Abstract
Background and objectives: Intravenous immunoglobulins (IVIg) may induce acute renal failure associated with tubular vacuolization. Although the use of IVIg is increasing in kidney transplantation, their impact on graft histology and function remains unknown.
Design, setting, participants, & measurements: Twenty-seven kidney transplant recipients who had high immunologic risk and were treated with four courses of IVIg after transplantation were studied retrospectively at a transplant center, and findings were compared with those of 27 control subjects. Protocol kidney biopsies were performed at time of transplantation and at 3 mo and 1 yr after transplantation.
Results: No episode of IVIg-related acute renal failure occurred. Nevertheless, screening biopsies revealed the presence of “microvacuoles” and “macrovacuoles.” Widespread microvacuolizations were often detected (70%) on preimplantation biopsy and not associated with IVIg. Macrovacuoles, which were absent on preimplantation biopsies, were observed exclusively in IVIg-treated patients. Macrovacuoles among IVIg-treated patients were seen in kidneys from older donors and were associated with chronic tubulointerstitial changes at 3 mo, with similar trends at 1 yr. Macrovacuoles were associated with lower creatinine clearance at last follow-up in IVIg-treated patients.
Conclusions: IVIg frequently induce tubular macrovacuoles in kidney transplant recipients. These are more frequently observed in grafts from older donors, suggesting a higher vulnerability to IVIg. These data suggest a deleterious impact of IVIg-induced macrovacuoles on chronic tubulointerstitial changes and long-term renal function.
Intravenous immunoglobulins (IVIg) have proved effective in the treatment of primary or secondary antibody deficiencies and various autoimmune and inflammatory disorders (1,2) and have become widely used since the 1980s. IVIg are also useful for pretransplantation desensitization of patients who have high levels of preformed anti-HLA antibodies and are awaiting renal transplantation (3) and, in combination with plasmapheresis, for the treatment of acute humoral rejection (4,5). Recently, our group also reported that the use of IVIg as a prophylactic therapy in patients at high immunologic risk was associated with good 1-yr outcome and a profound decrease in level of panel reactive antibodies (6). IVIg are usually considered to be safe and well tolerated, but the risk for IVIg-induced acute renal failure has recently been highlighted (7,8). The incidence of IVIg-related acute renal failure has been estimated to be approximately 6% in patients who are treated for autoimmune or infectious diseases (9), but because preexisting kidney disease seems to be a risk factor (10), IVIg-related renal toxicity is likely to occur more frequently in kidney transplant recipients. Although the mechanism of renal injury associated with IVIg use has not been clearly established, kidney biopsies performed in patients with IVIg-induced acute renal failure have demonstrated extensive vacuolization of proximal tubules, suggestive of osmotic nephrosis (10,11); however, the evolution of these acute histologic kidney lesions and their long-term impact on tubulointerstitial changes and renal function remain unknown. Tubular vacuolizations are commonly observed on renal transplant biopsies, including those obtained from patients who have not received IVIg. Other medications, such as calcineurin inhibitors (12–14), iodinated contrast media (15,16), and hydroxyethylstarch (17), are also potential inducers of tubular vacuolizations. To our knowledge, however, no attempt has been made to describe precisely these tubular vacuolizations and their long-term effects.
At our center, protocol kidney biopsies are performed systematically at time of transplantation (day 0) and at 3 mo and at 1 yr after -transplant, allowing longitudinal follow-up and monitoring of histologic changes. We present here the results of a study undertaken with the aim of describing tubular vacuolizations observed in kidney transplant biopsies, identifying those related to IVIg, and evaluating the impact of IVIg-induced vacuolizations on tubulointerstitial damage and renal function over time.
Materials and Methods
Study Population
We retrospectively studied all patients who were at high immunological risk and received posttransplantation prophylactic IVIg after undergoing kidney transplantation from a deceased donor at our center during 2006 and 2007. Patients were considered to be at high immunologic risk when they had previously exhibited positive T cell anti-human globulin-enhanced complement-dependent cytotoxicity cross-match and/or donor-specific anti-HLA antibodies. Patients who did not undergo protocol graft biopsy at day 0 and at 3 mo and 1 yr after transplantation were excluded. The control group consisted of patients who underwent deceased-donor kidney transplantation during the same period (2006 through 2007); received a similar immunosuppressive regimen; and underwent protocol biopsies at day 0, 3 mo, and 1 yr but were not given IVIg therapy.
Immunosuppression
The immunosuppressive regimen consisted of induction by polyclonal antibodies (rabbit ATG [Thymoglobulin; Genzyme, Lyon, France]) or basiliximab (Simulect; Novartis Pharma AG, Basel, Switzerland), a calcineurin inhibitor (cyclosporine [Neoral; Novartis Pharma AG, Basel, Switzerland] or tacrolimus [Prograf; Astellas, La Celle Saint Cloud, France]), mycophenolate mofetil (CellCept; Roche Pharmaceuticals, Basel, Switzerland), and corticosteroids. In addition, all patients received four courses of IVIg at a dosage of 2 g/kg administered over a 96-h period. The type of IVIg differed according to availability. The first course was started before reperfusion, with subsequent courses being given on days 21, 42, and 63. Other than during the first course, IVIg were administered in association with low-dosage heparin to prevent thrombotic complications.
Acute cellular rejection episodes were treated with high-dosage corticosteroids. Patients who experienced acute humoral rejection underwent plasma exchange therapy and received rituximab and high-dosage corticosteroids.
Data Collection
Medical records were used to collect data on recipient gender, recipient and donor age, use of iodinated contrast agent for diagnosis of donor brain death, cold ischemia time, delayed graft function, type and dosage of IVIg administered, number of IVIg courses, immunosuppressive regimen, blood concentration of calcineurin inhibitors, serum creatinine levels and proteinuria at 3 mo and 1 yr, and the last available serum creatinine level. Estimated creatinine clearance (CrCl) was calculated by Cockcroft and Gault formula (18).
Histologic Analysis
All protocol biopsies were reviewed by a renal pathologist (L.H.N.) and a nephrologist (G.B.), who were blinded to clinical information. Biopsy specimens were stained with Masson's trichrome stains, periodic acid-Schiff, methenamine silver, and hematoxylin-phloxin-safran. Histologic changes were graded according to the Banff 2005 classification (19).
Biopsy specimens were also examined for the presence of tubular vacuolizations. The morphologic features of vacuolizations (size and swelling of tubular epithelial cells, nuclear changes, localization in fibrotic areas or not) were accurately described in all biopsy specimens. The extent of vacuolizations was scored on the basis of the fraction of tubules with vacuoles at ×10 magnification in the most involved area (0, absent; 1, <25%; 2, 25 to 50%; 3, >50%).
Statistical Analyses
Results are expressed as numerical values and percentages for categorical variables and as mean ± SD for continuous variables. Comparisons were based on the χ2 test for categorical data and the nonparametric Mann-Whitney U test for continuous data. Statistical analyses were performed using Statview 5.0.1 software (Abacus Concepts, Berkeley, CA). P < 0.05 was regarded as statistically significant.
Results
Patient Characteristics
Twenty-seven patients met the inclusion criteria and were included in the study. Table 1 summarizes the baseline characteristics, immunosuppressive regimen, and graft outcomes in this group and in 27 control patients. Donor age was similar between groups, but in accordance with a high level of allosensitization, the IVIg-treated patients included more female recipients and mean cold ischemia time was longer as a result of the frequent need for day 0 serum cross-match.
Table 1.
Baseline characteristics, immunosuppression, and graft outcome in IVIg-treated patients and control subjectsa
| Parameter | IVIg-Treated Patients(n = 27) | Control Group(n = 27) | P |
|---|---|---|---|
| Recipient characteristics | |||
| age (yr; mean ± SD) | 45 ± 10.5 | 48 ± 9.4 | NS |
| weight (kg; mean ± SD) | 58.7 ± 10.6 | 68.9 ± 14.6 | <0.010 |
| female (n [%]) | 13 (48.1) | 5 (19.5) | <0.020 |
| Transplant characteristics | |||
| donor age (yr; mean ± SD) | 46.4 ± 14.6 | 50.2 ± 10.6 | NS |
| cold ischemia time (h; mean ± SD) | 25.2 ± 8.2 | 19.7 ± 6.0 | <0.005 |
| delayed graft function (n [%]) | 18 (66.7) | 15 (55.6) | NS |
| cyclosporine at 3 mo (n [%]) | 4 (14.8) | 7 (25.9) | NS |
| cyclosporine C2 at 3 mo (ng/ml; mean ± SD) | 1143 ± 328 | 869 ± 423 | NS |
| cyclosporine at 1 yr (n [%]) | 3 (11.1) | 6 (22.2) | NS |
| cyclosporine C2 at 1 yr (ng/ml; mean ± SD) | 1298 ± 509 | 743 ± 169 | 0.050 |
| tacrolimus at 3 mo (n [%]) | 23 (85.2) | 20 (74.1) | NS |
| tacrolimus C0 at 3 mo (ng/ml; mean ± SD) | 11.1 ± 3.0 | 10.4 ± 3.2 | NS |
| tacrolimus at 1 yr (n [%]) | 24 (88.9) | 21 (77.8) | NS |
| tacrolimus C0 at 1 yr (ng/ml; mean ± SD) | 7.7 ± 1.9 | 9.2 ± 2.4 | <0.050 |
| Graft outcome | |||
| serum creatinine at 3 mo (μmol/L; mean ± SD) | 133 ± 44 | 132 ± 39 | NS |
| CrCl at 3 mo (ml/min per 1.73 m2)a | 50 ± 13 | 60 ± 18 | <0.050 |
| proteinuria at 3 mo (g/d; mean ± SD) | 0.28 ± 0.40 | 0.18 ± 0.20 | NS |
| serum creatinine at 1 yr (μmol/L; mean ± SD) | 143 ± 65 | 131 ± 36 | NS |
| CrCl at 1 yr (ml/min per 1.73 m2)a | 50 ± 21 | 59 ± 21 | NS |
| proteinuria at 1 yr (g/d; mean ± SD) | 0.31 ± 0.70 | 0.18 ± 0.32 | NS |
| acute rejection at 3 mo (n [%]) | 7 (25.9) | 2 (7.4) | NS |
| acute rejection 3 mo to 1 yr (n [%]) | 2 (7.4) | 2 (7.4) | NS |
| acute rejection at 1 yr (n [%]) | 8 (29.6) | 4 (14.8) | NS |
CrCl, creatinine clearance calculated by the Cockcroft-Gault formula (18); IVIg, intravenous immunoglobulin.
Blood concentrations of calcineurin inhibitors at 3 mo were not different between the IVIg-treated group and control subjects (Table 1). At 3 mo and 1 yr, 23 (85.2%) IVIg-treated patients and 20 (74.1%) control patients were receiving tacrolimus; at 1 yr, this had increased slightly to 24 (88.9%) and 21 (77.8%) patients. The remaining patients were receiving cyclosporine. Tacrolimus trough concentration (C0) at 1 yr was lower in IVIg-treated patients than control subjects (7.7 ± 1.9 versus 9.2 ± 2.4 ng/ml; P < 0.05), but cyclosporine C2 concentration was higher in IVIg-treated patients (1298 ± 509 versus 743 ± 169 ng/ml; P < 0.05). In terms of IVIg treatment, the majority of patients (n = 20, 74.1%) received only Endobuline (Baxter, Maurepas, France; 5% solution of Ig with glucose as excipient); two (7.4%) patients received only Octagam (Octapharma, Lachen, Switzerland; 5% solution with maltose); three (11.1%) patients received both Endobuline and Octagam successively; and two (7.4%) patients received Endobuline, Octagam, and Tegeline (LFB, Les Vlis, France; 5% solution with saccharose).
The incidence of delayed graft function and acute rejection rate was similar in the IVIg-treated and control groups. Creatinine clearance, however, was lower in IVIg-treated patients than in control subjects at 3 mo (50 ± 13 versus 60 ± 18 ml/min per 1.73 m2; P < 0.05) with a NS trend to lower values at 1 yr (50 ± 21 versus 59 ± 21 ml/min per 1.73 m2; NS). No acute renal failure episode related to IVIg administration was observed.
Tubular Vacuolizations
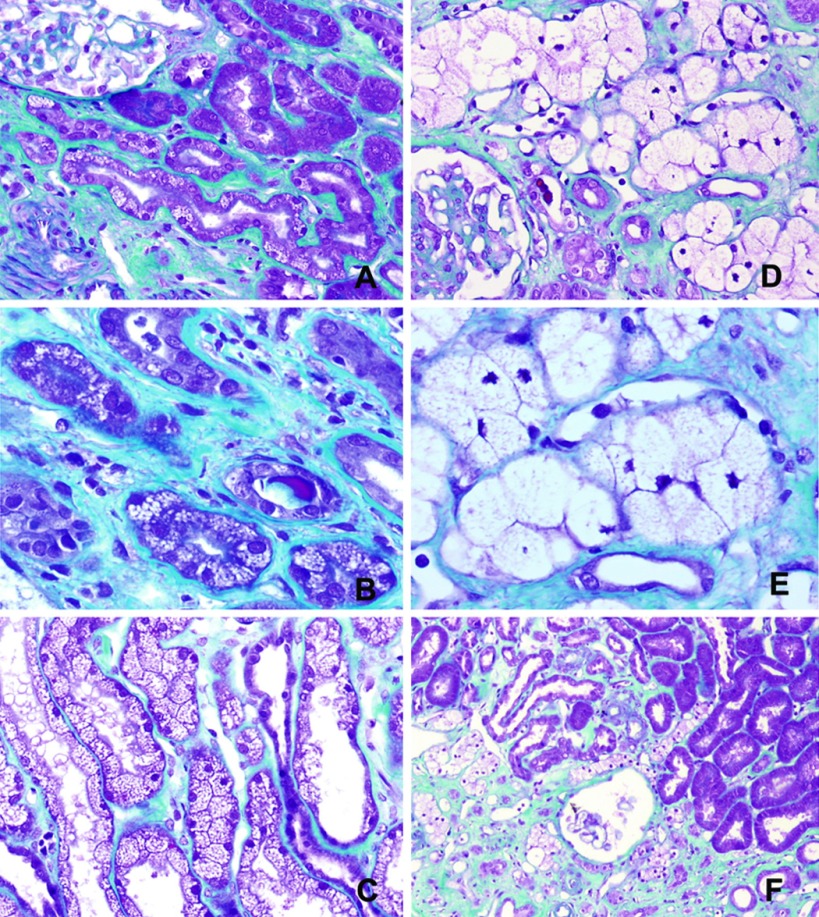
Two distinct patterns of tubular vacuolizations were frequently observed on 3-mo biopsies from IVIg-treated patients (Figure 1). Some tubules presented with voluminous vacuoles, resulting in cytoplasmic swelling of epithelial cells. The nucleus of these cells was pushed back to the basal part of the cell and was often pycnotic. As a result of the important deformation of these cells, we were unable to determine whether tubules involved were proximal, distal, or both. This pattern was termed “macrovacuoles.” Other tubular epithelial cells showed small isometric vacuoles called “microvacuoles,” which, unlike macrovacuoles, did not result in cytoplasmic swelling or nuclear changes. Both macrovacuoles and microvacuoles were often but not always observed in the subcapsular area. Macrovacuoles but not microvacuoles were preferentially located in fibrotic zones, even when the overall chronic tubulointerstitial lesions were mild or moderate (interstitial fibrosis/tubular atrophy [IF/TA] grade I or II). Macrovacuoles and microvacuoles were commonly grouped together, forming islets.
Figure 1.
Vacuole patterns in biopsies at 3 mo (Masson's trichrome staining). (A) Some tubular epithelial cells presented with small isometric vacuoles termed microvacuoles. (B) Microvacuoles resulted in neither cytoplasmic swelling nor nuclear changes. (C) Widespread microvacuoles were seen frequently on preimplantation biopsy specimens. (D) In follow-up protocol biopsies performed at 3 mo and 1 yr in intravenous immunoglobulin (IVIg)-treated patients, some tubules showed voluminous vacuoles called macrovacuoles, characterized by cytoplasmic swelling of epithelial cells. (E) The nucleus of tubular cells with macrovacuoles was pushed back to the basal part and was often pycnotic. (F) Macrovacuoles were preferentially located in fibrotic areas. Both macrovacuoles and microvacuoles were often grouped in islets and located in the subcapsular area. Magnifications: ×20 in A and D; ×40 in B and E; ×10 in F.
Tubular vacuolizations (including both macrovacuoles and microvacuoles) were observed in 21 (77.8%) IVIg-treated patients and in nine (33.3%) control patients (P = 0.001) on 3-mo biopsies (Table 2). The prevalence of tubular microvacuoles was similar in both groups, but macrovacuoles were overrepresented in IVIg-treated patients compared with the control group (17 [63.0%] versus 0 [0.0%]; P < 0.0001). Both macrovacuoles and microvacuoles were observed concurrently in four (14.8%) IVIg-treated patients. No macrovacuoles were observed in the preimplantation biopsies of the 27 IVIg-treated patients, whereas widespread microvacuolizations was a common finding (19 [70.4%] of 27 patients).
Table 2.
Characteristics of tubular vacuoles on biopsy at 3 moa
| Parameters | IVIg-Treated Patients(n = 27) | Control group(n = 27) | P |
|---|---|---|---|
| Tubular vacuoles (any type) | |||
| n (%) | 21 (77.8) | 9 (33.3) | 0.0010 |
| mean score at 3 mo (n [%]) | 0.96 ± 0.65 | 0.30 ± 0.54 | 0.0007 |
| Tubular microvacuoles | |||
| n (%) | 10 (37.0) | 9 (33.3) | NS |
| mean score at 3 mo (n [%]) | 0.41 ± 0.57 | 0.41 ± 0.64 | NS |
| score >1 (n [%]) | 1 (3.7) | 2 (7.4) | |
| Tubular macrovacuoles (n [%]) | |||
| n (%) | 17 (63.0) | 0 (0.0) | <0.0001 |
| mean score at 3 mo (n [%]) | 0.74 ± 0.66 | 0.00 ± 0.00 | <0.0001 |
| score >1 (n [%]) | 3 (11.1) | 0 (0.0) |
Tubular vacuoles were divided into microvacuoles and macrovacuoles, according to their morphologic features (size/swelling of tubular epithelial cells and nuclear changes). The extent of vacuolization was scored on the basis of the fraction of tubules with vacuoles at ×10 magnification in the most involved area (0, absent; 1, <25%; 2, 25 to 50%; 3, >50%).
Microvacuoles and macrovacuoles were usually focally distributed, occurring in <25% of the tubular sections. On 3-mo biopsies, three (11.1%) IVIg-treated patients presented with macrovacuoles in >25% of the tubular sections, and three patients (one IVIg-treated and two controls) had microvacuoles in 25 to 50% of tubular sections (Table 2).
Factors Associated with Presence of Macrovacuoles among IVIg-Treated Patients
IVIg-treated patients with and without macrovacuoles at 3 mo were compared to identify risk factors for macrovacuole development (Table 3). Control subjects were excluded from this analysis because they were likely to differ from IVIg-treated patients in other factors, mainly immunologic, that might have an impact on histopathologic changes and renal function. Macrovacuoles were more frequent in kidneys from older donors (mean age 51 ± 11 versus 38 ± 16 yr; P < 0.003). Recipient age, cold ischemia time, and delayed graft function were not associated with macrovacuoles at 3 mo. The cumulative dosage of IVIg, even when compared in terms of body weight or body surface area, was not associated with macrovacuoles; neither was calcineurin inhibitor exposure. The number of plasma exchange treatments for antibody-mediated acute rejection was also similar in the two groups. Data on use of iodinated contrast agent for diagnostic purposes in the donor was available for 19 patients, and the occurrence of macrovacuoles at 3 mo was similar with contrast agent (five [55.6%] of nine patients) or without (six [60.0%] of 10 patients). No patient was exposed to iodinated contrast agent during the first 3 mo after transplantation. For firmly ruling out the hypothesis of higher exposure to calcineurin inhibitors in patients with macrovacuoles during the first 3 mo, calcineurin inhibitor whole-blood concentrations were recorded at 7 d, 14 d, 1 mo, 2 mo, and 3 mo after transplantation. Drug concentrations were always similar in the two groups (data not shown).
Table 3.
Factors associated with presence of tubular macrovacuoles on biopsy at 3 mo among IVIg-treated patients
| Parameters | Macrovacuoles at 3 mo |
P | |
|---|---|---|---|
| Yes (n = 17) | No (n = 10) | ||
| Pretransplantation parameters | |||
| donor age (yr; mean ± SD) | 51.4 ± 11.2 | 38 ± 16.2 | <0.003 |
| cold ischemia time (h; mean ± SD) | 25.6 ± 9.2 | 26.0 ± 6.7 | NS |
| iodinated contrast agent before transplantation (n [%]) | 5/9 (55.6) | 6/10 (60.0) | NS |
| female recipient (n [%]) | 8 (47) | 5 (50) | NS |
| Posttransplantation parameters | |||
| recipient age (yr; mean ± SD) | 44.6 ± 11.2 | 45.7 ± 9.9 | NS |
| recipient weight (kg; mean ± SD) | 60.7 ± 11.9 | 55.5 ± 7.4 | NS |
| body surface area (m2; mean ± SD) | 1.7 ± 0.2 | 1.6 ± 0.1 | NS |
| IVIg cumulative dosage (g; mean ± SD) | 373 ± 170 | 330 ± 145 | NS |
| delayed graft function (n [%]) | 12 (70.6) | 6 (60.0) | NS |
| posttransplantation plasma exchange therapy (mean ± SD) | 7.6 ± 9.2 | 3.5 ± 6.1 | NS |
| acute rejection at 3 mo (n [%]) | 5 (29.4) | 2 (20.0) | NS |
| cyclosporine at 3 mo (n [%]) | 3 (17.6) | 1 (10.0) | NS |
| cyclosporine C2 at 3 mo (ng/ml; mean ± SD) | 1296 ± 148 | 686 | NS |
| tacrolimus at 3 mo (n [%]) | 14 (82.4) | 9 (90.0) | NS |
| tacrolimus C0 at 3 mo (ng/ml; mean ± SD) | 10.7 ± 3.1 | 11.3 ± 3.3 | NS |
Relation among Macrovacuoles, Histologic Changes, and Renal Function among IVIg-Treated Patients
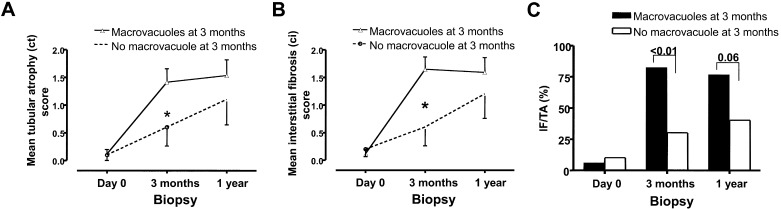
The impact of macrovacuoles on histologic changes and renal function at 3 mo to 1 yr after transplantation was evaluated among IVIg-treated patients. As previously, control subjects were excluded from this analysis. Although donor age was significantly higher in patients with macrovacuoles at 3 mo, chronic Banff scores were very similar on preimplantation biopsies in patients with or without macrovacuoles (Table 4). Marked chronic tubulointerstitial lesions were absent in preimplantation biopsies for all but two patients with grade I IF/TA score, one of whom showed macrovacuoles at 3 mo (NS); however, despite the similarity of day 0 Banff scores, 3-mo acute rejection rate, and incidence of delayed graft function, 3-mo biopsies showed that patients with macrovacuoles had a greater incidence of interstitial fibrosis and tubular atrophy, higher IF/TA scores, and a greater prevalence of IF/TA lesions than patients without macrovacuoles (Table 4, Figure 2).
Table 4.
Chronic tubulointerstitial changes associated with macrovacuoles on biopsy at 3 mo among IVIg-treated patientsa
| Parameters | Macrovacuoles at 3 mo |
P | |
|---|---|---|---|
| Yes (n = 17) | No (n = 10) | ||
| Banff scores on pretransplantation biopsy | |||
| interstitial fibrosis (mean ± SD) | 0.13 ± 0.35 | 0.20 ± 0.42 | NS |
| tubular atrophy (mean ± SD) | 0.12 ± 0.33 | 0.10 ± 0.32 | NS |
| IF/TA (n [%]) | 1 (5.9) | 1 (10.0) | NS |
| IF/TA score (mean ± SD) | 0.06 ± 0.24 | 0.10 ± 0.32 | NS |
| Banff scores at 3 mo | |||
| glomerulitis (mean ± SD) | 0.00 ± 0.00 | 0.00 ± 0.00 | NS |
| interstitial infiltrate (mean ± SD) | 0.41 ± 0.51 | 0.00 ± 0.00 | NS |
| tubulitis (mean ± SD) | 0.41 ± 0.79 | 0.30 ± 0.48 | NS |
| vasculitis (mean ± SD) | 0.41 ± 0.79 | 0.30 ± 0.48 | NS |
| allograft glomerulopathy (mean ± SD) | 0.00 ± 0.00 | 0.00 ± 0.00 | NS |
| interstitial fibrosis (mean ± SD) | 1.65 ± 0.93 | 0.60 ± 1.07 | <0.010 |
| change in interstitial fibrosis (day 0 to 3 mo; mean ± SD) | 1.53 ± 0.94 | 0.40 ± 1.17 | <0.010 |
| tubular atrophy (mean ± SD) | 1.41 ± 1.00 | 0.60 ± 1.07 | <0.010 |
| change in tubular atrophy (day 0 to 3 mo; mean ± SD) | 1.29 ± 0.85 | 0.50 ± 1.18 | <0.040 |
| IF/TA lesions (n [%]) | 14 (82.4) | 3 (30.0) | <0.007 |
| IF/TA mean score (mean ± SD) | 1.40 ± 1.00 | 0.60 ± 1.10 | <0.030 |
| change in IF/TA score (day 0 to 3 mo; mean ± SD) | 1.40 ± 0.90 | 0.50 ± 1.20 | <0.030 |
IT/TA, interstitial fibrosis/tubular atrophy.
Figure 2.
(A through C) Association of IVIg-induced macrovacuoles with histopathologic progression of tubular atrophy (A), interstitial fibrosis (B), and interstitial fibrosis/tubular atrophy (C) in protocol biopsies of IVIg-treated patients. *P < 0.01.
Macrovacuoles at 1 yr (n = 12, 44.4%) were more frequent among patients who already had macrovacuoles on 3-mo biopsy (seven [58.3%] of 12 versus three [20.0%] of 15; P < 0.05). It is interesting that patients with macrovacuoles at 1 yr had received significantly higher cumulative dosages of IVIg (442 ± 143 versus 286 ± 143 g; P < 0.02). Progression of tubulointerstitial damages from 3 mo to 1 yr tended to be higher in patients without macrovacuoles at 3 mo, despite that these patients were less severely affected at 3 mo. Nevertheless, chronic tubulointerstitial lesion scores and the proportion of patients with IF/TA remained slightly higher at 1 yr in patients who had macrovacuoles on 3-mo biopsy (NS; Figure 2).
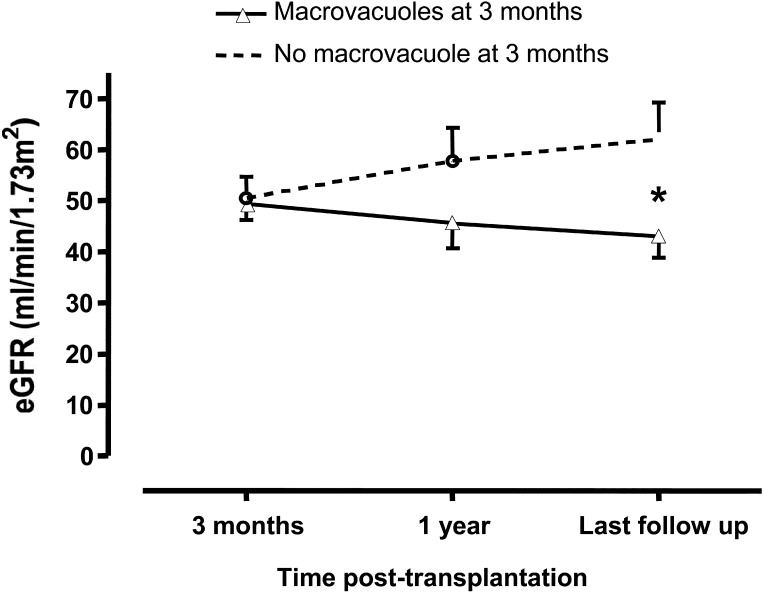
At 1 yr after transplantation, patients with macrovacuoles at 3 mo tended to have increased serum creatinine levels (155 ± 70 versus 122 ± 51 μmol/L; NS) and reduced CrCl (46 ± 20 versus 58 ± 20 ml/min per 1.73 m2; NS) compared with patients without macrovacuoles. Serum creatinine at last follow-up was also evaluated in patients with or without macrovacuoles at 3 mo. Last follow-up was 32 ± 18 mo in patients with macrovacuoles at 3 mo versus 22 ± 7 mo in those without macrovacuoles (NS). One patient was excluded from the analysis because of ESRD, which was secondary to thrombotic microangiopathy (corresponding to recurrence of the primary renal disease). CrCl was markedly lower in patients with macrovacuoles (43 ± 17 ml/min per 1.73 m2) than in those without (62 ± 22 ml/min per 1.73 m2; P < 0.03; Figure 3).
Figure 3.
Association of IVIg-induced macrovacuoles with estimated GFR (eGFR) in IVIg-treated patients, evaluated using the Cockcroft and Gault formula. *P < 0.05.
Discussion
IVIg treatment has been reported to induce acute renal failure associated with tubular vacuolization, suggesting an osmotic injury (10,11). It remains unknown, however, whether IVIg-induced vacuoles persist or disappear, and the long-term effects of IVIg-related vacuoles on the kidney are unclear. To our knowledge, this is the first study to undertake clinical and histologic follow-up of kidney transplant patients who were treated by high-dosage IVIg. Our results show that IVIg treatment frequently induces tubular macrovacuoles in this population even in the absence of any acute kidney dysfunction and is strongly associated with chronic tubulointerstitial damage. These changes have potentially a deleterious impact on long-term kidney function.
Two distinct patterns of tubular vacuolization were observed, termed microvacuoles and macrovacuoles. Microvacuoles were clearly unrelated to IVIg, because their prevalence was similar among control subjects and IVIg-treated patients, and their occurrence probably relates to tubular toxicity associated with calcineurin inhibitor therapy (12–14). Widespread microvacuoles, however, were often observed on preimplantation biopsies, suggesting that microvacuoles may also reflect nonspecific tubular injury. The significance of microvacuoles was beyond the scope of our study. In contrast, the presence of macrovacuoles did seem to be linked to IVIg administration for several reasons. First, macrovacuoles were not detected on preimplantation biopsies (i.e., before administration of IVIg). Second, macrovacuoles were observed at 3 mo in almost two thirds of IVIg-treated patients but in none of the control patients. Third, the presence of macrovacuoles on 1-yr biopsy was associated with higher cumulative dosages of IVIg. Fourth, the morphologic appearance of macrovacuoles was comparable to previous descriptions in IVIg-related acute renal failure (11,20). Fifth, macrovacuoles were not linked to blood concentration of cyclosporine or tacrolimus or to administration of iodinated contrast agent administration in donors or recipients.
It is notable that no patient experienced acute renal failure after IVIg administration. This may be at least partly due to the type of IVIg used at our center. IVIg products vary considerably in their composition, and these differences can have clinical implications. Several studies have shown that most cases of IVIg-related acute renal failure occurred after infusion of sucrose-based products (8,10), although not exclusively (21).
It remains unclear why only some IVIg-treated patients developed macrovacuoles. Electron microscopy study in patients with osmotic nephrosis and light microscopy patterns quite that were similar to our findings showed a number of lysosomes and endocytic vacuoles in proximal tubular cells (20). Sucrose and other agents implicated in osmotic nephrosis enter the tubular cells by pinocytosis, then pinocytic vacuoles fuse with lysosomes (22). Lysosomal tubular dysfunction has been described secondary to toxic agents such as cyclophosphamide (23), leading in some cases to accumulation of abnormal amounts of proteins and tubular cell swelling. Several reports have emphasized that preexisting kidney disease (10,21) is a risk factor for acute renal failure after IVIg, and it is conceivable that this vulnerability is related to lysosomal function impairment. It is interesting that among IVIg-treated patients, mean donor age was higher in those with macrovacuoles at 3 mo in our study. An attractive hypothesis would be that patients who developed macrovacuoles could have subtle age-related tubular impairment in their donor grafts despite similar pretransplantation histologic findings, making them more vulnerable to IVIg-related toxicity. Kidney transplant recipients may be particularly likely to develop renal side effects after IVIg administration. Because IVIg nephrotoxicity seems to be mainly due to lysosomal accumulation of sugar, the fact that transplant patients have only one functional kidney may increase the osmotic load, a factor that is likely to promote IVIg-related tubular injury. This hypothesis highlights the critical importance of preventive measures to limit IVIg toxicity in the transplant population, primarily by accompanying IVIg infusion with adequate parenteral hydration.
It is interesting that macrovacuoles were still detectable at 1 yr in more than half of the patients who exhibited macrovacuoles at 3 mo. To date, whether macrovacuoles disappear or persist over time has not been addressed in articles dealing with osmotic injury related to IVIg or iodinated contrast agent, probably because kidney biopsies were rarely repeated; however, persistence of vacuoles observed in our study is consistent with the experience of others, recently reported in a review article (20) and was previously described in kidney transplants from donors who were administered hydroxyethylstarch, another sugar-containing product that can induce macrovacuoles (17). Tubular impairment, related to donor age and/or other undetermined factors, might lead to macrovacuole persistence. Accumulation of tubular lysosomes could reveal toxic over time, leading to cell death and promoting interstitial fibrosis in surrounding areas; however, we must acknowledge that the exact mechanisms involved remain elusive.
Surprising, small amounts of macrovacuoles at 1 yr were found in two patients who showed no macrovacuoles at 3 mo, probably as a result of sampling bias. Neither patient had received iodinated contrast agent between 3 mo and 1 yr.
After linking macrovacuoles to IVIg, the second step of our study was to determine whether histologic changes (especially ci and ct) and renal function over time differed depending whether macrovacuoles were present at 3 mo. We then compared IVIg-treated patients who had macrovacuoles versus IVIg-treated patients who did not have macrovacuoles at 3 mo. These two groups of patients at high immunologic risk were quite similar regarding factors that could have induced chronic tubulointerstitial lesions at year 1 (including preimplantation biopsy findings, delayed graft function rate, Banff scores for acute rejection [glomerulitis, interstitial inflammation, tubulitis, arteritis] at 3 mo or 1 yr, acute rejection rate, and whole-blood concentrations of calcineurin inhibitors). In these IVIg-treated patients at high immunologic risk, the presence of macrovacuoles was significantly associated with a higher incidence of interstitial fibrosis and tubular atrophy, and greater severity of IF/TA lesions at 3 mo. Moreover, macrovacuoles were often observed in fibrotic areas, even in patients with only mild or moderate overall tubular atrophy and interstitial fibrosis. At 1 yr, patients with macrovacuoles at 3 mo no longer had significantly greater tubulointerstitial changes than patients without macrovacuoles at 3 mo, but this may have been because the chronic tubulointerstitial score was already high at 3 mo with little capacity to increase further. Importantly, the tubulointerstitial changes observed with macrovacuoles seemed to be associated with a progressive worsening of renal function, suggesting that IVIg-related macrovacuoles are associated with long-term effects. Thus, our data suggest for the first time that IVIg-related tubular toxicity can be insidious, manifesting in the form of chronic tubulointerstitial lesions. This raises questions about the safety of IVIg in recipients of grafts from marginal donors, who may be more prone to chronic tubulointerstitial damage.
Our study has several limitations and sources of potential bias. We selected control patients who were administered similar immunosuppressive drugs other than IVIg, and characteristics of the two groups were very similar; however, we cannot definitively exclude the possibility that the groups differed in terms of other factors that could increase the likelihood of macrovacuoles, although this seems unlikely. The other limitations concern the IVIg-treated group. First, the number of IVIg-treated patients included was small, preventing multivariate analysis. Multivariate analysis would have been helpful for investigating whether chronic tubulointerstitial changes were associated with macrovacuoles independent of other known factors that promote such lesions; however, no difference in these factors was detected by univariate analysis. Second, our study does not clarify the mechanisms by which IVIg treatment induces macrovacuoles or how macrovacuoles are associated with increased interstitial fibrosis and tubular atrophy. Last, we cannot definitively exclude that tubulointerstitial lesions and macrovacuoles are two distinct consequences of factors that injure tubules, without causative link. The presence of macrovacuoles in areas with most severe tubulointerstitial lesions could also reflect a higher vulnerability of tubules to lysosomal accumulation rather than a toxic effect of macrovacuoles per se. Especially, it is possible that factors related to donor age may account, in part, for the more severe tubulointerstitial changes observed in patients with macrovacuoles at 3 mo, independent of an IVIg effect.
Conclusions
Our results demonstrate that IVIg treatment of kidney transplant recipients frequently induces tubular macrovacuoles similar to those observed in IVIg-related acute renal failure. These can be distinguished from other types of tubular vacuoles and can occur in the absence of acute renal dysfunction. Macrovacuoles are more often observed in grafts from older donors, suggesting a higher vulnerability to IVIg toxicity. The presence of macrovacuoles is strongly associated with chronic tubulointerstitial damage. Although the long-term prognostic significance of IVIg-induced macrovacuoles needs to be further clarified, our data suggest that they have a potentially deleterious impact on graft histopathology and renal function.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kazatchkine MD, Kaveri SV: Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N Engl J Med 345 :747 –755,2001 [DOI] [PubMed] [Google Scholar]
- 2.Looney RJ, Huggins J: Use of intravenous immunoglobulin G (IVIG). Best Pract Res Clin Haematol 19 :3 –25,2006 [DOI] [PubMed] [Google Scholar]
- 3.Jordan SC, Pescovitz MD: Presensitization: The problem and its management. Clin J Am Soc Nephrol 1 :421 –432,2006 [DOI] [PubMed] [Google Scholar]
- 4.Ibernon M, Gil-Vernet S, Carrera M, Seron D, Moreso F, Bestard O, Cruzado JM, Grinyo JM: Therapy with plasmapheresis and intravenous immunoglobulin for acute humoral rejection in kidney transplantation. Transplant Proc 37 :3743 –3745,2005 [DOI] [PubMed] [Google Scholar]
- 5.Lehrich RW, Rocha PN, Reinsmoen N, Greenberg A, Butterly DW, Howell DN, Smith SR: Intravenous immunoglobulin and plasmapheresis in acute humoral rejection: Experience in renal allograft transplantation. Hum Immunol 66 :350 –358,2005 [DOI] [PubMed] [Google Scholar]
- 6.Anglicheau D, Loupy A, Suberbielle C, Zuber J, Patey N, Noel LH, Cavalcanti R, Le Quintrec M, Audat F, Mejean A, Martinez F, Mamzer-Bruneel MF, Thervet E, Legendre C: Posttransplant prophylactic intravenous immunoglobulin in kidney transplant patients at high immunological risk: A pilot study. Am J Transplant 7 :1185 –1192,2007 [DOI] [PubMed] [Google Scholar]
- 7.Orbach H, Tishler M, Shoenfeld Y: Intravenous immunoglobulin and the kidney: A two-edged sword. Semin Arthritis Rheum 34 :593 –601,2004 [DOI] [PubMed] [Google Scholar]
- 8.Vo AA, Cam V, Toyoda M, Puliyanda DP, Lukovsky M, Bunnapradist S, Peng A, Yang K, Jordan SC: Safety and adverse events profiles of intravenous gammaglobulin products used for immunomodulation: A single-center experience. Clin J Am Soc Nephrol 1 :844 –852,2006 [DOI] [PubMed] [Google Scholar]
- 9.Levy JB, Pusey CD: Nephrotoxicity of intravenous immunoglobulin. QJM 93 :751 –755,2000 [DOI] [PubMed] [Google Scholar]
- 10.Centers for Disease Control and Prevention (CDC): Renal insufficiency and failure associated with immune globulin intravenous therapy—United States, 1985–1998. MMWR Morb Mortal Wkly Rep 24 :518 –521,1999 [PubMed] [Google Scholar]
- 11.Soares SM, Sethi S: Impairment of renal function after intravenous immunoglobulin. Nephrol Dial Transplant 21 :816 –817,2006 [DOI] [PubMed] [Google Scholar]
- 12.Haas M, Sonnenday CJ, Cicone JS, Rabb H, Montgomery RA: Isometric tubular epithelial vacuolization in renal allograft biopsy specimens of patients receiving low-dose intravenous immunoglobulin for a positive crossmatch. Transplantation 78 :549 –556,2004 [DOI] [PubMed] [Google Scholar]
- 13.Mihatsch MJ, Thiel G, Basler V, Ryffel B, Landmann J, von Overbeck J, Zollinger HU: Morphological patterns in cyclosporine-treated renal transplant recipients. Transplant Proc 17 :101 –116,1985 [PubMed] [Google Scholar]
- 14.Randhawa PS, Saad RS, Jordan M, Scantlebury V, Vivas C, Shapiro R: Clinical significance of renal biopsies showing concurrent acute rejection and tacrolimus-associated tubular vacuolization. Transplantation 67 :85 –89,1999 [DOI] [PubMed] [Google Scholar]
- 15.Moreau JF, Noel LH, Droz D: Proximal renal tubular vacuolization induced by iodinated contrast media, or so-called “osmotic nephrosis.” Invest Radiol 28 :187 –190,1993 [PubMed] [Google Scholar]
- 16.Tervahartiala P, Kivisaari L, Kivisaari R, Vehmas T, Virtanen I: Structural changes in the renal proximal tubular cells induced by iodinated contrast media. Nephron 76 :96 –102,1997 [DOI] [PubMed] [Google Scholar]
- 17.Cittanova ML, Leblanc I, Legendre C, Mouquet C, Riou B, Coriat P: Effect of hydroxyethylstarch in brain-dead kidney donors on renal function in kidney-transplant recipients. Lancet 348 :1620 –1622,1996 [DOI] [PubMed] [Google Scholar]
- 18.Cockcroft DW, Gault MH: Prediction of creatinine clearance from serum creatinine. Nephron 16 :31 –41,1976 [DOI] [PubMed] [Google Scholar]
- 19.Solez K, Colvin RB, Racusen LC, Sis B, Halloran PF, Birk PE, Campbell PM, Cascalho M, Collins AB, Demetris AJ, Drachenberg CB, Gibson IW, Grimm PC, Haas M, Lerut E, Liapis H, Mannon RB, Marcus PB, Mengel M, Mihatsch MJ, Nankivell BJ, Nickeleit V, Papadimitriou JC, Platt JL, Randhawa P, Roberts I, Salinas-Madriga L, Salomon DR, Seron D, Sheaff M, Weening JJ: Banff '05 Meeting Report: Differential diagnosis of chronic allograft injury and elimination of chronic allograft nephropathy (‘CAN’). Am J Transplant 7 :518 –526,2007 [DOI] [PubMed] [Google Scholar]
- 20.Dickenmann M, Oettl T, Mihatsch MJ: Osmotic nephrosis: Acute kidney injury with accumulation of proximal tubular lysosomes due to administration of exogenous solutes. Am J Kidney Dis 51 :491 –503,2008 [DOI] [PubMed] [Google Scholar]
- 21.Chacko B, John GT, Balakrishnan N, Kirubakaran MG, Jacob CK: Osmotic nephropathy resulting from maltose-based intravenous immunoglobulin therapy. Ren Fail 28 :193 –195,2006 [DOI] [PubMed] [Google Scholar]
- 22.Schwartz SL, Johnson CB: Pinocytosis as the cause of sucrose nephrosis. Nephron 8 :246 –254,1971 [DOI] [PubMed] [Google Scholar]
- 23.Abraham P, Indirani K, Sugumar E: Effect of cyclophosphamide treatment on selected lysosomal enzymes in the kidney of rats. Exp Toxicol Pathol 59 :143 –149,2007 [DOI] [PubMed] [Google Scholar]