Abstract
Background and objectives: Glomerular lesions in allografts in recipients with end-stage nephritis resulting from systemic lupus erythematosus (SLE) were examined to determine the spectrum of glomerular pathology in recurrent glomerulonephritis (GN).
Design, setting, participants, & measurements: A total of 156 biopsy samples, from 49 serial allografts in 43 recipients with end-stage lupus nephritis, were examined by light microscopy, and by immunofluorescence and electron microscopy in selected cases. These were compared with control allografts (n = 35).
Results: Glomerular lesions best explained by recurrent lupus nephritis were observed in 19 of 49 allografts (38.8%) in lupus recipients. Three categories of glomerulopathies were identified: 1) immune complex glomerulopathies, including mesangial GN (28%) and membranous GN (4%); 2) atypical glomerulopathies, including acute proliferative GN (32%) and focal segmental glomerulosclerosis (12%), with scant immune deposits in glomerular capillaries, frequent endothelial tubuloreticular inclusions, and thrombotic microangiopathy; and 3) transplant-associated glomerulopathies (24%).
Conclusions: Allografts from recipients with SLE had typical immune complex-mediated GN and atypical pauci-immune, proliferative GN and segmental glomerular sclerosis. Atypical glomerulopathies like these suggest a role for nonimmune complex-mediated glomerular injury in recurrent lupus GN.
Glomerulonephritis (GN) is a cause of end-stage kidney disease in 20% to 40% of renal allograft recipients (1). Nephritis in systemic lupus erythematosus (SLE) progresses to end-stage renal failure in up to 50% of patients (2). Diagnosis of recurrent lupus nephritis is based on similarity of pathologic features of disease in the transplant to that of the native kidney (3–9). Lupus GN has been described in renal allografts in the absence of clinical and serologic evidence of recurrent SLE (7,8). The frequency of recurrent lupus nephritis is estimated at between 2% and 10% of allografts (3–7); however, recurrence rates of 30% (8,9) and 43.8% (10) have been described. Reported recurrent lupus GN includes classes II, III, IV, and V lesions (3–10), by the International Society of Nephrology/Renal Pathology Society (ISN/RPS) criteria (11). Lesions described in these studies resembled lupus GN of the native kidney histologically and had ′full house′ immunoglobulin (Ig) deposition in glomeruli.
Biopsy evaluation of the renal allograft may provide a window for observation of factors potentially important in the development of glomerular injury in early phases of recurrent lupus nephritis. Observation of incidental glomerular lesions may allow identification of early patterns of renal injury before development of clinical nephritis. To determine the spectrum of proliferative glomerular lesions potentially attributable to recurrent lupus GN, we retrospectively examined glomerular pathology in a sample of renal transplants in recipients with SLE and compared them with nonlupus controls.
Materials and Methods
All patients at the University of Chicago Hospitals with a diagnosis of end-stage lupus nephritis who underwent renal transplantation between 1991 and 2005 (n = 49) were included in this retrospective review and are designated the lupus group. The primary diagnosis of SLE was established when clinical and serologic features met criteria of the American College of Rheumatology (12). Extensive clinical and laboratory data were tabulated. Thirty-five of 49 recipients had lupus nephritis in native biopsies (5 class III, 17 class IV, 10 class V, 3 class VI, using 1982 WHO criteria (13)), 2 patients did not have native kidney biopsies, and data were unavailable in 12 recipients. A total of 156 biopsies were obtained from 49 of 55 allografts in 43 of 49 recipients with underlying SLE. The median number of biopsies was 2 per allograft with a range from 1 to 14. The mean time of biopsy was 21.9 mo with a range from 1 d to 114 mo. Kidney allograft biopsies demonstrating glomerular lesions by light microscopy were selected for analysis. Acute transplant glomerulitis was defined by intracapillary mononuclear cells with endothelial swelling in more than one third of the glomerular area. The mesangium was intact, and there was no podocyte proliferation or crescent formation. Chronic transplant glomerulopathy was defined by double contours of the glomerular capillary basement membrane in 10% or more of the glomerular capillaries (14), with or without interposition, mesangial lysis, or sclerosis. Thrombotic microangiopathy was defined as platelet-fibrin thrombi in one or more glomeruli or arterioles, with or without mesangial lysis or obliterative arteriolopathy. Acute proliferative GN was defined as endocapillary or extracapillary hypercellularity, with infiltrating mononuclear cells and/or neutrophils, with mesangial widening and lobular expansion. Focal segmental glomerulosclerosis was defined as segmental consolidation or collapse of the glomerular tuft, with accumulation of extracellular matrix and prominence or proliferation of visceral epithelium.
A nonlupus control group was chosen from contemporaneous renal allograft recipients, matched by age and gender distribution and with comparable immunosuppressive regimens to the lupus recipients. The controls consisted of 35 allografts in 34 recipients. End-stage renal disease in this group was caused by glomerular disease (n = 15), hypertensive nephrosclerosis (n = 8), congenital anomalies (n = 4), diabetic nephropathy (n = 3), sickle cell nephropathy (n = 2), and interstitial nephritis (n = 2). A total of 124 biopsies were included in this nonlupus control group. The biopsy tissues for light microscopic evaluation met Banff criteria for adequacy (14) in 155 of 156 (99.4%) in the lupus group and in 122 of 124 (98.3%) in the nonlupus controls.
From 1991 to 2002, kidney transplant tissue was not routinely saved for immunofluorescence or electron microscopy. Nineteen allografts in the lupus group and seven from the controls had additional tissue saved for immunofluorescence or for electron microscopy at biopsy. Three graft biopsies from the lupus group with glomerular lesions were subjected to paraffin immunofluorescence using published protocols (15). IgG, IgA, IgM, and fibrin were detected by direct immunofluorescence (DIF) on frozen or paraffin tissue sections. Complement components C3 and C1q were detected on frozen sections and were not reliably detectable on paraffin sections by DIF. C4d was sought by indirect immunofluorescence on frozen section in 12 allografts from the lupus recipients and in 6 controls. Immunoperoxidase was used for C4d detection in 3 allografts from lupus recipients and in 2 controls. The intensity of immunofluorescence staining was scored on a 0 to 4+ scale. Electron microscopic examination of 1 or more glomeruli was performed in 21 of the lupus group and in 4 controls. Differences in frequencies of histologic parameters were determined using χ2 analysis and the Fisher exact test.
Results
A comparison of the demographic and clinical details of all recipients with lupus who were biopsied (n = 43) and the controls (n = 34) is presented in Table 1. Forty-nine allografts in 43 recipients with SLE were biopsied. Twenty-five of 49 allografts in lupus recipients (51%) had glomerular pathology compared with 10 of 35 (28.6%) of the nonlupus control group (Table 2). The glomerulopathies in lupus recipients were classified as 1) immune complex GN, including mesangial GN (n = 7) and membranous GN (n = 1); 2) atypical glomerulopathies, including acute proliferative GN (n = 8) and de novo focal segmental glomerulosclerosis (FSGS) (n = 3); and 3) transplant-associated glomerulopathies (transplant glomerulopathy (n = 3), thrombotic microangiopathy (TMA) (n = 2), and nonspecific mesangial proliferation (n = 1). Tables 3 and 4 present the pathologic features, and Table 5 presents clinical and laboratory features of the lupus recipients with immune complex and atypical glomerulopathies unique to this group. Two lupus recipients had hepatitis C infection, but neither had identifiable glomerulopathy on biopsy. In addition to transplant-associated glomerulopathies, the controls had recurrent IgA nephropathy with necrotizing and crescentic GN (n = 1) and de novo FSGS (n = 2), with arteriolopathic changes suggestive of calcineurin inhibitor toxicity.
Table 1.
Demographic data for SLE recipients biopsied from 1991 to 2005 and controls
| Recipient characteristics | Lupus (n = 43) | Nonlupus (n = 34) |
|---|---|---|
| Age at transplant (yr (mean ±SD)) | 35 ± 13.1 | 34.2 ± 11.2 |
| Gender (M:F) | 10:33 | 8:26 |
| Transplant number | ||
| First | 38 | 29 |
| Second | 4 | 4 |
| Third | 1 | 1 |
| Donor source | ||
| Deceased | 30 | 24 |
| Living | 13 | 10 |
| Maintenance immunosuppression | ||
| Prednisone | 40 | 34 |
| Tacrolimus | 25 | 22 |
| Cyclosporine | 18 | 12 |
| Mycophenolate | 24 | 20 |
| Rapamycin | 0 | 1 |
| Azathioprine | 16 | 13 |
| Induction | 23 | 24 |
| Anti-IL-2R | 14 | 10 |
| ATGAM | 7 | 6 |
| Thymoglobulin | 0 | 3 |
| OKT3 | 2 | 5 |
| HLA mismatches (mean ±SD) | 3.1 ± 1.7 | 3.6 ± 1.7 |
| Hepatitis C virus antibodies | 2 | 0 |
Table 2.
Glomerular pathology in allografts from lupus and controls
| Lupus |
Nonlupus |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Total allografts biopsied | 49 | 35 | ||
| Time of biopsy (mo) (mean) | 12.9 | 11.1 | ||
| Range | 1 d to 114 mo | 3 d to 96 mo | ||
| Immune complex GN | ||||
| Mesangial (I or II) | 7 | 14.3 | 0 | 0 |
| Membranous | 1 | 2 | 0 | 0 |
| Atypical glomerulopathies | ||||
| Acute proliferative GN | ||||
| With IgG immune deposits | 4 | 8.2 | 0 | 0 |
| With no IgG | 4 | 8.2 | 0 | 0 |
| Focal segmental glomerulosclerosis de novo | 3 | 6.1 | 2 | 5.8 |
| Transplant related glomerulopathies | ||||
| Transplant glomerulopathy | 3 | 6.1 | 3 | 8.5 |
| Thrombotic microangiopathy | 2 | 4.1 | 4 | 11.4 |
| Nonspecific mesangial | 1 | 2 | 0 | 0 |
| Other glomerular lesions | ||||
| Crescentic GN with IgA immune deposits | 0 | 0 | 1 | 2.9 |
| Total with glomerulopathy | 25 | 51 | 10 | 28.6 |
Table 3.
Light microscopic findings in immune complex and pauci-immune glomerulopathies
| Case no. | No. of glomeruli | Hypercellularity |
Necrosis/karyorrhexis | Crescents | Foam cells | Glomerular sclerosis |
Glomerulopathy | ISN/RPS class | Native biopsy | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocapillary | Mesangial | Segmental | Global | ||||||||
| Mesangial | |||||||||||
| 1 | 12 | 0 | 70.0% | 0 | 0 | 0 | 0 | 0 | Mesangial GN | II | NA |
| 2 | 22 | 0 | 4.5% | 0 | 0 | 0 | 0 | 14.0% | Mesangial GN | II | No biopsy |
| 3 | 11 | 0 | 0 | 0 | 0 | 0 | 0 | 27.0% | Mesangial GN | I | V |
| 4 | 18 | 0 | 11.0% | 0 | 0 | 0 | 0 | 0 | Mesangial GN | II | IV |
| 5 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Mesangial GN | I | VI |
| 6 | 31 | 0 | 0 | 0 | 0 | 0 | 0 | 6.5% | Mesangial GN | I | IV |
| 7 | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | Mesangial GN | I | No biopsy |
| Membranous GN | |||||||||||
| 8 | 12 | 0 | 41.6% | 0 | 0 | 0 | 0 | 58.0% | Membranous GN | V | V |
| Acute proliferative GN | |||||||||||
| 9 | 13 | 38.5% | 15.3% | 15.4% | 7.7% | 0 | 23.1% | 23.0% | FS GN | III (A/C) | NA |
| 10 | 19 | 36.8% | 26.3% | 5.3% | 10.5% | 5.3% | 5.3% | 11.0% | FS GN | III (A/C) | IV |
| 11 | 13 | 24.0% | 0 | 8.0% | 8.0% | 8.0% | 0 | 15.0% | FG GN | III (A/C) | NA |
| 12 | 15 | 20.0% | 53.0% | 6.6% | 6.6% | 13.2% | 13.4% | 20.0% | FG GN | III (A/C) | IV |
| 13 | 16 | 19.0% | 30.0% | 19.0% | 18.8% | 0 | 6.3% | 44.0% | FS GN | III (A/C) | IV |
| 14 | 18 | 83.0% | 20.0% | 33.0% | 16.7% | 28.0% | 11.0% | 0 | DS GN | IV-S (A/C) | V |
| 15 | 18 | 38.9% | 11.1% | 16.7% | 5.5% | 0 | 11.1% | 28.0% | FS GN | III (A/C) | IV |
| 16 | 32 | 0.0% | 30.0% | 3.0% | 3.1% | 0 | 9.0% | 3.0% | FS GN | III (A/C) | IV |
| Focal segmental glomerulosclerosis | |||||||||||
| 17 | 22 | 32.0% | 0 | 0 | 0 | 18.2% | 45.0% | 0 | FSGS, CG | — | IV |
| 18 | 18 | 0 | 94.0% | 0 | 0 | 0 | 11.0% | 0 | FSGS | — | III, V |
| 19 | 13 | 0 | 50.0% | 0 | 0 | 15.40% | 8.0% | 0 | FSGS | — | V |
D, diffuse; F, focal; S, segmental; G, global; GN, glomerulonephritis, MH, mesangial hyperplasia; IF, interstitial fibrosis; TA, tubular atrophy; TMA, thrombotic microangiopathy; ATN, acute tubular necrosis; NA, not applicable.
Table 4.
Pathologic findings in immune complex GN and atypical glomerulopathies in lupus recipients
| Case no. | Immunofluorescence |
Electron microscopy |
Other pathology | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG | IgA | IgM | C3 | C1q | Fibrin | Glomerular location | PTC C4d | Electron dense deposits | TRI | ||
| Mesangial | |||||||||||
| 1 | 1 | 1 | 1 | NA | NA | 0 | m | NA | m | + | Old infarct |
| 2 | NA | NA | NA | NA | NA | NA | NA | NA | m | + | Diffuse IF and TA |
| 3 | 1 | 0.5 | 0.5 | 1 | 1 | 0 | m | − | m, sendo | + | Polyoma virus nephropathy |
| 4 | 1.5 | 1 | 1 | 0 | 0 | 0 | m | − | m | + | — |
| 5 | 2 | 0 | 0 | 0.5 | 0 | 0 | m | − | NA | NA | Focal IF and TA |
| 6 | 1 | 1 | 0 | 0 | 0 | 0 | m | − | 0 | + | Polyoma virus nephropathy |
| 7 | 1 | 1 | 0 | 0 | 0 | 0 | m | − | 0 | + | Acute rejection, type 1B |
| Membranous GN | |||||||||||
| 8 | 2 | 0 | 0 | 2 | 2 | 0 | cw | − | sepi, IM | + | Diffuse IF and TA |
| Acute proliferative GN | |||||||||||
| 9 | 0 | 0 | 0.5 | 0 | NA | 0 | cw, m | NA | 0 | + | Borderline infiltrate |
| 10 | 0 | 0 | 1 | 1 | 0 | 0 | cw, m | − | NA | NA | Borderline infiltrate, TMA |
| 11 | 0 | 0 | 0 | 0 | NA | 0 | − | − | 0 | 0 | Acute rejection, type 1B |
| 12 | 1 | 0 | 0.5 | 0 | 0 | 0 | cw, m | − | m, sendo | 0 | TMA |
| 13 | 1.5 | 0 | 1 | 1 | 1 | 2 | cw, m | − | sepi | + | — |
| 14 | 0.5 | 1 | 2 | NA | NA | 2 | cw, m | − | m, sendo | + | Borderline infiltrate, TMA |
| 15 | 1 | 0 | 0.5 | 0 | NA | 0 | cw, m | − | m, sendo, sepi | + | Arteriosclerosis |
| 16 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | TMA |
| Focal segmental glomerulosclerosis | |||||||||||
| 17 | 0 | 0 | 0.5 | 0 | 0 | 0 | cw | − | 0 | + | ATN |
| 18 | 0 | 0 | 1 | NA | NA | 0 | m | NA | 0 | + | TMA, ATN |
| 19 | 2 | 0 | 2 | 1.5 | 1.5 | 0 | m | − | 0 | + | TMA, ATN |
GN, glomerulonephritis; PTC, peritubular capillary; TRI, tubuloreticular inclusions; m, mesangial, cw, capillary wall; sendo, subendothelial; sepi, subepithelial; IF, interstitial fibrosis; TA, tubular atrophy; TMA, thrombotic microangiopathy; ATN, acute tubular necrosis; NA, not applicable.
Table 5.
Clinical data in immune complex GN and atypical glomerulopathies in lupus recipients
| Case no. | Time of biopsy (mo) | At presentation |
Follow-up time (mo) | SCr | Proteinuria | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SCr | Proteinuria | Hematuria | Anti-ANA | dsDNA | C3 | C4 | |||||
| Mesangial | |||||||||||
| 1 | 18 | 2.1 | 0 | 0 | <40 | <10 | 133 | 37 | 2 | 8 | NA |
| 2 | 36 | 1.5 | 0.1 | 1+ | NA | <10 | 150 | 28 | 36 | 1.5 | NA |
| 3 | 30 | 4.5 | 0.3 | 1+ | 1280 | <10 | 78 | 24 | 12 | 9.4 | NA |
| 4 | 7 | 1.2 | 0 | 0 | 1280 | 20 | 105 | 21 | 16 | 1.2 | NA |
| 5 | 36 | 1.4 | 0.3 | 1+ | 160 | <10 | 123 | 43 | 9 | 1.6 | 0.15 |
| 6 | 42 | 1.7 | 0.2 | 1+ | 320 | <10 | NA | NA | 6 | 1.6 | 0.08 |
| 7 | 1 | 2.0 | 0.2 | 1+ | 320 | <10 | NA | NA | 12 | 1.5 | 0.2 |
| Membranous GN | |||||||||||
| 8 | 68 | 2.5 | 1.1 | 1+ | NA | <10 | 106 | 39 | 48 | 5 | 0.44 |
| Acute proliferative GN | |||||||||||
| 9 | 23 | 2.2 | 4+ | 1+ | NA | NA | NA | NA | 12 | 11.1 | NA |
| 10 | 29.5 | 3.3 | 4.1 | NA | <40 | <10 | NA | NA | 6 | 7 | 3.2 |
| 11 | 12 | 3.5 | 0.5 | tr | 320 | <10 | 107 | 25 | 24 | 3.3 | 0.4 |
| 12 | 95 | 1.3 | 4.3 | NA | 160 | <10 | 208 | 77 | 4 | 2 | NA |
| 13 | 114 | 3.1 | 3.8 | 1+ | 1280 | NA | 63 | 16 | NA | NA | NA |
| 14 | 5.9 | 2.1 | 10.5 | 2+ | 1280 | <10 | 168 | 20 | 4 | 3 | 0.8 |
| 15 | 92 | 2.5 | 1.5 | 0 | + | ± | 139 | 40 | 3 | 2.8 | 1.35 |
| 16 | 5 | 1.3 | NA | NA | NA | NA | NA | NA | 26 | 13.6 | 3+ |
| Focal segmental glomerulosclerosis | |||||||||||
| 17 | 1.6 | 1.9 | 3.6 | 4+ | <40 | <10 | 56 | 11 | 59 | 1.8 | 1.2 |
| 18 | 26 | 3.9 | 3+ | 3+ | <40 | <10 | 62 | 42 | 2 | 3.1 | NA |
| 19 | 36 | 1.2 | 0.2 | 0 | 2560 | <10 | 107 | 31 | 60 | 1.3 | 0.2 |
NA, not applicable; SCr, serum creatinine (mg/dl; reference range, C3, 88–201 mg/dl; C4, 16–47 mg/dl). Proteinuria is given by urinary protein to creatinine ratio or urinary dipstick analysis (0–4+). Hematuria is given by dipstick analysis (0–4+).
Immune Complex GN
Mesangial immune complex GN was identified in cases 1 to 7. Three of 7 had mild mesangial hypercellularity in 4.5%, 11%, and 70% of glomeruli, respectively (Table 3). In the remaining four, the glomeruli were unremarkable by light microscopy. Mesangial deposits containing IgG, IgA, IgM, C3, and C1q were evident by DIF (Table 4). By electron microscopy, six had endothelial tubuloreticular inclusions, four had mesangial electron dense deposits, one had occasional subendothelial deposits, and four had focal effacement of the podocytes.
Membranous GN was identified in case 8. The allograft biopsy had diffuse glomerular basement membrane thickening with moderate interstitial fibrosis, tubular atrophy, and arteriosclerosis. Diffuse granular staining of the glomerular capillaries for IgG, C3, and C1q was evident by DIF. Electron microscopy revealed global subepithelial electron dense deposits with spike formation, and segmental intramembranous deposits. Occasional mesangial electron dense deposits were also identified. Endothelium had no identifiable tubuloreticular inclusions. Subsequent biopsy had membranous GN with deposits containing IgG, IgM, C3, C1q, and C4d. Electron microscopy revealed sparse subepithelial and intramembranous electron dense deposits.
Atypical Glomerulopathies
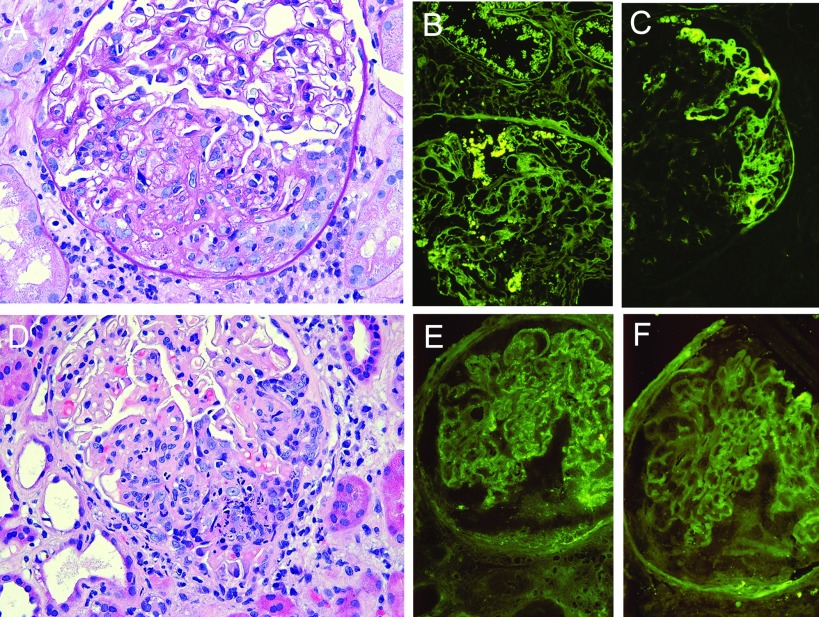
Acute proliferative GN was observed in cases 9 to 16. Seven biopsies had exudative and proliferative endocapillary GN in 19% to 83% of glomeruli (Table 3). Four had glomerular fibrinoid necrosis, four others had segmental marked endocapillary karyorrhexis, and eight had focal crescent formation (Figures 1, A and D, 2D). Seven had segmental sclerosis of glomerular tufts in addition to proliferative lesions. Three had focal arteriolar TMA and one additional case had focal glomerular thrombi. Wire loops, hyaline pseudothrombi, and hematoxyphil bodies were not evident. Hyaline arteriolosclerosis was evident in 6 of 8 biopsies: severe in 2 and mild in 4. Mild to moderate interstitial fibrosis and tubular atrophy were evident in 5 biopsies. Four patients with glomerular endocapillary foam cells, a finding described in cytomegalovirus viremia in renal allograft recipients (16), had negative cytomegalovirus cultures of blood and urine at the time of the biopsy. There were occasional segmental glomerular basement membrane double contours in three biopsies; however, diagnostic features of chronic transplant glomerulopathy were not observed.
Figure 1.
Representative examples of acute proliferative glomerulonephritis. (A) Case 14 demonstrating segmental proliferative and exudative glomerulonephritis, fibrinoid necrosis, and a cellular crescent. (B) Segmental staining of the mesangium and of protein droplets in podocytes and tubular epithelium for IgG and (C) segmental endocapillary and extracapillary fibrin/fibrinogen deposition by direct immunofluorescence. (D) Case 13 demonstrating segmental endocapillary and extracapillary proliferative and exudative glomerulonephritis with abundant karyorrhexis (apoptosis). (E) Direct immunofluorescence for IgG with segmental 1+ granular staining of the glomerular capillary walls and (F) albumin control.
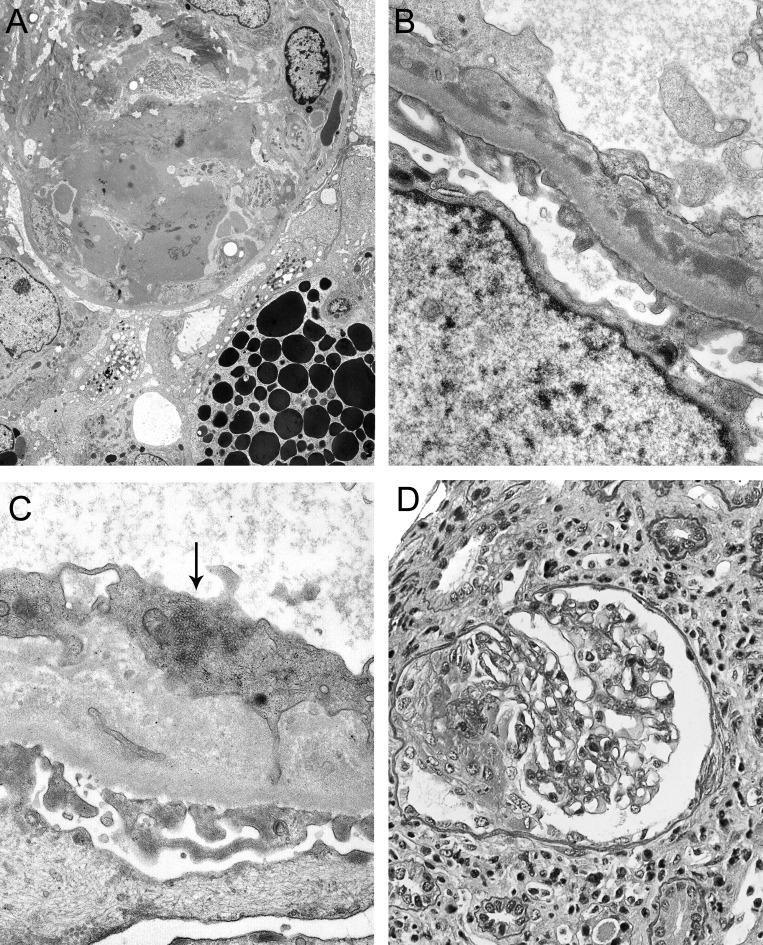
Figure 2.
Ultrastructural and other features in acute proliferative glomerulonephritis. (A) Electron micrograph of a capillary loop with severe endothelial injury, exudates with fibrin tactoids, inflammatory cells, and epithelial injury with detachment and new basement membrane in the absence of electron dense deposits (case 14). Detachment podocytopathy indicates severe podocyte injury and may herald segmental sclerosis. (B) Segmental subendothelial electron dense deposits were identifiable in less inflamed capillary segments (case 14) and associated with subendothelial new basement membrane formation. (C) Tubuloreticular inclusion in endothelium (case 9). (D) Case 16 demonstrating crescentic glomerulonephritis.
By DIF (n = 7), four biopsies had mesangial and focal segmental capillary wall immune deposits containing IgG at low intensity (0.5 to 1.5+) (Figure 1, B and E) and two had fibrin deposits (Figure 1C). None of six biopsies evaluated had diffuse peritubular capillary C4d staining. Electron microscopy (n = 6) revealed electron dense deposits in the mesangium and scant segmental subendothelial deposits in four biopsies (Figure 2, A and B), and four had endothelial tubuloreticular inclusions (Figure 2C). Effacement of podocyte foot processes was diffuse in three, focal in two, and absent in one. Three biopsies had no detectable immune deposits, and two of these had endothelial tubuloreticular inclusions. Case 16 had no immunofluorescence or electron microscopy but had focal segmental glomerular necrosis and cellular crescents (Figure 2D), without endocapillary hypercellularity, in serial biopsies 2 mo apart, and is included in this category because of features not explicable by transplant-associated glomerulopathies.
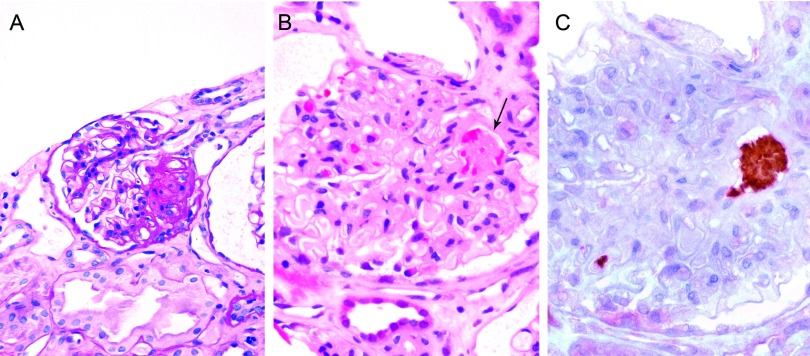
De novo focal segmental glomerulosclerosis was identified in cases 17 to 19. The predominant lesion was FSGS, including collapsing and hyalinizing variants (Figure 3A). FSGS affected 45%, 11%, and 8% of glomeruli in each biopsy, respectively, and 2 had mesangial hypercellularity. Case 17 had endocapillary mononuclear cells in 32% of glomeruli. Cases 18 and 19 had focal TMA involving arterioles and glomeruli, with fibrin platelet thrombi (Figure 3, B and C). Case 19 had mesangial IgG and complement components C3 and C1q, and the other cases had diffuse granular mesangial IgM only by DIF. C4d and fibrin were not evident. Electron microscopy revealed endothelial tubuloreticular inclusions and no electron dense deposits. Podocyte effacement was diffuse in two and focal in one biopsy. Patients 17 and 18 had renal allograft biopsies 1 and 2 mo before the index, revealing mild acute transplant glomerulitis. Patient 17 had a subsequent biopsy at 57 mo, revealing hyalinizing FSGS. Autopsy at 28 mo, in case 18, revealed focal segmental glomerular proliferation and sclerosis, TMA, and acute tubular necrosis in the kidney transplant.
Figure 3.
Focal segmental glomerulosclerosis in lupus recipients. (A) Case 18 demonstrating segmental glomerular sclerosis with hyalinization and adhesion. (B) Occlusive thrombus at the glomerular hilus (arrow). (C) Immunohistochemical staining for CD61 confirms glomerular platelet thrombosis.
Clinical Features
The biopsies with mesangial GN were obtained from seven patients at 1 to 42 mo (median, 30 mo) after transplantation (Table 5). The mean serum creatinine at biopsy was 2.06 mg/dl (range, 1.2 to 4.5 mg/dl). Five of 7 had proteinuria and hematuria. Membranous GN was identified in one renal allograft at 68 mo, with a serum creatinine of 2.5 mg/dl and proteinuria and hematuria. Acute proliferative GN was identified in 8 allograft biopsies procured from 6 patients at 5 to 114 mo (median, 26.3 mo) after transplantation. The mean serum creatinine at biopsy was 2.4 mg/dl (range, 1.3 to 3.5 mg/dl). Proteinuria and hematuria were detected in 4 of 5 patients with data. The three biopsies with de novo FSGS were obtained at 1.6, 26, and 36 mo after transplantation, with creatinine values of 1.2 to 3.9 mg/dl and proteinuria with hematuria in two of three. None of the lupus recipients had clinical manifestations of SLE at the time of biopsy of the allograft. All were HBsAg negative and were HIV and HCV antibody negative. One of 3 patients tested in the mesangial GN group had circulating IgG anticardiolipin antibodies detectable. One patient, of five tested in the acute proliferative GN group, had IgM anticardiolipin antibodies. Of four patients with thrombotic microangiopathy on biopsy in the acute proliferative GN group, two tested negative for anticardiolipin antibodies and two lacked data. The antineutrophil cytoplasmic autoantibody titer was within normal limits in 2 patients (cases 14 and 15) tested. Two patients with FSGS had thrombotic microangiopathy on biopsy: one had IgG anticardiolipin antibodies and one tested negative for these antibodies. None of the FSGS group had exposure to pamidronate or other bisphosphonates, and none was massively obese.
After follow-up periods of 6 to 36 mo, the mean serum creatinine for 5 patients with mesangial GN was 1.48 mg/dl (range, 1.2 to 1.6 mg/dl). Two grafts were lost from acute rejection at 20 and 42 mo. The patient with membranous GN excreted 440 mg of urinary protein per day and the serum creatinine was 5.0 mg/dl at 116 mo after transplantation. After the index biopsy, 4 patients with acute proliferative GN had stable serum creatinine values of 2.0 to 3.3 mg/dl at follow-up periods of 3 to 26 mo. Three patients had marked elevation of serum creatinine (7 to 13.6 mg/dl) at follow-up periods of 6 to 26 mo attributable to GN in two and GN with megalocytic interstitial nephritis in one. Two patients died during the follow-up period, at 60 and 120 mo after transplantation. Patient 13 was lost to follow-up. In the FSGS group, one patient had persistent graft dysfunction and died 2 mo after the index biopsy from necrotizing bronchopneumonia. Patients 17 and 19 had serum creatinine values of 1.8 and 1.3 mg/dl and low-grade proteinuria at 59 and 60 mo of follow-up, respectively.
Transplant-associated Glomerulopathies
The frequency of transplant glomerulopathy was similar in the lupus group and controls (3 of 49, 6.1% versus 3 of 35, 8.5%, P = 0.49). Calcineurin inhibitor-associated TMA was more frequent in controls than in the lupus group (2 of 49, 4.1% versus 4 of 35, 11.4%, P = 0.4). Eighteen of 49 biopsies (36.7%) in the lupus group and 15 of 35 (42.9%) in the controls had hyaline arteriolopathy (P = 0.65). These observations indicate that patients in the lupus group did not appear to have a greater propensity for development of these lesions.
Relationship of Glomerulopathies in the Lupus Group to Acute Rejection and TMA
In the lupus group, allografts with acute proliferative GN had concurrent or prior acute rejection in 4 of 8 cases (50%), compared with 12 of 24 allografts without identifiable glomerulopathy (50%). Controls had concurrent or prior acute rejection in 9 of 10 (90%) with glomerulopathy and in 18 of 25 (72%) with no identifiable glomerulopathy. An association of proliferative GN and acute rejection was not observed. TMA was evident in the lupus group with and without glomerulopathy (6 of 23, 26.1% versus 2 of 26, 7.7%, P = 0.12), and in controls without, but not with, glomerulopathy (4 of 29, 13.8% versus 0 of 6, 0%). The frequency of TMA observed in the proliferative GN and FSGS groups combined (6 of 11, 55%) was significantly greater than in the lupus group without glomerulopathy (P = 0.038) and the controls (P = 0.02).
Recurrence Rate of Lupus Glomerulonephritis
Glomerular lesions best explained by recurrence of lupus nephritis were observed in 19 of 49 allografts (38.8%) in lupus recipients. Four met criteria for class I and three for class II lesions by the ISN/RPS criteria (11). Seven biopsies had focal GN and morphologic similarities to class III lupus GN, with minimal IgG deposits in three, no detectable IgG in three, and no immunofluorescence data in one. One had diffuse segmental GN and met morphologic criteria for class IV-S lupus GN, with weak staining for IgG. One had membranous nephropathy (class V). Three had segmental glomerular sclerosis, with few or no immune deposits, and were unclassifiable by ISN/RPS criteria (11).
Glomerulopathy and Graft Survival
There were no significant differences in graft loss rates in the lupus group with glomerulopathy compared with those without glomerulopathy (death censored graft loss, 32% versus 20.7%, P = 0.37), with follow-up periods from 4 to 153 mo. Graft loss was highest at 37.5% in the acute proliferative GN subgroup and lower in the mesangial GN subgroup at 28.6%.
Discussion
Glomerular lesions were observed in just over half of allografts in lupus recipients and could be divided into three groups: 1) immune complex-mediated glomerulopathies with mesangial changes resembling class I and class II lupus GN, and recurrent membranous GN; 2) atypical lesions consisting of acute proliferative GN and de novo FSGS each with scant immune deposits; and 3) lesions considered unrelated to SLE, including transplant glomerulopathy and CI-associated TMA. Atypical glomerular lesions with segmental glomerular inflammation, necrosis, sclerosis, tubuloreticular inclusions, and scant immune complex deposition are the most striking finding in the study group. Glomerular morphology was inconsistent with acute transplant glomerulitis or chronic transplant glomerulopathy, and other types of GN were excluded. The exclusive presence of these active and sclerosing glomerular lesions in the lupus group is consistent with recurrent lupus GN. Absence of clinical or serologic criteria for SLE does not exclude recurrent lupus nephritis (7,8). A possible vasculitic mechanism of glomerular injury is raised by the observation of glomerular necrosis (50%), karyorrhexis (50%), and cellular crescents (100%) with relatively little glomerular capillary immune complex deposition, in the acute proliferative GN group. Two patients tested had negative ANCA titers; however, ANCA were not studied systematically. The paucity of immune deposits may potentially be an effect of immunosuppressive therapy; however, the observations suggest that severe glomerular injury may arise in the absence of immune deposits.
Current models of the pathogenesis of lupus nephritis give central importance to the deposition of immune complexes as the trigger for the development of glomerular inflammation (17). Generally, the severity of glomerular injury correlates with the severity of immune complex accumulation in proliferative lupus GN of native kidney (18–21). Microvascular thrombosis (22), pauci-immune vasculitis-like lesions (23–27), and podocytopathy (28,29) are additional forms of glomerular injury described in native lupus nephritis. Examples of pauci-immune, necrotizing GN have been reported in SLE (23–25), in one series associated with a high frequency of TMA (25). Recent systematic analyses of diffuse proliferative lupus nephritis identified segmental exudative and necrotizing GN with predominantly mesangial immune deposits (26,27,30). These studies provide evidence of disproportionately severe glomerular injury with scant subendothelial immune complex deposition and thus may be considered atypical forms of lupus GN. Identification of pathologic facsimiles in allografts of lupus recipients in this study provides further evidence of a role for nonimmune complex mechanisms of glomerular injury in SLE.
TMA and segmental glomerular sclerosis were observed significantly more frequently in lupus recipients than controls, an observation raising the possibility of a pathogenetic link between these lesions. The possible contribution of antiphospholipid nephropathy associated with SLE (31) is suggested by the observation of anticardiolipin antibodies in 3 of 7 patients tested; however, these antibody titers were not systematically studied in our patients. Glomerular thrombosis may be a frequent observation in proliferative lupus GN of native kidneys (22). TMA also raises the differential diagnosis of hepatitis C infection-associated antiphospholipid nephropathy (32), humoral rejection, and CI toxicity. None of the recipients with TMA had hepatitis C infection. Peritubular capillary C4d deposition was undetectable and TMA is therefore unlikely to be attributable to humoral rejection. Although the frequency of hyaline arteriolosclerosis was similar in lupus and control groups, indicating no apparent increased risk of CI toxicity in the lupus group, a possible direct or synergistic effect of CI toxicity in the development of TMA cannot be excluded.
The mesangial lesions in this series were discovered incidentally in biopsies obtained for allograft dysfunction from other causes. The observed frequency of recurrent ISN/RPS class I mesangial lupus nephritis in these allografts is undoubtedly an underestimate because there was limited availability of frozen tissue for DIF. These lesions appear to have a benign course and graft loss was attributable to other causes. Transformation of these lesions was not observed. Use of light microscopy to identify glomerulopathies underestimates the frequency of class I and pure class V lupus nephritis.
Conclusion
A spectrum of glomerular lesions arising in renal allografts for end-stage lupus nephritis that includes pauci-immune GN, microvascular thrombosis, podocytopathy, and immune complex deposition is described. The findings suggest a variety of possible mechanisms of injury in recurrent lupus glomerulopathy, many of which arise in the absence of much detectable immune complex deposition. These observations support the hypothesis that nonimmune complex-mediated inflammation, thrombosis, and podocytopathy are significant mechanisms of glomerular injury in SLE.
Disclosures.
There were no competing financial interests.
Acknowledgments
The authors thank Dr. Melvin M. Schwartz for helpful insights in preparation of the manuscript and Denise Wiler for her photographic skills.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Chadban S: Glomerulonephritis recurrence in the renal graft. J Am Soc Nephrol 12 :394 –402,2002 [DOI] [PubMed] [Google Scholar]
- 2.Appel GA, Radhakrishnan J, D'Agati VD. Secondary glomerular disease. In: The Kidney, 4th ed, edited by Brenner BM, Philadelphia, Saunders,2004. , pp1381 –1481
- 3.Mojcik CF, Klippel JH: End stage renal disease and systemic lupus erythematosus. Am J Med 101 :100 –107,1996 [DOI] [PubMed] [Google Scholar]
- 4.Azevedo LS, Romao JE Jr, Malheiros D, Saldhana LB, Ianhez LE, Sabbaga E: Renal transplantation in systemic lupus erythematosus: a case control study of 45 patients. Nephrol Dial Transplant 13 :2894 –2898,1998 [DOI] [PubMed] [Google Scholar]
- 5.Grimbert P, Frappier J, Bedrossian J, Legendre C, Antoine C, Hiesse C, Bitker MO, Sraer JD, Lang P: Long term outcome of kidney transplantation in patients with systemic lupus erythematosus: a multicenter study. Transplantation 66 :1000 –1003,1998 [DOI] [PubMed] [Google Scholar]
- 6.Moroni G, Tantardini F, Gallelli B, Quaglini S, Banfi G, Poli F, Montagnino G, Meroni P, Messa P, Ponticelli C: The long-term prognosis of renal transplantation in patients with lupus nephritis. Am J Kidney Dis 45 :903 –911,2005 [DOI] [PubMed] [Google Scholar]
- 7.Stone JH, Millward CL, Olson JL, Amend WJ, Criswell LA: Frequency of recurrent lupus nephritis among ninety-seven renal transplant patients during the cyclosporine era. Arthritis Rheum 41 :678 –686,1998 [DOI] [PubMed] [Google Scholar]
- 8.Goral S, Ynares C, Shappell SB, Snyder S, Feurer ID, Kazancioglu R, Fogo AB, Helderman JH: Recurrent lupus nephritis in renal transplant recipients revisited: it is not rare. Transplantation 75 :651 –656,2003 [DOI] [PubMed] [Google Scholar]
- 9.Weng F, Goral S: Recurrence of lupus nephritis after renal transplantation: if we look for it will we find it? Nat Clin Pract (Nephrol) 1 :62 –63,2005 [DOI] [PubMed] [Google Scholar]
- 10.Nyberg G, Blohme I, Perrson H, Olausson M, Svalander C: Recurrence of SLE in transplanted kidneys: a follow up transplant biopsy study. Nephrol Dial Transplant 7 :1116 –1123,1992 [PubMed] [Google Scholar]
- 11.Weening JJ, D'Agati VD, Schwartz MM, Seshan SV, Alpers CE, Appel GB, Balow JE, Bruijn JA, Cook T, Ferrario F, Fogo AB, Ginzler EM, Hebert L, Hill G, Hill P, Jennette JC, Kong NC, Lesavre P, Lockshin M, Looi LM, Makino H, Moura LA, Nagata M; International Society of Nephrology Working Group on the Classification of Lupus Nephritis; Renal Pathology Society Working Group on the Classification of Lupus Nephritis: The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int 65 :521 –530,2004 [DOI] [PubMed] [Google Scholar]
- 12.Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfiled NF, Schaller JG, Talal N, Winchester RJ: The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 25 :1271 –1277,1982 [DOI] [PubMed] [Google Scholar]
- 13.Churg J, Sobin LH: Renal Disease: Classification and Atlas of Glomerular Disease, Tokyo, Igaku-Shoin, Tokyo,1982
- 14.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P, Gaber LW, Gibson IW, Glotz D, Goldberg JC, Grande J, Halloran PF, Hansen HE, Hartley B, Hayry PJ, Hill CM, Hoffman EO, Hunsicker LG, Lindblad AS, Yamaguchi Y, et al: The Banff 97 working classification of renal allograft pathology. Kidney Int 55 :713 –723,1999 [DOI] [PubMed] [Google Scholar]
- 15.Dorsett B, Ioachim H: A method for the use of immunofluorescence on paraffin embedded tissues. Am J Clin Pathol 69 :66 –72,1978 [DOI] [PubMed] [Google Scholar]
- 16.Richardson WP, Colvin RB, Cheeseman SH, Tolkoff-Rubin N, Herrin JT, Cosimi AB, Collins AB, Hirsch MS, McCluskey RT, Russell PS, Rubin RH: Glomerulopathy associated with cytomegalovirus viremia in renal allografts. N Engl J Med 305 :57 –63,1981 [DOI] [PubMed] [Google Scholar]
- 17.Shlomchik MJ, Madaio MP: The role of antibodies and B cells in the pathogenesis of lupus nephritis. Springer Semin Immunopathol 24 :363 –375,2003 [DOI] [PubMed] [Google Scholar]
- 18.D'Agati VD: Renal disease in systemic lupus erythematosus, mixed connective tissue disease, Sjogrens syndrome, and rheumatoid arthritis. In: Heptinstall's Pathology of the Kidney, 6th ed, edited by Jennette JC, Olson JL, Schwartz MM, Silva F, Philadelphia, Lippincott-Raven,1998. , pp541 –624
- 19.Koffler D, Agnello V, Carr RI, Kunkel HG: Variable patterns of immunoglobulin and complement deposition in kidneys of patients with systemic lupus erythematosus. Am J Pathol 56 :305 –316,1969 [PMC free article] [PubMed] [Google Scholar]
- 20.Andres GA, Accinni L, Beiser SM, Christian CL, Cinotti CA, Erlanger BF, Hsu KC, Seegal BC: Localization of fluorscein labeled antinucleoside antibodies in glomeruli of patients active systemic lupus erythematosus. J Clin Invest 49 :2106 –2118:1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dujovne I, Pollak VE, Pirani CL, Dillard MG: The distribution and character of glomerular deposits in systemic lupus erythematosus. Kidney Int 2 :33 –50,1972 [DOI] [PubMed] [Google Scholar]
- 22.Kant KS, Polak VE, Weiss MA, Glueck HI, Miller MA, Hess EV: Glomerular thrombosis in systemic lupus erythematosus: prevalence and significance. Medicine 60 :71 –85,1981 [DOI] [PubMed] [Google Scholar]
- 23.Schwartz MM, Roberts JL, Lewis EJ: Necrotizing glomerulitis of systemic lupus erythematosus. Hum Pathol 14 :158 –167,1983 [DOI] [PubMed] [Google Scholar]
- 24.Akhtar M, Al-Dalaan A, El-Ramahi KM: Pauci-immune necrotizing lupus nephritis: report of two cases. Am J Kidney Dis 23 :320 –325,1994 [DOI] [PubMed] [Google Scholar]
- 25.Charney DA, Nassar G, Truong L, Nadasdy T: ′Pauci-immune′ proliferative and necrotizing glomerulonephritis with thrombotic microangiopathy in patients with systemic lupus erythematosus and lupus-like syndrome. Am J Kidney Dis 35 :1193 –1206,2000 [DOI] [PubMed] [Google Scholar]
- 26.Hill GS, Delahousse M, Nochy D, Bariety J: Class IV-S versus class IV-G lupus nephritis: clinical and morphologic differences suggesting different pathogenesis. Kidney Int 68 :2288 –2297,2005 [DOI] [PubMed] [Google Scholar]
- 27.Najafi CC, Korbet SM, Lewis EJ, Schwartz MM, Reichlin M, Evans J: Significance of histologic patterns of glomerular injury upon long-term prognosis in severe lupus glomerulonephritis. Kidney Int 59 :2156 –2163,2001 [DOI] [PubMed] [Google Scholar]
- 28.Kraft SW, Schwartz MM, Korbet SM, Lewis EJ: Glomerular podocytopathy in patients with systemic lupus erythematosus. J Am Soc Nephrol 16 :175 –179,2005 [DOI] [PubMed] [Google Scholar]
- 29.Hertig A, Droz D, Lesavre P, Grunefeld J-P, Rieu P: SLE and idiopathic nephrotic syndrome: coincidence or not? Am J Kidney Dis 40 :1179 –1184,2002 [DOI] [PubMed] [Google Scholar]
- 30.Mittal B, Hurwitz S, Rennke H, Singh AK: New subcategories of class IV lupus nephritis: are there clinical, histologic, and outcome differences? Am J Kidney Dis 44 :1050 –1059,2004 [DOI] [PubMed] [Google Scholar]
- 31.Daugas E, Nochy D, Huong DLT, Duhaut P, Beaufils H, Caudwell V, Bariety J, Piette J-C, Hill G: Antiphospholipid syndrome nephropathy in systemic lupus erythematosus. J Am Soc Nephrol 13 :42 –52,2002 [DOI] [PubMed] [Google Scholar]
- 32.Baid S, Pascual M, Williams WW, Tolkoff-Rubin N, Johnson SM, Collins B, Chung RT, Delmonico FL, Cosimi AB, Colvin RB: Renal thrombotic microangiopathy associated with anticardiolipin antibodies in hepatitis C positive renal allograft recipients. J Am Soc Nephrol 10 :146 –153,1999 [DOI] [PubMed] [Google Scholar]