.6%). The dural attachment was completely resected if the spinal menigioma was located dorsally or dorsolaterally. In these cases, duraplasty was performed with autologeous fascia obtained during the operative approach. In ventrally located tumours the dural attachment was not excised but extensively bipolar cauterized (Figs. 2, 3, 4, 5).
Fig. 2.
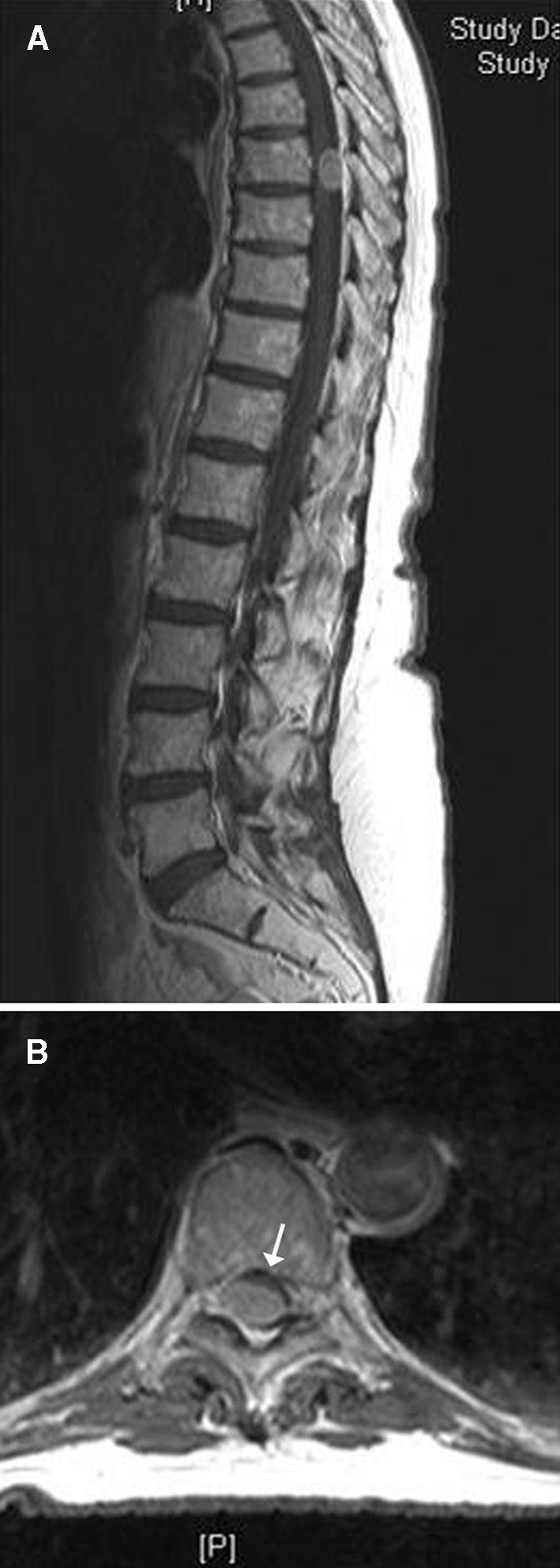
Preoperative gadolinium enhanced T1-weighted sagittal (a) and axial (b) MRI scans showing an extensive spinal menigioma with dorsal attachment at the level T8. Note the ventro-lateral displacement of the spinal cord (arrow)
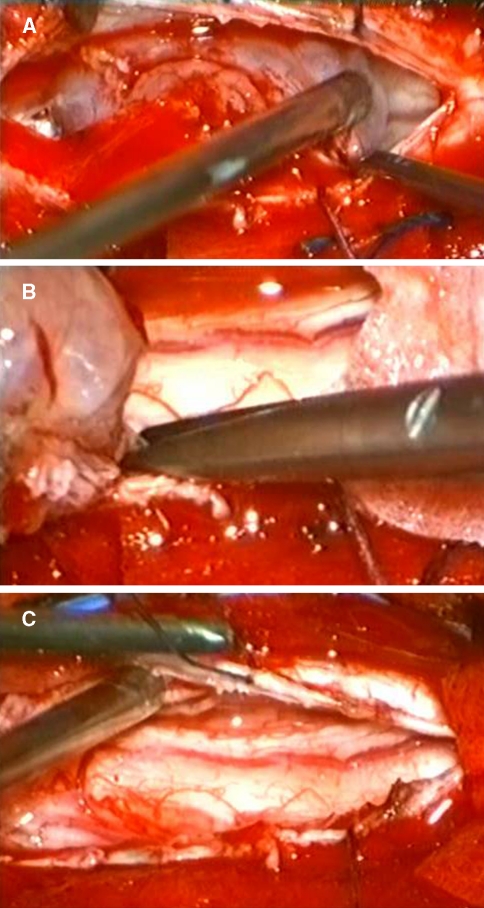
Fig. 3.
Intraoperative photographs showing the dorsal meningioma after dura opening (a) and following complete resection including the dural attachment (b). After tumour removal the ventral displacement of the spinal cord due to the extensive tumour growth is visible (c)
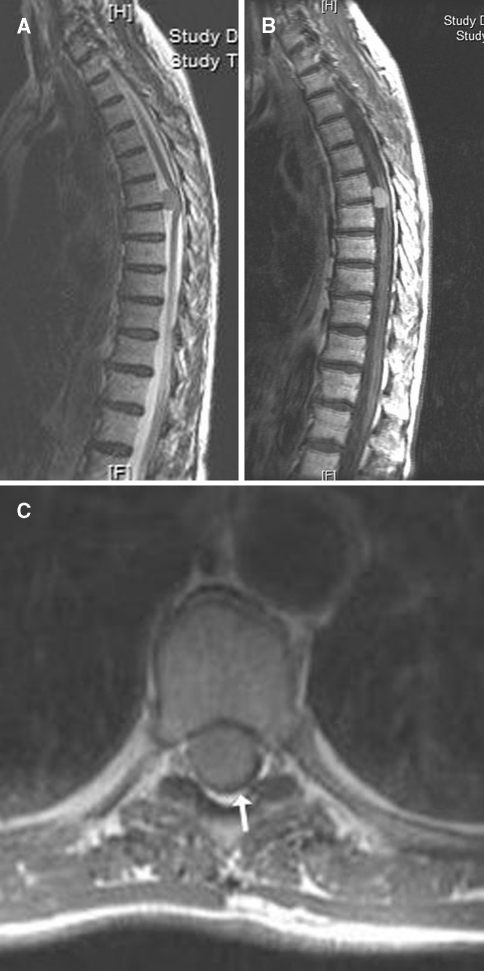
Fig. 4.
Illustration of a ventrally located spinal meningioma at the level T6/7 on T2 weighted (a) and gadolinium enhanced T1 weighted sagittal MRI scans (b). On axial gadolinium enhanced T1 weighted scan (c) the extensive tumour growth and dorsal displacement of the spinal cord are demonstrated (arrow)
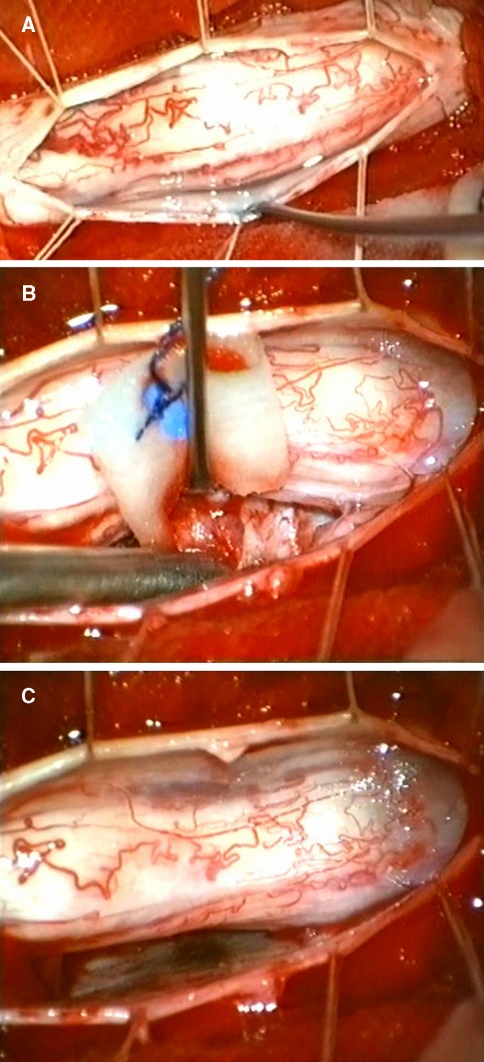
Fig. 5.
After dura opening the spinal cord is exposed and bulged due to the ventrally located tumour (a). Debulking of the tumour and piecemeal removal are mandatory to avoid additional neurological deficits (b). The dura is preserved and coagulated after complete tumour removal (c)
Surgical outcome
Compared to the preoperative neurological condition, the postoperative state at the last follow-up was improved or unchanged in 126 patients (96.2%) and worse in 4 patients (3%). One patient died postoperatively due to a severe myocardial infarction. Thus, the morbidity and mortality rates were 3 and 0.8%, respectively. The preoperative and postoperative Frankel grades are shown in Table 2.
Table 2.
Pre- and postoperative neurological status according to the Frankel classification
| Frankel-grade | Preoperative | Postoperative |
|---|---|---|
| A. No motor or sensory function below the level of injury | 2 | 1 |
| B. Some preserved sensory function | 5 | 1 |
| C. Some preserved motor function, unable to walk | 44 | 14 |
| D. Preserved useful motor function, able to walk | 71 | 77 |
| E. Normal motor and sensory function | 9 | 37 |
| Total | 131 | 130a |
aOne patient died due to a severe myocardial infarction
We observed a remarkable improvement with respect to the preoperative existing gait disturbances. Whereas preoperatively 39% of the patients were not able to walk independently according to Frankel grade A–C, postoperatively this rate diminished to only 12%.
Factors leading to poor outcome
Analysis of factors leading to neurological deterioration revealed that in particular elderly patients were affected. Age ranges between 76 to 80 years. All patients were female and had histologically grade I tumours (Table 3). In 3 of these cases, intraoperatively complete calcification of the tumour was observed, thus, this feature proved to be statistically significant (P < 0.0001) The localization of the dural attachment of the spinal meningioma, the tumour extension or the resection grade showed no correlation with the postoperative functional outcome.
Table 3.
Overview of patients with postoperative neurological deterioration
| Patients | Age (years) | Gender | Localisation and dural attachment | Histological grade | Intraoperative findings | Preoperative Frankel-grade | Postoperative Frankel-grade |
|---|---|---|---|---|---|---|---|
| 27 | 78 | F | T8/9 ventro-lateral | Grade I | Complete calcification | C | Ca |
| 46 | 77 | F | T6/7 ventro-lateral | Grade I | Complete calcification | C | A |
| 104 | 80 | F | T9/10 dorso-lateral | Grade I | Complete calcification | C | B |
| 131 | 76 | F | C7 lateral | Grade I | C | Ca |
F female; T thoracic; C cervical
aPatients with postoperative neurological deterioration, but unchanged grade on functional scale
Complications
A postoperative venous thrombosis occurred in two cases, a cerebrospinal fluid fistula in one case and a prolonged wound healing in one case. None of these patients required surgical repair and all recovered completely with conservative treatment. As mentioned above, one patient succumbed due to a severe myocardial infarction.
Recurrence rate
We observed a recurrence rate of 3% (4 patients) after a mean follow-up period of 76.5 months (range 36–116 months). Among these patients, a tumour recurrence occurred in 3 cases, which had a complete tumour resection initially. One patient showed a regrowth after incomplete tumour resection initially. Histological examination confirmed grade I tumours in these patients without evidence of increased cell proliferation. Tumour recurrence showed no correlation with the localization of the dural attachment. All patients with recurrent tumour underwent a second operation. The tumour was resected completely in 3 of these cases and incompletely in one case.
Discussion
Meningiomas are benign tumours arising from arachnoid cells and mostly located in the intracranial compartment. Spinal meningiomas are rare and account about 1.2% of all meningiomas and 25% of all spinal cord tumours [8, 18]. The first case of a successful removal was accomplished by Victor Horsley more than a century ago [11]. Since the first surgical series until now, surprisingly, there are only few reports with a higher number of patients [1, 6–8, 13, 18].
The primary goal of surgery is to achieve complete tumour removal and to avoid additional neurological damage. In the era before the introduction of the microscope in neurosurgery, good results were reported although some postoperative transient neurological deterioration was observed by some authors [7, 18]. In the last decades, safety of neurosurgical procedures increased due to technical developments for tumour resection and the routine use of neurophysiological intraoperative monitoring [14, 20, 22].
The aim of our study was to critically review our patients operated on a spinal meningioma in order to analyze factors with impact on postoperative outcome and to compare our results with the literature (Table 4).
Table 4.
Literature review on surgical series of spinal meningiomas
| Study | No. of patients | Complete resection (%) | Morbidity (%) | Mortality (%) | Recurrence rate (%) |
|---|---|---|---|---|---|
| Levy et al. 1982 [8] | 97 | 82 | 7.2 | 4 | 4 |
| Solero et al. 1989 [18] | 174 | 96.5 | 10 | 1.4 | 6.4 |
| Roux et al. 1996 [13] | 54 | 92.6 | 2 | 0 | 3.7 |
| King et al. 1998 [6] | 78 | 98 | 4 | 1 | 1.3 |
| Klekamp et Samii 1999 [7] | 117a | 89 | 0.8 | 1.5 | 14.7 |
| Schaller 2005 [15] | 33 | 85 | 21 | 0 | 3 |
| Presented series | 131 | 97 | 3 | 0.8 | 3 |
a117 patients with 130 spinal meningiomas
Clinical presentation
As spinal meningiomas are slow growing benign tumours, neurological deficits or gait disturbances appear from significant spinal cord compression mostly at an advanced stage of the disease [4, 8, 10, 13, 18]. Unspecific symptoms as local pain are often misinterpreted, until diagnosis is confirmed by neuroimaging procedures. Thus, patients mostly were referred to our institution for operative treatment not until the existence of progressive neurological deficits and gait disturbances. Delay of diagnosis as the result of failure to consider a slow growing spinal tumour responsible for longstanding back pain or neurological deficits was already observed by Pena et al. [12]. Magnet resonance imaging (MRI) is doubtlessly the diagnostic tool of choice [4, 16]. At the time of diagnosis, however, a considerable number of patients were not able to walk independently. In our series the rate of patients unable to walk was 39%. This is in accordance with the literature. Klekamp et Samii reported that 59% demonstrated gait ataxia as predominant neurological symptom on admission, and a total of 31 out of 117 patients (26.5%) were unable to walk [7]. In other series, the rate of patients unable to walk independently ranges between 21 and 53% [4–6, 8, 10, 15, 18].
Resectibility and functional outcome
In our series, the rate of complete resection of spinal meningiomas was 97%. This rate is in accordance with the literature, where the rates of complete tumour removal are reported to be between 82 and 98% [4–8, 13, 15, 18]. We have to stress that predominantly complete resection according to Simpsons grade II was performed in our study group as well as in other series. In contrast to meningiomas of other localization, e.g. meningiomas of the cranial convexity, resection of the dural attachment is less radical and not routinely performed in spinal meningiomas. The rate of resection of the dural attachment is reported to be between 14 and 58% [8, 13, 15, 18]. This is the result of the posterior operative approach in almost all cases and the difficulty of dural reconstruction ventral to the spinal cord. Tumour resection and following bipolar coagulation is generally considered to be adequate and effective.
The functional results of surgically treated spinal meningiomas are generally good. In our series the outcome was improved or unchanged in 96.2% at the time of last follow-up. Before surgery only 61% of our patients were able to walk independently, after surgery significant improvement was observed and this rate increases up to 88%. Similar results were reported by others. In Klekamp et. Samii’s series, 31 out of 117 patients were unable to walk preoperatively. Of these, 29% could walk again before discharge and 57% within 3 months of rehabilitation. This figure rose up to 80% after 1 year. They observed no difference between a recent series compared to a former series with respect on functional outcome [7]. In earlier series, others observed that the immediate postoperative course was characterized by a more-or-less pronounced transient neurological deterioration [18]. After the introduction of the operative microscope and microneurosurgical procedures, this observation was not longer reported. Excellent or good results range between 79 and 98% [4, 7, 8, 13, 15, 18].
However, there are some factors with potential risk for permanent neurological deterioration. In our series as well as in others, the existence of tumour calcification bears an increased risk for poor neurological outcome [8]. The evidence of calcification makes surgical removal difficult and increases the risk for neurological damage. Among 4 patients with poor outcome, 3 patients showed severe tumour calcification intraoperatively and tumour resection was demanding. All these patients were elderly. However, in a more recent study Morandi et al. evaluated 30 patients over 70 years and reported improved functional outcome in all cases. They observed in the first instance a correlation between the preoperative neurological status and the postoperative outcome, which eventually was not significant [10]. Furthermore, they concluded that surgery should be performed whenever there is an acceptable risk from an anesthesiological point of view.
Recurrence rate
The recurrence rate for spinal meningiomas is generally low ranging between 0 and 13% although the grade of tumour resection was Simpson’s grade II in the majority of the reported cases [4, 8–10, 18], as well as in the presented series. Mirimanoff et al. evaluated the recurrence rate of meningiomas and their progression after neurosurgical resection. They observed no tumour recurrence within the first 5 years, but a rate of 13% at the 10 years follow-up. Probably, this higher percentage is due to the low number of patients with spinal meningiomas (n = 18) included in their analysis [9]. In the literature, there seems to be no doubt that there is no correlation between the extension of dural resection and the risk for tumour recurrence. Some authors rather observed a higher recurrence rate in patients with radical tumour resection compared to patients, where the dural attachment was coagulated only [8, 18].
Reoperation with total tumour resection is generally possible, but remains a surgical challenge, in particular for en plaque or infiltrating meningiomas [7].
Some authors advocate adjuvant radiotherapy for incompletely resected spinal meningiomas or recurrent tumours, but there is still a lack of evidence that radiotherapy reduces the risk of tumour regrowth [4, 9, 13]. Thus, in our opinion adjuvant radiation treatment should be preserved for malignant spinal meningiomas only.
Despite the retrospective character of this study and its limitations we can conclude that resection of spinal meningiomas is a safe and most effective procedure. There might be a higher surgical risk for calcified tumours in elderly patients.
References
- 1.Bret P, Lecuire J, Lapras C, Deruty R, Dechaume JP, Assaad A. Intraspinal meningiomas. A series of 60 cases. Neurochirurgie. 1976;22:5–22. [PubMed] [Google Scholar]
- 2.Cohen-Gadol AA, Zikel OM, Koch CA, Scheithauer BW, Krauss WE. Spinal meningiomas in patients younger than 50 years of age: a 21-year experience. J Neurosurg. 2003;98:258–263. doi: 10.3171/spi.2003.98.3.0258. [DOI] [PubMed] [Google Scholar]
- 3.Frankel HL, Hancock DO, Hyslop G, Melzak J, Michaelis LS, Ungar GH, Vernon JD, Walsh JJ. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 4.Gezen F, Kahraman S, Canakci Z, Beduk A. Review of 36 cases of spinal cord meningioma. Spine. 2000;25:727–731. doi: 10.1097/00007632-200003150-00013. [DOI] [PubMed] [Google Scholar]
- 5.Gottfried ON, Gluf W, Quinones-Hinojosa A, Kan P, Schmidt MH. Spinal meningiomas: surgical management and outcome. Neurosurg Focus. 2003;14:e2. doi: 10.3171/foc.2003.14.6.2. [DOI] [PubMed] [Google Scholar]
- 6.King AT, Sharr MM, Gullan RW, Bartlett JR. Spinal meningiomas: a 20-year review. Br J Neurosurg. 1998;12:521–526. doi: 10.1080/02688699844367. [DOI] [PubMed] [Google Scholar]
- 7.Klekamp J, Samii M. Surgical results for spinal meningiomas. Surg Neurol. 1999;52:552–562. doi: 10.1016/S0090-3019(99)00153-6. [DOI] [PubMed] [Google Scholar]
- 8.Levy WJ, Jr, Bay J, Dohn D. Spinal cord meningioma. J Neurosurg. 1982;57:804–812. doi: 10.3171/jns.1982.57.6.0804. [DOI] [PubMed] [Google Scholar]
- 9.Mirimanoff RO, Dosoretz DE, Linggood RM, Ojemann RG, Martuza RL. Meningioma: analysis of recurrence and progression following neurosurgical resection. J Neurosurg. 1985;62:18–24. doi: 10.3171/jns.1985.62.1.0018. [DOI] [PubMed] [Google Scholar]
- 10.Morandi X, Haegelen C, Riffaud L, Amlashi S, Adn M, Brassier G. Results in the operative treatment of elderly patients with spinal meningiomas. Spine. 2004;29:2191–2194. doi: 10.1097/01.brs.0000141173.79572.40. [DOI] [PubMed] [Google Scholar]
- 11.Mulholland RC. Sir William Gowers 1845–1915. Spine. 1996;21:1106–1110. doi: 10.1097/00007632-199605010-00024. [DOI] [PubMed] [Google Scholar]
- 12.Pena M, Galasko CS, Barrie JL. Delay in diagnosis of intradural spinal tumors. Spine. 1992;17:1110–1116. doi: 10.1097/00007632-199209000-00017. [DOI] [PubMed] [Google Scholar]
- 13.Roux FX, Nataf F, Pinaudeau M, Borne G, Devaux B, Meder JF. Intraspinal meningiomas: review of 54 cases with discussion of poor prognosis factors and modern therapeutic management. Surg Neurol. 1996;46:458–463. doi: 10.1016/S0090-3019(96)00199-1. [DOI] [PubMed] [Google Scholar]
- 14.Sandalcioglu IE, Wiedemayer H, Secer S, Asgari S, Stolke D. Surgical removal of brain stem cavernous malformations: surgical indications, technical considerations, and results. J Neurol Neurosurg Psychiatry. 2002;72:351–355. doi: 10.1136/jnnp.72.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schaller B. Spinal meningioma: relationship between histological subtypes and surgical outcome? J Neurooncol. 2005;75:157–161. doi: 10.1007/s11060-005-1469-4. [DOI] [PubMed] [Google Scholar]
- 16.Schroth G, Thron A, Guhl L, Voigt K, Niendorf HP, Garces LR. Magnetic resonance imaging of spinal meningiomas and neurinomas. Improvement of imaging by paramagnetic contrast enhancement. J Neurosurg. 1987;66:695–700. doi: 10.3171/jns.1987.66.5.0695. [DOI] [PubMed] [Google Scholar]
- 17.Simpson D. The recurrence of intracranial meningiomas after surgical treatment. J Neurol Neurosurg Psychiatry. 1957;20:22–39. doi: 10.1136/jnnp.20.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Solero CL, Fornari M, Giombini S, Lasio G, Oliveri G, Cimino C, Pluchino F. Spinal meningiomas: review of 174 operated cases. Neurosurgery. 1989;25:153–160. doi: 10.1097/00006123-198908000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Weil SM, Gewirtz RJ, Tew JM., Jr Concurrent intradural and extradural meningiomas of the cervical spine. Neurosurgery. 1990;27:629–631. doi: 10.1097/00006123-199010000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Wiedemayer H, Fauser B, Sandalcioglu IE, Schafer H, Stolke D. The impact of neurophysiological intraoperative monitoring on surgical decisions: a critical analysis of 423 cases. J Neurosurg. 2002;96:255–262. doi: 10.3171/jns.2002.96.2.0255. [DOI] [PubMed] [Google Scholar]
- 21.Wiedemayer H, Sandalcioglu IE, Aalders M, Wiedemayer H, Floerke M, Stolke D. Reconstruction of the laminar roof with miniplates for a posterior approach in intraspinal surgery: technical considerations and critical evaluation of follow-up results. Spine. 2004;29:E333–342. doi: 10.1097/01.BRS.0000134592.07941.5E. [DOI] [PubMed] [Google Scholar]
- 22.Wiedemayer H, Sandalcioglu IE, Armbruster W, Regel J, Schaefer H, Stolke D. False negative findings in intraoperative SEP monitoring: analysis of 658 consecutive neurosurgical cases and review of published reports. J Neurol Neurosurg Psychiatry. 2004;75:280–286. [PMC free article] [PubMed] [Google Scholar]