Abstract
Background and objectives: Anti-endothelial cell antibody (AECA) can cause hyperacute rejection and immediate graft loss after renal transplantation; however, its prevalence and significance during acute rejection are unknown. Previous studies suggested that AECA may be detected in recipients with acute vascular rejection (AVR).
Design, setting, participants, & measurements: We retrospectively analyzed 653 cadaveric renal transplant recipients; circulating AECA was positive in 13 of 47 cases of AVR; another two cases of hyperacute rejection also had detectable AECA. Twenty-six cases of AVR without circulating AECA were selected as controls.
Results: AECA-positive AVR usually occurred within 1 yr after transplantation and mostly was resistant to steroid treatment. Compared with the control group, the AECA-positive group was associated with a significantly lower 1-yr graft survival rate (46.7 versus 80.5%; P = 0.038), and more patients had histologic interstitial plasma cell infiltration (53.8 versus 11.5%; P = 0.005). More patients with AECA-positive AVR experienced another one or more episodes of acute rejection during 1 yr of follow-up (75.0 versus 13.0%; P = 0.003). AECA-positive AVR with C4d deposition in peri-tubular capillaries had the worst outcome in this cohort, and it accounted for 38.5% graft loss in AVR. AECA in turn accounted for 71.4% of graft loss in C4d+ AVR.
Conclusions: Circulating AECA is associated with poor outcome in renal allograft recipients with acute rejection and should be monitored regularly.
In organ transplantation, vascular endothelial cells express tissue-specific antigens, which serve as targets for an immune response (1). Endothelial cell damage often induces tissue injury and inflammation, which usually leads to graft loss. Anti-endothelial cell antibodies (AECA) represent a heterogeneous group of non-HLA antibodies directly against a variety of antigenic determinants expressed on endothelial cells and can be detected in a variety of autoimmune and connective tissue diseases, especially in vascular disorder diseases (2–4). The presence of AECA has been previously reported in HLA-identical kidney transplants, indicating that AECA might be associated with transplant rejection (5,6). AECA can cause hyperacute rejection (HAR) and immediate graft loss (7–11). In the revised Banff criteria of 2001, it is believed that AECA can cause AMR in addition to antibodies to donor HLA (12).
Although it seems that AECA plays an important role in renal transplantation, previous reports were mainly focused on the association of AECA with HAR (7–9) and chronic rejection (11). The clinical and histologic characteristics of AR mediated by AECA are undefined, and the association between AECA and AMR is still unclear.
In a previous report (13), we found that AECA may be associated with histologic interstitial endoarteritis. This is consistent with traditional acute vascular rejection (AVR; met Banff 97 grade IIA, IIB, or III) (14). In this study, we reviewed AVR cases of 653 cadaveric renal allograft recipients and screened the serum samples for AECA. Clinical and histologic features of AECA-positive AVR were investigated.
Materials and Methods
Patients
All patient samples were collected from 653 cadaveric renal allograft recipients who underwent transplantation between April 1997 and August 2003 in Jinling Hospital, Nanjing University School of Medicine. Forty-seven cases of biopsy-proven AVR had been diagnosed, and two cases of HAR were also examined for AECA. Since 1997, we have stored serum samples of every patient for future study when renal allograft biopsies were performed. Stored sera were retrospectively screened for AECA.
Patients with AVR during the same period of time but without circulating AECA were assigned as the control group. The inclusion criteria were (1) biopsy-proven AR, (2) meeting the diagnosis of acute vascular rejection according to the classical Banff 97 criteria, (3) no history of vascular disorders before transplantation, and (4) serum AECA negative. Twenty-six patients who met these inclusion criteria were set as control group.
Pretransplantation cytotoxic cross-match (CDC) testing was performed according to the protocol of the Eurotransplant Organization. Pretransplantation cytotoxic panel reactive antibody (PRA) reactivity was assessed by using a panel of cells from 50 phenotyped donors before 2000; <20% was considered negative. Flow-PRA screening test was performed before transplantation since 2001 according to the guidelines provided by the supplier (One Lambda Inc., Canoga Park, CA). Sera with >10% flow-PRA class I and/or II reactivity were considered anti-HLA antibody positive. Flow-PRA technique was also used to detect posttransplantation HLA I and II reactive antibodies.
The research meets the ethical guidelines, including the legal requirements of China. The Human Subjects Committee of Jinling Hospital, Nanjing University School of Medicine, approved all study protocols.
Renal Allograft Pathology and C4d Staining
All AR episodes were proved by renal allograft biopsy. The diagnostic biopsies were performed immediately after the onset of rejection. Two needle biopsy cores were obtained from each renal allograft for morphologic study, and they were divided into two parts: One for formalin fixation and one for snap-freezing. Hematoxylin and eosin, periodic acid-Schiff, Methenamine-silver, and Masson stainings were routinely stained in formalin-fixed tissue. Fresh-frozen tissue was analyzed by immunofluorescence microscopy using a conventional panel of antibodies against IgG, IgM, IgA, C3, C4, and C1q. The remaining tissue was stored for further investigation. C4d staining was performed in frozen tissue using an indirect immunofluorescence technique as described previously (13). C4d staining was routinely performed since 2002. As to AVR episodes that occurred before 2002, C4d staining was performed on stored frozen sections. All biopsies met the Banff criteria for adequacy. No fewer than 10 glomeruli and two arterial sections were available in each slide.
AECA Detection
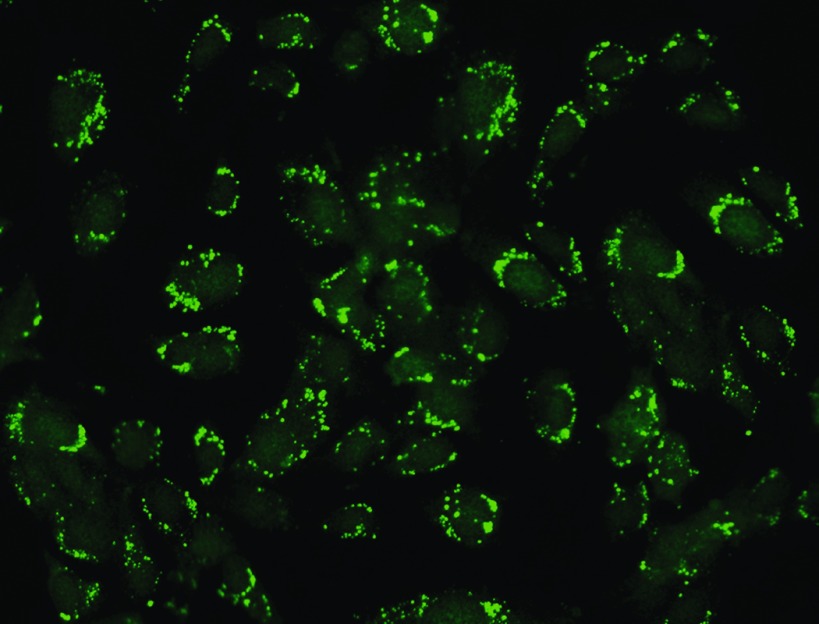
AECA titer was detected with indirect immunofluorescence using a diagnosis reagent kit from EUROIMMUN (Medizinische Labordiagnostika AG, Luebeck, Germany) with a TITERPLANE technique developed by EUROIMMUN. Serum species were taken on the biopsy day. Cultivated endothelial cells from umbilical vein covered the reaction areas of a BIOCHIP. Slides were incubated with a diluted serum sample. Positive reactions were marked by granular staining in cytoplasm of human umbilical vein endothelial cells with fluorescein-labeled anti-human antibodies and were visualized by fluorescence microscopy (Figure 1). The reference range is titer 1: <10. To evaluate the prevalence of AECA in general population after kidney transplantation, we screened AECA in 30 renal allograft recipients with stable graft function (16 to 45 d after transplantation), and none of them were positive for AECA in the serum.
Figure 1.
Circulating anti-endothelial cell antibodies (AECA) detection. Positive result is manifested as granular fluorescence in cytoplasm in cultivated endothelial cells from umbilical vein (HUVEC). Magnification, ×400.
Initial Immunosuppression
Primary immunosuppression consisted of cyclosporin A (CsA) or tacrolimus (TAC) + mycophenolate mofetil (MMF) + steroids. The initial dosage of MMF was 1.5 g/d, and the dosage of TAC and CsA was adjusted to trough levels (TAC 6 to 12 ng/ml from 0 to 6 mo and 5 to 10 ng/ml from 7 to 12 mo; CsA 180 to 250 ng/ml from 0 to 6 mo and 150 to 200 ng/ml from 7 to 12 mo). A standard corticosteroid tapering regimen that consisted of an intravenous bolus of methylprednisolone 500 mg on day 0 and day 2 was used, followed by oral prednisone 80 mg/d on day 3. The dosage was tapered 10 mg each day to 20 mg/d and then tapered slowly to 5 mg/d thereafter.
Judgments of Rescue Therapy Effect
The effect of rescue therapy was categorized into three groups: Reversal (graft function recovered to lower value within 1 mo of rescue therapy), controlled (graft function did not improve but remained stable without dependence on dialysis in the first month), and graft lost (recipient returned to dialysis owing to rejection within 1 mo after the rejection).
Statistical Analyses
Descriptive statistical values are expressed as the means ± SD. Between-group differences in frequencies of clinical characteristics were determined using the Fisher exact test. The analysis was done using the Stata 6.0 (Stata Corp., College Station, TX). P ≤ 0.05 was considered significant. Analysis of graft survival was performed using the Kaplan-Meier method using SPSS 10.0 for Windows (SPSS, Chicago, IL).
Results
Baseline Characteristics
In total, 15 patients had experienced AECA-positive AVR (n = 13) or HAR (n = 2). The baseline clinical characteristics are outlined in Table 1. There were eight men and seven women with an average age of 40.2 ± 8.5 (range 25 to 60). Only two patients had received a renal allograft before. All transplantations were ABO compatible. Ten episodes occurred within 1 mo after transplantation. Pretransplantation CDC cross-matches all were negative. Two patients had positive PRA before transplantation; however, pretransplantation PRA both were negative for the two cases of HAR. As to the baseline diseases, 73% were primary chronic glomerulonephritis, and none of them had history or signs of systemic diseases such as joint complain, fever, etc. before transplantation.
Table 1.
Detailed information of patients with acute/hyperacute rejection accompanied with circulating AECAa
| Patient | Gender/Age | Underlying Renal Disease | PRA (HLA I/II; %) |
Diagnostic Biopsyb | Banff Grade | C4d | AECA Titer | Response to Steroid | Other Treatment | Outcome | Current SCr (mo) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pretransplantation | On Rejection | |||||||||||
| 1 | F/25 | CGN | 3/0 | NA | 0 | HAR | + | 1:80 | Excised | No | Lost graft | Dialysis |
| 2 | F/33 | CGN | 15 | 3.28/0.09 | 0 | HAR | + | 1:80 | Excised | No | Lost graft | Dialysis |
| 3 | M/39 | CGN | 10 | 3.60/0.65 | 7 d | III | + | 1:10 | No | M+F | Controlled | Died of pneumonia |
| 4 | F/38 | CGN | 18 | 3.35/0.32 | 9 mo | IIA | + | 1:10 | No | IA+M+F | Lost graft | Dialysis |
| 5 | F/46 | Hypertension | 50/31c | 6.90/1.17 | 4 d | IIA | − | 1:10 | Yes | No | Reversed | 0.72 (78) |
| 6 | M/41 | CGN | 17 | 6.95/0.95 | 7 d | IIA | − | 1:20 | No | M+F | Lost graft | Dialysis |
| 7 | M/38 | Polycystic kidney | 8 | 2.66/2.74 | 55 d | IIA | + | 1:10 | Yes | No | Reversed | Dialysis |
| 8 | M/30 | CGN | 47c | NA | 34 d | IIB | − | 1:10 | No | ATG | Lost graft | Dialysis |
| 9 | M/44 | CGN | 16.7 | 23.2/0.35 | 2 d | IIA | − | 1:10 | No | ATG | Reversed | 1.24 (46) |
| 10 | M/34 | CGN | 2.08/1.71 | 4.25/1.06 | 6 d | IIB | − | 1:20 | Yes | M+F | Reversed | 0.98 (80) |
| 11 | F/39 | CGN | 1.61/9.8 | 38.4/14.9 | 4 d | IIB | + | 1:40 | No | IA+F+M | Reversed | 0.77 (41) |
| 12 | M/40 | Interstitial nephritis | 0.74/0.78 | 3.14/3.72 | 32 d | IIB | + | 1:10 | No | PP+F+M | Lost graft | Died of pneumonia |
| 13 | F/51 | CGN | 3.22/0.42 | 9.74/0.45 | 8 d | III | + | 1:80 | No | IA+F+M | Lost graft | Dialysis |
| 14 | F/60 | CGN | 4.20/2.12 | 5.96/0.28 | 45 d | IIA | − | 1:20 | No | M+F | Reversed | 1.86 (36) |
| 15 | M/45 | CGN | 0.86/6.60 | 2.73/7.86 | 7 d | IIB | − | 1:20 | No | M+F | Reversed | 2.58 (38) |
AECA, anti-endothelial cell antibody; C, cyclosporin; CGN, primary chronic glomerulonephritis; F, FK506 (tacrolimus); IA, immunoadsorption; M, mycophenolate mofetil; P, prednisolone; PP, plasmapheresis; PRA, panel reactivity antibody, it was assessed by using a panel of cells from 50 phenotyped donors before 2000, <20% was considered negative, flow-PRA screening test was performed before transplantation since 2001, sera with >10% flow-PRA class I and/or II reactivity were considered anti-HLA antibody-positive; SCr, serum creatinine; Z, CD25mAb (zanapax, Roche).
Measured by days after transplantation.
Transplantation was performed when PRA were negative in these patients.
Among the other 34 cases of AVR in the same period, 26 cases were assigned as the control group according to the aforementioned criteria; eight cases were excluded because of insufficient serum sample volume. There were no significant differences in recipient age, gender, pretransplantation PRA, CDC cross-match, and warm ischemia time for patients with AECA-positive AVR compared with AECA-negative ones (Table 2).
Table 2.
Clinical data of patients with AVR accompanied by circulating AECA or nota
| Parameter | AECA(+)(n = 13) | AECA(−)(n = 26) | P |
|---|---|---|---|
| Age (yr) | 41.9 ± 7.6 | 38.5 ± 6.6 | 0.161 |
| Gender (male/female) | 8/5 | 15/11 | 1.000 |
| Previous renal transplantation (%) | 2 (15.4) | 0 (0.0) | 0.105 |
| Pretransplantation CDC positive (%) | 0 | 0 | – |
| PRA positive (n [%]) | |||
| before transplantation | 2 (15.4) | 4 (15.4) | 1.000 |
| on rejection | 2/12 (16.7) | 7 (26.9) | 0.689 |
| Baseline immunosuppressants (n [%]) | |||
| MMF+CsA+Pred | 5 (38.5) | 10 (38.5) | 1.000 |
| MMF+TAC+Pred | 6 (46.2) | 14 (53.8) | 0.741 |
| Aza+CsA+Pred | 2 (15.4) | 2 (7.7) | 0.589 |
| IL-2mAb induction | 5 (38.5) | 10 (28.5) | 1.000 |
AVR, acute vascular rejection; Aza, azathioprine; CDC, complement-dependent cytotoxicity cross-match; CsA, cyclosporine; MMF, mycophenolate mofetil; Pred, prednisone; TAC, tacrolimus.
AECA Detection
In the AECA-positive AVR group, AECA titers ranged from 1:10 to 1:80. The two patients with HAR both had a titer of 1:80. The variation of AECA was monitored for patient 13, who experienced rising titer (1:10, 1:20, and 1:80) in the progress of graft loss. Pretransplantation circulating AECA was assayed in three patients (3, 6, and 7), and all were negative. In the control group, to exclude AECA-positive patients, AECA was reassayed and all were negative.
Posttransplantation HLA Reactive Antibodies Screening
Posttransplantation HLA class I and class II antibodies were screened with a flow cytometric method (Flow-PRA). Two patients were positive in the AECA-positive group; one patient who developed HAR had no anti-HLA antibodies either (Table 1). The incidence of positive Flow-PRA as well as the levels of HLA I and II reactive antibodies was similar in both groups (Table 2).
Clinical Manifestations
Except for the two patients with HAR, there were no significant differences in clinical manifestations of renal allograft between the two AVR groups. All patients with AVR experienced an initial graft function (reflected by decrease of serum creatinine level) before the rejection occurred. Fever, decrease of urine volume, and allograft dysfunction were observed in most recipients. Ultrasound examination revealed an enlarged renal allograft and increased vessel resistance index in each recipient (Table 3).
Table 3.
Histologic lesion of graft with AVR accompanied by circulating AECA or not
| Parameter (n [%]) | AECA(+)(n = 13) | AECA (−)(n = 26) | P |
|---|---|---|---|
| Mononuclear cell infiltration in the peritubular capillary area | 9 (69.2) | 16 (61.5) | 0.733 |
| Neutrophil infiltration in the peritubular capillary area | 7 (53.8) | 11 (42.3) | 0.520 |
| Glomerulitis with mononuclear cells (>5 mononuclear cells) | 7 (53.8) | 8 (30.8) | 0.185 |
| Tubulitis | 8 (61.5) | 12 (46.2) | 0.501 |
| Interstitial infiltration | 13 (100.0) | 26 (100.0) | – |
| Interstitial plasma cell infiltration | 7 (53.8) | 3 (11.5) | 0.005 |
| Intimal ateritis | 13 (100.0) | 26 (100.0) | – |
| Interstitial hemorrhage | 5 (38.5) | 7 (26.9) | 0.486 |
| C4d in peritubular capillary area | 6 (46.2) | 8 (30.8) | 0.482 |
| Vascular necrosis | 2 (15.4) | 2 (7.7) | 0.589 |
| Banff 97 grade | |||
| II | 11 (84.6) | 23 (88.5) | 1.000 |
| III | 2 (5.4) | 3 (11.5) | 1.000 |
Pathology Findings
Regarding the 15 episodes of AECA-positive rejection, two HAR episodes were proved by pathologic evidences from the excised grafts; other episodes of rejection had been shown by biopsies. All patients had varying degrees of endoarteritis, accompanied by interstitial infiltration. According to Banff 97 criteria (14), seven met the criteria of IIA, four met IIB, and two met III. Compared with AECA-negative AVR, significantly more patients had interstitial plasma cell infiltrations (53.8 versus 11.5%; P = 0.005) in renal allografts of patients with AECA-positive AVR (patients with AHR were excluded from this group). There were no significant differences in the interstitial hemorrhage, cell infiltration in peri-tubular capillaries (PTC), and glomerulitis. We did not find significant differences between the incidence of C4d staining between the two groups. C4d staining was positive in eight (53.3%) patients in all AECA-positive patients (including HAR). Eight (30.8%) patients in the control group had C4d deposition in PTC (seven of them were PRA positive when rejection occurred). There was no significant difference in the incidence of other lesions, such as monocyte interstitial infiltration, interstitial hemorrhage, glomerulitis, and tubulitis. According to the Banff ’97 classification, the grades of rejection were also comparable.
Rescue Treatment and Clinical Outcome
Bolus corticosteroid therapy (methylprednisolone 500 mg/d for 3 d) was selected as the first-line treatment. Concomitantly, all of the patients were given MMF (1.5 g/d) and TAC (trough levels maintained at 8 to 15 ng/ml) before 1999. Grafts were excised immediately from the two patients who had HAR, and they returned to dialysis. Only three of the 13 patients with AVR responded to the pulse methylprednisolone treatment. Three patients received two or three sessions of immunoadsorption, one received five sessions of plasmapheresis, and two received antithymocyte-globulin; however, five patients directly lost renal grafts as a result of rejection and returned to dialysis, including three patients who had received immunoadsorption or plasmapheresis. In the control group, 15 patients had response to pulse methylprednisolone, and only one needed immunoadsorption. Finally, only three lost renal allografts, and one had not received any rescue treatment because of severe pneumonia. Significantly more recipients developed another episode of AR in the AECA-positive group (75.0 versus 13.0%; P = 0.003) within 1 yr. One patient died of cytomegalovirus pneumonia 9 mo after the episodes (Table 4). The 1-yr graft survival rate was 53.8 versus 80.8% in the AECA-positive and -negative AVR groups. AECA was associated with a significantly lower 1-yr graft survival (46.7 versus 80.8%; P = 0.038).
Table 4.
Treatments and outcomesa
| Parameter (n [%]) | AECA(+)(n = 13) | AECA (−)(n = 26) | P |
|---|---|---|---|
| Steroid sensitive rejection | 3 (23.1) | 15 (57.7) | 0.051 |
| Patients received IA or PP | 4 (30.8) | 1 (3.8) | 0.035 |
| Patients received ATG | 2 (15.4) | 4 (15.4) | 1.000 |
| Patients lost renal graft as a result of AR | 4 (30.8) | 3 (11.5)b | 0.194 |
| Patients experienced another episode of AR during 1 yr follow-up | 6/8 (75.0) | 3/23 (13.0) | 0.003 |
| 1-yr graft survival | 7 (53.8) | 21 (80.8) | 0.131 |
| 1-yr patient survival | 11 (84.6) | 25 (96.2) | 0.253 |
ATG, antithymocyte globulin.
One of the three patients had not received any antirejection therapy for accompanied severe pulmonary infection and lost his graft at last.
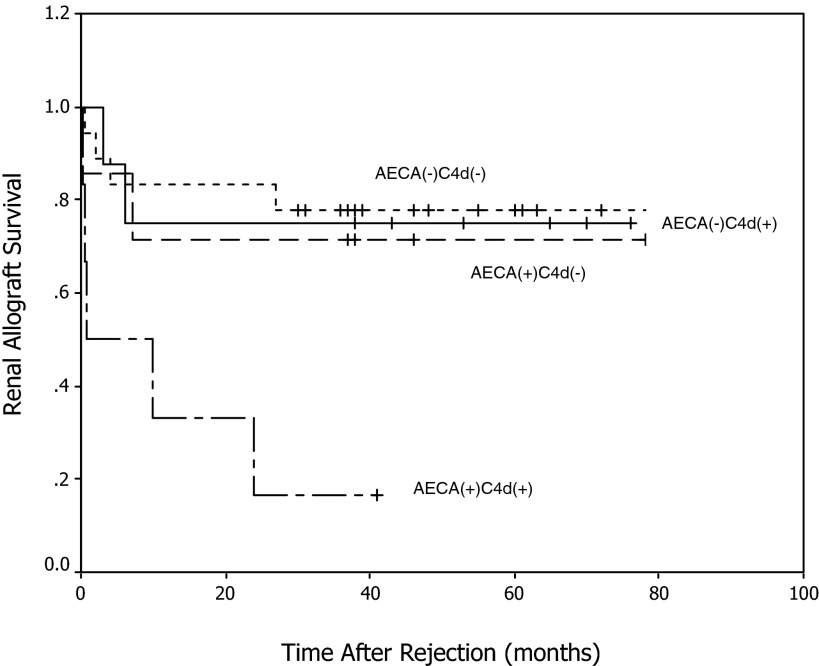
Thirteen patients lost grafts during 3 yr of follow-up (not including the two patients with HAR). The 3-yr graft survival was 16.7, 71.4, 75, and 77.8% in AECA(+)C4d(+) (n = 6), AECA(+)C4d(−) (n = 7), AECA(−)C4d(+) (n = 8), and AECA(−)C4d(−) (n = 18) AVR, respectively (Figure 2). C4d-positive AECA-positive AVR had the worst outcome in this cohort and accounted for 38.5% of graft loss in AVR. AECA-positive cases in turn accounted for 71.4% of graft loss in C4d-positive AVR.
Figure 2.
Renal allograft survival in patients who had acute vascular rejection (AVR) with different C4d and AECA status.
Discussion
AECA was shown to be a very important non-HLA antibody in organ transplantation; however, the incidence of AECA-positive AR is very low, and relevant investigations are limited. In this study, we report 13 renal allograft recipients with AECA-positive AR, and it suggests that circulating AECA is associated with graft loss in renal allograft recipients with AR and should be monitored regularly.
In clinical manifestations AECA-positive AVR was associated with a poor response to the treatment and poor outcomes. Significantly more patients of AECA-positive AVR were resistant to steroid. Although they received more aggressive treatment protocols, they had worse outcomes with a 1-yr survival rate of only 46.7%. This is even worse than that of AHR, for which as we reported that 1-yr survival rate is nearly 80% (15), and early episodes can be easily reversed by treatment of MMF combined with TAC in a Chinese population with negative pretransplantation PRA (16,17).
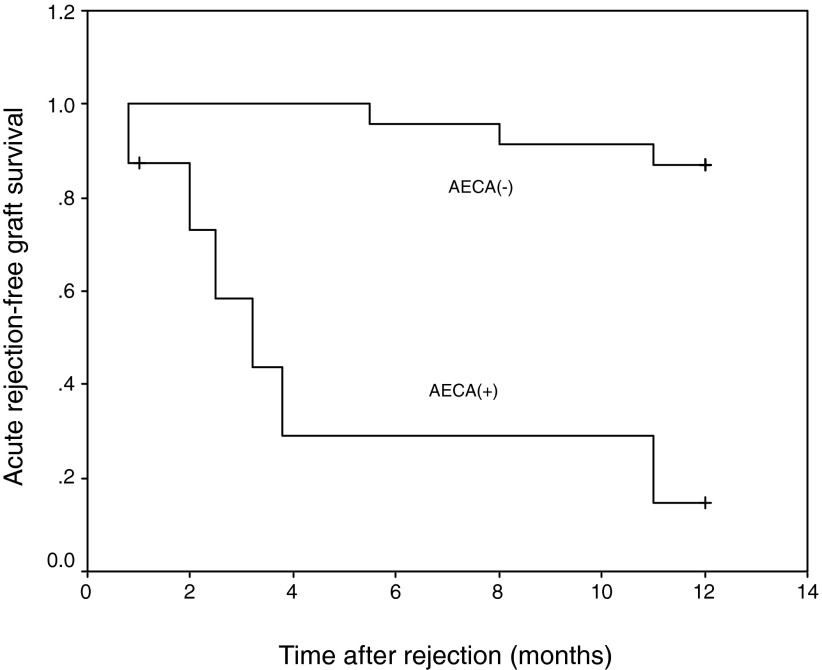
During the follow-up, it is very interesting that AECA-positive cases were associated with multiple episodes of AR (Figure 3). It has been reported that pretransplantation AECA is associated with multiple AR episodes after transplantation (18). Our finding suggests that posttransplantation AECA is associated with multiple AR episodes as well. This finding also suggests that these patients need more aggressive maintenance immunosuppressive agents, because they are at high risk for AR. This finding also supports the hypothesis that AECA plays a role in the development of AR. It is necessary to point out that the two cases of HAR during the period of study were AECA positive. They were negative for pretransplantation PRA and the CDC cross-match, but they had high titers of AECA when HAR occurred, which also indicates that AECA might play some role in the development of HAR. This finding is consistent with several previous reports (8,9). We previously reported (13) three renal allograft recipients who developed C4d-positive AR with detectable circulating AECA; the variation of the titer in these patients during the course of AR reflected that AECA might play an important role in the development of AR.
Figure 3.
Acute rejection–free survival rate after the previous AVR.
We previously found that most patients have some degree of vascular injury, and all met the traditional diagnosis of AVR (13). Interestingly, when compared with AECA-negative AVR, significantly more patients of AECA-positive AVR (patients with HAR not included) had interstitial plasma cell infiltrations in renal allografts. It seems that most AECA-positive AR is plasma cell–rich rejection; however, although plasma cell infiltration was reported to be associated with vascular rejection, it is not a strong predictor to the outcome (19,20). The role of plasma cells and their relationship with AECA remains to be defined.
Some patients had AECA and rejected very early (<7 d), suggesting that the antibodies might have been preformed before transplantation; however, in patients with kidney disease, mostly AECA is seen in systemic disease, especially in vascular disorder diseases (2–4); none of these patients had history or signs of systemic diseases, such as joint pain, or fever, before transplantation. Patients 3, 6, and 7 were tested for AECA before transplantation, and all were negative. This might suggest that AECA may emerge after transplantation. These will be confirmed by a prospective study that monitors AECA before and after transplantation. Actually, in another ongoing study, we are noticing that preexisting AECA is not always related to posttransplantation AR, but posttransplantation AECA does predict poor outcome for AR.
It is speculated that circulating AECA can directly lead to AMR after renal transplantation (12,13,21); however, because the prevalence of AECA is usually correlated with HLA alloimmunization (22), it is difficult to determine whether AECA is the original cause or only one of the mediators of tissue injuries. Excluding the role of anti-HLA antibodies should be of help, but we also need to exclude many kinds of non–HLA-type antibodies (23,24). We retrospectively assayed serum samples of patients when rejection occurred using a flow cytometry technique (Flow-PRA) (25). We found that only two patients were positive for HLA-reactive antibodies in the AECA-positive group, which was comparative to the AECA-negative group. The level of HLA I and II reactive antibodies was similar in both groups. HLA class II antibodies were even lower in AECA-positive groups, although there was no statistical significance; however, there is some possibility that HLA reactive antibodies were adsorbed by the graft; this can be excluded in a prospective study by analyzing the serum after removal of graft.
Circulating AECA can be detected in many methods. In one study (22), the investigators did not find any significant effect of pretransplantation AECA on AR or 5-yr graft survival rate. This negative result may be associated with the small patient numbers as the authors suggested. Another difference is that we used a different method to detect AECA. It is clear that different detection methods for AECA have different sensitivity and specificity; we used indirect immunofluorescence to detect the AECA titers in our center. In vasculitis and connective tissue diseases, this method has high specificity, and it seems to have obvious connections with clinicohistologic features. This suggests that the effect of AECA might depend on the amount in the sera.
Conclusions
AECA-positive AVR is associated with interstitial plasma cell infiltration, steroid resistance, poor outcomes, and a high risk for one or more episodes of AR. Circulating AECA in C4d-positive AVR predicts a very high incidence of graft loss, even more so than C4d deposition. All of these findings suggest that circulating AECA is associated with poor outcome in renal allograft recipients with acute rejection; therefore, AECA should be monitored among patients who developed allograft rejection after transplantation.
Disclosures
None.
Acknowledgments
This work was supported in part by a grant from the General Program of National Natural Science Foundation of China (30600572). Q.S. is supported by a grant from the American Society of Transplantation.
We thank Prof. Gregg Hadley and Dr. Bryan Anthony at Ohio State University and Dr. Yichao Wu at Harvard Medical School for valuable comments and assistance in proofing the language and Dr. Chunxia Zheng and Ms. Hong Zhou for technical support.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Pober JS, Orosz CG, Rose ML, Savage CO: Can graft endothelial cells initiate a host anti-graft immune response? Transplantation 61 :343 –349,1996 [DOI] [PubMed] [Google Scholar]
- 2.Meroni PL, Del Papa ND, Raschi E, Tincani A, Balestrieri G, Youinou P: Antiendothelial cell antibodies (AECA): From a laboratory curiosity to another useful autoantibody. In: The Decade of Autoimmunity, edited by Shoenfeld Y, Amsterdam, Elsevier Science BV,1999. , pp285 –294
- 3.Youinou P: New target antigens for antiendothelial cell antibodies. Immunobiology 210 :789 –797,2005 [DOI] [PubMed] [Google Scholar]
- 4.Kallenberg CGN, Tervaert JW: Systemic vasculitis. In: The Molecular Pathology of Autoimmune Diseases, edited by Theofilopoulos AN, New York, Taylor & Francis,2002. , pp483 –505
- 5.Cerilli J, Brasile L, Galouzis T, Lempert N, Clarke J: The vascular endothelial cell antigen system. Transplantation 39 :286 –289,1985 [DOI] [PubMed] [Google Scholar]
- 6.Paul LC, Baldwin WM 3rd, van Es LA: Vascular endothelial alloantigens in renal transplantation. Transplantation 40 :117 –123,1985 [DOI] [PubMed] [Google Scholar]
- 7.Rose ML: Role of MHC and non-MHC alloantibodies in graft rejection. Curr Opin Organ Transplant 9 :16 –22,2004 [Google Scholar]
- 8.Jordan SC, Yap HK, Sakai RS, Alfonso P, Fitchman M: Hyperacute allograft rejection mediated by anti-vascular endothelial cell antibodies with a negative monocyte crossmatch. Transplantation 46 :585 –587,1988 [DOI] [PubMed] [Google Scholar]
- 9.Sumitran-Karuppan S, Tyden G, Reinholt F, Berg U, Moller E: Hyperacute rejection of two consecutive renal allografts and early loss of third transplant caused by non-HLA antibodies specific for endothelial cells. Transpl Immunol 5 :321 –327,1997 [DOI] [PubMed] [Google Scholar]
- 10.Perrey C, Brenchley PE, Johnson RW, Martin S: An association between antibodies specific for endothelial cells and renal transplant failure. Transpl Immunol 6 :101 –106,1998 [DOI] [PubMed] [Google Scholar]
- 11.Ferry BL, Welsh KI, Dunn MJ, Law D, Proctor J, Chapel H,Yacoub MH, Rose ML: Anti-cell surface endothelial antibodies in sera from cardiac and kidney transplant recipients: Association with chronic rejection. Transpl Immunol 5 :17 –24,1997 [DOI] [PubMed] [Google Scholar]
- 12.Racusen LC, Colvin RB, Solez K, Mihatsch MJ, Halloran PF, Campbell PM, Cecka MJ, Cosyns JP, Demetris AJ, Fishbein MC, Fogo A, Furness P, Gibson IW, Glotz D, Hayry P, Hunsickern L, Kashgarian M, Kerman R, Magil AJ, Montgomery R, Morozumi K, Nickeleit V, Randhawa P, Regele H, Seron D, Seshan S, Sund S, Trpkov K: Antibody-mediated rejection criteria: An addition to the Banff ’97 Classification of Renal Allograft Rejection. Am J Transplant 3 :708 –714,2003 [DOI] [PubMed] [Google Scholar]
- 13.Sun Q, Liu Z, Yin G, Chen H, Chen J, Li L: Detectable circulating antiendothelial cell antibodies in renal allograft recipients with C4d-positive acute rejection: a report of three cases. Transplantation 79 :1759 –1762,2005 [DOI] [PubMed] [Google Scholar]
- 14.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, et al.: The Banff 97 working classification of renal allograft pathology. Kidney Int 55 :713 –723,1999 [DOI] [PubMed] [Google Scholar]
- 15.Sun Q, Liu ZH, Yin G, Chen H, Chen J, Ji S, Li LS: Tacrolimus combined with mycophenolate mofetil can effectively reverse C4d-positive steroid-resistant acute rejection in Chinese renal allograft recipients. Nephrol Dial Transplant 21 :510 –517,2006 [DOI] [PubMed] [Google Scholar]
- 16.Sun Q, Liu ZH, Cheng Z, Chen J, Ji S, Zeng C, Li LS: Treatment of early mixed cellular and humoral renal allograft rejection with tacrolimus and mycophenolate mofetil. Kidney Int 71 :24 –30,2007 [DOI] [PubMed] [Google Scholar]
- 17.Sun Q, Liu ZH, Ji S, Chen J, Tang Z, Zeng C, Zheng C, Li LS: Late and early C4d-positive acute rejection: Different clinico-histopathological subentities in renal transplantation. Kidney Int 70 :377 –383,2006 [DOI] [PubMed] [Google Scholar]
- 18.Nakagawa Y, Saito K, Morioka T, Tomita Y, Takahashi K, Oite T: The clinical significance of antibody to vascular endothelial cells after renal transplantation. Clin Transplant 16 [Suppl 8]:51 –57,2002 [DOI] [PubMed] [Google Scholar]
- 19.Meehan SM, Domer P, Josephson M, Donoghue M, Sadhu A, Ho LT, Aronson AJ, Thistlethwaite JR, Haas M: The clinical and pathologic implications of plasmacytic infiltrates in percutaneous renal allograft biopsies. Hum Pathol 32 :205 –215,2001 [DOI] [PubMed] [Google Scholar]
- 20.Gartner V, Eigentler TK, Viebahn R: Plasma cell-rich rejection processes in renal transplantation: Morphology and prognostic relevance. Transplantation 81 :986 –991,2006 [DOI] [PubMed] [Google Scholar]
- 21.Takemoto SK, Zeevi A, Feng S, Colvin RB, Jordan S, Kobashigawa J, Kupiec-Weglinski J, Matas A, Montgomery RA, Nickerson P, Platt JL, Rabb H, Thistlethwaite R, Tyan D, Delmonico FL: National conference to assess antibody-mediated rejection in solid organ transplantation. Am J Transplant 4 :1033 –1041,2004 [DOI] [PubMed] [Google Scholar]
- 22.Le Bas-Bernardet S, Hourmant M, Coupel S, Bignon JD, Soulillou JP, Charreau B: Non-HLA-type endothelial cell reactive alloantibodies in pre-transplant sera of kidney recipients trigger apoptosis. Am J Transplant 3 :167 –177,2003 [DOI] [PubMed] [Google Scholar]
- 23.Dragun D, Müller DN, Bräsen JH, Fritsche L, Nieminen-Kelhä M, Dechend R, Kintscher U, Rudolph B, Hoebeke J, Eckert D, Mazak I, Plehm R, Schönemann C, Unger T, Budde K, Neumayer HH, Luft FC, Wallukat G: Angiotensin II type 1-receptor-activating antibodies in renal-allograft rejection. N Engl J Med 352 :558 –569,2005 [DOI] [PubMed] [Google Scholar]
- 24.Zou Y, Stastny P, Süsal C, Döhler B, Opelz G: Antibodies against MICA antigens and kidney-transplant rejection. N Engl J Med 357 :1293 –1300,2007 [DOI] [PubMed] [Google Scholar]
- 25.Pei R, Wang G, Tarsitani C, Rojo S, Chen T, Takemura S, Liu A, Lee J: Simultaneous HLA class I and class II antibodies screening with flow cytometry. Hum Immunol 59 :313 –322,1998 [DOI] [PubMed] [Google Scholar]