Abstract
When quality of life questionnaires are used as measures of treatment outcomes, it is essential to know how well these can respond to clinical changes. The objective of this study is to examine the responsiveness of the Brazilian–Portuguese version of the Oswestry Disability Index (ODI-Brazil) in subjects with chronic low back pain submitted to a physical therapy program. Thirty subjects with chronic low back pain completed the ODI-Brazil questionnaire, along with an 11-point pain visual analogue scale (Pain VAS), and the Brazilian–Portuguese version of Roland–Morris disability questionnaire before and after the program. All patients also completed a global perception of change Likert scale in condition after the program. This scale was collapsed to produce a dichotomous variable outcome: improved and non-improved. Responsiveness was determined using effect size statistics and receiver operating characteristic curve (ROC curve), with best cut-point analysis. The best change score cut-off was identified when equally balanced sensitivity and specificity was found, as an expression of the minimum clinically important difference (MCID). After treatment, 19 patients considered themselves improved. Both the effect size (0.37) and the area under the ROC curve (0.73) for ODI-Brazil score in relation to global outcome after program indicated that the ODI-Brazil showed responsiveness. The ROC curve for ODI-Brazil was distributed at the upper corners of the diagonal line, indicating that the questionnaire presents discriminative ability. The best cut-off point for ODI-Brazil was approximately 4.45 points (63.2% sensitivity, 81.8% specificity). The Brazilian–Portuguese version of ODI has comparable responsiveness to other commonly used functional status measures and is appropriate for use in chronic low back pain patients receiving conservative care.
Keywords: Low back pain, Disability, Oswestry Disability Index, Responsiveness
Introduction
Low back pain (LBP) is an important cause of disability and work absenteeism [5]. Approximately 60–80% of the population will have at least one episode of LBP and related conditions in some moment of their lives [14]. In the UK, the rate prevalence of the LBP in adults is estimated at 19%, and in Canada, 28.7% [19].
To evaluate the quality of life in individuals with LBP the Oswestry Disability Index (ODI) is one of the most common instruments [6, 10]. This instrument consists of a 10-item questionnaire which assesses the impact of LBP on various functional activities [9]. Each item can receive a value from 0 to 5, with high values representing greater disability [10, 28]. The final result represents the sum of all items and expressed as percentage. The original version of the ODI was developed in the English language [9], with the Brazilian–Portuguese version being recently validated [28].
However, when instruments of quality of life are used as measures of treatment outcome, it is essential to know whether they are able to detect small, but important clinical changes, that is, their responsiveness [3, 4]. Responsiveness is a useful information not only for clinical decision making, but also to determine the importance of size of the effect in clinical trials, and to ensure that the questionnaires are appropriate to detect differences between treatments groups [1, 23].
In this study, responsiveness was determined by the effect size (ES) and by the receiver operating characteristics curve (ROC) [3, 4, 8]. The objective of this work is to examine the responsiveness of the Brazilian–Portuguese version of the Oswestry Disability Index in subjects with chronic low back pain.
Methods
Participants
A total of 30 subjects with non-specific chronic low back pain (LBP) were selected from physical therapy services at the Centro Universitário UNI-BH and Universidade Federal de Minas Gerais. To be included, patients needed to have back complaints for at least 3 months, and aged between 18 and 60 years.
Subjects were excluded if they were pregnant, presented with signs or symptoms suggesting cauda equine syndrome, progressive paresia, fracture, ankylosing spondylitis, inflammatory arthritis or other inflammatory illnesses, tumor or local infection. The study was approved by the Ethics Committee on Research, at the Pontifical Catholic University of Minas Gerais.
The treatment of all subjects in the two services of physical therapy was composed of manual therapy, electrotherapy, specific exercises of stabilization, therapeutic exercises and physical conditioning.
Procedures
The Brazilian–Portuguese version of the ODI (ODI-Brazil) was applied to all subjects prior to, and after a 6-week treatment programme. The ODI-Brazil was administered to all the patients as part of a comprehensive assessment. This comprehensive assessment consisted of socio-demographic data health-related and, instruments to evaluate patient’s perception of pain and quality life. Pain and quality of life was again assessed after the 6-week treatment period. Subjects also completed a global perception of change Likert scale on this condition after 6 weeks of treatment. The assessment was performed by the same assessor, on all individuals.
Instruments
Patients reported their pain intensity perception on the lumbar spine using a visual analogue scale (100-mm VAS).
To assess quality of life, subjects filled out the ODI-Brazil as well as the Brazilian–Portuguese version of Roland–Morris Disability Questionnaire (RMDQ-Brazil) [21]. This questionnaire consists of 24 items which reflect their disability at different activities and situations of daily life. Subjects marked each item that applied to their current status; each marked item received a score of 1. The final result of the RMDQ-Brazil consists of the sum of the marked items, varying from 0 (no disability) to 24 (maximum disability). The validation study of the Brazilian–Portuguese RMDQ has shown high test-retest reliability (0.88; P < 0.01).
A seven-level Likert scale on global perception of change in condition was used after 6 weeks of treatment [13, 25]. The scale has four improvement levels of the condition (completely better, much better, better and a little better), one no change level (approximately the same) and two worsening levels of the condition (a little worse and very much worse). The levels of the global perception of the condition scale were collapsed to produce a dichotomous variable outcome: improved group (that includes the levels completely better, much better and better) and non-improved group (including the conditions little better, approximately the same thing, a little worse and very much worse).
Statistical analyses
The responsiveness of the questionnaires was determined by calculating the ES and the ROC. The ES for each instrument was calculated by mean change in scores of the instrument’s applied (pre- and post-treatment) divided by the pooled standard deviation of this change [3]. The ES was also calculated for each instrument in relation to the seven categories of the global perception of change scale.
The ROC curve is a graph of “true positives” (sensitivity) versus “false positives” (1-specificity) for each of several cut-off points in change score [8]. Therefore, the ROC curve can be interpreted as the probability of correctly discriminating between improved and non-improved groups. This area theoretically ranges from 0.5 (no accuracy in discriminating improved from non-improved) to 1.0 (perfect accuracy). The ROC curve can provide an indication of what change score represents the best cut-off threshold to discriminate between improved or non-improved patients [7].
It has been suggested that the responsiveness of an instrument can be considered analogous to the evaluation of a diagnostic test, in which the instrument is the diagnostic test and the global result represents the gold standard [7]. The ROC curve synthesizes the information on sensitivity and specificity to identify alteration of the condition in agreement with an external dichotomized result. The Likert scale was collapsed and used as the external dichotomized instrument. We calculated the ROC area and the best cut-off change score for all the questionnaires. The optimal cut-off change score was identified when equally balanced sensitivity and specificity was found, and considered as an expression of the minimum clinically important difference (MCID) [11]. Statistical significance was accepted at P < 0.05. The SPSS (version 13.0, 2005, SPSS, Inc.) computer program was used.
Results
Table 1 shows socio-demographic characteristics of subjects of this study. The average values and effect size of the instruments are presented in Table 2. The results showed a reduction in pain intensity perception of 0.9 point (SD = 2.28) after 6 weeks of treatment, while the Brazilian–Portuguese version of Roland–Morris Disability Questionnaire showed a reduction in disability of 2.39 points (SD = 4.63) and the reduction of disability measured by Brazilian–Portuguese version of Oswestry Disability Index was at 6.14 points (SD = 13.61). Table 3 presents the effect sizes for each level of global perception of change scale. Using the dichotomized results of the scale as an external criterion, the ES for the improved patients (0.78; P < 0.01) is substantially larger and more significant than the ES for the non-improved patients (0.09; P < 0.05). When the levels of global perception of the condition scale were dichotomized, the majority of patients (19) reported an improved outcome. Thus, all three instruments showed good sensitivity to change.
Table 1.
Demographic and clinical characteristics of the study participants
| Variable | |
|---|---|
| Mean age in years (SD) | 38.07 (14.05) |
| Sex | |
| Male | 20 |
| Female | 10 |
| Occupation level (%) | |
| Student | 7 (23.3) |
| Housewife | 1 (3.3) |
| Not employed | 2 (6.7) |
| Not working | 4 (13.3) |
| Retired | 2 (6.7) |
| Employment | 14 (46.7) |
| Duration of symptoms in years (SD) | 3.4 (2.5) |
| Use of medication (%) | 12 (40) |
| Sedentary (%) | 21(70) |
Table 2.
Instruments scores before and after 6 weeks of treatment
| Instrument | Baseline mean (SD) | Post-treatment (SD) | Effect size | P value |
|---|---|---|---|---|
| ODI-Brazil | 32.85 (18.87) | 26.71 (16.44) | 0.37 | P < 0.01 |
| RMDQ-Brazil | 11.06 (5.67) | 8.67 (5.38) | 0.44 | P < 0.01 |
| Pain VAS | 4.15 (2.71) | 3.26 (2.47) | 0.36 | P < 0.01 |
ODI-Brazil Brazilian–Portuguese version of the Oswestry Disability Index
RMDQ-Brazil Brazil–Portuguese version of Roland–Morris Disability Questionnaire Pain VAS 100-mm visual analogue scale of pain
Pain VAS 100-mm visual analogue scale of pain
Table 3.
Mean change in ODI-Brazil, RMDQ-Brazil and Pain VAS scores in relation to the Likert Scale of the change condition after 6 weeks of treatment
| Categories of Likert Scale | Number of patients (%) | Mean change in ODI-Brazil (SD) | Effect Size ODI-Brazil | Mean change in RMDQ-Brazil (SD) | Effect size RMDQ-Brazil | Mean change Pain VAS (SD) | Effect Size Pain VAS |
|---|---|---|---|---|---|---|---|
| Much better | 10 (33.33) | 7.47c (11.01) | 0.68 | 4.70 (5.85) | 0.80 | 1.10 (1.63) | 0.68 |
| Better | 9 (30) | 13.40 (15.35) | 0.87 | 3.44c (2.92) | 1.17 | 2.11 (2.89) | 0.73 |
| Little better | 5 (16.67) | 1.69 (4.40) | 0.38 | 1.20 (2.28) | 0.53 | −0.14 (1.89) | 0.16 |
| About the same | 5 (16.67) | 2.10 (9.19) | 0.23 | −1.80 (3.03) | 0.59 | 0.24 (0.54) | 0.44b |
| Little worse | 1 (3.33) | –a | –a | –a | –a | –a | –a |
| Improved group | 19 (63.33) | 10.28c (13.21) | 0.78 | 4.10c (4.61) | 0.89 | 1.58 (2.30) | 0.69c |
| Non-improved group | 11 (37.67) | −1.00b (11.58) | 0.08 | −0.54b (2.94) | 0.18 | −0.31 (1.73) | 0.17 |
ODI-Brazil Brazilian–Portuguese version of the Oswestry Disability Index
RMDQ-Brazil Brazil–Portuguese version of Roland–Morris Disability Questionnaire
Pain VAS 100-mm visual analogue scale of pain
aChange is scores and effect size are not calculated because the case is less than or equal to 1
bCorrelation is significant at the 0.05 level
cCorrelation is significant at the 0.01 level
The difference in ODI-Brazil and RMDQ-Brazil score between improved and non-improved groups was approximately 11.2 points (SD = 13.21) and 4.6 points (SD = 4.61), respectively.
For the two disability questionnaires, the effect sizes for the improvement group were similar [(0.78 for ODI-Brazil (SD = 0.11) and 0.89 for RMDQ-Brazil (SD = 0.15)]. Both disability questionnaires showed good specificity, i.e., the ES for the patients in the non-improved group was minimal [0.08 for ODI-Brazil (SD = 0.14) and 0.18 for RMDQ-Brazil (SD = 0.11)]. In contrast, pain scale showed a smaller ES (0.17) for the non-improvement group.
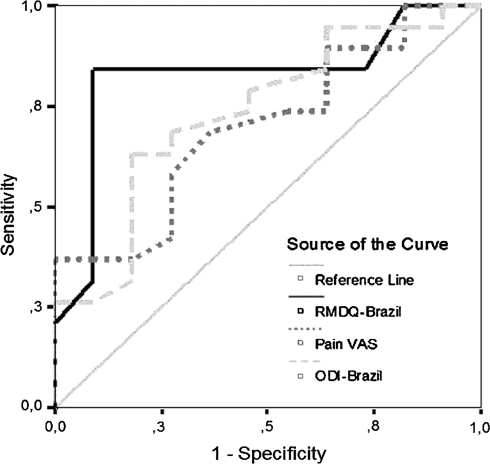
Using the dichotomized outcomes of global perception of change scale as external criterion, the ROC curves for the three instruments were concentrated at the upper corner of the diagonal line, indicating that all instruments present adequate discriminative abilities. The ROC areas for ODI-Brazil, RMDQ-Brazil and pain scale were 0.73, 0.82 and 0.70, respectively. Figure 1 presents ROC curves for all three instruments. The optimal cut-off points were approximately 4.45 points for ODI-Brazil (63.2% sensitivity, 81.8% specificity), 1 point for RMDQ-Brazil (60.4% sensitivity, 81.8% specificity) and 1.7 point for VAS (36.8% sensitivity, 100% specificity). These optimal cut-off points can be considered to represent the minimum clinically important difference (MCID).
Fig 1.
ROC curves of the change-scores for ODI, RMDQ and Pain VAS using the outcome of global perception of the condition scale dichotomized as the external criterion. Pain VAS 100-mm visual analogue scale of pain. RMDQ-Brazil Brazil–Portuguese version of Roland–Morris Disability Questionnaire ODI-Brazil Brazil–Portuguese version of the Oswestry Disability Index
Discussion
The results showed similar effect sizes for ODI-Brazil (0.37) and RMDQ-Brazil (0.44). These results are smaller than those reported in the literature for Oswestry Disability Index (0.80 [3], 0.84 [20], 0.45 [26], 0.82 [29]) and for Roland–Morris Disability Questionnaire (0.84 [20]). Therefore, different subject populations (conservative/surgical vs. conservative only) [18, 20], study design [2, 3, 17], instruments (different global perception of change scales) [16, 18], chronicity of patients [15], therapeutic modalities used [3, 18], and different instrument versions used could explain the small value of ODI-Brazil and discrepancy in results [18].
Table 3 shows that for the disability instruments employed in this study, the effect sizes for the improved group are significantly larger than for non-improved group. This has been previously identified in others studies [3, 20, 22, 27]. In the improved group, the difference between scores of the instruments before and after treatment is larger, producing a larger ES. On the other hand, when the difference between scores is small, the ES is small.
The improved patients group presented larger scores, suggesting that ODI-Brazil is a sensitive scale [3, 6, 18]. However, the concept of specificity is also important, since changes without clinical relevance may occur in function scale scores [3]. For example, the ES for the pain scale in the improvement group (0.69) was similar to that in the level about the same of non-improvement group (0.44). This comparison between improved group and non-improved group indicates that a number of patients who were not improved according to the global outcome criterion still showed an important reduction in their pain scores and suggesting that the Pain VAS is less specific than the disability instruments [3, 20]. We found a difference in ODI-Brazil mean change score between the improved and non-improved groups of 11.2 points. This value is similar to that in others studies (9.9 points [18] and 8 points [27]).
Our results showed that the area below the ROC curve was of 0.73 for ODI-Brazil, 0.82 for RMDQ-Brazil and 0.70 for pain scale, showing that all instruments presenting a high discriminative ability. These results are similar to the results of other studies on patients with LBP submitted to a treatment: for Oswestry Disability Index (0.76 [3]; 0.80 [6]; 0.72 [29]), Roland–Morris Disability Questionnaire (0.77 [6]; 0.79 [25]) and VAS (0.77 [29]).
The cut-off point of the ROC curve is a frequent method used to measure the MCID [11]. The best cut-off change score was identified for equally balanced sensitivity and specificity. The MCID values were approximately 4.45 points for ODI-Brazil (63.2% sensitivity, 81.8% specificity), 1 point for RMDQ-Brazil (60.4% sensitivity, 81.8% specificity) and 1.7 point for VAS (36.8% sensitivity, 100% specificity). The reported MCID values for the ODI range from 4 to 23 points [3, 20, 29]. The United States Food and Drug Administration (FDA) has chosen a minimum 15-point change in Oswestry Disability Index in patients who undergo spinal fusion before surgery and at follow-up [10, 12].
The results of this study should be interpreted in light of some potential limitations. After 6 weeks of treatment, patients showed a mean reduction in pain intensity of 0.9 point and a mean reduction of ODI’s score of 6.14 points. This value is well below the accepted minimum clinically-important differences [8, 24]. These results and ES values may be more due to patient’s chronicity, the treatment effectiveness and short period of time between clinical evaluations than the responsiveness of ODI-Brazil. Research with more subjects and subjects with bigger level of disability, measured by RMDQ-Brazil and ODI-Brazil, submitted to only a treatment and with a bigger time between clinical assessments can inform better the ES of ODI-Brazil.
Conclusion
The Brazilian–Portuguese version of the Oswestry Disability Index showed to be responsive to detect clinical changes in subjects with chronic low back pain after a physical therapy program.
References
- 1.Anderson G. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–585. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Beaton DE. Understanding the relevance of measured change through studies of responsiveness. Spine. 2000;25:3192–3199. doi: 10.1097/00007632-200012150-00015. [DOI] [PubMed] [Google Scholar]
- 3.Beurskens AJ, Vet HC, Koke AJ. Responsiveness of functional status in low back pain: a comparison of different instruments. Pain. 1996;65:71–76. doi: 10.1016/0304-3959(95)00149-2. [DOI] [PubMed] [Google Scholar]
- 4.Bombardier C. Outcome assessments in the evaluation of treatment of spinal disorders: summary and general recommendations. Spine. 2000;25:3100–3103. doi: 10.1097/00007632-200012150-00003. [DOI] [PubMed] [Google Scholar]
- 5.Chibnall JT, Tait RC, Andresen EM. Race and socioeconomic differences in post-settlement outcomes for African-American and Caucasian workers compensation claimants with low back injuries. Pain. 2005;114:462–472. doi: 10.1016/j.pain.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 6.Davidson M, Keating J. A comparison of five low back disability questionnaires: reliability and responsiveness. Phys Ther. 2002;82(1):8–24. doi: 10.1093/ptj/82.1.8. [DOI] [PubMed] [Google Scholar]
- 7.Deyo RA, Centor RM. Assessing the responsiveness of functional scales to clinical change: an analogy to diagnostic test performance. J Chronic Dis. 1986;39:897–906. doi: 10.1016/0021-9681(86)90038-X. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Diehr P, Patrick DL. Reproducibility and responsiveness of health status measures. Statistics and strategies for evaluation. Control Clin Trials. 1991;12(Suppl 4):S142–S158. doi: 10.1016/S0197-2456(05)80019-4. [DOI] [PubMed] [Google Scholar]
- 9.Fairbank JC, Couper J, Davies JB, O’Brien JP. The Oswestry low back pain questionnaire. Physiotherapy. 1980;66:271–273. [PubMed] [Google Scholar]
- 10.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine. 2000;25:2940–2952. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 11.Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 12.A prospective, randomized clinical investigation of recombinant human bone morphogenetic protein-2 and compression resistant matrix with the CD Horizon spinal system for posterolateral lumbar fusion in patients with symptomatic degenerative disc disease. Memphis: Medtronic Sofamor Danek; 2002. [Google Scholar]
- 13.Fischer D, Stewart AL, Bloch DA, Lorig K, Laurent D, Holman H. Capturing the patient’s view of change as a clinical outcome measure. JAMA. 1999;282:1157–1162. doi: 10.1001/jama.282.12.1157. [DOI] [PubMed] [Google Scholar]
- 14.Frymoyer JW, Pope MH, Clements JH. Risk factors in low-back pain: an epidemiological survey. J Bone Joint Surg Am. 1983;65:213–218. doi: 10.2106/00004623-198365020-00010. [DOI] [PubMed] [Google Scholar]
- 15.Grotle M, Brox JI, Vollestad NK. Concurrent comparison of responsiveness in pain and functional status measurements used for patients with low back pain. Spine. 2004;29:E492–E501. doi: 10.1097/01.brs.0000143664.02702.0b. [DOI] [PubMed] [Google Scholar]
- 16.Grotle M, Brox JI, Vollestad NK. Functional status and disability questionnaires: what do they assess? A systematic review of back-specific outcome questionnaires. Spine. 2005;30:130–140. [PubMed] [Google Scholar]
- 17.Hägg O, Fritzell P, Nordwall A. The clinical importance of changes in outcome scores after treatment for chronic low back pain. Eur Spine J. 2003;12:12–20. doi: 10.1007/s00586-002-0464-0. [DOI] [PubMed] [Google Scholar]
- 18.Lauridsen HH, Hartvigsen J, Manniche C, Korsholm L, Grunnet-Nilsson N. Danish version of the Oswestry Disability Index for patients with low back pain. Part 2: sensitivity, specificity and clinically significant improvement in two low back pain populations. Eur Spine J. 2006;15:1717–1728. doi: 10.1007/s00586-006-0128-6. [DOI] [PubMed] [Google Scholar]
- 19.Loney PL, Stratford PW. The prevalence of low back pain in adults: a methodological review of the literature. Phys Ther. 1999;79:384–396. [PubMed] [Google Scholar]
- 20.Mannion AF, Junge A, Grob D, Dvorak J, Fairbank JC. Development of a German version of the Oswestry Disability Index. Part 2: sensitivity to change after spinal surgery. Eur Spine J. 2006;15(1):66–73. doi: 10.1007/s00586-004-0816-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nusbaum L, Natour J, Ferraz MB, Goldenberg J. Translation, adaptation and validation of the Roland–Morris questionnaire-Brazil Roland Morris. Braz J Med Biol Res. 2001;34:203–210. doi: 10.1590/S0100-879X2001000200007. [DOI] [PubMed] [Google Scholar]
- 22.Perillo M, Bulbulian R. Responsiveness of Bournemouth and Oswestry Questionnaires: a prospective pilot study. J Manipulative Physiol Ther. 2003;26:77–86. doi: 10.1067/mmt.2003.6. [DOI] [PubMed] [Google Scholar]
- 23.Rantanen P. Physical measurements and questionnaires as diagnostic tools in chronic low back pain. J Rehabil Med. 2001;33:31–35. doi: 10.1080/165019701300006515. [DOI] [PubMed] [Google Scholar]
- 24.Roland M, Fairbank JC. The Roland–Morris disability questionnaire and the Oswestry disability questionnaire. Spine. 2000;25:3115–3124. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 25.Stratford PW, Binkley JM, Riddle DL, Guyatt GH. Sensitivity to change of the Roland–Morris back pain questionnaire: part 1. Phys Ther. 1998;78:1186–1196. doi: 10.1093/ptj/78.11.1186. [DOI] [PubMed] [Google Scholar]
- 26.Stratford PW, Binkley JM, Solomon P, Gill C, Finch E. Assessing change over time in patients with low back pain. Phys Ther. 1994;74:528–533. doi: 10.1093/ptj/74.6.528. [DOI] [PubMed] [Google Scholar]
- 27.Suarez-Almazor ME, Kendall C, Johnson JA, Skeith K, Vincent D. Use of health status measures in patients with low back pain in clinical settings. Comparison of specific, generic and preference-based instruments. Rheumatology. 2000;39:783–790. doi: 10.1093/rheumatology/39.7.783. [DOI] [PubMed] [Google Scholar]
- 28.Vigatto R, Alexandre NMC, Filho HR. Development of a Brazilian Portuguese version of the Oswestry Disability Index—cross-cultural adaptation, reliability, and validity. Spine. 2007;32(4):481–486. doi: 10.1097/01.brs.0000255075.11496.47. [DOI] [PubMed] [Google Scholar]
- 29.Walsh TL, Hanscom B, Lurie JD, Weinsteim J. Is a condition-specific instrument for patients with low back pain/leg symptoms really necessary? Spine. 2003;28(6):607–615. doi: 10.1097/00007632-200303150-00017. [DOI] [PubMed] [Google Scholar]