Abstract
It has been reported that in patients undergoing posterolateral lumbar fusion (PLF), the fusion status is not related to the short-term operative results. To determine whether the fusion status influences the long-term operative results of PLF, we retrospectively examined the surgical outcomes of uninstrumented PLF for a minimum of 8 years (average, 9.5 years), by comparing cases exhibiting union with those exhibiting nonunion. Uninstrumented PLF was performed for the treatment of lumbar canal stenosis (LCS) with degenerative spondylolisthesis. Since nine patients were lost to final follow-up, the study included 42 patients, and the follow-up rate was 82.4%. The mean age of the patients was 64.1 years (range 46–77 years). Eight patients exhibited fusion at the L3–4 level and 34 patients, at the L4–5 level. The fusion status was assessed using plain radiographs. The clinical outcomes were evaluated using the Japanese Orthopaedic Association (JOA) scores. Nonunion was noted in 26% (11/42) of the patients. There were no statistically significant differences between the groups exhibiting union and nonunion with respect to age, sex, preoperative JOA score, or preoperative lumbar instability. The union group achieved better operative results than the nonunion group at the 5-year and final follow-up (P = 0.006 and 0.008, respectively) although there was no significant difference in the percent recovery at 1 and 3-year follow-up (P = 0.515 and 0.506, respectively). A stepwise regression analysis revealed that the best combination of predictors for percent recovery at the time of final follow-up included the fusion status and the presence of comorbid disease. The results indicate that the fusion status following PLF is a critical factor influencing the long-term but not short-term operative results in the treatment of LCS with degenerative spondylolisthesis.
Key words: Lumbar spinal fusion, Posterolateral lumbar fusion, Lumbar canal stenosis, Degenerative spondylolisthesis
Introduction
Lumbar spine fusion is indicated as a primary procedure or as an adjunct to decompression for patients with degenerative spinal disorders for securing spinal stability and preventing postoperative instability [5, 17, 20]. Although several techniques of lumbar fusion exist, posterolateral lumbar fusion (PLF) is considered to be the gold standard for lumbar spinal fusion [1, 21]. PLF involves placing a bone graft harvested from the iliac crest between the transverse processes; this restricts spinal motion by bridging the posterolateral portion of the lumbar spine. In the surgical treatment of lumbar canal stenosis (LCS) associated with degenerative spondylolisthesis, a randomized trial and a study with alternating treatment assignments indicated better outcomes for laminectomy plus PLF than for laminectomy alone [7]. Fusion as an adjunct prevents the progression of spondylolisthesis after decompression and improves operative results possibly due to decreased postoperative back pain by the elimination of instability [7, 14]. These results have prompted many surgeons to recommend concomitant spinal fusion in the management of LCS associated with degenerative spondylolisthesis.
Nonunion following PLF may lead to changes in alignment, spinal instability and potential neurological injury; however, asymptomatic nonunion has also been documented. In a 3-year prospective study comparing laminectomy alone to laminectomy combined with PLF in the treatment of LCS with degenerative spondylolisthesis, Herkowitz and Kurz [7] reported that the nonunion rate of PLF was 36% as observed on plain radiographs and that the clinical results were excellent or good for all patients who underwent PLF, including those demonstrating nonunion. In another report, Fischgrund et al. [6] published a 2-year prospective randomized study comparing the results of decompression and PLF alone with those of decompression and instrumented PLF in 67 patients with degenerative spondylolisthesis. This study demonstrated that in the uninstrumented PLF group, the clinical outcome was noted to be excellent or good in 83% of the patients who developed a pseudoarthrosis. Both studies indicated that the fusion status following uninstrumented PLF does not affect the short-term clinical outcome.
However, whether nonunion following uninstrumented PLF maintains good operative results on long-term follow-up remains an unresolved problem. In this study, we retrospectively studied the long-term operative results of uninstrumented PLF in patients having LCS with degenerative spondylolisthesis. The goals of the study were (1) to assess the outcome of uninstrumented PLF over time in terms of the fusion status and (2) to attempt to identify the demographic and clinical factors of the patients that were predictive of surgical outcomes.
Patients and methods
Patients
This study included patients who underwent decompressive surgery and a single-level PLF at the authors’ institution for LCS with grade 1 degenerative spondylolisthesis. The patient exclusion criteria were as follows: (1) prior surgery to the lumbar spine, (2) isthmic spondylolisthesis, (3) segmental kyphosis at the listhetic segment and (4) completely collapsed disc height at the listhetic level. The charts of the patients who underwent the surgery between January 1995 and December 1997 were retrospectively reviewed. Spondylolisthesis was confirmed when the percentage of slip (%slip) was 5% or more on a lateral radiograph in a neutral standing position [15]. The total amount of angular motion between the adjacent vertebral endplate (dynamic angulation) and the total extent of the vertebral slip (dynamic translation) between the lateral radiographs taken in flexion and extension in the standing position were used to determine the dynamic motion at the fusion site [4]. In order to eliminate the X-ray magnification factor, the amount of translation was calculated as a percentage of the vertebral body width. Diagnosis was based on plain radiographic findings together with myelography, computed tomography (CT) and magnetic resonance imaging of the lumbar spine.
Operative technique
The decompression of the cauda equina and nerve roots was achieved by laminotomy (fenestration) or laminectomy with partial facetectomy (<50% on both sides). Discectomy was included when indicated. The transverse processes of the levels above and below the fusion segment were decorticated to expose the bone marrow. The autogenous iliac crest bone was placed so as to bridge the gap between the decorticated transverse processes. Concomitant spinal instrumentation was not used. Each patient was fitted with a lumbosacral brace and instructed to wear the brace when out of bed for 6 months following surgery.
Follow-up study
Radiographs (AP, oblique, lateral and flexion-extension) obtained at the final follow-up were examined to determine the fusion status of PLF. PLF was defined as union if radiographs demonstrated a bilateral continuity in the fusion mass between the cephalad and caudad transverse processes with less than 2° of angular motion and no translation between the vertebrae at the level of PLF on lateral flexion-extension radiographs [6, 7, 13]. The absence of continuity in the fusion mass at any point between the transverse process on one or both sides, greater than 2° of angular motion or any translation was considered a failure of fusion with nonunion [6, 7, 13]. The Japanese Orthopaedic Association’s (JOA) scores for the assessment of low back pain were reviewed to evaluate the conditions before surgery and the clinical results at 1, 3 and 5 years after surgery and at the final follow-up. All the patients were followed up at each time interval. The JOA score comprises nine points assigned for subjective symptoms, six points for clinical signs and 14 points for the restriction of activity of daily living (ADL); the total score is thus 29 points [9]. Among the subjective symptoms, low back pain (LBP) and leg pain were also evaluated using the JOA score. Both scores ranged from 0 (indicating continuous severe pain) to 3 (no pain). The percent recovery of the JOA score that indicated the degree of normalization after surgery was calculated using the formula specified by Hirabayashi et al. [8] which was as follows:
Percent recovery (%) = [(postoperative JOA score − preoperative JOA score)/(29 − preoperative JOA score)] × 100.
The outcome was graded ‘4’ for an improvement in the recovery rate of 75% or more, ‘3’ for 50–74% improvement, ‘2’ for 25–49% improvement and ‘1’ for 24% improvement or less and for when revision surgery was required.
Statistical analysis
All radiological data and clinical charts of these patients were retrospectively reviewed by examiners other than the treating surgeons in a blinded manner. The results are expressed as the mean ± SD. To determine what factors might be associated with the operative result at the final follow-up, stepwise regression analysis was used to determine the best multiple regression models of the postoperative percent recovery and potential predictors assessed. All variables with F values below 4 were excluded from the regression analysis. The factors included age at the time of surgery (continuous), gender (categorical: 1 = male, 2 = female), the severity of preoperative symptoms (preoperative JOA score; continuous), preoperative %slip (continuous), preoperative dynamic translation (continuous), preoperative dynamic angulation (continuous), postoperative %slip (continuous), fusion status (categorical: 1 = union, 2 = nonunion) and presence of comorbid diseases (categorical: 1 = present, 2 = absent). For each radiological and clinical parameter, the statistical differences between union and nonunion or those obtained before and after surgery were compared by using Fisher’s exact probability test, Mann–Whitney test, or Wilcoxon signed-ranks test. Statistical analyses were performed with the StatView program (version 5.0; Abacus Concept Inc., Berkeley, CA). P < 0.05 was considered statistically significant.
Results
Between January 1995 and December 1997, 51 patients who underwent the surgery met the selection criteria. One patient died due to causes unrelated to the surgical procedure, and eight patients could not be located; therefore, these nine patients were excluded from the study. Thus, we retrospectively reviewed 42 patients (25 female and 17 male, follow-up rate: 82.4%) for an average follow-up period of 9.5 years (range 8–10 years). No new neurological deficits were observed after surgery. At the time of surgery, the mean age was 64.1 years (range 46–77 years). Eight patients exhibited fusion at the L3–4 level and 34 patients, at the L4–5 level. Of the 42 patients studied, 8 (19%) had a comorbid disease influencing their walking ability. The comorbid diseases included advanced osteoarthrosis of the hip or knee joint necessitating arthroplasty (n = 4), Parkinson’s disease (n = 2), cervical or thoracic myelopathy (n = 4) and rheumatoid arthritis (n = 1). Three patients suffered from two comorbid diseases.
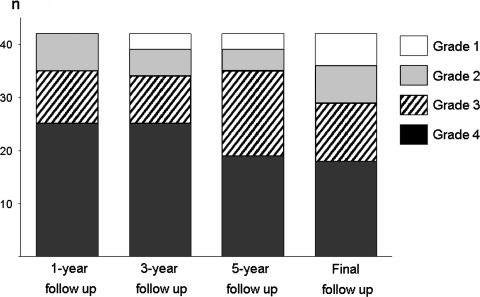
The averaged JOA score was 13.2 (range 3–20 points) before surgery and 23.5 (range 11–29 points) at the final follow-up. At the final follow-up, the percent recovery was greater than 3 in 69.0% (29/42) of the patients (Fig. 1). Of the six patients with a grade 1 percent recovery at the final evaluation, four patients belonged to the nonunion group and two patients belonged to the union group. Among them, two patients underwent a revision surgery at least 1-year after the initial operation. One patient in the union group suffered from recurrent leg pain due to lumbar disc herniation at the adjacent level below the fused segment 18 months after the surgery and subsequently underwent facetectomy and spinal instrumentation. The other patient with persistent LBP and leg pain underwent PLIF and spinal instrumentation at the same level due to nonunion.
Fig. 1.
Time-course changes in percent recovery (n = 42). The grades include grade 4 (percent recovery 75–100%), grade 3 (percent recovery 50–74%), grade 2 (percent recovery 25–49%) and grade 1 (percent recovery <25%, revision surgery)
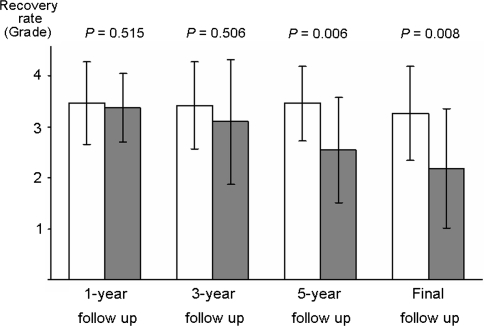
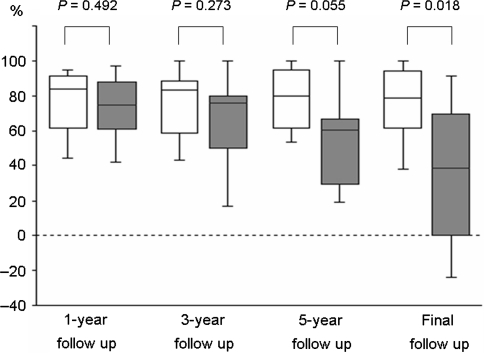
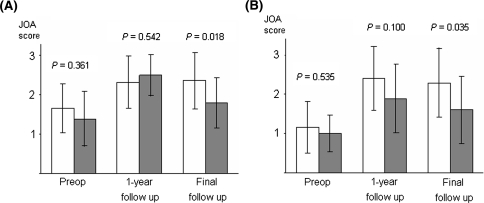
Nonunion developed in 26.2% (11/42) of the patients (Table 1). There were no significant differences between the union and nonunion groups with regard to age, gender, fusion level, preoperative %slip, preoperative dynamic translation, preoperative dynamic angulation, preoperative JOA scores and number of patients with comorbid diseases. Figures 2 and 3 show the comparative clinical results for the two groups. At 1 and 3-year follow-up, there was no significant difference in the overall percent recovery between the two groups (union vs. nonunion: 3.5 ± 0.8 vs. 3.4 ± 0.7, P = 0.515, and 3.4 ± 0.8 vs. 3.1 ± 1.2, P = 0.508, respectively) (Fig. 2). However, the percent recovery in the union group was significantly better than that of the nonunion group at 5-year and final follow-up (union vs. nonunion: 3.5 ± 0.7 vs. 2.5 ± 1.0, P = 0.006, and 3.3 ± 0.9 vs. 2.2 ± 1.2, P = 0.008, respectively) (Fig. 2). The averages of the preoperative LBP score and the leg symptoms score did not differ significantly between the union and nonunion groups (P = 0.361 and 0.535, respectively). One year after surgery, a significant improvement in these scores was noted in both the groups (LBP: P < 0.001 in the union group and P = 0.005 in the nonunion group; leg symptoms: P < 0.001 in the union group and P = 0.034 in the nonunion group). At the final follow-up, the LBP score and the leg symptoms score in the union group were significantly better than the scores in the nonunion group (P = 0.018 and 0.035, respectively) (Fig. 4).
Table 1.
Patients’ demographic data
| Union (n = 31) | Nonunion (n = 11) | P value | |
|---|---|---|---|
| Mean age at surgery (years) | 63.4 | 66.2 | 0.183 |
| range | 46–77 | 49–75 | |
| Sex (female/male) | 19/12 | 6/5 | 0.733 |
| PLF level | |||
| L3–4 | 6 | 2 | >0.999 |
| L4–5 | 25 | 9 | |
| Preoperative percent slip | 12.2 | 14.6 | 0.271 |
| range | 5.0–20.8 | 6.1–24.3 | |
| Dynamic translation (%) | 6.0 | 5.9 | 0.853 |
| range | 0–17.1 | 2.4–11.6 | |
| Dynamic angulation (°) | 8.3 | 7.9 | 0.698 |
| range | 1–17 | 0–20 | |
| Preoperative JOA score | 13.5 | 12.5 | 0.615 |
| range | 3–20 | 5–17 | |
| Comorbid disease [no. (%)] | 5 (16) | 3 (27) | 0.412 |
PLF posterolateral lumbar fusion, JOA Japanese Orthopaedic Association
Fig. 2.
Average percent recovery (grade) at different follow-up time points in the union and the nonunion groups. Open bars, union group (n = 31); filled bars, nonunion group (n = 11). The error bars represent standard deviation. The P values were calculated by using the Mann–Whitney test
Fig. 3.
Box plots showing the time-course changes in percent recovery (%) in the union and the nonunion groups. Patients who underwent revision surgery were excluded. Open boxes, union group (n = 30); filled boxes, nonunion group (n = 10). These plots illustrate the mean value as a horizontal line in a box. The vertical limits of the box define the 25th and 75th percentiles, and the error bars denote the 10th and 90th percentiles. The P values were calculated by using the Mann–Whitney test
Fig. 4.
Average scores of LBP (a) and leg symptoms (b) at different follow-up time points in the union and nonunion groups. Open bars, union group (n = 30); filled bars, nonunion group (n = 10). The error bars indicate standard deviation. The P values were calculated by using the Mann–Whitney test. Patients who underwent revision surgery were excluded. (Preop Preoperative values)
A stepwise regression analysis revealed that the best combination of the predictors for the percent recovery at the final follow-up included fusion status and the presence of comorbid disease. The multiple regression equations are as follows: percent recovery (grade) = 4.334 − 1.076 (fusion status: union = 1, nonunion = 2), (adjusted R2 = 0.172; P = 0.004), and percent recovery (%) = 48.927 − 31.309 (fusion status: union = 1, nonunion = 2) + 30.149 (comorbid disease: present = 1, absent = 2), (adjusted R2 = 0.311; P = 0.0004).
Discussion
A fundamental problem that arises while investigating spinal fusion is the lack of definitive methods for confirming solid fusion. The fusion status can by accurately evaluated only through surgical exploration and direct inspection of the fusion mass; however, these methods are impractical for routine use. CT scanning has become the preferred diagnostic imaging modality for evaluating spinal fusion. Carreon et al. [2] have demonstrated that the positive predictive value for solid fusion on CT scans was 89%, while that of nonunion was only 74% when PLF on both sides were not fused on fine-cut CT scans with reconstructions. Thus, CT evaluation does not seem to be very reliable for the diagnosis of nonunion. Additionally, due to the harmful effects of radiation exposure, CT is not currently used as a routine method for fusion-status evaluation in our hospital. Although plain radiography is not the best method for assessing the fusion status [11], plain radiographs, accompanied by those in the flexion and extension bending views, are commonly used for this purpose because they are relatively inexpensive and easy to obtain [19]. Static plain radiography is used to detect the presence of a bone bridge between the transverse processes, and functional radiography is used to detect motion at the fused segment. In this study, the overall fusion rate achieved by PLF was 74%, as evaluated by the method described by Fischgrund et al. [6] and Kornblum et al. [13].
Even though the data suggest some beneficial effects of concomitant spinal fusion in the treatment of patients with degenerative spondylolisthesis [7], there is no agreement concerning the association between fusion status and surgical outcomes. In this study, we have retrospectively examined a minimum of 8-year surgical outcomes of decompression and PLF in the treatment of LCS with degenerative spondylolisthesis by comparing cases demonstrating union with those exhibiting nonunion. The results demonstrated that the union group achieved better clinical results than the nonunion group at the 5-year and final follow-up although no significant difference was observed at the 1 and 3-year follow-up. Additionally, the scores of LBP and leg symptoms in the union group were better than those observed in the nonunion group at the final follow-up, while these scores were not significantly different between the two groups at 1-year follow-up. In a 3-year prospective study comparing decompression alone with decompression and uninstrumented PLF in the treatment of patients with LCS and degenerative spondylolisthesis, Herkowitz and Kurz [7] reported that patients undergoing concomitant arthrodesis demonstrated improved clinical results, regardless of the fusion status, when compared with the ‘decompression only’ group. Thereafter, Kornblum et al. [13] described the long-term outcomes (mean, 7.7 years) of the patients treated with uninstrumented PLF in the studies by Herkowitz and Kurz [7] and Fischgrund et al. [6]. They demonstrated that patients exhibiting nonunion experienced significant deterioration in the surgical outcome as compared to a more stable long-term relief for patients with a solid fusion. These results together suggest that an arthrodesis attempt, regardless of the fusion status, appears to play a key role in the treatment of LCS with degenerative spondylolisthesis in the short term; however, the fusion status is a critical factor influencing the long-term operative results. The cause of deterioration in the long-term operative results in the nonunion group with time is a matter of debate. One possible explanation is that instability at the fusion segment in the nonunion group causes greater degenerative changes, such as laminar regrowth and hypertrophy of the facet joints, than that observed in the union group. These changes might lead to the recurrence of the spinal stenosis, resulting in the deterioration of surgical outcomes with time [3, 18].
Degeneration that develops at mobile segments above or below a fused spinal segment is known as adjacent segment disease (ASD) [16]. In this study, plain radiographs obtained at the time of final follow-up revealed degenerative changes in the regions adjacent to the fused segment in the case of eight patients (herniated nucleus pulposus in one patient, disc space narrowing in 4, and instability in 3) belonging to the union group. ASD did not seem to be related to the surgical outcomes, except in the case of one patient, who required revision surgery at the adjacent level, below the fused segment, due to lumbar disc herniation.
Regarding the predictors of surgical outcomes, a multiple regression analysis revealed that the coexistence of comorbid conditions was also a key predictor of the long-term operative results, which was consistent with previous studies [10, 12, 23]. This result is possibly caused by the influence of comorbid diseases on gait and ADL, which comprise 17 points in the JOA score. In contrast, a multivariate analysis was unable to identify a significant correlation between percent recovery and age, gender, preoperative %slip, preoperative JOA score, preoperative dynamic motion at the listhetic segment and postoperative %slip.
The present study has some limitations. First, it was conducted based on a set of retrospective data, and the outcomes were measured solely based on the JOA score due to the unavailability of other scales such as the Visual Analogue Scale and the Oswestry Disability Index. Second, psychosocial factors which have been reported to influence the long-term surgical outcomes of spinal fusion [22] were not evaluated. Finally, the number of patients exhibiting nonunion was small (n = 11). Although the small sample size in the nonunion group clearly limited the results of the statistical analysis, we believe that this fact does not invalidate the main findings of our study.
Conclusions
In patients having LCS with degenerative spondylolisthesis who underwent uninstrumented PLF, the fusion rate evaluated by plain radiographs was 74%. The union group demonstrated better clinical results than the nonunion group in the long-term outcomes, while there were no significant differences in the short-term outcomes. Our results suggest that the fusion status in PLF and the coexistence of comorbid conditions are critical factors influencing the long-term operative results.
References
- 1.Boden SD. Overview of the biology of lumbar spine fusion and principles for selecting a bone graft substitute. Spine. 2002;27:S26–S31. doi: 10.1097/00007632-200208151-00007. [DOI] [PubMed] [Google Scholar]
- 2.Carreon LY, Djurasovic M, Glassman SD, et al. Diagnostic accuracy and reliability of fine-cut CT scans with reconstructions to determine the status of an instrumented posterolateral fusion with surgical exploration as reference standard. Spine. 2007;32:892–895. doi: 10.1097/01.brs.0000259808.47104.dd. [DOI] [PubMed] [Google Scholar]
- 3.Chen Q, Baba H, Kamitani K, et al. Postoperative bone re-growth in lumbar spinal stenosis. A multivariate analysis of 48 patients. Spine. 1994;19:2144–2149. doi: 10.1097/00007632-199410000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Dupuis PR, Yong-Hing K, Cassidy JD, et al. Radiologic diagnosis of degenerative lumbar spinal instability. Spine. 1985;10:262–276. doi: 10.1097/00007632-198504000-00015. [DOI] [PubMed] [Google Scholar]
- 5.Esses SI, Huler RJ (1992) Indications for lumbar spine fusion in the adult. Clin Orthop:87–100 [PubMed]
- 6.Fischgrund JS, Mackay M, Herkowitz HN, et al. 1997 Volvo Award winner in clinical studies. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective, randomized study comparing decompressive laminectomy and arthrodesis with and without spinal instrumentation. Spine. 1997;22:2807–2812. doi: 10.1097/00007632-199712150-00003. [DOI] [PubMed] [Google Scholar]
- 7.Herkowitz HN, Kurz LT. Degenerative lumbar spondylolisthesis with spinal stenosis. A prospective study comparing decompression with decompression and intertransverse process arthrodesis. J Bone Joint Surg Am. 1991;73:802–808. [PubMed] [Google Scholar]
- 8.Hirabayashi K, Miyakawa J, Satomi K, et al. Operative results and postoperative progression of ossification among patients with ossification of cervical posterior longitudinal ligament. Spine. 1981;6:354–364. doi: 10.1097/00007632-198107000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Izumida S, Inoue S. Assessment of treatment for low back pain. Japanese Orthopaedic Association. J Jpn Orthop Assoc. 1986;60:391–394. [Google Scholar]
- 10.Jonsson B, Annertz M, Sjoberg C, et al. A prospective and consecutive study of surgically treated lumbar spinal stenosis. Part II: five-year follow-up by an independent observer. Spine. 1997;22:2938–2944. doi: 10.1097/00007632-199712150-00017. [DOI] [PubMed] [Google Scholar]
- 11.Kant AP, Daum WJ, Dean SM, et al. Evaluation of lumbar spine fusion. Plain radiographs versus direct surgical exploration and observation. Spine. 1995;20:2313–2317. doi: 10.1097/00007632-199511000-00009. [DOI] [PubMed] [Google Scholar]
- 12.Katz JN, Lipson SJ, Larson MG, et al. The outcome of decompressive laminectomy for degenerative lumbar stenosis. J Bone Joint Surg (Am) 1991;73:809–816. [PubMed] [Google Scholar]
- 13.Kornblum MB, Fischgrund JS, Herkowitz HN, et al. Degenerative lumbar spondylolisthesis with spinal stenosis: a prospective long-term study comparing fusion and pseudarthrosis. Spine. 2004;29:726–724. doi: 10.1097/01.BRS.0000119398.22620.92. [DOI] [PubMed] [Google Scholar]
- 14.Mardjetko SM, Connolly PJ, Shott S, et al. Degenerative lumbar spondylolisthesis. A meta-analysis of literature 1970–1993. Spine. 1994;29:726–724. [PubMed] [Google Scholar]
- 15.Nagaosa Y, Kikuchi S, Hasue M, et al. Pathoanatomic mechanisms of degenerative spondylolisthesis. A radiographic study. Spine. 1998;23:1447–1451. doi: 10.1097/00007632-199807010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Park P, Garton HJ, Gala VC, et al. Adjacent segment disease after lumbar or lumbosacral fusion: review of the literature. Spine. 2004;29:1938–1944. doi: 10.1097/01.brs.0000137069.88904.03. [DOI] [PubMed] [Google Scholar]
- 17.Polly DW, Jr, Santos ER, Mehbod AA. Surgical treatment for the painful motion segment: matching technology with the indications: posterior lumbar fusion. Spine. 2005;30:S44–S51. doi: 10.1097/01.brs.0000174529.07959.c0. [DOI] [PubMed] [Google Scholar]
- 18.Postacchini F, Cinotti G. Bone regrowth after surgical decompression for lumbar spinal stenosis. J Bone Joint Surg (Br) 1992;74:862–869. doi: 10.1302/0301-620X.74B6.1447247. [DOI] [PubMed] [Google Scholar]
- 19.Resnick DK, Choudhri TF, Dailey AT, et al. Guidelines for the performance of fusion procedures for degenerative disease of the lumbar spine. Part 4: radiographic assessment of fusion. J Neurosurg Spine. 2005;2:653–657. doi: 10.3171/spi.2005.2.6.0653. [DOI] [PubMed] [Google Scholar]
- 20.Sengupta DK, Herkowitz HN. Degenerative spondylolisthesis: review of current trends and controversies. Spine. 2005;30:S71–S81. doi: 10.1097/01.brs.0000155579.88537.8e. [DOI] [PubMed] [Google Scholar]
- 21.Tajima N, Chosa E, Watanabe S. Posterolateral lumbar fusion. J Orthop Sci. 2004;9:327–333. doi: 10.1007/s00776-004-0773-8. [DOI] [PubMed] [Google Scholar]
- 22.Trief PM, Ploutz-Snyder R, Fredrickson BE. Emotional health predicts pain and function after fusion: a prospective multicenter study. Spine. 2006;31:823–830. doi: 10.1097/01.brs.0000206362.03950.5b. [DOI] [PubMed] [Google Scholar]
- 23.Zheng F, Sandhu HS, Cammisa FP, Jr, et al. Predictors of functional outcome in elderly patients undergoing posterior lumbar spine surgery. J Spinal Disord. 2001;14:518–521. doi: 10.1097/00002517-200112000-00011. [DOI] [PubMed] [Google Scholar]