Abstract
The sympathetic trunk is sometimes damaged during the anterior and anterolateral approach to the cervical spine, resulting in Horner’s syndrome. No quantitative regional anatomy in fresh human cadavers describing the course and location of the cervical sympathetic trunk (CST) and its relation to the longus colli muscle (LCM) is available in the literature. The aims of this study are to clearly delineate the surgical anatomy and the anatomical variations of CST with respect to the structures around it and to develop a safer surgical method that will diminish the potential risk of CST injury. In this study, 30 cadavers from the Department of Forensic Medicine were dissected to observe the surgical anatomy of the CST. The cadavers used in this study were fresh cadavers chosen at 12–24 h postmortem. The levels of superior and intermediate ganglions of cervical sympathetic chain were determined. The distance of the sympathetic trunk from the medial border of LCM at C6, the diameter of the CST at C6 and the length and width of the superior and intermediate (middle) cervical ganglion were measured. Cervical sympathetic chain is located posteromedial to carotid sheath and just anterior to the longus muscles. It extends longitudinally from the longus capitis to the longus colli over the muscles and under the prevertebral fascia. The average distance between the CST and medial border of the LCM at C6 is 11.6 ± 1.6 mm. The average diameter of the CST at C6 is 3.3 ± 0.6 mm. Superior ganglion of CSC in all dissections was located at the level of C4 vertebra. The length and width of the superior cervical ganglion were 12.5 ± 1.5 and 5.3 ± 0.6 mm, respectively. The location of the intermediate (middle) ganglion of CST showed some variations. The length and width of the middle cervical ganglion were 10.5 ± 1.3 and 6.3 ± 0.6 mm, respectively. The CST’s are at high risk when the LC muscle is cut transversely, or when dissection of the prevertebral fascia is performed. Awareness of the CST’s regional anatomy may help the surgeon to identify and preserve it during anterior cervical surgeries.
Keywords: Anatomy, Anterolateral approach, Cervical spine, Cervical sympathetic trunk
Introduction
The anterior approach to the subaxial cervical spine is commonly used in the surgical treatment of several diseases, including cervical disc herniation, cervical spondylitic myelopathy, and disease of cervical vertebral bodies caused by tumour, infection, or trauma.
During the anterior approach to the cervical vertebral bodies, cervical sympathetic trunk (CST) is at potential risk of injury. Horner’s syndrome (ptosis, ipsilateral miosis, anhydrosis) resulting from the anterior surgical approaches to the cervical spine or cervicothoracic junction has been documented [1, 9, 15, 16]. Incidence of Horner’s syndrome after anterior cervical spine surgeries has varied between 0.2 [18] and 4% [7, 8]. Bertalanffy and Eggert [2] reported that Horner’s syndrome was associated with anterior cervical discectomy in 1.1% of 450 consecutive cases, which was similar to the findings of others [3, 4].
There are very few reports related to the anatomy of the CST in the anterior cervical spine approach. In the current study, 30 fresh cadavers were dissected and measured to define the topography and location of the CST during surgical exposure at the lower cervical vertebral levels. This study describes the number and size of the cervical ganglia and measure the distances of the sympathetic trunk from the longus colli muscle (LCM).
Methods
Thirty cadavers from people of age 20–80 years (mean age 41, 23 men and seven women) from the Department of Forensic Medicine were used for dissection to observe surgical anatomy of the CST. The cadavers used in this study were fresh cadavers chosen at 12–24 h postmortem. Exclusion criteria in chosen cadavers were neck trauma and previous cervical operation. All dissections were performed by a single neurosurgeon in a private autopsy training room. Digital camera (Nikon, 6.0 megapixel), and some tools (tripod and electric light) were placed to take high-quality photographs during dissections.
The cadavers were placed on the autopsy table in the supine position. Both shoulders of the cadavers were supported with 7-cm high pads. The heads of the cadavers were fixed in the position of slight extension and rotated to 30° left. This position was preferred to make the sternocleidomastoid (SCM) muscle more prominent.
The incision was made on the right side of all cadavers and extended to manubrium to expose C6 and C7 vertebra and to the posterior of mastoid process to expose C3 and C4 vertebras. After exploring platysma muscle, it was opened along the incision. Along the anterior border of the SCM muscle, superficial lamina of cervical fascia was opened. When SCM muscle was moved laterally, internal jugular vein (IJV) and omohyoid muscle whose inferior belly crosses SCM muscle obliquely were exposed. The dissection field was widened with blunt dissection in between SCM muscle and IJV.
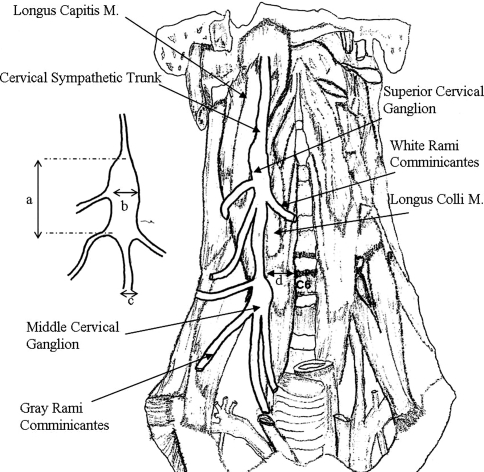
After IJV and other neurovascular structures were moved medially, a wide dissection field covered by adipose tissue was reached. Beneath the adipose tissue, prevertebral fascia and fascia of longus muscles were explored. Over the fascia of longus muscles, CST, and its superior and intermediate ganglions were exposed (Figs. 1, 2). White and gray rami communicantes which are the medial and lateral attachments of the CST were seen (Figs. 2, 3).
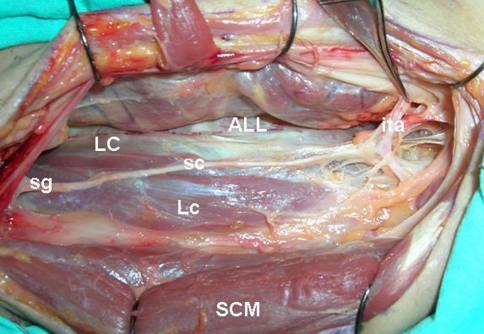
Fig. 1.
ALL anterior longitudinal ligament, LC longus colli muscle, Lc longus capitis muscle, sc sympathetic trunk, SCM sternocleidomastoid muscle, ita inferior thyroidal artery, sg superior ganglion of sympathetic trunk. (left side is rostral part and right side is caudal part of the neck)
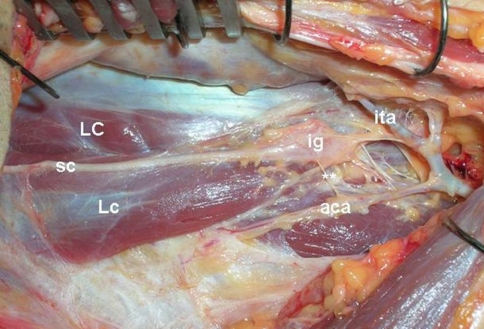
Fig. 2.
LC longus colli muscle, Lc longus capitis muscle, sc sympathetic trunk, ita inferior thyroidal artery, ig intermediate (middle) ganglion of sympathetic trunk, aca ascending cervical artery, double asterisks lateral attachment of sympathetic trunk (gray rami communicantes) (left side is rostral part and right side is caudal part of the neck)
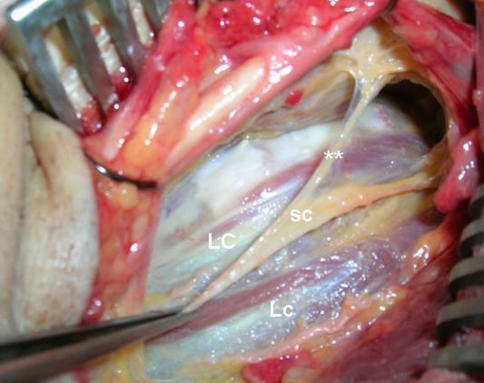
Fig. 3.
LC longus colli muscle, Lc longus capitis muscle, sc sympathetic trunk, double asterisks medial attachment of sympathetic trunk (white rami communicantes) (left side is rostral part and right side is caudal part of the neck)
In this stage of the dissection transverse processes of the vertebra were palpated and cervical vertebra and disc levels from C2 to C7 were exposed. The most prominent anterior tubercle was accepted as C6 (Fig. 4). The vertebrae and disc levels were also checked from the superior part by counting disc levels.
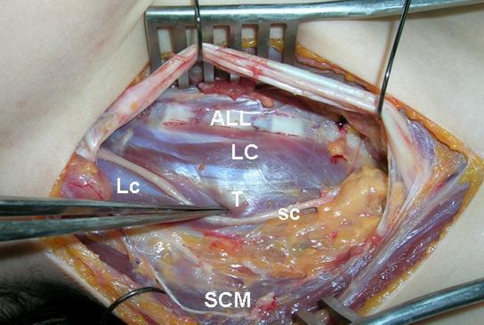
Fig. 4.
ALL anterior longitudinal ligament, LC longus colli muscle, Lc longus capitis muscle, T anterior tubercle of cervical 6th vertebrae (carotid tubercle or Chassaignac tubercle), sc sympathetic trunk, SCM sternocleidomastoid muscle (left side is rostral part and right side is caudal part of the neck)
The levels of superior and intermediate ganglions of CST were determined and the distances of the sympathetic trunk from the medial border of LCM at C6 level were recorded for each of the cadavers. Additionally, the diameter of the CST at C6 and the length and width of the superior and intermediate (middle) cervical ganglion were determined (Fig. 5).
Fig. 5.
a length of ganglion, b width of ganglion, c diameter of CST, d distance of CST from the medial border of longus colli muscle at C6 level
The surgical tools for a routine cervical spinal procedure were used in all of the dissections. All measurements were obtained using a manual caliper accurate to 0.1 mm (Mitutoyo Corporation, Tokyo, Japan). A single person performed each measurement and dissection. In order to minimize the errors inherent in a morphometric study of this nature, all measurements were taken three times and an average of the three observations was used for statistical evaluation. The statistical significance of the differences between men and women was calculated using the Student’s t test.
Results
Cervical sympathetic chain is located posteromedial to carotid sheath and runs over the longus muscles (Figs 1, 2). It extends longitudinally from longus capitis to longus colli over the muscles and under the prevertebral fascia. From superior to inferior, LCM diverge laterally, whereas the CST converges medially at C6. The average distance between the CST and medial border of the LCM at C6 is 11.6 ± 1.6 mm. The average diameter of the CST at C6 is 3.3 ± 0.6 mm. In all dissections, CST was found over the anterior surface of the longus muscles. Superior ganglion of CST in all dissections was located at the level of C4 vertebra. The length and width of the superior cervical ganglion were 12.5 ± 1.5 and 5.3 ± 0.6 mm, respectively. The location of the intermediate (middle) ganglion of CST was showing some variations (Table 1). It was located in ten cadavers (33%) at the level of C6 vertebra, in eight cadavers (26%) at the level of C5, in four cadavers (13%) at the level of C7 vertebra, and in the remaining eight cadavers (26%) it was not seen. The length and width of the middle cervical ganglion were 10.5 ± 1.3 and 6.3 ± 0.6 mm, respectively. In the cadavers whose intermediate ganglions of CST were not seen, it was probably located in the lower segments of vertebral column. There were no statistical differences between men and women for any of the parameters (p > 0.05).
Table 1.
Levels of the intermediate ganglion of the cervical sympathetic trunk
| Level of intermediate ganglion | Number | % |
|---|---|---|
| C6 | 10 | 33 |
| C5 | 8 | 26 |
| C7 | 4 | 13 |
| Not seen | 8 | 26 |
Discussion
The anterolateral approach offers a direct route to the transverse foramen with less retraction of the carotid sheath neurovascular bundle. However, extensive anterolateral cervical dissection or severance of the LC muscle to expose the transverse foramens or uncovertebral joint seems to be associated with occurrence of Horner’s syndrome. Therefore, the CST is more at risk using this approach.
The current anatomic dissection demonstrates that the CSTs are situated at 11.6 ± 1.6 mm lateral to the medial border of the LCM. Therefore, to avoid injury, exposure of the CSTs is important during the approach to the lower cervical spine. To avoid inadvertent damage to the ST, attention should be paid when transverse severance of the LCM or extensive dissection over or below the LCM is performed. To protect the ST, a blunt tip of the retractor should be placed securely beneath rather than on the surface of the LCM. Hence, compression of CST by the retractor is the other reason for CST injury. In the anterolateral approach, although the CST located over longus colli and longus capitis muscles might be injured, this may be prevented using the remaining two-third of LCM for retraction and to pull the sympathetic trunk medially [12]. At this stage of operation, one or two gray rami communicantes, which are the lateral branches of CST to the nerve roots, can be sacrificed. The medial ones (white rami communicantes), which are laringopharyngeal, cardiac, and thyroidal branches, should be protected (Figs. 2, 3). George reported that the only morbidity directly related to this technique was Horner’s syndrome, which cannot be avoided, in the immediate postoperative period [6]. However, controlling and displacing it medially have preserved the main sympathetic trunk. Horner’s syndrome resolves in 2–3 months. Whatever the importance of the Horner’s syndrome, it has no functional consequences but only creates slight cosmetic problems. The probability of occurrence of the Horner’s syndrome was reported as 20% and it changes depending on the surgeon’s experience [6].
The CST is 3.3 ± 0.6 mm in diameter at the level of C6 vertebra where the transverse process is an important surgical landmark. The diameter at this site was reported to be 3–4 mm by Lyons and Mills [12], 2.7 ± 0.6 mm by Ebraheim et al. [5], and 2.2 ± 0.7 mm by Kıray et al. [11].
The CST passes over the LCM [20]. The CST can pass within the posterior wall of the carotid sheath. A CST in this location was reported by Lyons and Mills [13] in 16.7% of their specimens. In this study, there was no such variation. Such a variation may cause a stretching injury of the CST during lateral retraction of the carotid artery, even during the anterior approach to the cervical spine.
The SG is the most consistent and largest ganglion of the CST. The length and width of the superior cervical ganglion were 12.5 ± 1.5 and 5.3 ± 0.6 mm, respectively. Tubbs [19] reported that the superior cervical ganglion rests posterior to the internal carotid artery and lies anterior to the second and third cervical vertebrae. This level is approximated by the hyoid bone. In this study, superior ganglion of CST was located at the level of C4 vertebrae in all dissections. In all other studies, location of the ganglion varies between C2 and C3 levels [5, 14, 19]. In our opinion, this difference is because fresh cadavers were dissected. This ganglion is not commonly at risk of injury during routine cervical spine procedures.
The intermediate cervical ganglion (MG) lies at the C6 vertebral level approximated by the cricoid cartilage or carotid tubercle. In all dissections, CST was found over the anterior faces of the longus muscles. The location of the intermediate ganglion of CST showed some variations (Table 1). The MG was observed in 74% of our cases. It was observed in 39.3% cases by Ebraheim et al. [5] and in 53.2% by Katritsis et al. [10]. If present, it was located in ten cadavers (33%) at the level of C6 vertebrae, in eight cadavers (26%) at the level of C5, in four cadavers (13%) at the level of C7 vertebrae. In the series of Ebraheim et al. [5], it measured 9.7 ± 2.1 mm in length and 5.2 ± 1.3 mm in width. In this study, the length and width of the middle cervical ganglion were 10.5 ± 1.3 and 6.3 ± 0.6 mm, respectively. The inferior thyroid artery may cross the MG anteriorly or posteriorly. The close approximation of the MG and the medial border of LCM should be taken into account during dissection or retraction of LCM in anterior lower cervical spine surgery.
Conclusion
The CSTs are at high risk when the LCM is cut transversely, or when dissection of the prevertebral fascia is performed. Awareness of the CST’s regional anatomy may help the surgeon to identify and preserve it during anterior cervical surgeries. All studies of this subject in the literature were conducted upon formalin-fixed cadavers or cervical spines that were processed with chemicals [17]. Formalin-fixed techniques render the soft tissue structures, such as ligament, muscle, and adipose tissue [14]. Therefore, identification of the structures is more difficult. Our measurements are different from the studies in the literature that used formalin-fixed cadavers. In contrast to related studies, this study was conducted on fresh cadavers; therefore, we believe that the data given here were more accurate and helpful to the surgical approach.
References
- 1.An HS, Vaccaro A, Cotler JM, Lin S. Spinal disorders at the cervicothoracic junction. Spine. 1994;19:2557–2564. doi: 10.1097/00007632-199411001-00011. [DOI] [PubMed] [Google Scholar]
- 2.Bertalanffy H, Eggert HR. Complications of anterior cervical discectomy without fusion in 450 consecutive patients. Acta Neurochir (Wien) 1989;99:41–50. doi: 10.1007/BF01407775. [DOI] [PubMed] [Google Scholar]
- 3.Cuatico W. Anterior cervical discectomy without interbody fusion: an analysis of 81 cases. Acta Neurochir (Wien) 1981;57:269–274. doi: 10.1007/BF01664843. [DOI] [PubMed] [Google Scholar]
- 4.Dohn DF. Anterior interbody fusion for treatment of cervical disc conditions. JAMA. 1966;197:897–900. doi: 10.1001/jama.197.11.897. [DOI] [PubMed] [Google Scholar]
- 5.Ebraheim NA, Lu J, Yang H, Heck BE, Yeasting RA. Vulnerability of the sympathetic trunk during the anterior approach to the lower cervical spine. Spine. 2000;25:1603–1606. doi: 10.1097/00007632-200007010-00002. [DOI] [PubMed] [Google Scholar]
- 6.George B, Lot G. Oblique transcorporeal drilling to treat anterior compression of the spinal cord at the cervical level. Minim Invas Neurosurg. 1994;37:48–52. doi: 10.1055/s-2008-1053448. [DOI] [PubMed] [Google Scholar]
- 7.Giombini S, Solero CL. Considerations on 100 anterior cervical discectomies without fusion. In: Grote W, Brock M, Clar HE, Klinger M, Nau HE, editors. Advances in neurosurgery. Berlin: Springer; 1980. pp. 302–307. [Google Scholar]
- 8.Hankinson HL, Wilson CB. Use of the operating microscope in anterior cervical discectomy without fusion. J Neurosurg. 1975;43:452–456. doi: 10.3171/jns.1975.43.4.0452. [DOI] [PubMed] [Google Scholar]
- 9.Johnston FG, Crockard A. One-stage internal fixation and anterior fusion in complex cervical spinal disorders. J Neurosurg. 1995;82:234–238. doi: 10.3171/jns.1995.82.2.0234. [DOI] [PubMed] [Google Scholar]
- 10.Katritsis ED, Lykaki-Anastopoulou G, Papadopoulos NJ. Anatomical observations on the intermediate ganglion of the cervical sympathetic trunk. Anat Anz. 1983;154:33–38. [PubMed] [Google Scholar]
- 11.Kıray A, Arman C, Naderi S, Guvencer M, Korman E. Surgical anatomy of the cervical sympathetic trunk. Clin Anat. 2005;18:179–185. doi: 10.1002/ca.20055. [DOI] [PubMed] [Google Scholar]
- 12.Kiris T. Anterolateral surgical approach in cervical spondilotic myelopathy and surgery of spinal column. In: Zileli M, Ozer AF, editors. Omurilik ve omurga cerrahisi, Cilt 1. Bornova: Meta Basim Matbaacilik Hizmetler Publishing; 2002. pp. 605–623. [Google Scholar]
- 13.Lyons AJ, Mills CC. Anatomical variants of the cervical sympathetic chain to be considered during neck dissection. Br J Oral Maxillofac Surg. 1998;36:180–182. doi: 10.1016/S0266-4356(98)90493-4. [DOI] [PubMed] [Google Scholar]
- 14.Pait TG, Killefer JA, Arnautovic KI. Surgical anatomy of the anterior cervical spine: the disc space, vertebral artery, and associated bony structures. Neurosurgery. 1996;39(4):769–776. doi: 10.1097/00006123-199610000-00026. [DOI] [PubMed] [Google Scholar]
- 15.Saunders RL, Bernini PM, Shirreffs TG, et al. Central corpectomy for cervical spondylotic myelopathy: a consecutive series with long-term follow-up evaluation. J Neurosurg. 1991;74:163–170. doi: 10.3171/jns.1991.74.2.0163. [DOI] [PubMed] [Google Scholar]
- 16.Smith GW, Robinson RA. The treatment of cervical spine disorders by anterior removal of the intervertebral disc and interbody fusion. J Bone Joint Surg [Am] 1958;40:607–624. [PubMed] [Google Scholar]
- 17.Tanaka N, Fujimoto Y, Howard A, Ikuta Y, Yasuda M. The Anatomic relation among the nerve roots, intervertebral foramina, and intervertebral discs of the cervical spine. Spine. 2000;25(3):286–291. doi: 10.1097/00007632-200002010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Tew JM, Mayfield FH. Complications of surgery of the anterior cervical spine. Clin Neurosurg. 1976;23:424–434. doi: 10.1093/neurosurgery/23.cn_suppl_1.424. [DOI] [PubMed] [Google Scholar]
- 19.Tubbs RS, Salter EG, Oakes WJ. Anatomic landmarks for nerves of the neck: a Vade Mecum for neurosurgeons. Oper Neurosurg. 2005;2(56):256–260. doi: 10.1227/01.NEU.0000156541.78020.DA. [DOI] [PubMed] [Google Scholar]
- 20.Williams PL, Bannister LH, Berry MM, Collins P, Dyson M, Dussek JE, Ferguson MWJ. Gray’s Anatomy. 38. London: Churchill Livingstone; 1995. pp. 1298–1312. [Google Scholar]