Abstract
The nonsurgical treatment of thoracolumbar (TLB) and lumbar burst (LB) fractures remains to be of interest, though it is not costly and avoids surgical risks. However, a subset of distinct burst fracture patterns tend to go with a suboptimal radiographic and clinical long-term outcome. Detailed fracture pattern and treatment-related results in terms of validated outcome measures are still lacking. In addition, there are controversial data on the impact of local posttraumatic kyphosis that is associated, in particular, with nonsurgical treatment. The assessment of global spinal balance following burst fractures has not been assesed, yet. Therefore, the current study intended to investigate the radiographical and clinical long-term outcome in neurologically intact patients with special focus on the impact of regional posttraumatic kyphosis, adjacent-level compensatoric mechanisms, and global spine balance on the clinical outcome. For the purpose of a homogenous sample, strong in- and exclusion criteria were applied that resulted in a final study sample of 21 patients with a mean follow-up of 9.5 years. Overall, clinical outcome evaluated by validated measures was diminished, with 62% showing a good or excellent outcome and 38% a moderate or poor outcome in terms of the Greenough Low Back Outcome Scale. Notably, vertebral comminution in terms of the load-sharing classification, posttraumatic kyphosis, and an overall decreased lumbopelvic lordosis showed a significant effect on clinical outcome. A global and segmental curve analysis of the spine T9 to S1 revealed significant alterations as compared to normals. But, the interdependence of spinopelvic parameters was not disrupted. The patients’ spinal adaptability to compensate for the posttraumatic kyphotic deformity varied in the ranges dictated by pelvic geometry, in particular the pelvic incidence. The study substantiates the concept that surgical reconstruction and maintenance of a physiologically shaped spinal curve might be the appropriate treatment in the more severely crushed TLB and LB fractures.
Keywords: Burst fracture, Thoracolumbar spine, Nonsurgical treatment, Spinal balance, Clinical outcome
Introduction
Despite a sizable amount of literature, the ideal management of thoracolumbar burst (TLB) and lumbar burst (LB) fractures without neurological compromise remains controversial [72, 84, 87, 89]. In particular, although several studies using validated outcome measures exist [14, 31, 46, 48, 51, 67, 77, 79, 97, 99], distinct outcome variables that can be expected for individual fracture patterns according to the AO-classification or the Load Sharing Classification remain scarce [4, 79]. A significant but unsolved question is which burst fracture patterns can be treated nonsurgically, and which demand surgical reconstruction to avoid a posttraumatic kyphotic deformity which in turn can be associated with serious long-term sequelae [28, 87]. Rarely, the initial treatment may be inadequate, resulting in acute instability and leading to early painful deformity or neurologic deficit. More commonly, because of the occult instability and exposure to chronic physiologic stresses, a gradual deformity may become apparent [87] with symptoms related to loss of spinal alignment and compensatory adjacent-level adjustments. Most previous prospective trials on the management of burst fractures have failed to provide large and homogenous samples with sufficient long-term surveillance [72, 79]. Prospective studies are ongoing [47], but until these results are available, systematic reviews of the literature remain a valuable tool to seek out evidence-based conclusions. Unfortunately, although homogeneous groups are indicated to delineate prognostic factors that have impact on clinical outcome [5], current meta-analyses suffer from a small number of articles with homogeneous cohorts, fracture patterns, and long-term outcome [89].
Thoracic and lumbar burst fractures often result in an abrupt change of in the quality of the patient’s life, with significant impact on the ability to work, perform sports, and, with remaining pain, can result in a gradual but persistent loss of function that may lead to the development of chronic complications over time [4, 14, 15, 45, 46, 51, 70, 80, 81, 87, 98]. In TLB and LB fractures, regional kyphosis increases during the clinical course following casting or posterior-only instrumentations [6, 62, 70, 71, 77, 78]. Whether long-standing sequelae, such as muscle fatigue and capsular insufficiencies with burned-out adjacent-level adaptabilities might be associated with a measureable spinal imbalance and alteration of the sagittal curve of the fractured spine is yet to be answered. The objective of the current study was to investigate the clinical and radiographical long-term results of nonsurgically treated TLB and LB fractures in a strongly homogeneous patient sample. The authors sought to determine the influence of regional and global sagittal spinal alignment on long-term clinical outcome. Emphasis was placed on the correlation of radiographic measures of global spinal deformity and validated patient-based quality of life, functional, and health status measures.
Material and methods
The current study represents a retrospective review of a nonsurgically treated case series of neurologically intact patients with TLB and LB fractures.
Data sampling
Electronic and hard-copy medical files were reviewed to detect all thoracolumbar (T11–L2) and lumbar (L3–L5) compression and burst fractures that had been treated with closed reduction and casting between 1.1.1990 and 31.12.2001 at the author’s institution. The search resulted in 146 fractures and patients’ radiographs and medical charts were reviewed with the following inclusion criteria for entrance into final analysis: documented evidence of (a) single-level thoracolumbar (T11–L2) or lumbar (L3–5) compression or burst fracture; (b) absence of neurological injury (Frankel A–D); (c) age between 18 and 60 years at injury; (d) understanding of the author’s language; (e) ≤10 days between injury and index treatment; (f) full set of injury lateral and antero–posterior (a–p) radiographs. CT-scans were not necessary for inclusion. Exlusion criteria were as following : (a) medical illness that precluded operative treatment; (b) prior thoracic, lumbar, abdominal or genitourinary surgery; (c) major organ system or musculoskeletal injuries; (d) spinal disorders in the patient’s medical history requiring a specific medical treatment; (e) chronic drug and alcohol abuse; (f) concomittant serious head, thoracic, or abdominal injuries; (g) proven evidence of osteoporosis; (h) serious mental disorders leading to medical intervention; (i) pregnancy; (j) end-stage medical diseases; (k) lower extremity injuries affecting gait or limb length; (m) failure to comply in wearing the brace; (n) worker’s compensation claims; or (o) pre-existing spinal deformity. After the selection process, patients’ demographics and medical characteristics were recorded. Forty-seven patients with compression or burst fractures remained for further investigation and fracture classification.
Fracture classification
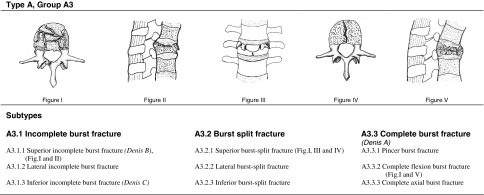
Burst fractures were characterized using the AO-C according to Magerl et al. [55] (Fig. 1) and LSC according to McCormack et al. [57] (Fig. 2). Fractures included were only those of AO type A3. Equivalent fracture pattern according to the Denis classification are templated in Fig. 1. Radiographic and CT-based characterization of distinct AO subtypes was performed as recommended [12, 52, 55, 76]. At the beginning of the study period CT-scans were not performed on a regular basis, and therefore were available in only 64% of the patients in this study. Six orthopedic surgeons evaluated all 47 fractures on lateral and ap-radiographs according to the AO-C and an a priori interobserver analysis was performed. The observers were asked to select the AO types first, then group and subtype. Fractures were excluded if more than two observers differed regarding type or group. Final subtype classification (e.g. A3.2.1) was carried out on common agreement within all observers, including available CT-scans. The interobserver reliability was calculated using Cohen’s weighted kappa and interpretation of strength of agreement was done according to the criteria of Landis and Koch [50]. Complete agreement including subtype classification within all observers occurred in 60% and the Kappa value was 0.55, delineating moderate agreement. Following the interobserver assessment, 15 cases were excluded because of differences concerning the suggested AO type (type A or B) or group (type A1.2.1 vs type A3.1.1). A previous study [19] demonstrated that the overall kappa value of the interobserver assessment of the LSC was 0.82, representing almost perfect interobserver reliability. Therefore, assessment of the LSS on CT-scans was performed by two of the authors (H.K., M.T.) on agreement.
Fig. 1.
AO-classification of burst fractures according to Magerl et al. [55]
Fig. 2.
Load-sharing classification (LSC) according to McCormack et al. [57]
Nonsurgical treatment
During the study interval, patients with TLB and LB fractures were hospitalized after obtaining supine radiographs with increasing use of CT-scans as the study time interval progressed. The decision whether to treat surgically depended on the assumed fracture instability taking into account the patient’s expectations, compliance, associated trauma, and pain, with an increasing rate of surgical interventions for A3 fractures since the late 90s. Fractures subjected to nonsurgical treatment were managed with a body cast. Patients were placed in the supine position on a Risser-like cast table with a canvas belt temporarily wrapped around the waist at the level of the fracture. An anterior force was applied to reduce the fracture kyphosis as described [20, 86]. The cast was worn for 24 h a day for 3 months. Associated injuries were managed as indicated, neurologic status evaluated serially and patients discharged when their pain was controlled and families comfortable with their care. Patients were prohibited from engaging in heavy work and sports for 3 months, but were allowed to perform activities of daily living and sedentary work. They were followed twice a month for 3 months, at 6 months, and then scheduled for annual follow-up.
Radiographic assessment
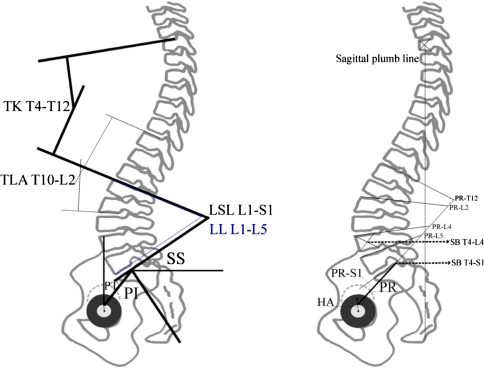
All measurement techniques applied are illustrated in Figs. 3 and 4. Abbreviations are explained in Table 1. Injury spine films were taken in supine position. Serial follow-up radiographs were not evaluated in the current study as the early radiographic course of nonsurgically treated burst fractures with their loss of reduction is already documented [60, 70, 71, 95]. The injury RKA was indicated as the Cobb angle on supine lateral and on standing full-length radiographs at final follow-up in all patients. With AO type A3.1 fractures not affecting both endplates the SKA and the RKA were measured on injury radiographs. At follow-up, all those fractures, including A3.2 and A3.3 fractures, in which the cortical anatomical boundaries could be reliably tracked were also assessed with the SKA. In the coronal plane, injury and posttraumatic scoliosis were assessed using the Cobb method. The TLA was defined from T10 to L2 as measured with the Cobb method. All parameters at follow-up were measured on digital full-length standing lateral and ap-radiographs. Patients were asked to stand in an erect but comfortable posture with the hands resting on the shoulders, the knees held in extension, and horizontal gaze [33]. Digital copies of the radiographs were imported into software for analysis (EMV, Escape Medical, Thessaloniki/Greece). The software has the capability to magnify and enhance visual details in regions of interest in order to improve the accuracy of locating certain anatomical landmarks on the film.
Fig. 3.
Measurement techniques for assessment of spinal balance and spinopelvic parameters. Figure left: PI pelvic incidence, PT pelvic tilt, SS sacral slope, LSL lumbosacral lordosis L1-S1, LL lumbar lordosis L1-L5, TLA thoracolumbar junction angle T10–L2, TK thoracic kyphosis T4–T12. Figure right: SB T4–L4/S1 sagittal balance w/plumb line from T4 to reference point at center of L4 and posterior corner of S1, respectively. HA hip axis, PR pelvis radius, PR–S1 pelvic morphology, PRT12–L5 lumbopelvic lordosis according to pelvis radius technique [35]
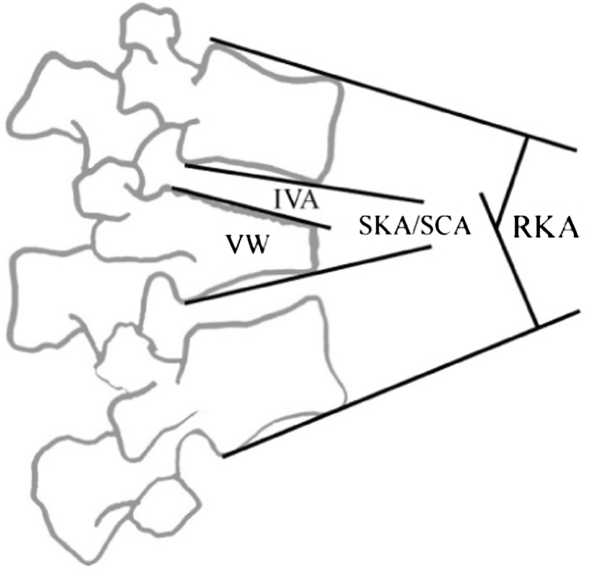
Fig. 4.
Measurement techniques for assessment of regional deformity (RKA/SKA) and sagittal curve analysis (SCA). IVA intervertebral angle, VW vertebral wedging, SKA segmental kyphosis angle/SCA segmental Cobb angle. IVA + VW = SCA
Table 1.
Abbreviated items and angular measurements
| AO-C | AO-classification |
| AUC | Area under the curve |
| IVA | Intervertebral angle |
| LB | Lumbar burst |
| LBOS | Greenough low back outcome scale |
| LL | Lumbar lordosis L1-L5 |
| LSC | Load sharing classification |
| LSL | Lmbosacral lordosis L1-S1 |
| LSPI | Long-segment posterior transpedicular instrumentation |
| LSS | Load sharing score |
| MLL | Maximum lumbar lordosis |
| MTK | Maximum thoracic kyphosis |
| ODI | Oswestry disability index |
| PCS/MCS | Physical/mental component summary |
| PI | Pelvic incidence |
| PKD | Posttraumatic kyphotic deformity |
| PR | Pelvic radius |
| PR-L2, -L4, -L5 | Regional lumbopelvic lordosis |
| PR-S1 | Pelvic morphology |
| PR-T12 | Total lumbopelvic lordosis |
| PT | Pelvic tilt |
| RKA | Regional kyphosis angle |
| RMDQ | Roland Morris Disability Spine Questionnaire |
| SBT4-S1 | Sagittal spinal balance T4-S1 |
| SB T-L4 | Sagittal spine balance T4-L4 |
| SCA | Segmental Cobb angle |
| SF-36-v2 | Short-form 36 questionary, version 2 |
| SKA | Segmental kyphosis angle |
| SS | Sacral slope |
| SSPI | Short-segment posterior transpedicular instrumentation |
| TK | Toracic kyphosis T4-T12 |
| TLA | Thoracolumbar junction angle T10-L2 |
| TLB | Thoracolumbar burst |
| TLSLT12-S1 | Thoracolumbo-sacral lordosis |
| VW | Vertebral wedging |
Sagittal plane assessment
Pelvic parameters included in the analysis were the pelvic incidence (PI), the sacral slope (SS), and the pelvis tilt (PT). The PI is a key anatomical parameter for determining the spinal balance [13]. It is the angle between the line perpendicular to the middle of the cranial sacral endplate and the line joining the middle of the cranial sacral endplate to the center of the bicoxofemoral axis, that is, the line between the geometric center of both femoral heads (hip axis). The SS corresponds to the angle between the horizontal line and the cranial sacral endplate. The PT is the angle between the vertical line and the line joining the middle of the sacral plate and the center of the bicoxofemoral axis. The SS and PT are positional parameters, varying according to the pelvis position, affected by changes in the alignment of the lower extremities. The PI is a morphological parameter, considered a constant independent of the spatial orientation of the pelvis and by body posture [7, 49, 74, 90].
Spinal parameters included in the analysis were the lumbar lordosis L1–L5 (LL), lumbosacral lordosis L1–S1 (LSL), and the thoracic kyphosis T4–T12 (TK). The LSL was defined using Cobb’s method from the sacral endplate to the upper endplate of L1, the LL corresponds to the angle between the cranial endplate of L1 and the caudal endplate of L5, and the TK was measured using Cobb’s method from the cranial endplate of T4 to the caudad endplate of T12. According to Jackson [35], we assessed the pelvic radius (PR), pelvic morphology (PR–S1, similar to the PI), total lumbopelvic lordosis (PR–T12) and regional lumbopelvic lordosis (PR–L2, PR–L4, PR–L5). With lumbopelvic regional angulations according to the pelvis radius technique, the corresponding angles are formed between a line connecting the center of the hip axis with the posterior corner of the S1 endplate (pelvis radius) and the superior endplate of S1 (PR–S1) or the superior endplates of the lumbar vertebrae (PR–L2, L4, -L5) and the T12 vertebrae (PR–T12). Thoracolumbo-sacral lordosis was measured from the inferior T12 endplate to the superior endplate of S1 (TLSL T12–S1).
Assessment of sagittal spinal balance (SB) involved horizontal perpendicular distances measured in millimeters between the plumb line from the center of the T4 vertebral body to the reference point at the center of the L4 vertebral body (SB T4–S1) and to the reference point at the posterior aspect of the S1 endplate (SB T4–S1). The T4–S1 and T4–L4 sagittal vertical axes have a positive/negative value when the vertical plumb line is anterior/posterior to the sacral and lumbar reference point, respectively [49]. Sagittal imbalance was assumed if the SB was ≥5 cm positive or negative [54]. We noted the transitional vertebra at the junction of the lumbar lordosis and the thoracic kyphosis in order to measure the maximum lordosis (the angle between the cranial endplate of the transitional vertebra and the cranial endplate of S1) and the maximum kyphosis (the angle between the cranial endplate of T4 and the caudad endplate of the transitional vertebra), delineated by maximum lumbar lordosis (MLL) and maximum thoracic kyphosis (MTK), respectively.
With all measurements, kyphosis is indicated by a positive value whereas lordosis is indicated by a negative value. The evaluation and interpretations of the distinct measurement techniques are described elsewhere [29, 35, 40, 49, 74, 90]. The intra- and inter-observer reliability of measurements are known and reviewed elsewhere [13].
Sagittal spinal curve assessment
To assess any alteration of the global sagittal curvature, the segmental Cobb angle (SCA), which is the sum of the vertebral wedging (VW) and the intervertebral angle (IVA) was measured from T10 to S1 according to Vialle et al. [90] on follow-up radiographs (Fig. 4). With the measurement of each SCA, sagittal curves for each patient were reconstructed and compared algebraically with a curve reconstructed from data of normals (including T10–S1) [90]: First of all, the percentage deviation of the SCA at each level (T10–S1) from the physiological standard [90] was calculated. Next, the percentage deviation of the global curve T10–S1 from the physiological standard was calculated (model 1). Then, the deviation of the global sagittal curves of the fractured spines from the physiological curve was calculated as a difference of the area under both curves (AUC; model 2). For both models, 1 and 2, the global curve deviation was calculated as a factor of fracture-level. Finally, for both models, 1 and 2, the global percentage deviation from normal was plotted against outcome parameters (RMDQ, LBOS, SF-36, VAS-Spine-Score). For the purpose of curve calculations, the SCA of S1 was defined as the angle formed by the endplate of S1 and the caudad of L5. The SS was defined as the start of the sagittal spinal curves.
To outline those levels contributing the most to spinal adjustments adjacent to the fracture-level, the percentage deviation of the IVAs from the physiological curve at each level was calculated for each patient. For a measurable difference, we assumed an IVA with at least 100% deviation from normalcy as significantly altered. With the graphical plotting of the data, an increase of a physiologically lordotic IVA or a decrease of a physiologically kyphotic IVA as compared to normalcy was denoted as a ‘negative’ percentage deviation. A decrease of a physiologically lordotic IVA or a reversal of a physiologically kyphotic IVA to a lordotic IVA was denoted as ‘positive’ percentage deviation.
Coronal plane assessment
Any scoliotic curve was documented at final follow-up. Spinal balance in coronal plane was assessed dropping a vertical plumb line from the center of the C7 vertebra to the sacrum (C7-SPL). The reference point used inferiorly was the mid-sagittal line of S1. Deviation of the C7-SPL to the right and left off the coronal axis was indicated by a positive and negative value, respectively [49]. Coronal imbalance was assigned if the C7-SPL was ≥3 cm [54].
Clinical outcome measures
For correlative analysis of spinal parameters and fracture patterns with clinical outcome, standardized self-assessment measures were used: With the Greenough low back outcome scale (LBOS), a maximum score of 75 can be yielded [73]. An excellent result is assigned for 65–75 points, good for 50–64 points, fair for 30–49 points, and poor for 0–29 points [32]. Restrictions in daily activities were measured using the Roland Morris Disability Spine Questionnaire (RMDQ). Scores can vary between 0 and 24; a lower score indicating less impairment [73]. The Short-form 36 questionary, version 2 (SF-36-v2) was used to assess restrictions in participation/quality of life. The SF-36 scale contains nine sub-scales measuring physical functioning. Results can be expressed as total sum or grouped as physical and mental component summary (PCS and MCS). Total scores can vary from 0 to 100; higher scores indicate better results and good quality of life. The patients’ subjective state and back function were ascertained using a visual analogue scale specific to the spine (VAS-Spine-Score) [46]. The scale contains 19 questions on back pain and function limitation of the spine or back. The total score is between 0 and 100. Higher scores indicate better results. All outcome measures applied have been validated [25, 46, 73, 96]. Finally, the Denis work (W1-5) and pain (P1-5) scales were used for global outcome assessment [22]. The Oswestry disability index (ODI) was submitted, but several patients were not comfortable with the questionnaire and did not complete it. Thus, the ODI could not be included in the analysis.
Follow-up cohort
Following the interobserver assessment 32 patients were invited for follow-up. In all, three patients had died due to general diseases. Although extensive efforts were made to track patients, four patients living abroad were lost to follow-up. Another patient had survived a suicide trial 5 years after index treatment and was excluded afterwards. One patient submitted the sent questionnaires, but rejected radiological follow-up. The patient suffered an A3.3.2 fracture of L1 with a LSS of 8 and poor 12-year outcome (RMDQ = 7, VAS-Spine-Score = 46, LBOS = 30). Another patient with a 10-year follow-up after an A3.1.1 fracture of T12 (LSS = 4) showed good clinical outcome. She declined follow-up and answering questionnaires. One 28-year old patient with an A3.2.1 fracture of L1 (LSS = 7) had moderate outcome and declined follow-up. The patient had open abdominal surgery for paralytic ileus while in the cast. Finally, one 65-year-old patient with T12 fracture (A3.2.1, LSC not applicable) showed up for an 8-year follow-up. But the patient depicted a collapsing spine with a significant PKD, adjacent osteoporotic fractures in T6 to L4 causing severe pain and positive sagittal imbalance. Due to the underlying osteoporosis causing the global deformity, the patient was excluded from the follow-up sample. At all, 87.5% of patients were tracked successfully; follow-up rate was 70.4% that is 21 patients.
Statistical analysis
Statistical analyses included along with descriptive statistics, parametric methods (independent two-sided Student’s t-test, Pearson’s correlation coefficient) as well as nonparametric tests (Wald–Wolfowitz test, Mann–Whitney U-test, Spearman’s correlations coefficient). A P-value less than 5% indicated a statistical significant result. All analyses were done using SPSS 11.0 (SPSS for Windows, SPSS Inc., Chicago), Statistica 6.1 (StatSoft, Tulsa) and StatXact (Cytel Software, Cambridge). For the purpose of separated reporting of TLB and LB fractures, statistical analysis was repeated for the main measurements in TLB (group 2, n = 16) and LB fractures (group 3, n = 5). The complete sample (n = 21) was defined as group 1.
Results
The patients’ main characteristics are summarized in Tables 2, 3, 4, 5, 6, 7. Case examples are with Figs. 5, 6, 7, 8.
Table 2.
Main clinical and radiographic characteristics of 21 patients with thoracolumbar and lumbar burst fractures
| No | Sex | Level | AgeFU | FU (mo) | AO-class | LSC | FU RKA | FU SKA | RMDQ | LBOS | VAS | SF-36 PCS | SF-36 MCS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | f | T12 | 27 | 102 | A3.1.2 | 5 | 17.5 | 12.5 | 1 | 47 | 63 | 47.9 | 46.7 |
| 2 | m | T12 | 65 | 122 | A3.3.1 | 6 | 15.2 | 12 | 14 | 22 | 49 | 35.8 | 26.9 |
| 3 | m | T12 | 65 | 81 | A3.2.1 | 7 | 14.8 | 16.9 | 3 | 66 | 77 | 54.1 | 52.7 |
| 4 | m | T12 | 28 | 93 | A3.2.1 | 4 | 9.1 | 7.9 | 0 | 62 | 76 | 47.4 | 58.7 |
| 5 | m | T12 | 25 | 75 | A3.1.1 | 3 | 3.5 | 4.2 | 0 | 65 | 95 | 55.8 | 53.3 |
| 6 | f | L1 | 65 | 201 | A3.3.3 | 4 | 14.1 | 18.1a | 0 | 65 | 94 | 52.4 | 57.4 |
| 7 | m | L1 | 49 | 204 | A3.1.1 | 6 | 24.2 | 18.8 | 0 | 57 | 73 | 43.5 | 55.5 |
| 8 | m | L1 | 47 | 114 | A3.1.1 | 4 | 13.3 | 16.6 | 0 | 60 | 75 | 43.9 | 61.6 |
| 9 | m | L1 | 52 | 72 | A3.1.1 | 5 | 6.7 | 14.1 | 4 | 41 | 53 | 29.8 | 42.2 |
| 10 | f | L1 | 62 | 85 | A3.1.1 | N/a | 3.4 | 12.8 | 3 | 22 | 64 | 47.9 | 46.7 |
| 11 | m | L1 | 37 | 175 | A3.1.1 | N/a | 0.7 | 6.4 | 0 | 62 | 86 | 50.5 | 60.7 |
| 12 | m | L1 | 65 | 60 | A3.2.1 | 6 | 9.3 | 15.5 | 6 | 26 | 64 | 38.9 | 41.4 |
| 13 | f | L1 | 34 | 89 | A3.1.1 | N/a | 6.5 | 9.5 | 2 | 54 | 89 | 56.3 | 54.2 |
| 14 | m | L1 | 54 | 89 | A3.2.1 | 7 | 6.1 | 12.9 | 1 | 61 | 74 | 56.6 | 57.7 |
| 15 | m | L2 | 69 | 161 | A3.1.2 | N/a | 5.7 | 5 | 1 | 49 | 83 | 48.6 | 57.0 |
| 16 | m | L2 | 71 | 192 | A3.2.1 | 6 | −4.6 | 6.3 | 3 | 44 | 89 | 48.3 | 30.9 |
| 17 | f | L3 | 54 | 75 | A3.1.1 | 4 | 1.8 | 6 | 1 | 67 | 71 | 53.2 | 56.4 |
| 18 | m | L3 | 39 | 130 | A3.2.2 | 6 | −10.3 | 2.7a | 2 | 54 | 79 | 54.2 | 44.7 |
| 19 | m | L4 | 32 | 69 | A3.2.1 | 6 | −10 | 2.4a | 19 | 10 | 42 | 20.5 | 29.6 |
| 20 | f | L4 | 30 | 118 | A3.1.1 | 6 | −6.5 | 9 | 0 | 72 | 94 | 55.1 | 56.4 |
| 21 | m | L4 | 62 | 62 | A3.2.1 | 7 | −22.1 | 4.3 | 8 | 33 | 69 | 47.9 | 46.7 |
| Mean | 49.1 | 112.8 | 5.4 | 4.7 | 12.1 | 3.2 | 49.5 | 74 | 47.1 | 49.4 | |||
| SD | 15.7 | 47 | 1.2 | 10.9 | 6.3 | 5.0 | 17.6 | 15 | 9.3 | 10.3 | |||
| Median | 1 | 54 | 75 | 48.3 | 53.3 | ||||||||
| 25–75% quartile | 0–3 | 41–62 | 64–84 | 43.9–54.1 | 44.7–57 |
N/a LSC not applicable because CT-scans not available
aMarked SKA values were not included in statistical analysis (radiographic parameters vs outcome measures)
Table 3.
Radiographic spinal and spinopelvic parameters and comparison to data in literature
| TKT4–T12 | T10–L2 | T12–S1 TLSL | LLL1–L5 | LSLL1–S1 | PI | SL | PT | SB T4–S1 | SB T4–L4 | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current, group 1 | 33.7 ± 13.4 | 5.0 ± 10.3 | −57.6 ± 9.4 | −39.6 ± 9.2 | −51.4 ± 10.9 | 52.2 ± 9.7 | 38.6 ± 7.2 | 18.3 ± 6.9 | −23.5 ± 29.1 | −48.5 ± 31.8 |
| (T11–L5, n = 21) | (9.5–54.4) | (−12.5 to 27.5) | (−44.4 to −77.6) | (−24.1 to 51.7) | (−26.4 to −69.10) | (39.1−75.5) | (24.3−53.5) | (4.8−34.60) | (54 to −67.8) | (26.2 to −90.4) |
| Current, group 2 | 34.9 ± 14.9 | 7.6 ± 7.3 | −59.5 ± 9.1 | −41.5 ± 9.2 | −54.0 ± 12.3 | 59.0 ± 11.7 | 39.9 ± 7.5 | 18.3 ± 7.2 | −24.7 ± 24.8 | −47.5 ± 30.2 |
| (T11–L2, n = 16) | (9.5–54.4) | (−3.6 to 27.5) | (−45.2 to −74.1) | (−24.5 to −51.7) | (−26.4 to 69.1) | (39.1–75.5) | (24.3–53.5) | (4.8–34.6) | (16 to −67.8) | (26.2 to −90.4) |
| Current, group 3 | 35.1 ± 15.1 | −7.8 ± 4 | −55.1 ± 13.5 | −29.3 ± 4.6 | −42.0 ± 5.2 | 52.4 ± 5.8 | 34.7 ± 4.7 | 18.1 ± 2.7 | −5.4 ± 38 | −32.5 ± 38.5 |
| (L3–5, n = 5) | (16.2–50.8) | (−2.7 to −12.5) | (−44.4 to −77.6) | (−24.1 to −35.9) | (−36.1 to −48.2) | (44.1–57.7) | (28.3–40.5) | (14.9–21.9) | (54 to −40.5) | (24.6 to −69.7) |
| Jackson et al. [35] | 38.5 ± 12 | 6.3 ± 7.9 | −62.5 ± 12.0 | −31.3 ± 23.0 | −59.5 ± 21.8 | |||||
| Roussouly et al. [74] | – | – | – | – | – | 51.9 ± 10.7 | 39.9 ± 8.2 | 12.0 ± 6.5 | – | – |
| Kuntz et al. [49]a | 41 ± 11 | 9 ± 7 | −62 ± 11 | −44 ± 11 | – | 54 ± 10 | 41 ± 8 | 13 ± 6 | – | – |
| Boulay et al. [13] | 53.8 ± 10.1 | 53.1 ± 9.0 | 41.2 ± 7.0 | 12.0 ± 6.4 | ||||||
| Vialle et al. [91] | 40.6 ± 10 | −43 ± 11.2 | 54.7 ± 10.6 | 41.2. ± 8.4 | 13.2 ± 6.1 |
aData derived from literature review
Table 4.
Radiographic spino-pelvic parameters according to Jackson et al. [35]
| PR–T12 | PR–L2 | PR–L4 | PR–L5 | PR–S1 | |
|---|---|---|---|---|---|
| Current, group 1 | −84.0 ± 7.8 | −81.2 ± 9.2 | −66.6 ± 6.0 | −51.8 ± 9.2 | −29 ± 9.1 |
| (T11–L5, n = 21) | (−73.8 to −101.5) | (−66.4 to −98.9) | (−55.4 to −77.2) | (−33.3 to −55.5) | (−12.7 to −44.5) |
| Current, group 2, | −85.2 ± 9.2 | −83.4 ± 8.8 | −66.8 ± 7.3 | −50.0 ± 8.8 | −26.9 ± 10.5 |
| (T11–L2, n = 16) | (−73.8 to −101.5) | (−71.3 to −98.9) | (−55.4 to −77.2) | (−33.3 to −63.3) | (−12.7 to −44.5) |
| Current, group 3 | −82.9 ± 6 | −70.2 ± 3.7 | −66.2 ± 3−5 | −57.9 ± 7.2 | −35.9 ± 4.4 |
| (L3–5, n = 5) | (−24.1 to −35.9) | (−66.4 to 75.0) | (−62.1 to 71.6) | (−46 to −63.5) | (−31.5 to −41) |
| Jackson et al. [35] | −92.2 ± 9.7 | −88.8 ± 8.5 | −71.2 ± 9.7 | −55.2 ± 10.4 | 32.3 ± 9.8 |
Table 5.
Results of the SF-36-v2
| SF-36 subscales | Current study | Controls (from [15]) |
|---|---|---|
| Physical functioning | 74.5 | 89.0 |
| Social functioning | 87.5 | 89.4 |
| Role physical | 67.9 | 87.5 |
| Role-emotional | 79.8 | 91.5 |
| Mental health | 71.9 | 73.7 |
| Vitality | 61.0 | 64.1 |
| Bodily Pain | 67.9 | 78.9 |
| General health | 67.9 | 68.0 |
Table 6.
Results of clinical outcome measures
| RMDQ | LBOS | VAS-Spine-Score | Denis P | Denis W | SF-36 MCS | SF-36 PCS | |
|---|---|---|---|---|---|---|---|
| Current, group 1 | 3.2 ± 5.0 | 49.5 ± 17.6 | 74.3 ± 14.9 | 2.7 ± 1.0 | 2.3 ± 0.8 | 49.4 ± 10.4 | 47.0 ± 9.3 |
| (T11–L5, n = 21) | (0–19) | (10–72) | (42.4–95.3) | (2–5) | (1–5) | (26.9–61.6) | (20.5–56.6) |
| Current, group 2, | 2.4 ± 3.6 | 50.2 ± 15.4 | 75.2 ± 14.0 | 2.6 ± 0.8 | 2.3 ± 0.8 | 50.2 ± 10.4 | 47.4 ± 7.6 |
| (T11–L2, n = 16) | (0–14) | (22–66) | (48.5–95.3) | (2–5) | (1–4) | (26.9–61.6) | (29.8–56.57) |
| Current, group 3 | 6.0 ± 7.9 | 47.2 ± 25.7 | 71.2 ± 18.9 | 3 ± 1.4 | 2.5 ± 1 | 46.8 ± 11.0 | 46.2 ± 14.6 |
| (L3–5, n = 5) | (0–19) | (10–72) | (42.4–94.4) | (2–5) | (2–4) | (29.6–56.4) | (20.5–55.13) |
Table 7.
Results of segmental Cobb angle measurements T9–S1 for curve calculations
| SCAT9 | SCAT10 | SCAT11 | SCAT12 | SCAL1 | SCAL2 | SCAL3 | SCAL4 | SCAL5 | SCAS1a | |
|---|---|---|---|---|---|---|---|---|---|---|
| Current, n = 21, | 2.9 ± 2.9 | 1.2 ± 2.9 | 1.9 ± 3.3 | 1.9 ± 5.8 | 3.8 ± 8.7 | −6.2 ± 4.9 | −7.9 ± 5.6 | −11.3 ± 8.2 | −20.0 ± 6.0 | −13.6 ± 6−5 |
| T10–L5 | (8.1 to −2.2) | (6.2 to −4.7) | (9.7 to −3.3) | (16.9 to −3.4) | (18.8 to −7.9) | (6.3 to −12.6) | (6 to −15.2) | (9 to −22.4) | (−6.3 to −32.4) | (−1.8 to −28.5) |
| Current, n = 16, | 2.7 ± 3.0 | 1.3 ± 3.0 | 2.1 ± 2.8 | 2.5 ± 6.5 | 6.0 ± 8.9 | −5.2 ± 5.1 | −9.2 ± 4.0 | −14.2 ± 4.5 | −20.4 ± 5.4 | −14.6 ± 6.3 |
| T10–L2 | (8.1 to −2.2) | (6.2 to −4.7) | (7 to −1.8) | (16.9 to −3.4) | (18.8 to −7.9) | (6.3 to −11.1) | (−3.5 to −15.2) | (−7.8 to −22.4) | (−10.8 to −32.4) | (−1.8 to −28.5) |
| Current, n = 5, | 3.7 ± 2.5 | 0.7 ± 2.9 | 1.3 ± 4.9 | −0.16 ± 2.3 | −3.0 ± 2.2 | −9.5 ± 2.3 | −3.5 ± 8.1 | −1.9 ± 10.6 | −18.5 ± 8.3 | −10.9 ± 6.7 |
| L3–L5 | (6.1 to −0.2) | (5.6 to −2.4) | (9.7 to −3.3) | (2.5 to −2.6) | (0.5 to −5) | (−7.3 to −12.6) | (6 to −14.1) | (9 to −17.1) | (−6.3 to −28) | (−1.8 to −17.4) |
| Vialle [91] | _ | 5.7 | 7 | 7.6 | 5.1 | −0.45 | −7.2 | −13 | −24.6 | −15.8 |
SCA Segmental Cobb angle
aCephalad endplate of S1 vertebral body to caudad endplate of L5 vertebral body
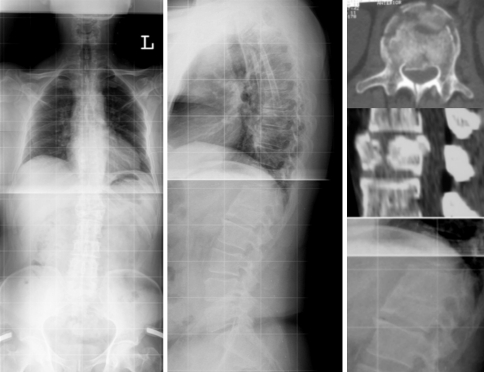
Fig. 5.
Case 14. A3.2.1 fracture of L1. 54-Year-old patient at 7.5 years follow-up showed sagittal balance with synostois of T12–L1 and adjacent-level gapping at L1–2 and T11–12. Patient complained on increasing pain during daily activities, particularly lifting issues, with the onset 3-years prior to follow-up
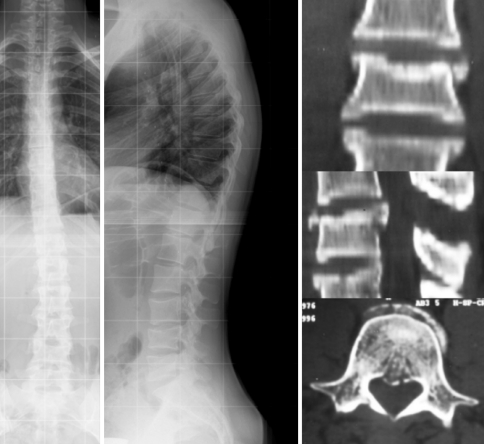
Fig. 6.
Case 20. 28-Year old patient at 10-year follow-up following A3.1.1 fracture (LSS = 6) showed balanced spine with lumbar kyphosis but straightened thoracolumbar junction and lower thoracic spine. Clinical self-rated outcome was good, but patient had severe restriction with prolonged sitting and lifting issues
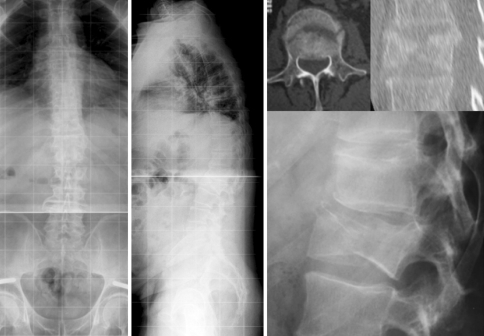
Fig. 7.
Case 17. AO 3.1.1 fracture (LSS = 4). At 6.3-years follow-up patient had good self-rated clinical outcome. Radiographs depicted a straightened but balanced spine and significant adjacent-level gapping
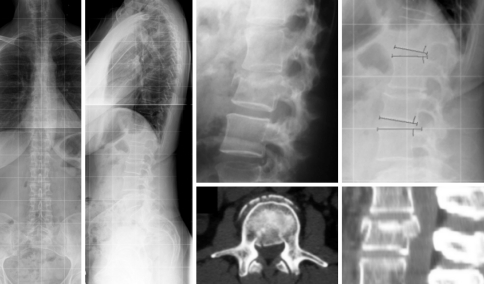
Fig. 8.
Case 12. A3.2.1 of L1 (LSS = 6). 65-year old patient at 5-year follow-up had moderate self-rated clinical outcome with increasing pain at the site of the kyphosis since 3-years after injury. Note, adjacent-level gapping at L1-2, but not at the degeneratively altered level T11-T12. Sagittal plumb line was displaced anteriorly to the S1 reference point
Patient sample (group 1)
Sample size was 21 including 6 female and 15 male patients. Mean age at index treatment was 39.6 ± 15.5 years (range 18–60 year) and 49.1 ± 15.6 years (range 25–71 year) at follow-up. Mean follow-up was 112.8 ± 47 months (range 60–204 months). That is, follow-up was 9.5 years on average with the longest lasting 17 years. Mean time of hospital stay was 12.5 ± 10.1 days (range 1–40 days). Patients with extended hospital stay had concomitant minor intracranial hemorrhage without sequelae, and foot, ankle or calf fractures treated operatively but not affecting lower extremity function at follow-up. Eight patients (38.1%) had injuries other than the spine. The most frequent injury mechanism was motor vehicle accident in nine patients (42.9%) and a fall from varying heights during hiking or doing the chores, in ten patients (47.6%). One patient crashed during paragliding and another was hit by a rock (9.6%).
Radiographic results (group 1)
Fracture morphology
Within the thoracolumbar spine (T11–L2) nine fractures occured at the L1 level, five at T12, and 2 at L2. In the lumbar spine (L3–5) there were two fractures at L3 and 3 at L4. AO type A3.1 fractures accounted for 52.4% (n = 11) of all fractures, type A3.2 for 38.1% (n = 8), and type A3.3 for 9.5% (n = 2). Subtypes are listed within Table 2. The mean LSS that was assessable in 17 patients (81% with CT-scans) was 5.4 ± 1.2 points (range 3–7 points). Mean injury RKA in all fractures was 4.0° ± 9.2 (range −17 to 18°). In type A3.1 fracture injury SKA was 9.6° ± 5.4 (range 2–18°). RKA increased until follow-up to 4.7° ± 10.9 on average (range −22.1 to 24.2°) as did the SKA with 12.8° ± 5.8 (range 4.9–25.4°). Loss of alignment either assessed with the RKA (A3.2 and A3.3 fractures) or SKA (A3.1 fractures) was 2.3° ± 8.3 on average (range −10.3 to 23.4°). Overall changes and therein merely a loss of alignment between injury and follow-up was not statistically significant for both RKA and SKA. However, statistical analysis revealed a significant inverse correlation between injury RKA and injury SKA and the increase of kyphosis for each individual (P = 0.04, r = −0.5; P = 0.04, r = −0.6). Statistically there was no significant correlation between follow-up RKA and SKA and the LSS or AO-subtypes. Concerning outcome measures, the injury LSS showed significant impact (P = 0.02, r = 0.55) on outcome in terms of increased RMDQ scores with higher LSS.
Spinal parameters
The SKA was used on injury radiographs for A3.1 fractures. In follow-up radiographs, anatomical landmarks indicated for the SKA could be safely refined in most patients (n = 18, 86% of patients). Follow-up SKA in all 21 patients with AO type A3.1 to 3.3 fractures was 12.1° ± 6.3 on average (range 2.4–18.8°). Except for the three individuals in whom bony landmarks were judged difficult to assess reliably because of endplate alterations or spondylosis (Table 2), the remaining SKAs were used for correlative analysis with outcome measures.
Statistical analysis revealed that the LL and LSL showed significant correlation with follow-up RKA (P = 0.02, r = −0.49; P = 0.002, r = −0.63) and the follow-up SKA was correlative with the LSL (P = 0.04, r = −0.49). In between the spinal parameters, the LL showed significant correlation with the LSL (P = 0.005, r = 0.59), whilst both the LL and the LSL were significantly correlated with the TLA (P = 0.02, r = −0.52; P = 0.02, r = −0.49). Increased kyphotic alignment at and adjacent to the injury levels in terms of the TLA caused compensatoric adaptions and increase of lumbar and lumbosacral lordosis.
Significant correlation existed between the SB T4-S1 and the follow-up RKA (P = 0.016, r = −0.52). That is, with increasing kyphotic RKA, the vertical axis shifted anteriorly. Except for four patients with a positive sagittal plumb line, it was negative. Altogether, three patients showed a negative sagittal imbalance and 1 a positive (≥5 cm).
The transitional vertebra noted was T12 in 42.9%, L1 in 23.8%, T10 and T11 each twice (9.5%), and L2 and T8 each once (4.8%). The associated MTK was 40.7° ± 14.9 on average (range 12.9–70.1°). It strongly correlated (P < 0.001, r = −0.8) with the MLL, that had a mean of −61.2° ± 9.6 (range −42.1 to −77.1°).
Coronal plane assessment showed a mean injury coronal Cobb angle of 2.0° ± 1.7 (range 0–6.9°) and 3.9° ± 3.0 (range 0.3–10.4°) at follow-up. The difference yielded significance (P = 0.005). Adjacent to the slight deformities in coronal plane, compensatory thoracolumbar or lumbar curves were observed in six patients (28.5%). Coronal trunk shift was −3.0 ± 14.0 mm on average (range −28.9 to 29.9 mm). No patient depicted a coronal balance ≥3 cm.
Spinopelvic parameters
The mean, SD, and ranges are illustrated in Tables 3 and 4. Main results of the correlative analysis are ranked in Table 8. As concerns pelvic parameters, strong correlations existed between the PI, PT, and SS, and the regional lumbopelvic lordosis at L4 (PR–L4). Of note, no patient had a fracture of L5. So, the geometric analysis of burst fractures echoed the physiological spatial interplay between the spinal and pelvic parameters as seen in normal subjects. Regarding spinopelvic interdependency, there was a substantial correlation between the lumbosacral lordosis and the SS, between the PT and the regional lumbopelvic lordosis at L2 and at T12 (PR–L2 and PR–T12). Both the lumbar and lumbosacral lordosis correlated with the PI and PR–S1, and the PR–L2 was stronlgy correlative with the TLA. The results reveal the distinct relationship between the spinal parameters (LL, LSL, PR–L2, PR–T12) and the pelvic parameters (PI, PT, SS, PR–S1) in which the regional lumbopelvic lordosis (PR–L2) can adjust for an increased kyphosis at the thoracolumbar junction T10-L2 (TLA measurement) following the burst fracture. Besides, the PR–T12 showed significant correlation with follow-up length (P = 0.04, r = 0.46; P = 0.02, r = 0.51).
Table 8.
Results of statistical analysis of spinopelvic parameters and geometric interplay for all patients (group 1) and for thoracolumbar fractures (group 2)
| group 1 (TLB and LB fractures, n = 21) | group 2 (TLB fractures, n = 16) | ||||
|---|---|---|---|---|---|
| Variables | Correlation coefficient | Level of statistical significance | Variables | Correlation coefficient | Level of statistical significance |
| PR–S1 and PI | r = 0.95 | P < 0.001 | PR–S1–PR–L4 | r = 0.82 | P = 0.001 |
| PT and PR–L4 | r = 0.77 | P < 0.001 | PR–L2 and PT | r = 0.69 | P = 0.013 |
| PI and PR–L4 | r = 0.73 | P < 0.001 | PI and SS | r = 0.62 | P = 0.01 |
| PI and SS | r = 0.67 | P = 0.001 | PR–L2 and PR–S1 | r = 0.62 | P = 0.033 |
| PI and PT | r = 0.65 | P = 0.001 | PR–L2 and PI | r = 0.62 | P = 0.031 |
| PR–S1–PR–L4 | r = 0.65 | P = 0.002 | PT–PR–T12 | r = 0.58 | P = 0.05 |
| TLA and PR–L2 | r = −0.62 | P = 0.003 | PI and LSL | r = −0.51 | P = 0.042 |
| LSL and SS | r = −0.58 | P = 0.006 | LSL and SS | r = −0.51 | P = 0.041 |
| PT–PR–T12 | r = 0.58 | P = 0.006 | TLSLT12–S1 and SS | r = −0.50 | P = 0.049 |
| PR–S1 and LL | r = −0.53 | P = 0.013 | |||
| LSL–PR–T12 | r = 0.49 | P = 0.026 | |||
| PT and PR–L2 | r = 0.48 | P = 0.03 | |||
| LL and SS | r = −0.44 | P = 0.045 | |||
| PR-S1 and LSL | r = 0.44 | P = 0.049 | |||
| PI and PR-T12 | r = 0.43 | P = 0.05 | |||
| PI and LL | r = −0.42 | P = 0.05 | |||
Outcome results (group 1)
Mean, SD, and ranges of outcome measures are listed in Tables 2, 5, and 6 for groups 1–3. In terms of the LBOS [32] clinical outcome was excellent in five patients, good in seven patients, fair in five patients, and poor in four patients. Four patients (19%) were did not have previous employment and one patient was unable to work at all.
If follow-up radiographic parameters were plotted against outcome measures, the statistical analysis revealed a significant correlation between higher SKAs at follow-up and decreased VAS-spine-scores (P = 0.03, r = −0.52) as well as decreased SF-36 PCS (P = 0.02, r = −0.53). In addition, PR–T12 significantly correlated with the LBOS (P = 0.03, r = −0.47). A more positive sagittal balance in terms of SB T4–S1 was correlated with a worse outcome in terms of increased RMDQ values (P = 0.044, r = 0.44) and decreased values for the SF-36 MCS (P = 0.01, r = −0.54). Similarly, SB T4-L4 showed significant correlation with the RMDQ (r = 0.46; P = 0.03) and the LBOS (P = 0.01, r = −0.52) as well as the SF-36 MCS (P = 0.008, r = −0.56). Significant correlation existed also between increasing TK and the SF-36 subscales for PCS (P = 0.03, r = −0.47). There were no associations between the outcome and the spinopelvic parameters (PT, SS, and PI).
Patients with TLB and LB fractures of AO type 3.1 were compared to those with AO type 3.2 and 3.3 regarding clinical outcome. In addition, patients with a LSS of <6 points were compared to those with a LSS of ≥6. Statistical calculations showed a tendency (24% difference in outcome measures) towards a decreased outome with an LSS of ≥6. But, nonparametric tests (Wald–Wolfowitz, Mann–Whitney U-test, Kolmogoroff–Smirnov) with a sample size of 17 patients (with CT-scans available) were not sufficient to detect significant differences between groups.
Radiographic results (groups 2 and 3)
Spinal parameters
The mean RKA and SKA in group 2 was 9.1° ± 7.1 (range −4.6 to 24.2°) and 13.9° ± 5.6 (range 4.9−25.4), respectively. In group 3, same measurements were −6.6° ± 9.1 (range −22° to 5.7°) and 5.1° ± 2.3 (range 2.4–9°), respectively. As mentioned, one SKA measurement (Case 12) in group 2, out of the three SKA measurements not at all judged to be reproducibly measured, was not included in statistical analysis, although exclusion of that case did not alter mean and SD significantly (mean SKA of 14.2° if ‘case 12’ was excluded). Radiographic characteristics of group 2 and 3 are listed in Tables 3 and 6. The follow-up RKA was significantly different (P < 0.001) with a mean of 9.1° in group 2 and −9.4° in group 3. Measurement with the RKA included two levels and 9° lordosis within the lumbar spine at L3–5 actually represents lumbar kyphosis. Significant differences between group 2 and 3 also existed for LL (P = 0.03; −42.2° vs −29.3°), LSL (P = 0.02; −54.3° vs −41.9°) and for the PR-L2 (P = 0.001; −84.5° vs −70.7°). In group 2, the thoracolumbar junction T10–L2 was kyphotic (mean TAL: 7.6°) whilst it was straightened in group 3 (mean TAL: −7.8°). The difference yielded significance (P < 0.001). As in group 1, the PR-T12 and, in addition, the PR-L2 showed significant correlation with follow-up (P = 0.04, r = 0.61; P = 0.02, r = 0.67).
Concerning the spinopelvic parameters of group 2 including 16 TLB fractures, statistically significant findings remained and the correlative analysis confirmed the interdependency in between the pelvic parameters (PI, SS, PT, PR–S1) and between the spinal and pelvic geometry (PR-T12, PR-L2, LL, LSL, TLSL) (Table 8). In group 2, the LSL and TLSL T12–S1 were correlative with the SS, and the PR-L2 showed strong correlation with the PR–S1 resembling the lumbopelvic lordotic adjustments.
The overall number of LB fractures was small (n = 5). Statistical analysis revealed no further significant findings except for the follow-up RKA, which showed a strong inverse correlation with the PR-L2 (P = 0.04, r = −0.9). That is, with increased, focal kyphotic posture due to the burst fractures at L2–4 there was loss of lumbopelvic lordosis in terms of a dereased PR–L2. In turn, the thoracic kyphosis was strongly correlative with the LL (P = 0.003, r = 0.98) and overall hypokyphotic (mean TK 35.3°), compensating for the lack of LL.
Outcome results (groups 2 and 3)
In terms of the LBOS, outcome in group 2 was excellent in three patients, good in six, fair in four, and poor in three patients. Statistical analysis for group 2 revealed a strong correlation between the follow-up RKA and SKA and the VAS-Spine-Scores (P = 0.01, r = −0.69; P = 0.0016, r = −0.8). The correlation between SKA and the clinical outcome measures was obviously stronger in the TLB fractures as compared to when analyzing all T11-L4 fractures (group 2: P = 0.0016/r = −0.8 vs group 1: P = 0.03/r = −0.5), which can be referred to the overall lower mean SKA in LB fractures as compared to the TLB fractures as well as to an overall decreased clinical outcome in the LB fractures (Table 6). In addition to final kyphosis, there was also significant correlation between the increase of kyphosis as assessed on injury and follow-up radiographs and the RMDQ (P = 0.03, r = 0.62). Increased kyphosis at the thoracolumbar junction T10–L2 (TAL) was also strongly correlated with lower VAS-Spine-Scores (P = 0.009, r = −0.71) and with a decreased PR-T12 there were significantly decreased LBOS values (P = 0.03, r = −0.62). With an increased positive sagittal balance in terms of SB T4-L4, the LBOS (P = 0.025, r = −0.64) and SF-36 MCS (P = 0.017, r = −0.67) decreased. On the other hand, increased TK T4–T12 was significantly related to higher RMDQ values (P = 0.04, r = 0.59) and lower VAS-Spine-Scores (P = 0.04, r = −0.59).
In group 3, outcome was excellent in two patients, good, fair and poor in each one patient according to the LBOS. Statistical analysis revealed no significant differences in outcome measures although means were smaller in group 3 compared to group 2. Further statistical analysis of group 3 did not show significant interdependencies.
Segmental and global curve analysis (group 1)
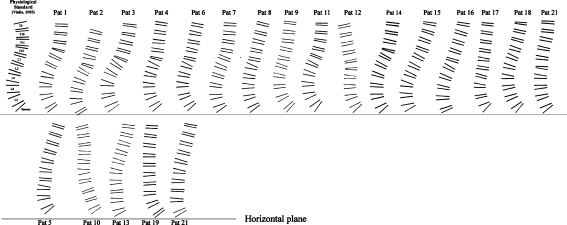
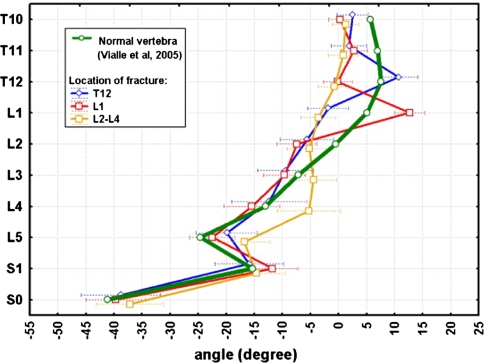
The mean, SD, and ranges of the SCA, which were used to reconstruct curves of the fractured spines for each patient, are listed in Table 7. The curves are illustrated in Fig. 9, giving an impression of the global deviation from the physiological standard. An example of a spinal curve reconstructed from the percentage deviations of the IVA at each spinal level was plotted graphically against the physiological standard of Vialle [90] (Graph 1). Visualization of the percentage deviation of the IVAs T10–S1 from normal was done by grouping T12, L1 and L2-L4 fractures (Graph 2). The plotted percentage deviations demonstrate that the compensatoric adjacent-level adjustments, that is lordosizing, was stressed at the caudad two and cephalad adjacent-level in T12 (Graph 3a) and L1 fractures (Graph 3b), respectively. Cephalad to the kyphosis, the compensatoric adjustments were more widely distributed including two to three adjacent-levels. With L2–L4 fractures (Graph 3c), significant adjustments occurred, albeit exclusively at the cephalad-adjacent levels to the fracture.
Fig. 9.
Drawings of spinal curves of all patients with TLB (upper row) and LB (lower row) fractures. Segmental data derived from vertebral wedging and intervertebral angulation summing the segmental cobb angle (SCA) from S1 to T9. Sacral slope denotes start of lumbosacral curve. First curve upper row resembles the spinal curve T9–S1 as reconstructed using data of normals [92]
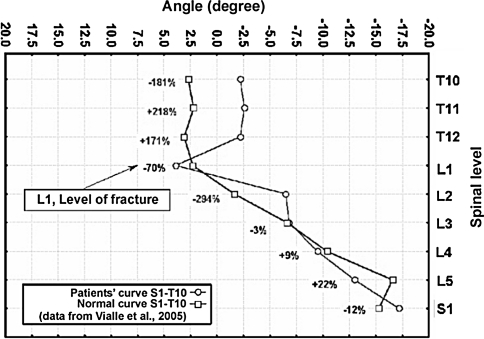
Graph 1.
Example of plotting a patient’s spinal curve (case 13) as percentage deviation of the IVA at each level against that of normals [92]
Graph 2.
Patients’ spinal curves are plotted as a factor of fracture level (blue line indicates T12-fractures, red line indicates L1-fractures, yellow-line indicates L2–4 fractures). The curves are reconstructed from the patients’ SCA S1–T10l and plotted against that of normals (green-line) [92]
Graph 3.
a–c Patients curves plotted as percentage deviation of the IVA at each level against the physiological standard (green line resembles baseline and data of normals, respectively, derived from Vialle et al. [92]). a displays curves of patients with fractures at the T12-level, b L1-level, and c L2-L4 levels
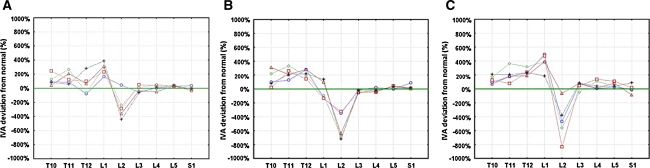
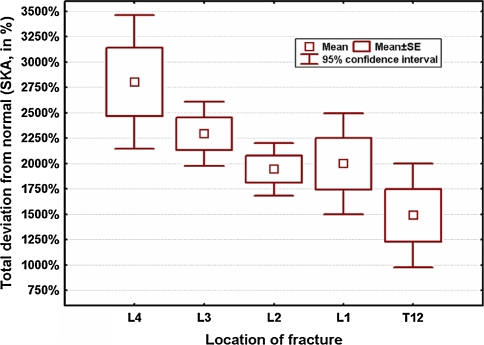
Curve analysis revealed that total percentage deviation of the fracture curves T10–S1 was significant for model 1 (P = 0.01) and model 2 (P = 0.001) comparing the fracture curves with the physiological standard. Maximum deviation from the physiological curve occurred if L4 was the fracture level, either calculated for model 1 (P = 0.0001), or model 2 (P < 0.00001, Graph 4).
Graph 4.
Global percentage deviation of the patients’ curves from the physiological standard with the percentage deviation as a factor of fracture-level. If L4 was the fracture level, curve alteration was the most
Using data of model 1 and 2 we plotted the fracture curves as well as the segmental percentage deviations in terms of the SCAs or IVAs against clinical outcome parameters. Summarizing, the algebraic analysis echoed the clinical observation that those patients showing spinal compensations adjacent to the fracture level did better, expressed in terms of the validated outcome measures applied, e.g., in L1-fractures there was significant correlation between the LBOS and the percentage deviation of the IVA and SCA of S1 (both P = 0.04, r = 0.7) and between the SF-36 PCS and the IVA of L3 and L4. Though of interest, we judged sample size too small and the extensive results preliminary for further exploration.
Follow-up and age (groups 1–3)
For group 1, the length of follow-up showed a significant inverse correlation with the RMDQ (P = 0.01, r = −0.53), Denis pain scale (P = 0.01, r = −0.54), and VAS-Spine-Scores (P = 0.03, r = 0.47), whereas age did not. In contrast, in group 2, age at follow-up was strongly related with higher RMDQ values (P = 0.02, r = 0.67), with a tendency also for the LBOS and RMDQ. If calculated only for the TLB fractures (group 2), length of follow-up showed no significant impact on functional outcome measures. Differences between the groups are put into perspective regarding the sample characteristics of group 1 and group 2 and 3 with a lower age at follow-up of those patients with LB fractures compared to that of TLB fractures (43.4 vs 50.9 years) and a shorter follow-up (mean 90.8 vs 119.6 months), but an overall decreased clinical outcome for LB fractures (Table 6).
Complications (group 1)
Most patients did well while casted. However, some complained on the 3-month casting at follow-up concerning discomfort, pain during the first 8 weeks of treatment and hygiene aspects. One patient suffered a pulmonar artery embolism while immobilized in the cast and underwent oral anticoagulative therapy without pulmonary long-term sequelae. Another patient with symptomatic lumbar PKD following burst fracture of L4 is scheduled for reconstructive surgery while this manuscript is under preparation.
Discussion
There is an increasing consensus that surgery for TLB and LB is indicated for patients with neurological deficit and/or three-column injuries [2, 9, 42, 64]. But treatment of other TLB and LB fractures remains part of considerable discussion. Therapeutical concepts include nonsurgical treatment for A1.2/A3.1 fractures with <15° of kyphosis [39, 64], anterior-only and combined surgery in A3.1 and 3.3 fractures, respectively [41], or if kyphosis ≥20° and/or anterior column collapse ≥50% [75]. The main reasons for continuing controversy refers to the fact that most published studies include heterogeneous groups of patients, fracture patterns, levels and numbers of fractures per patient, neurological function, treatment modalities, and outcome measures [2–4, 17, 31, 37, 39, 41, 48, 51, 53, 58–60, 62, 66–68, 70, 75, 77, 79, 83, 86, 89, 97–99]. Therefore, the current study emphasized a homogeneous group of patients with strict inclusion criteria and long-term follow-up, although this meant critical shortening of the sample size which was 21. Our sample sized compares well with previous, relatively homogeneous cohorts with a mean of 20 cases [2, 4, 14, 18, 21, 75, 79, 97]. The current study is unique in that it is the first long-term investigation of the global sagittal alignment following TLB and LB fractures managed non-operatively. The inclusion of validated outcome measures and distinct classifications to delineate fracture pattern extend the scope of findings to be discussed.
Fracture morphology
With the single use of the classification of Denis [61, 83, 86], Magerl [14, 53, 67, 71], and McCormack [19, 48] reporting of fracture pattern is limited. The single-usage of injury SKA/RKA as a decision making variable frequently fails, because supine position during morphological diagnostics can serve for reduction of the kyphosis with the real deformity masked [3, 20]. Accordingly, we observed the increase of kyphosis significantly pronounced in those burst fractures that had smaller injury RKA/SKA. That is, with higher injury RKA/SKA there was less increase of kyphosis until follow-up, and vice versa. Most TLB and LB fractures are of AO type A3.1.1 [4, 31, 55, 60, 62, 70, 71] with scarce information on related distinct outcome values [4, 79]. For detailed reporting on fracture pattern, the authors applied the AO-C addressing fracture morphology, the LSC addressing vertebral body comminution and characterization of the kyphosis by means of the RKA/SKA. With a mean LSS of 5.4 points the current sample represented mainly mild TLB and LB fractures. CT-based LSC was applicable in 81% of patients, being a known factor of long follow-up periods [39, 86]. Nonetheless, even with a small but homogenous sample we observed a significant correlation (P = 0.02) between increased LSS and decreased outcome in terms of the RMDQ (group 1). In contrast, the AO type showed no correlation with outcome. At times it can be difficult to distinguish AO types A1.2.1, A3.1.1, and B1.2.1 fractures because of nondisplaced posterior wall fragments or occult posterior ligamentous injury [12]. We assessed overall interobserver reliability and the kappa value, including subtype classification, was 0.55 resembling moderate agreement. The final AO-C based on interobserver agreement with a 5:1 majority-rule for definite selection of cases. Hence, included cases are deemed reliably selected.
Comparing SSPI and LSPI Altay [4, 31] showed that SSPI was preferable for A3.1/3.2 fractures and A3.3 fractures with a LSS <6 points at T12-L2. In turn, treatment with LSPI for A3.3 fractures was better than with SSPI concerning deformity prevention and clinical outcome. Parker et al. [65] reported success with SSPI and fusion for TLB fractures totaling a LSS of ≤6 points; patients who scored ≥7 points were supposed to do better if subjected to anterior column reconstruction. Findings concur with biomechanical data of Wang et al. [93]. Fractures that scored ≥7 points presented a significantly decreased stability than those with ≤6 points. In concurrence, Defino [21] showed correlation between an increased preoperative LSS and increased loss of correction using SSPI. Because a LSS of ≥6 points might be a numerical cut-off reflecting increased instability, statistical analysis was performed with grouping of fractures into those with LSS < 6points (&AO type A3.1) and those with LSS ≥ 6 (&AO type A3.2-A3.3). The analysis lacked significance due to statistical power, although a tendency existed. Nonetheless, the tabulated data will be valuable if pooled with the findings of upcoming studies.
Posttraumatic kyphosis and deformity
The TLB and LB fractures in our series were subjected to closed reduction and casting. Casting was judged useful for initial pain control; it allows for early mobilization, and after reduction allows for unloading of the posterior muscles with the cast supporting a lordotic posture. Nevertheless, because of the mechanical limits of external orthosis [6], collapse of the vertebra is not prevented [6, 77]. With and without reduction the loss does not differ significantly [70] averaging about 2–9° [62, 70, 71, 77]. It was 3.6° more compared to injury measures in our TLB fractures. The kyphotic progress refers to the collapse of the (mainly) upper disc and gradual settling of the burst vertebral body under physiologic loading [20, 21, 24, 64, 66, 71, 75, 79], irrespective of whether patients are treated nonsurgically or with SSPI/LSPI [2, 21, 24, 45, 48, 66, 79]. The amount of collapse refers to the index comminution of both vertebral body and disc [21]. Complete maintenance of spinal alignment is difficult [44]. For the nonsurgical, posterior-only, and anterior-only treatments, the authors found a mean final RKA of about 13° [2, 18, 27, 77, 79, 83, 86, 89, 97], 10° [14, 75, 77, 79, 89, 97, 98], and 8° [10, 44, 64, 89, 98], respectively. In this context it is striking that several authors failed to detect significant correlation between final kyphosis and outcome measures, regardless of surgical or nonsurgical treatment [2, 14, 18, 20, 48, 53, 62–64, 70, 75, 79, 97]. In contrast, we observed a strong correlation between decreased VAS-Spine-Scores, worse outcomes, and increased kyphosis in terms of follow-up SKA and RKA in group 2 (P = 0.01, r = −0.7; P = 0.002, r = −0.8) and follow-up SKA still in group 1 that included all fractures (P = 0.03, r = −0.5). Increased kyphosis (SKA) was also correlative with the SF-36 PCS in the larger group 1. Findings concur with some reports indicating correlations between pain and functional restrictions and a PKD exceeding 10° [24, 59], 20° [39, 85], 30° [16, 27, 87] or a sagittal index >15° [86]. Oner et al. [64] observed an association between the gradual increase of kyphosis and poor clinical outcome in nonsurgically treated patients. Rather, the increase in the kyphosis angle was found to be predictive of persistent pain than the final deformity. Similarly, we observed a significant correlation between the increase of kyphosis and higher RMDQ values. By causing a cleft in the anterior vertebra [41] after closed or posterior surgical reduction, additional loss of anterior stability and increased collapse may result. Overall, a PKD is a potential complication after spinal trauma as a result of malunion [1, 8, 11, 29, 30, 34, 51, 81]. In the worst case, it can be functionally limiting and be associated with severe pain [8, 10, 11, 29, 30, 51, 81]. Although burst fractures with posterior element involvement are more susceptible, even simple compression and burst fractures treated nonsurgically can cause a PKD necessitating surgical reconstruction [8, 42]. Notably, two of our patients, with one being excluded from final analysis because of multilevel osteoporotic fractures adjacent to a PKD, are scheduled for reconstructive surgery.
Sagittal spino-pelvic alignment and balance
Stoltze and Harms [81] recognized a focus on only the injured area of the spine in the literature concerning fracture treatment and follow-up evaluation. The consequences of not restoring normal anatomy on the global sagittal profile and clinical outcome were not fully evaluated. In contrast, there has been an increasing recognition of the importance of the sagittal plane contour in the normal function of the spine [29, 35, 36, 82, 90] and with reference to its various disease states [7, 10, 11, 29, 90, 91]. Physiological standards of sagittal alignment exist [7, 33, 87] and allowed for plotting them against our group (Tables 3 and 4). As the ranges of our groups show, the sagittal alignment of the spinopelvic unit is variable. But, several studies involving volunteers and patients with spinal disorders demonstrated a chain of associations between the spinal and pelvic parameters with the main role of the PI determining the organization of the lumbosacral spine [7, 13, 29, 35, 36, 74, 90]. Tanguay et al. [82] observed a close interdependence between lumbar lordosis and pelvic geometry that was maintained following posterior fusion. Significant correlations existed between PI and LL as well as SS and LL as it was in normal subjects [13] and the current study. In concurrence, after TLB and LB fractures the spinopelvic interplay was not disrupted. Strong correlations existed for the pelvic parameters (PI, PT, and SS) and between the spinal (LL, LSL) and pelvic parameters (PI, SS). Similarly, using Jackson’s techniques there was significant correlation between the pelvic (PR–S1) and spinal parameter (LL, LSL, PR–L2). The PR–T12, the angle incorporating the spinal curve from T12 to the pelvis radius, showed significant correlation with the PT and the PI, and the PR-L2 showed substantial correlation with the PR–S1. Our investigation of PI/PR–S1confirmed that as with the normal and other spinal pathologies [13, 35, 90, 91] the lumbar lordosis that can be achieved in the presence of a PKD is closely related to the orientation of the pelvis: an increased SS predisposes an increased LL in attempt to maintain the trunk centered over the femoral heads. If spinopelvic data were calculated for the TLB fractures, the correlations between the PI and the SS/LSL as well as between the SS and the LSL/TLSL T12–S1 were maintained. The PR–T12 showed significant correlation with the PT in group 2, but not with PI or PR-S1, as it was the case for the PI in group 1. Differences might refer to 67% of all fractures (group 1) occurring at the T12–L1 levels. Hence, with the usage of the PR-T12 in T12 fractures those adjacent-cephalad thoracic levels that also took part in the lordotic compensation were not assessed. If, i.e., a ‘PR–T9’ would have been taken as reference, the balancing adjustments of the adjacent thoracic segments could have been assessed, too. In contrast, with the TLB fractures, including two fractures at L2 only, the PR-L2 showed significant correlation with the PR–S1, the PI, and the PT. Overall, as in the study of Jackson et al. [35] we found the lumbar/lumbosacral lordosis dependant on the pelvic morphology quantitated by the pelvic radius technique, with the PR–S1 (similar to the PI) correlative with the spinopelvic parameters (PR–T12, PR–L2).
Concerning the interdependence of the spinal (LL, LSL, TLSL, PR–12, PR–L2) and the pelvic parameters (PI, PT, SS, PR–S1), our results show that the lumbosacral levels caudad to a fractured thoracolumbar vertebrae adjusted for the local kyphosis in the ranges that were set and ‘dictated’ by the PI/PR–S1, respectively. Both the RKA and SKA showed a significant inverse correlation with the LSL. That is, with the increase of the fracture kyphosis at the thoracolumbar junction, the LSL increased in ranges dictated by the individual PI and SS. Our algebraic results indicated that a spatial interplay between pelvic geometry and the lumbosacral spine still exist following TLB and LB fractures: The PI and PR–S1 are morphological parameters for which the value is fixed and invariable for each subject. Sagittal balance is obtained because of the adaption of other parameters to this fixed parameter. Though, in each individual the PI predicts a distinct lumbar lordosis [90], with the PI remaining similar pre- and posttrauma. Individuals establish their own standard due to the non-positional PI/PR-S1, which gives an adaption potential at two levels of positional compensation in normal people [13] and individuals with a PKD: thoracic and thoracolumbar hypokyphosis cephalad to the fracture level or increased LL/LSL and SS caudad to the fracture-level. To warrant balance the lower thoracic spine and thoracolumbar junction made significant adjustments if necessary, with the TLA T10–L2 in the TLB fractures still being kyphotic (Ø7.6°) but straightened in the LB fractures (Ø-7.8°). The transition vertebra was located the most at the T12-level (43%), compared to L1 (38%) and L2 (26%) in normal individuals [90]. Though, it shifted cranially along the spinal axis resembling the straightened upper lumbar and lower thoracic levels as illustrated in Fig. 9. Any change in the spinopelvic parameters induces a change in the others, except for PI. Thus, the ability of the spinopelvic unit to maintain physiological sagittal balance and upright posture depends both on the morphological parameter PI/PR–S1 and on the dispersion of the other positional parameters. The dispersion of these parameters squares with the adaptability of the lumbosacral curve. If the adaptability of the lumbosacral spine, with the range determined by the PI/SS, to compensate for a thoracolumbar PKD is scooped out, as it was seens in the LB fractures, than the thoracolumbar junction and lower thoracic levels take part in equilibrating global balance.
As in previous studies on non-traumatic spinal disorders [7, 11], loss of lordosis led to an anterior displacement of the plumb line. Increased fracture kyhosis caused a significant anterior displacement of the SB T4–S1. Nevertheless, the equilibrating capabilities of the spine to compensate for the merely mild PKD were sufficient in all patients except three with a negative and one with a positive sagittal imbalance. The mean MTK (40.7°) showed strong correlation with the MLL (−61.2°) again stressing the spinal adjustments to seek for sagittal balance.
Clinical impact of sagittal balance
The authors observed a significant correlation between increased lumbopelvic lordosis (PR–T12) and a better LBOS in group 1 and group 2. Results allow for conclusion that with the thoracolumbar/lumbosacral spine equilibrating balance through compensatory adjustments, the clinical outcome improves. Notably, as we investigated separately for patients with TLB fractures, it became evident that the individuals who could not fully compensate for their fracture kyphosis by adjusted lumbar lordosis (as far as possible within the ranges dictated by the PI), straightening of the lower thoracic levels or adjacent levels to the thoracolumbar junction, had a worse outcome: An increased TAL was found strongly correlative with a decreased outcome in terms of lower VAS-Spine-Scores. Conversely, an increased MTK was significantly related to worse clinical outcome in terms of higher RMDQ values and lower VAS-Spine-Scores. In this context it is of note that in the TLB fractures the thoracic spine was hypokyphotic (Ø34.9°), resembling those individuals who compensated for the thoracolumbar kyphosis by straightening of adjacent upper thoracic levels. Concerning all patients, increased positive shift of the vertical axis in terms of the SB T4–S1 and SB T4–L4 was correlated with worse outcome measures (RMDQ, SF–36 MCS, LBOS, SF–36 MCS) indicating that anterior displacement of the sagittal axis had negative impact on outcome. Results concur with that of Glassman et al. [29, 33] of a lumbar deformity population showing increased pain and decreased function as the magnitude of positive sagittal balance increased.
As concerns outcome measures of LB fractures, they did worse than the TLB fractures although having the less kyphosis. Although the differences were not statistically significant (Table 6), results substantiate previous findings that a similar amount of kyphosis is tolerated better in the upper thoracic region but worse in the lumbar spine [11, 29, 59, 87]. The current sample encountered only five LB fractures, but similar to Siebenga et al. [79], we included also the lumbar spine for increased sample size and the purpose of data pooling with future studies.
Coronal alignment
Lateral burst injuries may result in a posttraumatic coronal deformity [16], but reports on posttraumatic and postsurgical coronal deformity are sparse [10, 34, 45]. In the current study, six patients depicted slight but obvious compensatoric thoracic/thoracolumbar curves adjacent to the fracture-level with one malunion following an A3.1.2 fracture that initialized a symptomatic scoliotic deformity.
Segmental and global curve analysis
A postraumatic kyphosis can influence adjacent-level kinematics and posture [16]. In an in vivo sheep fusion model, Oda et al. [63] observed that regional kyphosis led to early compensatory hyperlordosis, contracture of the posterior ligamentous complex and facet arthrosis below the surgically reconstructed kyphotic level. In the early clinical setting, the deformity is compensated with gapping of the adjacent discs. But, with the advanced kyphosis across the mobile unsupported thoracolumbar/lumbar segments intradiscal pressures increases, the mechanical advantage of the erector spinae musculature is compromised and in the long-term run it can result in posterior overload, consumption or compensatory hyperlordosis [8, 10, 11, 29, 30, 51, 81]. With our visualization of the percentage deviation of the SCAs and the IVAs T10–S1 (Graphs 2 and 3), the authors demonstrated that the mobile segments between T10 and L2 showed highest deviation from normal, thereby compensating for the fracture kyphosis. In addition, the algebraic assessment of spinal curves T10–S1 with the SCA (Graph 4) displayed that fractures affecting the L4 vertebra caused maximum curve deviation from the physiological standard. These findings indicate that, although adjustments by the LL/LSL within the adoption potential and ranges dictated by the PI occurred, the adaptability of adjacent levels at the mid- and lower-lumbar spine were scooped out in several individuals (Fig. 7). Therefore, compensation had to take place at more cephalad levels, T12-L1 and the lower thoracic spine. The segmental characteristics of the remaining thoracic spine (T10-T1) could not be analyzed as normalized data for the IVA/SCA are not available, yet [92]. Overall, our findings reflect that effective segmental compensation mechanism develop in PKD, pronounced at the adjacent-levels, but distributed over the global spine to generate an acceptable sagittal balance [29].
The muscle envelope takes major part in the compensation as a dynamic stabilizer [20]. However, these muscles, particularly in the elderly and less active patients, are prone to fatigue. Therefore, the time until kyphotic deformity becomes symptomatic due to exaggerating of the altered segmental biomechanics varies for each individual. This might also explain while prior correlative analysis between clinical outcome and kyphosis failed [2, 14, 18, 20, 48, 53, 62, 63, 64, 70, 75, 79, 97, 98]. Some authors, however, noted that pain was found as one of the frequent symptoms accompanying (advanced) PKD [16, 26, 28] and in the current study all of our patients showed some degree of tenderness and pain at the fracture level. If present, the pain varied in magnitude but was constant and aching in quality, as described in previous series [16, 39]. Pain response was positive in testing the fracture and adjacent levels. With the pain, however, its source is difficult to identify [87]. Several authors lend credence to the theory of mechanical and motion-induced pain originating from the injured disc level [59, 70, 79]. The incidence of this pain might be reduced by yielding for a solid arthrodesis [69] as compared to SSPI alone or by an extension of instrumentation [4]. With nonsurgical treatment, mechanical stability can be achieved years after trauma by spontaneous synostosis at the fracture level (Fig. 5), resulting in improvement in pain, as was seen in three of our cases. Also, the consequences of exaggerated gapping of levels adjacent to a PKD, which might also be secondary to the analgesic posture of the patient to avoid further load-bearing onto the vertebral body and injured disc, contributes to the pain. If the compensatory mechanisms are scooped out pain arises, resulting as a consequence of abnormal forces placed on the capsula, ligaments, and muscles [16, 87, 87]. We plotted the segmental percentage deviations against the clinical outcome measures and noticed a statistical trend that segments with exaggerated lordosis were related to decreased outcome values. But, this issue demands increased sample size an increased sample homogeneity, i.e., fractures only at T12 and L1 as well as analyzing the global spine T4–S1. The latter demands collection of physiological standards of the SCA/IVA for T10–T4 that does not exist yet. Finally, with the decompensation of adjacent levels premature symptomatic degenerative changes can be associated [24, 87]. Although our follow-up radiographs displayed a slight increase in degenerative changes at adjacent levels (Fig. 8), we cannot substantiate the concept that symptoms resulted from a concurrence of deformity and degeneration without MRI assessment.
Global clinical outcome
Both the presence of neurologic injury [59, 70] and compensation claims [37, 66, 75, 77] were shown to have a greater impact on clinical outcome than any other variables [59, 70]. Many studies are difficult to compare and the current study yielded to be homogenous in regard to these issues. Overall, the TLB and LB fractures caused a remarkable reduction of patients’ function in terms of the RMDQ, LBOS, SF-36, and VAS-Spine-Score (Table 6). We observed a diminished outcome in terms of the SF-36 (Table 5) compared to that of controls [14, 94], particularly for the subscales ‘Role physical’ and ‘Physical function’. The results are similar to previous studies [14, 67], but difficult to compare owing to the fact of their heterogeneity. If results are melted down to a global outcome assessment, they compare well with that of Mumford et al. [62], reporting on 41 neurologically intact patients with 2-year follow-up after burst fractures at T11–L5, but with the treatment widely varying between strict recumbancy in bed and bracing. Outcome was excellent or good in 66%, and 62% in the current series. However, 34% of their series and 38% of the current had a fair or poor outcome. In view of the fact that a significant number of patients with TLB and LB fractures, whether treated surgically or nonsurgically, can show significant long-term sequelae [4, 14, 15, 45, 46, 51, 70, 80, 81, 87, 98], it is worth noting that most studies on TLB fractures cover a short to mid-term follow-up with a mean of about 2 years to a maximum of 5 [14, 18, 43, 58, 59, 66, 67, 4, 75, 77, 79, 86, 89, 97]. Long-term results are the important estimates to value different treatment concepts. Mean age in TLB fractures is about 40 years [2, 44]. Therefore, besides early recovery from injury tissue-pain, the outcome is of interest when patients age in the 5th–6th decade of their life, 10–20 years after injury. Though, in the current study with a follow-up of 9.5 years and the longest showing 17 years there was a strong correlation between age and a decreased outcome in terms of the VAS-Spine-Scores in the TLB burst fractures. Several of the elderly patients in the current series were doing fine in the first years following injury, but then noted increasing pain under physical loading at the fracture-level. Statistical analysis failed yielding significance for the follow-up length being a factor for decreased outcome. This probably refers to the aformentioned characteristics of group 2 and 3 as well as early fatigue in the elderly patients reducing the ability to compensate for the PKD. But, with a follow-up of 16 years Reinhold et al. [70] detected a follow-up related physical impairment (SF-36) in 43 nonsurgically treated TLB and LB fractures. Mean VAS-Spine-Score was 58.9 only. Although the cohort lacked homogeneity concerning neurological status, fracture types, levels, and number per patient, and co-morbidity variables, the authors could emphasize the correlation between pain, follow-up and age. Notably, the pain that can arise with a PKD causing pension claim and diminished ability to work was found relevant in 5–10 years following injury [81], a period that is not covered by most articles. A number of patients exist that are operated on later for the sequelae following a nonsurgical treated TLB burst fracture [18, 80, 81], which is the case with two of our cases. This might explain why previous studies with short- to mid-term follow-up frequently observed high rates of favorable outcomes with nonsurgical treatment [2, 20, 60–62, 70, 71, 77, 78, 83, 86, 95]. A few reports had a follow-up of about 6.5 [2, 83], 8 [53], 16 [70], and 20 years [95]. Moller et al. [60] reported a mean follow-up of 27 years with 22% of 27 patients showing a moderate to poor outcome. But their results were flawed by the sample’s heterogeneity concerning fracture levels (T5–L4), classification, and treatment (bed-rest/bracing). Weinstein et al. [95] reported on minimal or no pain in 72% of 42 patients 20 years after burst fractures at T10–L5. Advanced pain was present in 28% of patients and 57% of patients never achieved painlessness. The current study outcome in terms of the LBOS was fair or poor in 38% and overall four patients (19%) were not able to return to previous employment and one was unable to work at all. Return to work rate compares fairly with previous reports documenting that within neurologically intact patients at least 10% stop working following nonsurgical or surgical treatment [14, 21, 62, 67, 77, 98]. With some series on nonsurgical treatment, only 53 [62] and 62% [70] returned to the same or similar job or only 50% were able to do heavy work [18, 77]. In view of the clinical results the general success of nonsurgical treatment for burst fractures is questioned.
Proponents of the surgical approach indicated that patients fare better in terms of recuperation time, neurologic stability, progressive PKD, disabling back pain, earlier mobilization, and faster return to work [22, 41, 42, 62, 77]. A prospective randomized study on TLB fractures by Siebenga et al. [79] showed better results in favor to SSPI compared to bracing for 3 months at 4-year follow-up. In the nonsurgical group mean LSS was 6.1 points and mean RKA was 19.5° at follow-up. Hence, fracture severity was increased compared to our sample. Mean VAS-Spine-Score and RMDQ was 61 and 8.9, which was 75.2 and 2.4 in the current study (group 2). VAS-Spine-Score and RMDQ was significantly better in the surgical group (81 and 3.1). SSPI was advocated for A3 burst fractures. With a similar short-term study, Shen and Shen [77] observed no significant differences regarding outcome in terms of the LBOS between SSPI and bracing (61 vs 65). However, the LSS was low in both groups (3.9 vs 4.1 points) as compared to the nonsurgical of Siebenga et al. (6.1 points) and the current (5.1 points). Also, their number of compensation issues was high (72% vs 66%). The mean kyphotic angle at 2 years in the nonsurgical group was 24° compared to 12° in the surgical group raising the question ‘what will happen at 10-years’ follow-up? Our study substantiates that larger deviation from anatomical shape of the thoracolumbar/lumbar spine does have impact on clinical long-term outcome. But, the amount of surgery indicated in each burst fracture pattern is not yet defined. Notably, in the cited studies posterior fusion was not performed, but can have significant impact on clinical outcome [69] as does the usage of LSPI for the more seriously crushed vertebra [4, 31]. Several authors reported a failed outcome for SSPI in a subset of TLB and LB fractures [4, 39, 59, 76, 77, 79] and the literature provides some evidence that more seriously comminuted vertebral fractures, e.g., A3.2/3.3 fractures and that with LSS of ≥6 points [4, 31, 93], should undergo extended surgery. That is, using (1) SSPI/LSPI with intermediate transpedicular screw fixation of the fractured vertebrae [56, 77, 81, 92] providing three-point fixation and increasing stiffness of the construct [23, 56, 77] or (2) anterior-only reconstruction [31, 37, 41, 66], the trauma PLIF-technique [38] or antero-posterior surgery [14, 24, 45, 48, 51, 66, 80] that serve for reconstruction of the weight-bearing anterior column and less loss of reduction [31, 76, 98], particularly for the combined surgery [14, 24, 45, 48, 51, 66, 80]. Because of the suggested importance of maintaining the reconstructed lordosis as a crucial factor for the clinical long-term outcome [14, 28] the combined approach (including the trauma PLIF-technique [38]) might be appropriate showing superior radiological long-term outcome compared to the posterior- or anterior-only approach with albeit equal mid-term clinical outcomes [14, 45, 48, 66, 80, 98]. To adapt treatment options ranging from casting to 360° surgery in distinct burst fracture patterns, detailed treatment-related results and particularly long-term results are to be investigated.
Conclusions
With the assessment of the global spinopelvic alignment for the first time in a homogenous sample of TLB and LB fractures, the authors demonstrated that the global spine adjusted for the local posttraumatic kyphotic deformity within the ranges dictated by the spinopelvic geometry. The overall limited clinical outcomes measures correlated with the regional kyphosis and global deviations from normal. The current findings and review of literature suggest that from an anatomical standpoint, the ideal treatment of the more severely-crushed burst fractures (LSS ≥6 points) is complete kyphosis correction with long-term correction maintenance [28]. In sight of the global balance concept, further studies are indicated to delineate which burst fracture pattern(s) should be subjected to nonsurgical treatment or any of the reconstructive surgical concepts to yield a superior clinical outcome.
Acknowledgments
Conflict of interest statement We did not receive any funding or grants.
References
- 1.Acaroglu ER, Schwab FJ, Farcy JP. Simultaneous anterior and posterior approaches for correction of late deformity due to thoracolumbar fractures. Eur Spine J. 1996;5:56–62. doi: 10.1007/BF00307828. [DOI] [PubMed] [Google Scholar]
- 2.Agus H, Kayali C, Arslantas M. Nonoperative treatment of burst-type thoracolumbar vertebra fractures: clinical and radiological results of 29 patients. Eur Spine J. 2005;14:536–540. doi: 10.1007/s00586-004-0740-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al-Khalifa FK, Adjei N, Yee AJ, Finkelstein JA. Patterns of collapse in thoracolumbar burst fracture. J Spinal Disord Tech. 2005;18:410–412. doi: 10.1097/01.bsd.0000177957.11603.5c. [DOI] [PubMed] [Google Scholar]
- 4.Altay M, Ozkurt B, Nuri Aktekin C, Ozturk AM, Dogan Ö, Tabak AY. Treatment of unstable thoracolumbar junction burst fractures with short- or long-segment posterior fixation in magerla type A fractures. Eur Spine J. 2007;16:1145–1155. doi: 10.1007/s00586-007-0310-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angevine PD, McCormick PC. Editorial—the importance of sagittal balance: how good is the evidence? J Neurosurg Spine. 2007;6:101–103. doi: 10.3171/spi.2007.6.2.101. [DOI] [PubMed] [Google Scholar]
- 6.Axelsson P, Johnson R, Stromqvist B. Effect of lumbar orthosis on intervertebral mobility: a roentgen stereophotogrammetric analysis. Spine. 1992;17:678–681. doi: 10.1097/00007632-199206000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Barrey C, Jund J, Noseda O, Roussouly P. Sagittal balance of the pelvis–spine complex and lumbar degenerative diseases. A comparative study about 85 cases. Eur Spine J. 2007;16:1459–1467. doi: 10.1007/s00586-006-0294-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Been HD, Poolman RW, Ubags LH. Clinical outcome and radiographic results after surgical treamment of post-traumatic thoracolumbar kyphosis following simple type A fractures. Eur Spine J. 2004;13:101–107. doi: 10.1007/s00586-003-0576-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bence T, Schreiber U, Grupp T, Steinhauser E, Mittelmeier W. Two column lesions in the thoracolumbar junction: anterior, posterior or combined approach? A comparative biomechanical in vitro investigation. Eur Spine J. 2006;16:813–820. doi: 10.1007/s00586-006-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benli TI, Kaya A, Uruc V, Akalin S. Minimum 5-year follow-up surgical results of post-traumatic thoracic and lumbar kyphosis treated with anterior instrumentation. Spine. 2007;32:986–994. doi: 10.1097/01.brs.0000260796.77990.f7. [DOI] [PubMed] [Google Scholar]
- 11.Berven SH, Deviren V, Smith JA, Emami A, Hu SS, Bradford DS. Management of fixed sagittal plane deformity. Spine. 2001;26:2036–2043. doi: 10.1097/00007632-200109150-00020. [DOI] [PubMed] [Google Scholar]
- 12.Blauth M, Bastian L, Knop C, Lange U, Tusch G. Inter-observer reliability in the classification of thoraco-lumbar spinal injuries. Orthopade. 1999;28:1157–1162. doi: 10.1007/s001320050397. [DOI] [PubMed] [Google Scholar]
- 13.Boulay C, Tardieu C, Hecquet J, Benaim C, Mouilleseaux B, Marty C, Prat-Pradal D, Legaye Duval-Beaupere G, Pelissier J. Sagittal alignment of spine and pelvis: regulated by pelvic incidence: standard values and prediction of lordosis. Eur Spine J. 2006;15:415–422. doi: 10.1007/s00586-005-0984-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Briem D, Lehmann W, Rueckner AH, Windolf J, Rueger JM, Linhart W. Factors influencing the quality of life after burst fractures of the thoracolumbar transition. Arch Orthop Trauma Surg. 2004;124:461–468. doi: 10.1007/s00402-004-0710-5. [DOI] [PubMed] [Google Scholar]
- 15.Briem D, Behechtnejad A, Ouchmaev A, Morfeld M, Schermelleh-Engel K, Amling M, et al. Pain regulation and health-related quality of life after thoracolumbar fractures of the spine. Eur Spine J. 2007;16:1925–1933. doi: 10.1007/s00586-007-0395-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buchowski JM, Kuhns CA, Bridwell KH, Lenke LG (2007) Surgical management of posttraumatic thoracolumbar kyphosis. Spine J (E-pub: May 2007) (in press). doi:10.1016/j.spinee.2007.03.006 [DOI] [PubMed]
- 17.Butler JS, Fitzpatrick P, Mhaolain AMN, Synnot K, O’Byrne JM. The management and functional outcome of isolated burst fractures for the fifth lumbar vertebra. Spine. 2007;32:443–447. doi: 10.1097/01.brs.0000255076.45825.1e. [DOI] [PubMed] [Google Scholar]
- 18.Celebi L, Muratli HH, Dogan Ö, Yagmurlu MF, Aktekin CN, Bicimoglu A. The efficacy of non-operative treatment of burst fractures of the thoracolumbar vertebrae. Acta Orthop Traumatol Turc. 2004;38:16–22. [PubMed] [Google Scholar]
- 19.Dai L-Y, Jin W-J. Interobserver and intraobserver reliability in the load sharing classification of the assessment of thoracolumbar burst fractures. Spine. 2005;30:354–358. doi: 10.1097/01.brs.0000152095.85927.24. [DOI] [PubMed] [Google Scholar]
- 20.Daniaux H, Wagner M, Kathrein A, Lang T. Fractures of the thoracolumbar junction. The conservative treatment. Orthopade. 1999;28:682–691. doi: 10.1007/s001320050398. [DOI] [PubMed] [Google Scholar]
- 21.Defino HL, Canto FRT. Low thoracic and lumbar burst fractures: radiographic and functional outcomes. Eur Spine J. 2007;16:1934–1943. doi: 10.1007/s00586-007-0406-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denis F, Armstrong GWD, Searls K, Matta L. Acute thoracolumbar burst fractures in the absence of neurologic deficit. Clin Orthop Relat Res. 1984;189:142–149. [PubMed] [Google Scholar]
- 23.Dick JC, Jones MP, Zdeblick TA. A biomechanical comparison evaluating the use of intermediated screws and cross-linkage in lumbar pedicle fixation. J Spinal Disord. 1994;1994:402–407. [PubMed] [Google Scholar]
- 24.Eysel P, Hopf C, Fürderer S. Kyphotic deformities in fractures of the thoracolumbar spine. Orthopade. 2001;30:955–964. doi: 10.1007/s001320170009. [DOI] [PubMed] [Google Scholar]
- 25.Fairbank JC, Pynsent PB. The Oswestry disability index. Spine. 2000;25:2940–2953. doi: 10.1097/00007632-200011150-00017. [DOI] [PubMed] [Google Scholar]
- 26.Fürderer S, Wenda K, Thiem N, Hachenberger R, Peer E. Traumatic intervertebral disc lesion—magentic resonance imaging as a criterion for or against intervertebral fusion. Eur Spine J. 2001;10:154–163. doi: 10.1007/s005860000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gertzbein SD. Scoliosis Research Society. Multicentric spine fracture study. Spine. 1992;17:528–540. doi: 10.1097/00007632-199205000-00010. [DOI] [PubMed] [Google Scholar]
- 28.Gertzbein SD, Harris MB. Wedge osteotomy for the correction of post-traumatic kyphosis. A new technique and a report of three cases. Spine. 1992;17:374–379. doi: 10.1097/00007632-199203000-00025. [DOI] [PubMed] [Google Scholar]
- 29.Glassman SD, Bridwell K, Dimar JR, Horton W, Berven S, Schwab F. The impact of positive sagittal balance in adult spinal deformity. Spine. 2005;30:2024–2029. doi: 10.1097/01.brs.0000179086.30449.96. [DOI] [PubMed] [Google Scholar]
- 30.Heary FH, Bono CM. Pedicle subtraction osteotomy in the treatment of chronic posttraumatic kyphotic deformity. J Neurosurg Spine. 2006;5:1–8. doi: 10.3171/spi.2006.5.1.1. [DOI] [PubMed] [Google Scholar]
- 31.Hitchon PW, Torner J, Eichholz KM, Beeler SN. Comparison of anterolateral and posterior approaches in the management of thoracolumbar burst fractures. J Neurosurg Spine. 2006;5:117–125. doi: 10.3171/spi.2006.5.2.117. [DOI] [PubMed] [Google Scholar]
- 32.Holt AE, Shaw NJ, Shetty A, Greenough CG. The reliability of the low back outcome score for back pain. Spine. 2002;27:206–210. doi: 10.1097/00007632-200201150-00017. [DOI] [PubMed] [Google Scholar]
- 33.Horton WC, Brown CW, Bridwell KH, Glassman SD, Se-II Suk, Cha CW. Is there an optimal patient stance for obtaining a lateral 36’’ radiographs. Spine. 2005;30:427–433. doi: 10.1097/01.brs.0000153698.94091.f8. [DOI] [PubMed] [Google Scholar]
- 34.Illes T, Jonge T, Doman I, Doczi T. Surgical correction of the late consequences of posttraumatic spinal disorders. J Spinal Disord Tech. 2002;15:127–132. doi: 10.1097/00024720-200204000-00006. [DOI] [PubMed] [Google Scholar]
- 35.Jackson RP, Hales C, Tech COR. Congruent spinopelvic alignment on standing lateral radiographs of adult volunteers. Spine. 2000;25:2808–2815. doi: 10.1097/00007632-200011010-00014. [DOI] [PubMed] [Google Scholar]
- 36.Jackson RP, Kanemura T, Kawakami N, Hales C. Lumbopelvic lordosis and pelvic balance on repeated standing lateral radiographs of adult volunteers and untreated patients with constant low back pain. Spine. 2000;25:575–586. doi: 10.1097/00007632-200003010-00008. [DOI] [PubMed] [Google Scholar]
- 37.Kaneda K, Taneichi H, Abumi K, Hashimot T, Satoh S, Fujiya M. Anterior decompression and stabilization with the Kaneda device for thoracolumbar burst fractures associated with neurological deficits. J Bone Joint Surg. 1997;79-A:69–83. doi: 10.2106/00004623-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 38.Kathrein A, Waldegger M, Huber B, Sitte I, Schmid R, Blauth M, Daniaux H (2005) Transforaminal approach and interbody fusion in thoracolumbar spine fractures. Poster Presentation, Spineweek, Porto, Portugal
- 39.Katscher S, Verheyden P, Gonschorek O, Glasmacher S, Josten C. Thoracolumbar spine fractures after conservative and surgical treatment. Dependence of correction loss on fracture level. Unfallchirug. 2003;106:20–27. doi: 10.1007/s00113-002-0459-7. [DOI] [PubMed] [Google Scholar]
- 40.Keynan O, Fisher CG, Vaccaro A, Fehlings MG, Oner FC, Dietz J, et al. Radiographic measurement parameters in thoracolumbar fractures: a systematic review and consensus statement of the Spine Trauma Study group. Spine. 2006;31:E156–E165. doi: 10.1097/01.brs.0000201261.94907.0d. [DOI] [PubMed] [Google Scholar]
- 41.Khoo LT, Beisse R, Ptulski M. Thoracoscopic-asisted treatment of thoracic and lumbar fractures: a series of 371 consecutive cases. Neurosurg. 2002;51(Suppl 2):S104–S117. [PubMed] [Google Scholar]
- 42.Klöckner C, Hofmann A, Weber U. Post-traumatic kyphosis of the thoracic and lumbar spine. Orthopade. 2001;30:947–954. doi: 10.1007/s001320170008. [DOI] [PubMed] [Google Scholar]
- 43.Knight RQ, Stornelli DP, Chan DP, et al. Comparison of operative versus nonoperative treatment of lumbar burst fractures. Clin Orthop Relat Res. 1993;306:286–293. [PubMed] [Google Scholar]
- 44.Knop C, Blauth M, Bühren V, Hax PM, Kinzl L, Mutschler W, et al. Operative treatment of thoracolumbar fractures. Part II: operation and radiology. Unfallchirug. 2000;103:1032–1047. doi: 10.1007/s001130050667. [DOI] [PubMed] [Google Scholar]
- 45.Knop C, Blauth M, Bühren V, Arand M, Egbers HJ, Hax PM, Nothwang J, Oestern HJ, Pizanis A, Roth R, Weckbach A, Wentzensen A. Operative treatment of thoracolumbar fractures. Part III: follow-up. Results of a prospective multicenter study by the working group ‘spine’ of the German Society of Trauma Surgery. Unfallchirug. 2001;104:583–600. doi: 10.1007/s001130170089. [DOI] [PubMed] [Google Scholar]
- 46.Knop C, Oeser M, Bastian L, Lange U, Zdichavsky M, Blauth M. Development and validation of the Visual Analogue Scale (VAS). Spine score. Unfallchirug. 2001;104:488–497. doi: 10.1007/s001130170111. [DOI] [PubMed] [Google Scholar]
- 47.Knop C, Reinhold M, Roeder C, Staub L, Schmid R, Beisse R, et al. Internet based multicenter study for thoracolumbar injuries: a new concept and preliminary results. Eur Spine J. 2006;15:1687–1694. doi: 10.1007/s00586-006-0135-7. [DOI] [PubMed] [Google Scholar]
- 48.Korovessis P, Baikousis A, Zacharatos S, Petsinis G, Koureas G, Iliopoulos P. Combined anterior plus posterior stabilization versus posterior short-segment instrumentation and fusion for mid-lumbar (L2–L4) burst fractures. Spine. 2006;31:859–868. doi: 10.1097/01.brs.0000209251.65417.16. [DOI] [PubMed] [Google Scholar]
- 49.Kuntz C, Levin LS, Ondra SL, Shaffrey CI, Morgan CJ. Neutral upright sagittal spine alignement from the occiput to the pelvis in asymptomatic adults: a review and resynthesis of the literature. J Neurosurg Spine. 2007;6:104–112. doi: 10.3171/spi.2007.6.2.104. [DOI] [PubMed] [Google Scholar]
- 50.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. doi: 10.2307/2529310. [DOI] [PubMed] [Google Scholar]
- 51.Lange U, Edeling S, Knop C, Bastian L, Oeser M, Krettel C, et al. Anterior vertebral body replacement with titanium implant adjustable height: prospective clinical study. Eur Spine J. 2006;2:161–172. doi: 10.1007/s00586-005-0015-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee JY, Vaccaro AR, Schweitzer KM, Jr, Lim MR, Baron EM, Rampersaud R, et al. Assessment of injury to the throacolumbar posterior ligamentous complex in the setting of normal-appearing plain radiography. Spine J. 2007;7:422–427. doi: 10.1016/j.spinee.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Lombao D, Pellise F, Bago J, Villanueva C (2006) Quality of life in patients who did not undergo surgery for fracture of the thoracolumbar junction. Eur Spine J 15(Suppl 4):Abstract 25
- 54.Lowe T, Berven SH, Schwab FJ, Bridwell KH (2006) The SRS Classification for adult spinal deformity. Spine 31(Suppl):S119–S125. doi:10.1097/01.brs.0000232709.48446.be [DOI] [PubMed]
- 55.Magerl F, Aebi Gertzbein SD, Harms J, Nazarian S. A comprehensive classification of thoracic and lumbar injuries. Eur Spine J. 1994;3:184–201. doi: 10.1007/BF02221591. [DOI] [PubMed] [Google Scholar]
- 56.Mahar A, Kim C, Wedemeyer M, Mitsunga L, Odell T, Johnson B, et al. Short-segment fixation of lumbar burst fractures using pedicle fixation at the level of the fracture. Spine. 2007;32:1503–1507. doi: 10.1097/BRS.0b013e318067dd24. [DOI] [PubMed] [Google Scholar]
- 57.McCormack T, Karaikovic E, Gaines RW. The load sharing classification of spine fractures. Spine. 1994;19:1741–1744. doi: 10.1097/00007632-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 58.McDonough PW, Davis R, Tribus C, Zdeblick TA. The management of acute thoracolumbar burst fractures with anterior corpectomy and Z-plate fixation. Spine. 2004;29:1901–1909. doi: 10.1097/01.brs.0000137059.03557.1d. [DOI] [PubMed] [Google Scholar]
- 59.McLain RF. Functional outcomes after surgery for spinal fractures: reutrn to work and acitivity. Spine. 2004;15:470–477. doi: 10.1097/01.BRS.0000092373.57039.FC. [DOI] [PubMed] [Google Scholar]
- 60.Moller A, Hasserius R, Redlund-Johnell I, Ohlin A, Karlsson MK. Non-operatively treated burst fractures of the thoracic and lumbar spine in adults—a 23–41 years follow-up. Spine J. 2007;7:701–707. doi: 10.1016/j.spinee.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Moller A, Hasserius R, Redlund-Johnell Ohlin A, Karlsson M. Nonoperatively treated burst fractures of the thoracic and lumbar spine in adults—a 23–41 years follow-up. Eur Spine J. 2006;26:S459–S489. doi: 10.1016/j.spinee.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 62.Mumford J, Weinstein JN, Spratt KF, Goel VK. Thoracolumbar burst fractures. Spine. 1993;18:955–970. doi: 10.1097/00007632-199306150-00003. [DOI] [PubMed] [Google Scholar]
- 63.Oda I, Cunningham BW, Buckley RA, Goebel MJ, Haggerty CJ, Orbegoso CM, et al. Does spinal kyphotic deformity influencce the biomechanical characteristics of the adjacent motion segment? An in vivo model study. Spine. 1999;24:2139–2146. doi: 10.1097/00007632-199910150-00014. [DOI] [PubMed] [Google Scholar]
- 64.Oner FC, Gils AP, Faber JA, Dhert WJ, Verbout AJ. Some complications of common treatment schemes of thoracolumbar spine fractures can be predicted with magnetic resonance imaging: prospective study of 53 patients wit h71 fractures. Spine. 2002;27:629–636. doi: 10.1097/00007632-200203150-00012. [DOI] [PubMed] [Google Scholar]
- 65.Parker JW, Lane JR, Karaikovic EE, Gaines RW. Successful short-segment instrumentation and fusion for thoracolumbar spine fractures: a consecutive 4 1/2-years series. Spine. 2000;25:1157–1170. doi: 10.1097/00007632-200005010-00018. [DOI] [PubMed] [Google Scholar]
- 66.Payer M. Unstable burst fractures of the thoraco-lumbar junction: treatment by posterior bisegmental correction/fixation and staged anterior corpectomy and titanium cage implantation. Acta Neurochir (Wien) 2006;148:299–306. doi: 10.1007/s00701-005-0681-5. [DOI] [PubMed] [Google Scholar]
- 67.Post RB, Keizer HJE, Leferink VJM, Sluis CK. Functional outcome 5 years after non-operative treatment of type A spinal fractures. Eur Spine J. 2006;15:472–478. doi: 10.1007/s00586-005-0887-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Post RB, Leferink VJM. Sagittal range of motion after a spinal fracture: does ROM correlate with functional ooutcome? Eur Spine J. 2004;13:489–494. doi: 10.1007/s00586-003-0669-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Qian BP, Qiu Y, Wang B, Yu Y, Zhu ZZ. Effect of posterlateral fusion on thoracolumbar burst fractures. Chin J Traumatol. 2006;9:349–355. [PubMed] [Google Scholar]
- 70.Reinhold M, Knop C, Lange U, Bastian L, Blauth M. Non-operative treatment of thoracolumbar spinal fractures. Long-term clinical results over 16 years. Unfallchirug. 2003;106:565–576. doi: 10.1007/s00113-003-0607-8. [DOI] [PubMed] [Google Scholar]
- 71.Resch H, Rabl M, Klampfer H, Ritter E, Povacz (2000) Operative versus conservative treatment of fractures of the thoracolumbar spine. Unfallchirug 103:281–288. doi:10.1007/s001130050537 [DOI] [PubMed]
- 72.Roer N, Lange ESM, Bakker FC, Vet HCW, Tulder MW. Management of traumatic thoracolumbar fractures: a systematic review of the literature. Eur Spine J. 2005;14:527–534. doi: 10.1007/s00586-004-0847-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roland M, Fairbank J. The Roland Morris Disability questionnaire and the Oswestry Disability Questionnaire. Spine. 2000;25:3115–3124. doi: 10.1097/00007632-200012150-00006. [DOI] [PubMed] [Google Scholar]
- 74.Roussouly P, Gollogly S, Berthonnaud E, Dimnet J. Classification of the normal variation in the sagittal alignment of the human lumbar spine and pelvis in the standing position. Spine. 2005;30:346–353. doi: 10.1097/01.brs.0000152379.54463.65. [DOI] [PubMed] [Google Scholar]
- 75.Sanderson PL, Fraser RD, Hall DJ, Cain CM, Osti OL, Potter GR. Short segment fixation of thoracolumbar burst fractures without fusion. Eur Spine J. 1999;8:495–500. doi: 10.1007/s005860050212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sasso RC, Renkens K, Hanson D, Reilly T, Mcguire RA, Best NM. Unstable thoracolumbar burst fractures. J Spinal Disord Tech. 2005;19:242–248. doi: 10.1097/01.bsd.0000211298.59884.24. [DOI] [PubMed] [Google Scholar]
- 77.Shen WJ, Shen YS. Nonsurgical treatment of three-column thoracolumbar junction burst fractures without neurologic deficit. Spine. 1999;24:412–415. doi: 10.1097/00007632-199902150-00024. [DOI] [PubMed] [Google Scholar]
- 78.Shen W-J, Liu T-J, Shen Y-S. Nonoperative treatment versus posterior fixation for thoracolumbar burst fractures without neurologic deficit. Spine. 2001;26:1038–1045. doi: 10.1097/00007632-200105010-00010. [DOI] [PubMed] [Google Scholar]
- 79.Siebenga J, Leferink VJM, Segers MJM, Elzinga MJ, Bakker FC, Haarman HJTHM, et al. Treatment of traumatic thoracolumbar spine fractures: a multicenter prospective randomized study of operative versus nonsurgical treatment. Spine. 2006;31:2881–2890. doi: 10.1097/01.brs.0000247804.91869.1e. [DOI] [PubMed] [Google Scholar]
- 80.Stoltze D, Harms J. Kombinierte Stabilisationsverfahren an der thorakolumbalen Wirbelsäule. Osteosyn Int. 1998;6:157–171. [Google Scholar]
- 81.Stoltze D, Harms J. Correction of traumatic deformities. Principles and techniques. Orthopade. 1999;28:731–745. doi: 10.1007/s001320050403. [DOI] [PubMed] [Google Scholar]
- 82.Tanguay F, Mac-Thiong J-M, de Guise JA, Labelle H (2006) Relation between the sagittal pelvic and lumbar spine geometries following surgical correction of adolescent idiopathic scoliosis. Eur Spine J 16:531–536 Medline. doi:10.1007/s00586-006-0238-1 [DOI] [PMC free article] [PubMed]
- 83.Tetzer M, Erturer RE, Ozturk C, Ozturk I, Kuzgun U. Conservative treament of fractures of the thoracolumbar spine. Int Orthop. 2005;29:78–82. doi: 10.1007/s00264-004-0619-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Thomas KC, Bailey CS, Dvorak MF, Kwon B, Fisher C. Comparison of operative and nonoperative treatment for thoracolumbar burst fractures in patients without neurological deficit: a systematic review. J Neurosurg Spine. 2006;4:351–358. doi: 10.3171/spi.2006.4.5.351. [DOI] [PubMed] [Google Scholar]
- 85.Trojan E (1972) Langfristige Ergebnisse von 200 Wirbelbrüchen der Brust- und Lendenwirbelsäule ohne Lähmung. Z Unfallmed 65:122 (in German) [PubMed]
- 86.Tropiano P, Huang RC, Louis CA, Potout DG, Louis RP. Functional and radiographic outcome of thoracolumbar and lumbar burst fractures managed by closed orthopedic reduction and casting. Spine. 2003;28:2459–2465. doi: 10.1097/01.BRS.0000090834.36061.DD. [DOI] [PubMed] [Google Scholar]
- 87.Vaccaro AR, Silber JS. Post-traumatic spinal deformity. Spine. 2001;26(Suppl):S111–S118. doi: 10.1097/00007632-200112151-00019. [DOI] [PubMed] [Google Scholar]
- 88.Vaccaro AR, Lehman RA, Hurlbert J, Anderson PA, Harris M, Hedlund R, et al. A new classification of thoracolumbar injuries. Spine. 2005;30:2325–2333. doi: 10.1097/01.brs.0000182986.43345.cb. [DOI] [PubMed] [Google Scholar]
- 89.Verlaan JJ, Diekerhof CH, Buskens E, Tweel I, Verbout AJ, Dhert WJA, et al. Surgical treatment of traumatic fractures of the thoracic and lumbar spine. Spine. 2004;29:803–814. doi: 10.1097/01.BRS.0000116990.31984.A9. [DOI] [PubMed] [Google Scholar]
- 90.Vialle R, Levassor N, Rillardon L, Templier A, Skalli W, Guigui P. Radiographic analysis of the sagittal alignement and balance of the spine in asymptomatic subjects. J Bone Joint Surg. 2005;87-A:260–267. doi: 10.2106/JBJS.D.02043. [DOI] [PubMed] [Google Scholar]
- 91.Vialle R, Ilharreborde B, Dauzac C, Lenoir T, Rillardon L, Guigui P. Is there a sagittal imbalance of the spine in isthmic spondylolisthesis? A correlation study. Eur Spine J. 2007;16:1641–1649. doi: 10.1007/s00586-007-0348-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang S-T, Ma H-L, Liu C-L, Yu W-K, Chang M-C, Chen T-H. Is fusion necessary for surgically treated burst fractures of the thoracolumbar and lumbar spine? Spine. 2006;31:2646–2652. doi: 10.1097/01.brs.0000244555.28310.40. [DOI] [PubMed] [Google Scholar]
- 93.Wang X-Y, Dai L-Y, Xu H-Z, Chi Y-L. The load-sharing classification of thoracolumbar fractures. An in vitro biomechanical validation. Spine. 2007;32:1214–1219. doi: 10.1097/BRS.0b013e318053ec69. [DOI] [PubMed] [Google Scholar]
- 94.Ware JE, Kosinski M, Dewey JE. How to score version 2 of the SF-36 Health survey. Lincoln: Quality Metric Incorporated; 2000. [Google Scholar]
- 95.Weinstein JN, Collalto P, Lehmann TR. Thoracolumbar ‘burst’ fractures treated conservatively: a long-term follow-up. Spine. 1988;13:33–38. doi: 10.1097/00007632-198801000-00008. [DOI] [PubMed] [Google Scholar]
- 96.Wiesinger GF, Nuhr M, Quittan M, Ebenbichler G, Wölfl G, Fialka-Moser V. Cross-cultural adaption of the Roland-Morris questionnaire for German-speaking patients with low back pain. Spine. 1999;24:1099–1103. doi: 10.1097/00007632-199906010-00009. [DOI] [PubMed] [Google Scholar]
- 97.Wood K, Butterman G, Mehbod A, Garvey T, Jhanjee R, Sechriest V. Operative compared with nonoperative treatment of a thoracolumbar burst fracture without neurological deficit. J Bone Joint Surg. 2003;85-A:773–781. doi: 10.2106/00004623-200305000-00001. [DOI] [PubMed] [Google Scholar]
- 98.Wood KB, Bohn D, Mehbod A. Anterior versus posterior treatment of stable thoracic burst fractures without neurologic deficit. J Spinal Disord Tech. 2005;18(Suppl 1):S15–S23. doi: 10.1097/01.bsd.0000132287.65702.8a. [DOI] [PubMed] [Google Scholar]
- 99.Yurac R, Marre B, Urzua A, Munjin M, Lecaros MA. Residual mobility of instrumented and non-fused segments in thoracolumbar spine fractures. Eur Spine J. 2006;15:864–875. doi: 10.1007/s00586-005-0939-x. [DOI] [PMC free article] [PubMed] [Google Scholar]