Abstract
Neck pain is the cardinal symptom following whiplash injuries. The trauma mechanism could theoretically lead to both soft tissue and bone injury that could be visualised by means of MRI. From previous quite small trials it seems that MRI does not demonstrate significant tissue damage. Large prospectively followed cohorts are needed to identify possible clinically relevant MRI findings. The objective of this trial was to evaluate (1) the predictive value of cervical MRI after whiplash injuries and (2) the value of repeating MRI examinations after 3 months including sequences with flexion and extension of the cervical spine. Participants were included after rear-end or frontal car collisions. Patients with fractures or dislocations diagnosed by standard procedures at the emergency unit were not included. MRI scans of the cervical spine were performed at baseline and repeated after 3 months. Clinical follow-ups were performed after 3 and 12 months. Outcome parameters were neck pain, headache, neck disability and working ability. A total of 178 participants had a cervical MRI scan on average 13 days after the injury. Traumatic findings were observed in seven participants. Signs of disc degeneration were common and most frequent at the C5–6 and C6–7 levels. Findings were not associated with outcome after 3 or 12 months. The population had no considerable neck trouble prior to the whiplash injury and the non-traumatic findings represent findings to be expected in the background population. Trauma-related MRI findings are rare in a whiplash population screened for serious injuries in the emergency unit and not related to a specific symptomatology. Also, pre-existing degeneration is not associated with prognosis.
Keywords: Magnetic resonance imaging, Neck pain, Prognosis, Prospective studies, Whiplash injuries
Background
Whiplash injuries are defined as the consequences of acceleration–deceleration traumas with energy transfer to the cervical spine. This mechanism of injury most often occurs in relation to car collisions, and both in frontal and rear-end impacts the cervical spine is subject to non-physiological stress loading[12, 28]. The cardinal symptom following whiplash injuries is neck pain. Some of the patients presenting with acute pain recover spontaneously within relatively short time [35] while other individuals exposed to whiplash injuries develop long-lasting and sometimes disabling symptoms [13, 18, 30]. The aetiology behind this lasting pain is largely unknown and most patients with long-lasting whiplash related symptoms end up with a diagnosis no more specific than chronic whiplash-associated disorders (WAD).
Most likely, several different aetiologies exist behind WAD and it seems relevant to address all components of the bio-psycho-social model in order to understand chronic WAD [11, 36]. Also, cultural factors such as compensation systems [6, 7] and general expectations of long-lasting symptoms [10, 27] seems to influence the prognosis after whiplash.
To explore the biological component of neck pain following whiplash injuries, studies of magnetic resonance imaging (MRI) have been performed in order to visualise potential damage to the cervical spine [3, 4, 8, 17, 21–23, 29]. Generally, very few identifications of traumatic lesions have been described in the acute phase after whiplash injuries and findings do not seem to be associated with the prognosis [4, 17, 29]. In previous trials, most findings were signs of pre-existing degeneration [3, 4, 29], which has been observed to be associated with an increased risk of long-lasting symptoms [4, 5, 29].
In one cohort, a considerable part of patients with chronic WAD were observed to have lesions in the upper cervical ligaments as visualised on MRI of the craniovertebral junction [21–23]. This study indicated that the alar and transverse ligaments as well as tectorial and posterior atlanto-occipital membranes can be damaged by whiplash injuries. However, it is not known whether these findings represent the aetiology behind the development of chronic pain in these individuals.
It seems that standard MRI is not the answer to finding the aetiology of WAD, but existing results are based upon small trials [3, 4, 8, 17, 29], and only large prospectively followed series can potentially identify MRI findings that are clinically relevant in subgroups of patients. The objective of this trial was to assess (1) the predictive value of standard cervical MRI and (2) the value of repeating MRI after 3 month including sequences with flexion and extension of the cervical spine in a large consecutively enrolled patient population who had been exposed to whiplash injuries.
Methods
This study was part of a larger prospective trial carried out at two university centres. Participants in this present study were included in one of the centres after referral from emergency units and general practitioners in two counties with approximately 823.400 inhabitants [1]. Permission for the study was granted by the local ethics committee and for the database by the Danish Data Protection Agency. The study was conducted in accordance with the Helsinki II declaration.
Data collection
Persons who had acute symptoms after a rear-end or frontal car collision were considered potential participants. All eligible participants were visited by a project nurse in their home. If inclusion criteria were fulfilled, written consent to participate was obtained at this visit after verbal and written information about the study. Baseline self-reported data were obtained from a questionnaire filled in at this visit. Inclusion criteria were: age 18–70 years, debut of symptoms within 3 days after the motor vehicle accident (MVA) and maximum 10 days could pass from the MVA to inclusion. Exclusion criteria were fractures or dislocations of the cervical spine disclosed by standard procedures at the emergency unit, amnesia or unconsciousness in relation to the accident, injuries other than the whiplash injury, average neck pain during the preceding 6 months exceeding 5 on a box scale 0–10, where 0 = no pain and 10 = worst possible pain, significant pre-existing somatic or psychiatric disease, and known alcohol- or drug abuse. Subjects were also excluded if they could not read or understand Danish.
Participants with marked symptoms, and an expected increased risk of developing persistent symptoms, were allocated to a randomized clinical trial (RCT). The allocations was based upon risk factors for chronic WAD identified in previous trials. The risk factors were gender, pain, number of nonpainful complaints and cervical range of motion. These factors were combined into a “risk score” which determined allocation to the intervention trial. Pain above 4 or a summarised cervical range of motion of 240° or less across the six movement directions was considered high risk on its own. Participants in this RCT received one of three interventions: (1) immobilisation in a semi-rigid collar followed by exercises, (2) active mobilisation, or (3) advice to act as usual. There was no relevant difference in outcome after 1 year between these three treatment groups [20]. Those with milder symptoms were allocated to a study in which they were randomized to either written or oral advice to act as usual. Due to limited MRI capacity, participants in this study were randomly drawn from the main population such that about 2/3 from the 3-armed RCT and 1/3 from the advice-study were enrolled for MRI. In this way, participants with more severe symptoms were given higher priority. Within each of the two sub-trials the chance of being selected for MRI was the same within each treatment group. Clinical follow-up was performed 3 and 12 months after the injury. Participants were not informed about the result of the MRI in order to avoid a potential influence on the prognosis.
MRI procedures and variables
The MRI was performed with an open, low field 0.2 T magnetic resonance unit (Magnetom Open Viva, Siemens AG, Erlangen, Germany) at baseline and after 3 months. Imaging was performed using a neck surface coil. The following sequences were used:
Localizer sequence of four images in the sagittal plane 40/10 (TR/TE), 1 acquisition in 26 s.
Sagittal T1 weighted spin echo, 510/26 (TR/TE), 250 mm field of view, 4-mm section thickness, 4 acquisitions in 5 min 17 s.
Sagittal T2 weighted turbo spin echo, 6,000/114 (TR/TE), 250 mm field of view, 4-mm section thickness, 1 acquisition in 5 min 43 s.
Sagittal T2 weighted TRUFI 10.8/5/80° (TR/TE/flip angle), 240 mm field of view, 1.5 mm section thickness, 1 acquisition in 4 min 8 s.
Sagittal TIRM 3,900/48/107 (TR/TE/TI), 250 mm field of view, 4 mm section thickness, 4 acquisitions in 7 min 26 s.
Axial T2 weighted GE (fl 2D) 1,200/50/40° (TR/TE/flip angle), 220 mm field of view, 4 mm section thickness, 1 acquisition in 5 min 47 s.
After 3 months, the sequences (1), (2), (3), and (6) were repeated and in addition sagittal T2 weighted turbo spin echo, 4,762/134 (TR/TE), 230 mm field of view, 6-mm section thickness with flexion and extension of the cervical spine were performed.
The MRI readings followed a predefined protocol and the radiologist was blinded regarding symptoms and results of clinical examinations. The radiologist has previously demonstrated ability to do reliable readings of lumbar MRI [34]. For description of variables and gradings, see Table 1. Bulging and protrusions/extrusions were registered as traumatic in case of hyperintensity of the disc on T2 weighted images and/or visible lesion of paraspinal soft tissue or spinal cord at the level with the altered disc contour.
Table 1.
MRI findings at baseline
| MRI finding | Grading | Frequency (%) |
|---|---|---|
| Fracture or dislocation of the cervical spinea | 0 = No | 178 (100) |
| 1 = Present | 0 | |
| Seperation of disc from vertebral end-plate | 0 = Normal | 177 (99.6) |
| 1 = Hyperintensive zone adjacent to anterior part of the end-plate | 1 (0.4) | |
| 2 = Longitudinal hyperintensive zone parallel to the vertebratal end-plate | 0 | |
| Bleeding/oedema | 0 = No | 175 (98.3) |
| 1 = prevertebral, paravertebral or interspinal bleeding or oedema | 3 (1.7) | |
| Spinal cord injury | 0 = Normal signal intensity in the spinal cord | 176 (98.9) |
| 1 = Bleeding/oedema in the spinal cord | 2 (1.1) | |
| Compression of the spinal cord | 0 = Normal | 176 (98.9) |
| 1 = No visible subarachnoid space (no dislocation or compression of the spinal cord) | 1 (0.5) | |
| 2 = Dislocation or compression of the spinal cord < 50% of the unaffected spinal cord diameter | 1 (0.5) | |
| 3 = Dislocation or compression of the spinal cord ≥ 50% of the unaffected spinal cord diameter | 0 | |
| Foraminal spinal stenosis | 0 = Normal | 153 (86) |
| 1 = ≥ 50% reduced space as compared to the opposite side and adjacent levels | 25 (14) | |
| Disc height | 0 = Disc higher than the disc above (if normal). C6/7 higher or as high as C7/Th 1 | 103 (58) |
| 1 = Disc as high or more narrow than the above (if normal) | 75 (42) | |
| Signal intensity | 0 = Visual hyperintense area in the disc | 39 (22) |
| 1 = No hyperintense area in the disc | 139 (78) | |
| Disc contour | 0 = Normal | 133 (75) |
| 1 = Bulging | 36 (20)b | |
| 2 = Focal protrusion | 6 (3) | |
| 3 = Broad based protrusion | 1 (0.5) | |
| 4 = Extrusion | 5 (3)c | |
| 5 = Sequestration | 0 | |
| Modic changes [19] | 0 = Normal bone marrow signal | 168 (94) |
| 1 = Modic type 1 | 6 (3) | |
| 2 = Modic type 2 | 4 (2) | |
| 3 = Modic type 3 | 0 |
aAccording to inclusion criteria, patients with suspected fractures of dislocations were not referred to the study
b2/36 registered as traumatic
c1/5 registered as traumatic
Clinical outcome variables
Self reported clinical data were used as outcome measures. Follow-up data were collected from a mailed questionnaire. In case, subjects refused to fill in the 1-year questionnaire, they were asked to participate in a short telephone interview. The interview included whether symptoms related to the accident were still present and information about their working ability the preceding month.
Participants scored their average intensity of neck pain, radiating arm pain and headache the preceding week on a box scale 0–10 (0 = no pain and 10 = worst possible pain) [14]. Neck disability was measured by the 15-item Copenhagen neck functional disability scale (0 = no neck disability and 30 = extremely disabled) [16]. The scales for measuring pain and disability have been validated in other spinal pain populations [2, 16]. Self-reported working ability during the 12th month after the injury was registered by marking days with sick listing and reduced working hours in a calendar constructed for this trial. The answers of the calendars were controlled on a spot sample basis by a secretary who checked that participants understood how to fill them out.
Data analysis
Pain intensities were dichotomised into “mild” (0–4) and “considerable” (5–10) [9]. Disability scores from 0 to 6 were defined as “minimal” and scores >6 as “considerable” [16]. Missing items in the neck disability scale were replaced by worst case scores if a maximum 2/15 items were missing, and no disability-score was calculated if more items were missing. The ability to work was only analysed at 1-year follow-up. It had a dichotomous distribution and was split into (1) “unaffected working ability” if no days with sick-listing or reduced working hours were reported during the 12th month after the accident, and (2) “affected working ability” if any sick listing or days with reduced working hours were reported during that period, or if no longer working because of the accident.
The MRI findings were grouped from all levels into one dichotomised variable for each type of finding. This meant that, for example, disc bulging was analysed as “present” no matter if present at one or more levels. Because of few findings, these variables were afterwards combined into the explanatory variables: No abnormal findings, mild pre-existing disc degeneration, moderate/severe pre-existing degeneration and traumatic finding (Table 2).
Table 2.
Definition of explanatory variables
| No abnormal findings | Mild pre-existing degeneration | Moderate/severe pre-existing degeneration | Traumatic finding |
|---|---|---|---|
| No findings | Reduced disc height and/or signal | Foraminal spinal stenosis and/or | Bleeding/oedema (pre-, paravertebral tissue or in the spinal cord) and/or |
| Non-traumatic bulge/protrusion with or without compression of the spinal cord and/or | Seperation of the disc from the vertebral endplate and/or | ||
| Modic changes | Traumatic bulge/protrusion with or without compression of the spinal cord |
The predictive value of MRI was only analysed in relation to baseline MRI since 3-month MRI findings were almost similar (please refer to results). Associations were sought between the explanatory variables and each dichotomised outcome measure and presented as odds ratios (OR) with 95% confidence intervals (95 CI). The statistical package STATA 8 (release 8.2, Stata Corp., TX) was used for statistical analyses.
Results
Study sample
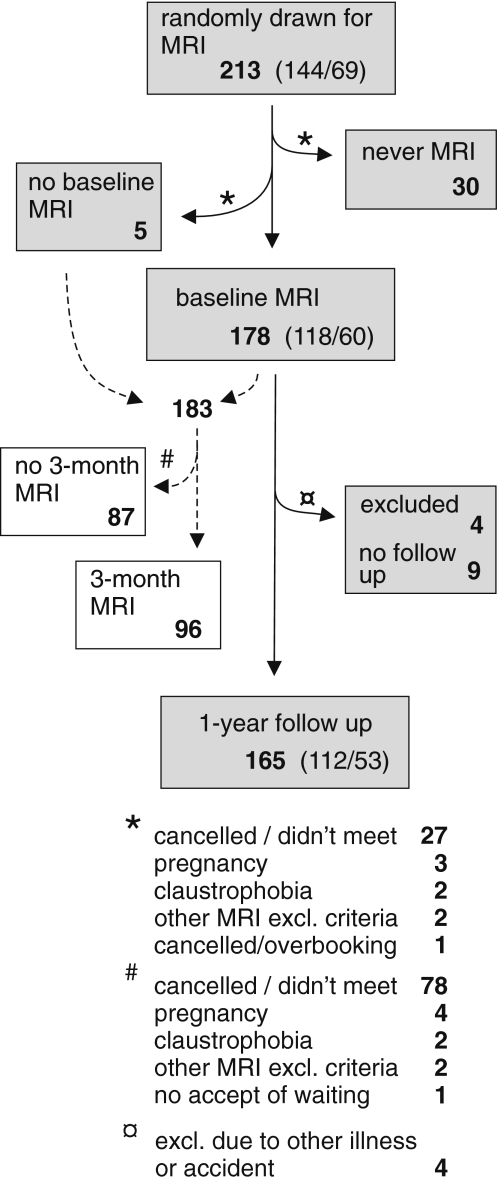
A total of 408 participants were included in the main trial. From these, 213 were randomly drawn for inclusion in the present MRI study and 178 had an MRI scan performed (=“MRI-population”). The main reason that not all included had the MRI scan was non-attendance to the appointment. Unfortunately, non-participation to the second MRI was frequent (Fig. 1). The MRI-population had slightly more severe symptoms and cervical range of motion was significantly reduced compared to those who were not selected for MRI, which was expected from the selection criteria. Other differences between participants who had an MRI scan and the remainder population were non-significant. Those included in the MRI study, but who did not have the MRI performed (“MRI-missed”) tended to have less severe symptoms and were less frequently sick listed at baseline. See Table 3 for baseline data.
Fig. 1.
Study flow chart. The flow of participants throughout the trial. Brackets divide (females/males)
Table 3.
Baseline data
| MRI scanned population (n = 178) | MRI-missed (n = 35) | Non-MRI (n = 195) | |
|---|---|---|---|
| Gender, % female | 66 | 74 | 63 |
| Age, median (IQR) | 33 (27–38) | 34 (25–40) | 33 (26–44) |
| Occupation, % | |||
| Self-employed | 6 | 6 | 5 |
| White collar | 44 | 37 | 46 |
| Blue collar | 15 | 27 | 18 |
| Student | 15 | 19 | 23 |
| Unemployed | 20 | 11 | 8 |
| Neck pain 0–10, median (IQR) | 5 (4–6) | 5 (3–6) | 4 (2–6) |
| Headache 0–10, median (IQR) | 5 (2–7) | 4 (2–6) | 3 (1–5) |
| Rediating arm pain 0–10, median (IQR) | 1 (0–3) | 0 (0–2) | 0 (0–2) |
| Sick listed at baseline, % (95 Cl) | 42 (34–49) | 29 (13–44) | 44 (37–51) |
| Cervical range of motion, degrees median (IQR) | 278 (226–306) | 286 (180–318) | 298 (242–334) |
Baseline data from participant in the present MRI study (MRI scanned + MRI-missed) and in participants in the main trial who were not selected for MRI (non-MRI)
IQR interquartile range, 95 CI 95% confidence interval
MRI findings-baseline
The baseline MRI was performed median 13 days after the injury (IQR 5–27 days). The most frequent findings were signs of pre-existing degeneration (Table 1). In 139/178 (78%) of participants, at least one disc was classified as having reduced signal intensity. Also, reduced disc height was frequent. Frequencies of MRI findings are listed in Table 1. Bulges or protrusions of one or more discs were present in 35/178 (20%) of the participants. According to the predefined criteria, this was assessed to be of traumatic origin in three cases. The level with disc bulges, protrusions/extrusions and Modic changes was most frequently C5–C6, and foraminal stenoses were most common at the C5–C6 and C6–C7 level (Table 4). A total of 56 participants had moderate/severe pre-existing degeneration. As expected, degeneration was related to age (Fig. 2). In 7 participants (6 females, 1 male), findings of possible traumatic origin were observed (Table 5).
Table 4.
Frequency of supposedly non-traumatic findings at baseline (n = 171)
| Foraminal stenosis Left/Right (n = 25) | Bulge (n = 36) | Protrusion (n = 12) | Modica | |
|---|---|---|---|---|
| C2–C3 | 1 | |||
| C3–C4 | 0/0 | 3 | 1 | 1 |
| C4–C5 | 2/3 | 3 | 1 | 2 |
| C5–C6 | 7/12 | 26 | 8 | 6 |
| C6–C7 | 7/7 | 14 | 4 | 2 |
| C7–Th1 | 0/0 | 0 | 0 | 0 |
Number of foraminal stesnoses, disc bulges/protrusions, and Modic changes at each cervical level
aModic type I or II described at one or both adjacent vertebrae
Fig. 2.
Age in relation to MRI findings. Age within the four MRI-groups. Boxes represent interquartile ranges. Dots are outlier values
Table 5.
Cases with supposedly traumatically induced findings
| Case | MRI findings | Baseline symptoms | 3 Months status | 1 Year status |
|---|---|---|---|---|
| Female 50 years | “Traumatic” bulge C6/7 | Neck pain = 5 | Neck pain = 7 | Neck pain = 1 |
| Headache = 5 | Headache = 2 | Headache = 1 | ||
| Radiating arm pain = 0 | Radiating arm | Radiating arm | ||
| Neck pain prior to MVA = 0 | Pain = 0 | Pain = 0 | ||
| Headache prior to MVA = 1 | Not sick listed | Unaltered working ability | ||
| Female 22 years | Paravertebral bleeding/oedema, left side C6 level | Neck pain = 6 | Neck pain = 0 | Neck pain = 0 |
| Headache = 5 | Headache = 3 | Headache = 0 | ||
| Radiating arm pain = 4 | Radiating arm | Radiating arm | ||
| Neck pain prior to MVA = 0 | Pain = 0 | Pain = 0 | ||
| headache proir to MVA = 5 | not sick listed | unaltered working ability | ||
| Female 45 years | Paravertebral bleeding/oedema, left side C5–Th1 level | Neck pain = 5 | Neck pain = 5 | Neck pain = 5 |
| Headache = 5 | Headache = 4 | Headache = 6 | ||
| Radiating arm pain = 0 | Radiating arm | Radiating arm | ||
| Neck pain prior to MVA = 0 | Pain = 0 | Pain = 0 | ||
| Headache prior to MVA = 3 | Not sick listed | Unaltered working ability | ||
| Female 20 years | Prevertebral bleeding/oedema C4–C6 level Oedema in the spinal cord C3–C5/6 level | Neck pain = 5 | Neck pain = 7 | Neck pain = 8 |
| Headache=6 | Headache=8 | Headache=7 | ||
| Radiating arm pain = 1 | Radiating arm | Radiating arm | ||
| Neck pain prior to MVA = 0 | Pain = 4 | Pain = 5 | ||
| Headache prior to MVA = 0 | Sick listed | Unaltered working ability | ||
| Female 34 years | “Traumatic” bulge and high intensity zone C5/6 disc. Modic I in upper vertebral end plate C6 | Neck pain = 2 | Missing | Missing |
| Headache = 8 | ||||
| Radiating arm pain = 0 | ||||
| Neck pain prior to MVA = 0 | Sick listed | |||
| Headache prior to MVA = 0 | ||||
| Female 37 years | “Traumatic” protrusion C5/6, compression of the spinal cord (grade 2) C5–C6 level | Neck pain = 6 | Missing | Headache = 2 |
| Headache = 8 | Unaltered | |||
| Radiating arm pain = 2 | Working ability | |||
| Neck pain prior to MVA = 0 | ||||
| Headache prior to MVA = 0 | Other variables missing | |||
| Male 40 years | Oedema in the spinal cord C3–C4 level | Neck pain = 3 | Neck pain = 5 | Neck pain = 2 |
| Headache = 9 | Headache = 7 | Headache = 8 | ||
| Radiating arm pain = 0 | Radiating arm | Radiating arm | ||
| Neck pain prior to MVA = 0 | Pain = 0 | Pain = 0 | ||
| Headache prior to MVA = 0 | Not sick listed | Unaltered working ability |
MRI findings: 3 months
Most findings were similar at the baseline and the 3-month MRI: 39 cases had no abnormal findings at the two examinations, 17 cases had mild degeneration at both MRI, and in 26 cases moderate/severe degeneration was present both times.
Three participants with no abnormal findings at baseline presented with findings after 3 months: one with mild degeneration, one with Modic type I and one showed minor instability with anterolisthesis in flexion.
Three cases with, respectively, Modic type II, a foraminal stenosis, and a bulge at baseline, normalised at 3 months. Only three of the seven participants with traumatic findings at baseline were re-examined at 3-month follow-up. One had a bulge as at baseline, the others with prior bleeding or oedema had no abnormal findings.
Five participants did only show up for the 3-month MRI. The MRI was normal in three of these cases, one had mild degeneration, and one a protrusion at C2–3.
Clinical outcome
At the 3-month follow-up, 40% of the MRI population reported considerable neck pain and/or headache. At the 1-year follow-up the corresponding number was 44%. The disability score indicated considerable neck disability in 39 and 50 % of the population at the 3- and 12-month follow-up. After 1 year, 12% had reduced working ability.
Associations between MRI findings and baseline symptoms
Descriptions of the cases with traumatic findings are summarised in Table 5. These participants had significantly more intense headache at baseline (Wilcoxon rank sum, P < 0.01). Intensity of neck pain and radiating arm pain at baseline did not differ between the participants with traumatic findings, those with pre-existing degeneration and participants with no abnormal MRI findings (Table 6).
Table 6.
Baseline and 1-year symptoms in relation to baseline MRI findings
| No abnormal findings | Pre-existing mild disc degeneration | Pre-existing moderate/severe degeneration | Traumatic findings | |
|---|---|---|---|---|
| Baseline (n = 178) | 91 | 21 | 56 | 7 |
| Neck pain | 5 (1–9) | 5 (2–8) | 4 (1–8) | 5 (2–6) |
| Headache | 4 (0–10) | 4 (0–10) | 4 (0–10) | 6 (5–9) |
| Radiating arm pain | 0 (0–7) | 0 (0–9) | 0 (0–7) | 0 (0–4) |
| 3-Months (n = 128) | 65 | 17 | 41 | 5 |
| Neck pain | 3 (0–6) | 2 (0–5) | 2 (0–5) | 5 (3–7) |
| Headache | 2 (0–7) | 3 (2–6) | 2 (0–6) | 4 (3–8) |
| Radiating arm pain | 0 (0–2) | 0 (0–2) | 0 (0–3) | 0 (0–3) |
| 1-Year (n = 131) | 64 | 17 | 45 | 5 |
| Neck pain | 3 (0–9) | 2 (0–9) | 1 (0–9) | 2 (0–8) |
| Headache | 3 (0–10) | 2 (0–10) | 1 (0–9) | 6 (0–8) |
| Radiating arm pain | 0 (0–9) | 0 (0–6) | 0 (0–7) | 0 (0–5) |
All numbers are median pain intensities with ranges in brackets
Associations between MRI findings and outcome
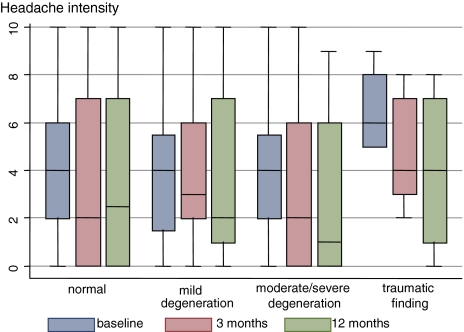
In cases with traumatic MRI findings, a trend towards more intensive neck pain and headache (Figs. 3, 4) and higher frequency of considerable neck pain at 3 months follow-up (OR 8; 95 CI 0.9–75) were observed compared to subjects without traumatic findings (Tables 6, 7).
Fig. 3.
Headache intensities in relation to MRI findings. Baseline, 3 months and 1 year headache intensities in relation to MRI findings at baseline. Boxes represent interquartile ranges
Fig. 4.
Neck pain intensities in relation to MRI findings. Baseline, 3 months and 1 year neck pain intensities in relation to MRI findings at baseline. Boxes represent interquartile ranges
Table 7.
Frequency of considerable symptoms at clinical follow-up in relation to baseline MRI
| 3 Months | 1 Year | |||
|---|---|---|---|---|
| Trauma-related finding on baseline MRI: | Yes, n = 7 | No, n = 171 | Yes, n = 7 | No, n = 171 |
| Neck pain > 3,% | 80 (4/5)a | 33 | 40 (2/5)a | 35 |
| Headache > 3, % | 40 (2/5)a | 33 | 60 (3/5)a | 35 |
| Radiating arm pain > 3, % | 0 | 4 | 20 (1/5)a | 13 |
| Considerable neck disability, % | 33 (1/3)a | 39 | 40 (2/5)a | 52 |
| Reduced working ability, % | 14 (1/7) | 12 | ||
Percentage of the MRI populaiotn with considerable pain, considerable disability, reduced working ability at 3 months and 1 year follow-up. Absolute numbers are presented in brackets if cases were few
aDiscrepancies from n = 7 are due to missing values
Also, after 1 year it was observed that considerable headache was more frequent in the group with traumatic findings (OR 2.8; 95 CI 0.4–17) and this group had a higher median headache intensity (Table 6). These above mentioned associations were not significant and the group did not differ otherwise from other participants (Table 7).
Pre-existing degeneration was not associated with the 3-month outcome. In relation to 1-year outcome moderate/severe pre-existing degeneration was associated with reduced risk of lasting pain (Table 6). This association was not significant when baseline pain was taken into account. No other potentially relevant associations between MRI findings and outcome were observed. Additional information from the 3-month examination was too sparse to warrant further analysis.
Discussion
This is to our best knowledge the largest study to date presenting MRI data from a prospectively followed whiplash population. The results of this study confirmed that traumatic findings visible at standard cervical MRI are rare following whiplash injuries, and no distinct symptomatology or prognosis was related to the findings present. However, a tendency towards more severe neck pain and headache was observed in subjects with traumatic findings. It should be noted that this population was screened by standard procedures in the emergency unit before referral to the trial, and patients were not included if fractures or dislocations were diagnosed at that time. The population, therefore, represents patients who are considered to have soft tissue injuries.
Our results were not in line with the prior studies’ observations of pre-existing degenerative findings being related to a poor prognosis [5, 31]. Signs of disc degeneration considered to be pre-existing were frequent but not related to more intense pain at neither baseline nor follow-up. One explanation for our results being contradictory to previous findings regarding this issue might be that we excluded persons with considerable neck pain prior to the accident. This way we may have excluded potential participants with painful degenerative changes. One could hypothesise that the associations previously observed between degenerative changes and long-lasting pain after whiplash were driven by inclusion of patients who had symptomatic degeneration prior to the accident. Contradictory results could also very well be a result of association found by chance in previous small trials.
We observed that reduced disc signal or lowered disc height is practically always present in individuals above 40 years. Further, disc bulges/protrusions at the C5–6 and C6–7 levels were common as observed also in asymptomatic subjects [24, 25]. The population had no considerable neck trouble prior to the whiplash injury and data on pre-existing findings most likely represent findings as in a general population. Modic changes that are shown to be highly related to pain when present in the lumbar spine [19], were also present in the cervical spine. Most Modic changes were observed at the end-plates adjacent to a herniated disc. It cannot be evaluated in the present study design whether Modic changes in the cervical spine are related to pain.
This was the first study presenting MRI data from examinations performed shortly after a whiplash injury and repeated in the subacute phase, and the first to include MRI performed in cervical flexion and extension. Although only half of the population was rescanned, it showed that the MRI performed at 3-month follow-up provided very little additional information, and minor signs of instability in flexion or extension were only visualised in one subject.
Some limitations of this study should be taken into consideration. First, the baseline examinations were generally performed later than planned. The interval between the accident and the MRI was on average 11 days and in 20 cases of more than 3 weeks. It is possible that signs of minor tissue damage visible on MRI could vanish before the MRI examinations were performed. However, in one previous study MRI was performed within 2 days after a whiplash injury and no signs of acute injury were seen [4]. Moreover, six of seven cases with traumatic findings in the present study had MRI performed between eight and 22 days after the injury. Hence, at least some traumatic findings are still visible that late. Second, it was a shortcoming of this study that a large number of included subjects did not complete the MRI planned at 3-month follow-up. About half of the population (108/213) did not meet for the second MRI. A large part of those who did not meet also did not return questionnaires, and their clinical status is thus unknown. It is a limitation of the study that all participants invited to have a MRI scan did not show up. It does, however, seem unlikely that persons who did not show for the scan had a more serious impact than the participants and the lack of such participation is not expected to result in ignorance of significant findings and hence did not alter conclusions. These patients would not be offered an MRI outside the research setting and it is very likely that the more severely hurt would be motivated for the MRI. Still, it should be recognised that no firm conclusions can be drawn concerning the value of introducing MRI including scans in flexion and extension after 3 months.
Third, we performed MRI using a low-field system. The better signal-to-noise ratio in high-field systems might reveal findings that were not visible in these scans. On the other hand, the low-field system have less chemical shift, susceptibility and flow artefacts, the evaluation of subchondral bones is better by low-field and the patient tolerance is slightly better [32]. Also one multi-centre study found comparable diagnostic accuracy in spinal disease using a low-field 0.2 T Magnetom Open as used in this study and a high-field MRI system[26]. To our knowledge low- and high-field have not been compared in relation to cervical trauma.
A fourth consideration is that having known more recent results demonstrating signs of injury to the upper cervical ligaments in chronic whiplash-associated disorders [15, 21–23] when planning this study, we would have focused more upon the upper cervical spine. The importance of upper ligament injuries should be evaluated in future prospective trials. Finally, concerning the examinations with flexion and extension it should be noted that these were carried out in the supine position and that pain in some instances hindered examinations in the outer range of motion. X-ray with patients standing or sitting could be a better choice for this part of the examination. However, pain provocation also limits the value of this examination. It should also be considered whether functional imaging, instead of traditional images in the outer range of motion, is relevant in this group of patients. It has been suggested that C1–2 instability can be diagnosed in some patients with chronic WAD by functional MRI [15, 38]. These results are not validated and ought to be elucidated further before any clinical consequence can be made from such findings. Another potentially relevant approach to these cervical spine injuries is multi-slice CT-scan [37] which may be superior to MRI in detecting bone avulsions.
We agree with previous statements that cervical MRI is not relevant as a standard procedure following whiplash injuries [4, 33] unless herniated disc is clinically suspected. So far, prospective studies have not focused upon upper cervical ligaments. This might disclose significant findings and ought to be evaluated. We observed a tendency towards more intense headache in participants with traumatic MRI findings, and it cannot be ruled out that the infrequent signs of tissue damage represent the aetiology behind lasting pain in some cases of chronic whiplash-associated disorders. In that case, this will explain symptoms only in a very small subgroup of patients.
Conclusions
In conclusion, MRI is not the answer to a diagnosis in the vast majority of patients developing long-lasting pain after a whiplash injury, and early MRI scans do not predict prognosis. It may be relevant to focus future trials upon imaging of the upper cervical spine including functional imaging.
Acknowledgments
The authors want to thank Danish Insurance Association, the IMK Foundation and The Foundation for Chiropractic Research and Postgraduate Education for unrestricted grants. We would also like to thank radiographers Jette Sanchez, Susanne Pedersen, Winnie Most and Steen Ahlman Nielsen, nurse Lene Kiertzner, and secretary Ann-Chrisitna Just Pedersen for skilful participation in the study. Further, we want to thank Professor Werner Vach and associate Professor Lars Korsholm, Department of Statistics, University of Southern Denmark for statistical advice regarding the design of the study. Thanks to Alan Jordan, PhD, for proofreading. The study complies with Danish laws and was approved by the local ethics committee.
References
- 1.(2007) Statistics Denmark. http://www.statbank.dk
- 2.Bolton JE, Wilkinson RC. Responsiveness of pain scales: a comparison of three pain intensity measures in chiropractic patients. J Manipulative Physiol Ther. 1998;21:1–7. [PubMed] [Google Scholar]
- 3.Bonuccelli U, Pavese N, Lucetti C, Renna MR, et al. Late whiplash syndrome: a clinical and magnetic resonance imaging study. Funct Neurol. 1999;14:219–225. [PubMed] [Google Scholar]
- 4.Borchgrevink G, Smevik O, Haave I, Haraldseth O, et al. MRI of cerebrum and cervical columna within two days after whiplash neck sprain injury. Injury. 1997;28:331–335. doi: 10.1016/S0020-1383(97)00027-2. [DOI] [PubMed] [Google Scholar]
- 5.Borchgrevink GE, Smevik O, Nordby A, Rinck PA, et al. MR imaging and radiography of patients with cervical hyperextension—flexion injuries after car accidents. Acta Radiol. 1995;36:425–428. doi: 10.3109/02841859509173401. [DOI] [PubMed] [Google Scholar]
- 6.Cassidy JD, Carroll LJ, Cote P, Lemstra M, et al. Effect of eliminating compensation for pain and suffering on the outcome of insurance claims for whiplash injury (see comments) N Engl J Med. 2000;342:1179–1186. doi: 10.1056/NEJM200004203421606. [DOI] [PubMed] [Google Scholar]
- 7.Cote P, Cassidy JD, Carroll L, Frank JW, et al. A systematic review of the prognosis of acute whiplash and a new conceptual framework to synthesize the literature. Spine. 2001;26:E445–E458. doi: 10.1097/00007632-200110010-00020. [DOI] [PubMed] [Google Scholar]
- 8.Davis SJ, Teresi LM, Bradley WG, Jr, Ziemba MA, et al. Cervical spine hyperextension injuries: MR findings. Radiology. 1991;180:245–251. doi: 10.1148/radiology.180.1.2052703. [DOI] [PubMed] [Google Scholar]
- 9.Fejer R, Jordan A, Hartvigsen J. Categorising the severity of neck pain: establishment of cut-points for use in clinical and epidemiological research. Pain. 2005;119:176–182. doi: 10.1016/j.pain.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari R, Obelieniene D, Russell A, Darlington P, et al. Laypersons’ expectation of the sequelae of whiplash injury. A cross-cultural comparative study between Canada and Lithuania. Med Sci Monit. 2002;8:CR728–CR734. [PubMed] [Google Scholar]
- 11.Ferrari R, Schrader H. The late whiplash syndrome: a biopsychosocial approach. J Neurol Neurosurg Psychiatry. 2001;70:722–726. doi: 10.1136/jnnp.70.6.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grauer JN, Panjabi MM, Cholewicki J, Nibu K, et al. Whiplash produces an S-shaped curvature of the neck with hyperextension at lower levels. Spine. 1997;22:2489–2494. doi: 10.1097/00007632-199711010-00005. [DOI] [PubMed] [Google Scholar]
- 13.Hildingsson C, Toolanen G. Outcome after soft-tissue injury of the cervical spine. A prospective study of 93 car-accident victims. Acta Orthop Scand. 1990;61:357–359. doi: 10.3109/17453679008993536. [DOI] [PubMed] [Google Scholar]
- 14.Jensen MP, Karoly P, O’Riordan EF, Bland F, Jr, et al. The subjective experience of acute pain. An assessment of the utility of 10 indices. Clin J Pain. 1989;5:153–159. doi: 10.1097/00002508-198906000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Johansson BH. Whiplash injuries can be visible by functional magnetic resonance imaging. Pain Res Manag. 2006;11:197–199. doi: 10.1155/2006/413757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jordan A, Manniche C, Mosdal C, Hindsberger C. The Copenhagen neck functional disability scale: a study of reliability and validity. J Manipulative Physiol Ther. 1998;21:520–527. [PubMed] [Google Scholar]
- 17.Karlsborg M, Smed A, Jespersen H, Stephensen S, et al. A prospective study of 39 patients with whiplash injury. Acta Neurol Scand. 1997;95:65–72. doi: 10.1111/j.1600-0404.1997.tb00071.x. [DOI] [PubMed] [Google Scholar]
- 18.Kasch H, Bach FW, Stengaard-Pedersen K, Jensen TS. Development in pain and neurologic complaints after whiplash: a 1-year prospective study. Neurology. 2003;60:743–749. doi: 10.1212/01.wnl.0000046661.82212.04. [DOI] [PubMed] [Google Scholar]
- 19.Kjaer P, Korsholm L, Bendix T, Sorensen JS, et al. Modic changes and their associations with clinical findings. Eur Spine J. 2006;15:1312–1319. doi: 10.1007/s00586-006-0185-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kongsted A, Qerama E, Kasch H, Bendix T, et al. A randomized early-intervention study on whiplash injuries comparing the effect of a neck collar, “act-as-usual” and active mobilization on one year outcomes. Spine. 2007;32:618–626. doi: 10.1097/01.brs.0000257535.77691.bd. [DOI] [PubMed] [Google Scholar]
- 21.Krakenes J, Kaale BR, Moen G, Nordli H, et al. MRI assessment of the alar ligaments in the late stage of whiplash injury—a study of structural abnormalities and observer agreement. Neuroradiology. 2002;44:617–624. doi: 10.1007/s00234-002-0799-6. [DOI] [PubMed] [Google Scholar]
- 22.Krakenes J, Kaale BR, Moen G, Nordli H, et al. MRI of the tectorial and posterior atlanto-occipital membranes in the late stage of whiplash injury. Neuroradiology. 2003;46(2):165–166. doi: 10.1007/s00234-003-1036-7. [DOI] [PubMed] [Google Scholar]
- 23.Krakenes J, Kaale BR, Nordli H, Moen G, et al. MR analysis of the transverse ligament in the late stage of whiplash injury. Acta Radiol. 2003;44:637–644. doi: 10.1046/j.1600-0455.2003.00134.x. [DOI] [PubMed] [Google Scholar]
- 24.Lehto IJ, Tertti MO, Komu ME, Paajanen HE, et al. Age-related MRI changes at 0.1 T in cervical discs in asymptomatic subjects. Neuroradiology. 1994;36:49–53. doi: 10.1007/BF00599196. [DOI] [PubMed] [Google Scholar]
- 25.Matsumoto M, Fujimura Y, Suzuki N, Nishi Y, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80:19–24. doi: 10.1302/0301-620X.80B1.7929. [DOI] [PubMed] [Google Scholar]
- 26.Merl T, Scholz M, Gerhardt P, Langer M, et al. Results of a prospective multicenter study for evaluation of the diagnostic quality of an open whole-body low-field MRI unit. A comparison with high-field MRI measured by the applicable gold standard. Eur J Radiol. 1999;30:43–53. doi: 10.1016/S0720-048X(98)00134-X. [DOI] [PubMed] [Google Scholar]
- 27.Obelieniene D, Schrader H, Bovim G, Miseviciene I, et al. Pain after whiplash: a prospective controlled inception cohort study (see comments) J Neurol Neurosurg Psychiatry. 1999;66:279–283. doi: 10.1136/jnnp.66.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panjabi MM, Cholewicki J, Nibu K, Grauer J, et al. Capsular ligament stretches during in vitro whiplash simulations. J Spinal Disord. 1998;11:227–232. doi: 10.1097/00002517-199806000-00009. [DOI] [PubMed] [Google Scholar]
- 29.Pettersson K, Hildingsson C, Toolanen G, Fagerlund M, et al. MRI and neurology in acute whiplash trauma. No correlation in prospective examination of 39 cases. Acta Orthop Scand. 1994;65:525–528. doi: 10.3109/17453679409000906. [DOI] [PubMed] [Google Scholar]
- 30.Radanov BP, Sturzenegger M. Predicting recovery from common whiplash. Eur Neurol. 1996;36:48–51. doi: 10.1159/000117200. [DOI] [PubMed] [Google Scholar]
- 31.Radanov BP, Sturzenegger M, Di Stefano G. Long-term outcome after whiplash injury. A 2-year follow-up considering features of injury mechanism and somatic, radiologic, and psychosocial findings. Medicine (Baltimore) 1995;74:281–297. doi: 10.1097/00005792-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 32.Rand T, Imhof H, Turetschek K, Schneider B, et al. Comparison of low field (0.2T) and high field (1.5T) MR imaging in the differentiation of torned from intact menisci. Eur J Radiol. 1999;30:22–27. doi: 10.1016/S0720-048X(98)00108-9. [DOI] [PubMed] [Google Scholar]
- 33.Ronnen HR, Korte PJ, Brink PR, Bijl HJ, et al. Acute whiplash injury: is there a role for MR imaging?—a prospective study of 100 patients. Radiology. 1996;201:93–96. doi: 10.1148/radiology.201.1.8816527. [DOI] [PubMed] [Google Scholar]
- 34.Sorensen SJ, Kjaer P, Jensen ST, Andersen P. Low-field magnetic resonance imaging of the lumbar spine: reliability of qualitative evaluation of disc and muscle parameters. Acta Radiol. 2006;47:947–953. doi: 10.1080/02841850600965062. [DOI] [PubMed] [Google Scholar]
- 35.Spitzer WO, Skovron ML, Salmi LR, Cassidy JD et al. (1995) Scientific monograph of the Quebec Task Force on whiplash-associated disorders: redefining “whiplash” and its management. Spine 20:1S–73S (erratum in Spine 1995, 20(21):2372) [PubMed]
- 36.Sterling M, Jull G, Kenardy J. Physical and psychological factors maintain long-term predictive capacity post-whiplash injury. Pain. 2006;122:102–108. doi: 10.1016/j.pain.2006.01.014. [DOI] [PubMed] [Google Scholar]
- 37.Goethem JW, Maes M, Ozsarlak O, van den HL, et al. Imaging in spinal trauma. Eur Radiol. 2005;15:582–590. doi: 10.1007/s00330-004-2625-5. [DOI] [PubMed] [Google Scholar]
- 38.Volle E, Montazem A. Strukturdefekte der Ligamenta alaria in der offenen Funktionskernspintomographie. Manuelle Medizin. 1997;35:188–193. doi: 10.1007/s003370050031. [DOI] [Google Scholar]