Abstract
Background and objectives: Management of hyperphosphatemia, a predictor of mortality in chronic kidney disease, is challenging. Nonadherence to dietary phosphate binders, in part, contributes to uncontrolled serum phosphorus levels. This phase IIIb trial assessed the efficacy of increased dosages (3000 to 4500 mg/d) of reformulated lanthanum carbonate (500-, 750-, and 1000-mg tablets) in nonresponders to dosages of up to 3000 mg/d.
Design, setting, participants, & measurements: This 8-wk study with a 4-mo open-label extension enrolled 513 patients who were undergoing maintenance hemodialysis. Patients who achieved serum phosphorus control at week 4 with ≤3000 mg/d lanthanum carbonate entered cohort A; nonresponders were randomly assigned to receive 3000, 3750, or 4500 mg/d (cohort B). The primary outcome measure was the control rate for predialysis serum phosphorus levels at the end of week 8, among patients in cohort B.
Results: At the end of week 4, 54% of patients achieved serum phosphorus control at dosages ≤3000 mg/d administered as one tablet per meal. Among patients who entered cohort B, control rates of 25, 38, and 32% for patients who were randomly assigned to 3000, 3750, or 4500 mg/d lanthanum carbonate, respectively, were achieved, with no increase in adverse events. Patients and physicians reported significantly higher levels of satisfaction with reformulated lanthanum carbonate compared with previous phosphate binders, partly because of reduced tablet burden with higher dosage strengths. Physicians and patients also expressed a preference for lanthanum carbonate over previous medication.
Conclusions: Reformulated lanthanum carbonate is an effective phosphate binder that may reduce daily tablet burden.
Decrease in renal phosphorus excretion is an important consequence of progressive chronic kidney disease (CKD) such that hyperphosphatemia is highly prevalent among patients who undergo maintenance dialysis. In patients with stage 5 CKD, numerous studies have now demonstrated that hyperphosphatemia is an independent predictor of all-cause mortality and fatal and nonfatal cardiovascular events (reviewed in reference [1]). Based, in part, on data linking hyperphosphatemia to cardiovascular disease, the 2003 Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommend maintaining serum phosphorus between 3.5 and 5.5 mg/dl (1.13 and 1.78 mmol/L) in patients with stage 5 CKD (2).
Not withstanding the recognition of its importance, the management of hyperphosphatemia in patients with stage 5 CKD remains challenging. It is widely believed that with conventional dialytic techniques, nonadherence to dietary recommendation, dialysis schedules, and phosphate-binder medications contributes significantly to this challenge. Phosphate binders are only one of many medications that patients with stage 5 CKD are prescribed. A survey of almost 4000 patients who were beginning maintenance dialysis therapy indicated that patients were prescribed a median of eight different medications (3). Among all incident maintenance hemodialysis patients in 1993, 34% were prescribed ≥10 different medications, and 8% were prescribed ≥15 different medications (3). A more recent survey of >10,000 prevalent maintenance hemodialysis patients who were undergoing treatment at Dialysis Clinics Inc. units reported that these patients were prescribed a median of 12 different medications (4). It seems reasonable to suggest that the high daily tablet burden associated with most phosphate binders, superimposed on that associated with other medications, may contribute to poor patient adherence to prescribed dosing (5). Thus, reducing tablet burden has the potential to improve adherence and, in turn, outcomes of maintenance dialysis patients.
Lanthanum carbonate (Fosrenol; Shire Pharmaceuticals, Wayne, PA) is a calcium-free phosphate binder used in the treatment of hyperphosphatemia. Extensive clinical data support its efficacy and tolerability in both short-term (6,7) and long-term (8,9) treatment of hyperphosphatemia in patients with stage 5 CKD. Reformulated (500 mg) and higher strength (750 and 1000 mg) lanthanum carbonate tablets have been available since 2006. The sizes of the tablets of the new formulation are smaller such that the 1000-mg tablet is comparable in size to the 500-mg tablet of the original formulation. The reduced tablet size may increase patient acceptance of the new formulation. Furthermore, the availability of a higher strength formulation translates into the need to prescribe fewer tablets for the same dosage. This, in turn, has the potential to reduce daily tablet burden, improve adherence, and potentially improve serum phosphorus control. The purpose of this study was to assess the efficacy of lanthanum carbonate in the control of serum phosphorus levels at higher daily dosages (3000 to 4500 mg/d) in patients who did not achieve target serum phosphorus levels (3.5 to 5.5 mg/dl) with dosages of up to 3000 mg/d.
Materials and Methods
Patients
Adult patients (≥18 yr) who had stage 5 CKD and required treatment for hyperphosphatemia (serum phosphorus >5.5 mg/dl [1.78 mmol/L] after washout) and were on a stable hemodialysis regimen three times per week for at least 2 mo before screening were eligible to participate in the study. Patients who required continued treatment with calcium-, aluminum-, or magnesium-based phosphate binders were excluded; however, limited use (≤200 mg/d) of calcium-based antacids was permitted. Phosphate binder–naive patients with a serum phosphorus level ≤5.5 mg/dl (1.78 mmol/L) at screening were excluded, as were patients with corrected serum calcium levels outside the range 8.4 to 10.2 mg/dl, biointact parathyroid hormone (PTH) ≥800 pg/ml, elevated serum transaminases (>three times upper limit of normal), or other abnormal laboratory values or uncontrolled concurrent illness. Pregnant and lactating women were also excluded.
The patients were withdrawn from the study when serum phosphorus was ≤5.5 mg/dl (1.78 mmol/L) after 2 wk of washout from previous phosphate binder; when the patient became pregnant or had a kidney transplant during the study; or when, on two consecutive visits, (1) patients had a calcium-phosphorus product (Ca × P) >86.7 mg2/dl2, (2) predialysis serum phosphorus was <2.0 or >8.5 mg/dl, or (3) serum calcium was <7.5 or >12.0 mg/dl.
This study was conducted in compliance with the ethical principles set forth in the Declaration of Helsinki and with local laws and regulations relevant to use of new therapeutic agents. All patients provided written informed consent before participation in the study.
Study Design
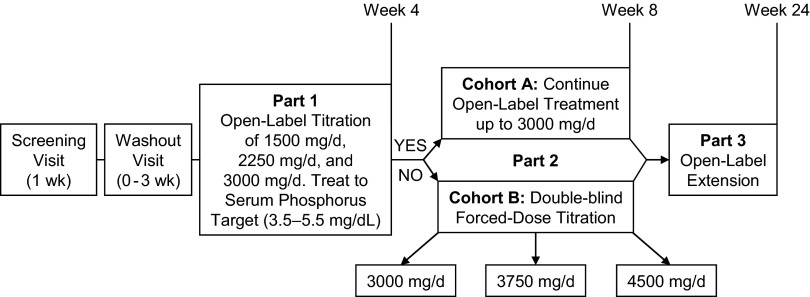
The study design is summarized in Figure 1; it consisted of two parts over 8 wk, followed by an open-label extension phase (part 3), for which all patients who completed part 2 were eligible. All enrolled patients were eligible to enter a washout period from their previous phosphate binder of up to 3 wk; they embarked on the study at any time during this 3-wk period (including time 0) when the serum phosphorus levels exceeded 5.5 mg/dl. During part 1 of the study, the dosage of lanthanum carbonate could be titrated up to 3000 mg/d in 750-mg/d increments at 1-wk intervals if serum phosphorus levels were not controlled. Dosages of ≥1500 mg/d in the final week of part 1 were required for study continuation; these dosages were meant to ensure that only patients with an ongoing need for phosphate binder would advance to the next stage of the study. However, patients in whom serum phosphorus was not controlled at the end of part 1 but who were receiving <3000 mg/d of lanthanum carbonate were withdrawn from the study (e.g., patients who were in the process of titration to an optimal dosage). Patients who entered part 2 were assigned to two separate cohorts. Patients with serum phosphorus in the target range at the end of part 1 entered a 4-wk open-label phase in which they continued on the same final daily dosage from part 1 (cohort A); the dosage could be titrated to maintain serum phosphorus control but had to remain within 1500 to 3000 mg/d. Patients whose serum phosphorus was not controlled with 3000 mg/d at the end of part 1 (cohort B) entered a double-dummy, double-blind, forced-dosage titration phase in which they received a final daily dosage of 3000, 3750, or 4500 mg of lanthanum carbonate. In part 3, all patients were eligible to continue in an open-label extension of lanthanum carbonate treatment for an additional 4 mo. The dosage could be adjusted within the range of 1500 to 4500 mg/d in 750-mg/d increments at weekly intervals. The study concluded with a telephone follow-up (used to provide flexibility for tracking patients far from satellite centers) 30 d after the last dose of study drug to determine ongoing adverse events (AE) or emergent serious AE.
Figure 1.
Schematic of study design. All patients in cohort B took the same number of pills (active + placebo) to maintain blinding.
Study Medication
Lanthanum carbonate was administered as reformulated 250-, 500-, 750-, and 1000-mg tablets. The chewable tablets were taken during each meal. Cohort A patients took from 1500 mg (three 500-mg tablets per day, one per meal) to 3000 mg (three 1000-mg tablets per day, one per meal). Blinding for cohort B in part 2 of the study was maintained by having patients take one tablet from each of three study medication bottles at every meal (three tablets per meal), for a total of nine tablets per day. One bottle contained 1000-mg tablets of lanthanum carbonate; the other two bottles contained either 250-mg tablets of lanthanum carbonate or placebo, depending on randomization group. In study part 3, cohort B patients returned to taking fewer, higher strength tablets, reducing their daily tablet burden.
Study Assessments
Screening visit assessments included a complete medical history, including renal disease history, physical examination, predialysis vital signs, 12-lead electrocardiogram, pre- and postdialysis weight, and information on concomitant medications. Laboratory assessments were carried out at designated times during the study. Predialysis serum phosphorus was measured at screening and then weekly for all patients.
Efficacy Assessments
The primary efficacy measure was the control rate for predialysis serum phosphorus levels among patients in cohort B. This was based on the proportion of patients with controlled serum phosphorus levels at the end of week 8. Secondary efficacy parameters, including weekly levels and control rates for serum phosphorus, calcium, Ca × P, and biointact PTH, were measured at screening and at treatment weeks 4, 8, and 24.
Other secondary efficacy parameters included satisfaction with the new formulation and preference questionnaires, pill count, and adherence. Patient and physician satisfaction with medication was assessed at washout (an assessment of previous medication) and treatment weeks 4, 8, and 24 using six-point Likert scale questionnaires designed with answers ranging from “strongly agree” to “strongly disagree.” Patient preference for phosphate-binder medication was measured at week 4 only; this timing allowed patients to recognize potential treatment differences while still recalling characteristics of previous treatment. Patients were asked whether they preferred the study or prestudy medication or considered them to be equal for the following: Number of tablets or capsules taken daily, ease of taking medications, medication adherence, symptom control, adverse effects, and overall preference. Similarly, physician preference was measured at week 4 and the following categories: Adherence with prestudy/study medications, dosage forms available, effectiveness, adverse effects reported by patient and/or family, clinical observation, and overall preference. Adherence to treatment was assessed by tablet counts at weekly intervals during parts 1 and 2 and at weeks 16 and 24.
Safety Assessments
Safety was assessed by physical examination and monitoring of vital signs, laboratory values, and AE throughout the study. Plasma lanthanum levels were measured at treatment weeks 5 and 8 for patients in cohort B only.
Statistical Analyses
All statistical analyses were performed using SAS/STAT 8.2 (SAS Institute, Cary, NC). Efficacy analyses were performed for the intention-to-treat (ITT) population, defined as all patients in the safety population who were given study medication. The proportion of patients in cohort B with serum phosphorus controlled at the end of part 2, the primary efficacy end point, was compared for the 3000-mg/d dosage with each of the higher dosages (3750 and 4500 mg/d) using the Fisher exact test. The odds ratio and 95% confidence interval for the controlled proportions of patients in the 3000-mg/d group versus each of the higher dosages were computed. The same comparison was made for the 3750- versus 4500-mg/d dosages.
Change in serum phosphorus over time in cohort A was analyzed using an ANOVA model with treatment week as a covariate. Changes in corrected calcium, Ca × P, and biointact PTH were summarized each week in cohort A and were analyzed using a one-sample t test. In cohort B, the proportion of patients with controlled serum phosphorus at each treatment week was analyzed in the same manner as the primary end point.
Physician and patient satisfaction questionnaires were summarized at baseline and at weeks 4, 8, and 24. The change from baseline was analyzed using the Cochran-Mantel-Haenszel test. Physician and patient preference questionnaires collected at week 4 were analyzed by comparing preference for the new medication versus preference for the previous medication and equal preference combined using binomial test with null hypothesis of proportion = 0.5. The difference in plasma lanthanum levels between weeks 5 and 8 was analyzed using a one-way ANOVA with change from week 5 as the dependent variable and dosage group as the independent variable.
Results
Patient Disposition
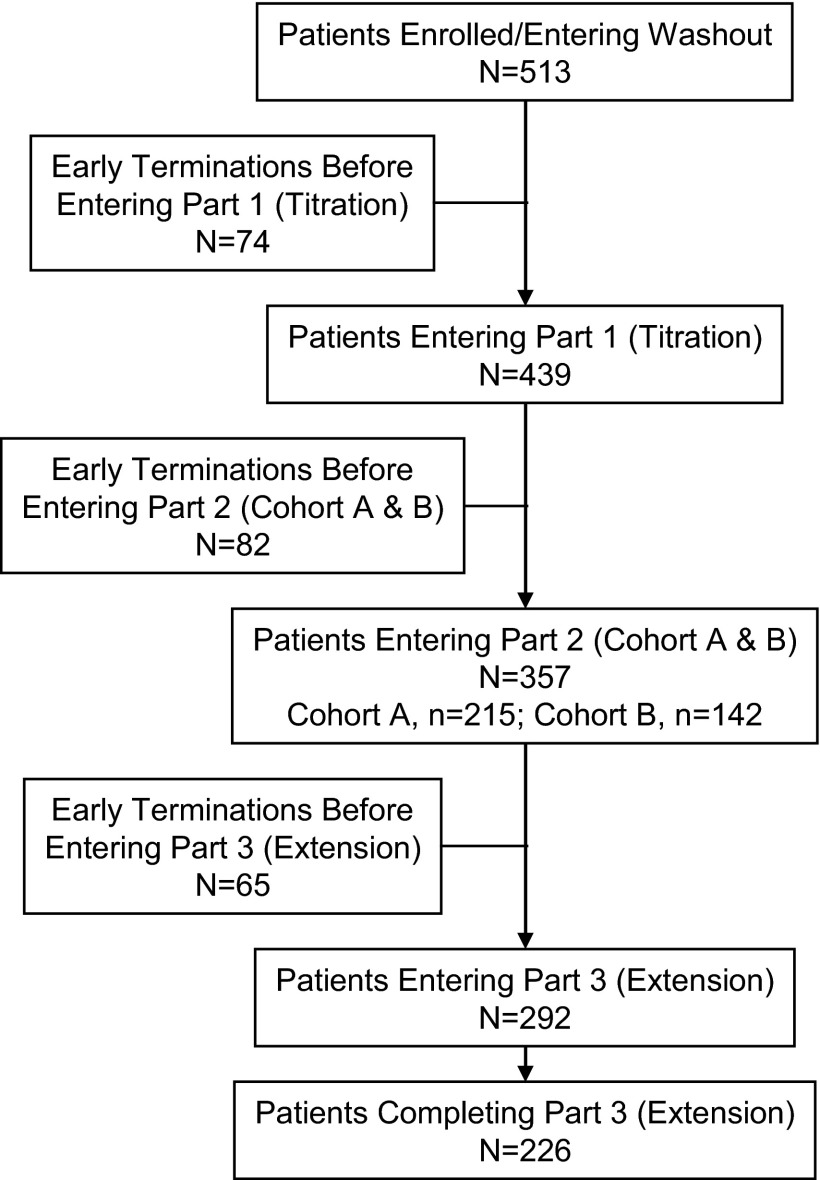
A total of 513 patients enrolled in the study, and 439 entered part 1, the 4-wk open-label titration phase (Figure 2). A total of 357 patients entered part 2 in cohort A (n = 215) and cohort B (n = 142). At screening, most patients had previously been treated with either sevelamer HCl (44%) or calcium-based phosphate-binding agents (41%). A detailed description of selected patient characteristics is provided in Table 1.
Figure 2.
Patient disposition throughout the study. The most common reasons for study discontinuation were adverse events (AE; 12.1%), protocol violation (10.3%), and withdrawal of consent (9.6%). Kidney transplantation, loss to follow-up, and death each led to discontinuation for <2% of patients. Similar trends were observed across various parts of the study.
Table 1.
Selected characteristics of study patients
| Characteristic | Enrolled (Entering Washout) | Part 1 | Part 2 |
Part 3 | |
|---|---|---|---|---|---|
| Cohort A | Cohort B | ||||
| Patients | 513 | 439 | 215 | 142 | 292 |
| Age (yr; mean [range]) | 54.9 (19.0 to 89.0) | 54.5 (19.0 to 89.0) | 56.7 (27.0 to 89.0) | 51.6 (19.0 to 87.0) | 55.4 (21.0 to 89.0) |
| Male gender (%) | 63 | 61 | 63 | 63 | 63 |
| Race/ethnicity (%) | |||||
| white | 36 | 35 | 37 | 30 | 34 |
| black | 48 | 49 | 46 | 54 | 50 |
| Hispanic | 13 | 13 | 14 | 13 | 12 |
| other | 3 | 4 | 3 | 4 | 3 |
| Weight (kg; mean ± SD) | 84.9 ± 22.7 | 84.6 ± 23.0 | 83.6 ± 23.6 | 88.7 ± 22.2 | 85.0 ± 22.1 |
| Dialysis vintage (yr; mean ± SD) | 3.9 ± 4.2 | 3.9 ± 4.0 | 3.8 ± 3.9 | 3.8 ± 4.5 | 3.8 ± 4.2 |
| Diabetes, yes (%) | 50 | 49 | 53 | 50 | 51 |
| Previous phosphate binder (%) | |||||
| sevelamer | 43.5 | ||||
| calcium acetate | 31.5 | ||||
| calcium carbonate | 9.1 | ||||
| other (mostly combination) | 16.0 | ||||
Efficacy
Primary Efficacy End Point
A total of 28 (5.5%) patients were withdrawn throughout the study as a result of persistent hyperphosphatemia (serum phosphorus >8.5 mg/dl on two consecutive visits). Fifty-four percent of patients achieved target serum phosphorus levels by the end of week 4 and entered cohort A. Twenty-eight (6.4%) patients who completed part 1 of the study were excluded from entering part 2 because of uncontrolled serum phosphorus on a dosage <3000 mg/d lanthanum carbonate. Those who were already titrated to 3000 mg/d but did not reach the target levels in part 1 entered cohort B. Patients in cohort B had dosage-dependent decreases in serum phosphorus from week 4, with changes of −0.23, −0.59, and −0.76 mg/dl at dosages of 3000, 3750, and 4500 mg/d, respectively. The achieved serum phosphorus levels in the three groups at week 8 were (mean ± SD) 6.5 ± 1.5, 6.0 ± 1.4, and 5.9 ± 1.5, respectively.
Twenty-five percent of patients in cohort B who were randomly assigned to receive 3000 mg/d and had not achieved the target at week 4 with an identical dosage did so by treatment week 8. Among patients who were randomly assigned to 3750 or 4500 mg/d, 38 and 32% of patients, respectively, achieved the target serum phosphorus levels. The difference between groups for rate of controlled serum phosphorus levels suggests a benefit of titrating to higher dosages, but statistical significance was not reached (Table 2).
Table 2.
Between-group comparison of patients who did not respond to 4 wk of lanthanum carbonate dosages of ≤3000 mg/d and were randomly assigned to higher dosagesa
| Parameter | Odds Ratio | 95% CI | P |
|---|---|---|---|
| 3000 versus 3750 mg | 0.55 | 0.22 to 1.40 | 0.25 |
| 3000 versus 4500 mg | 0.71 | 0.28 to 1.80 | 0.49 |
| 3750 versus 4500 mg | 1.29 | 0.55 to 3.01 | 0.67 |
CI, confidence interval.
Secondary Efficacy End Points
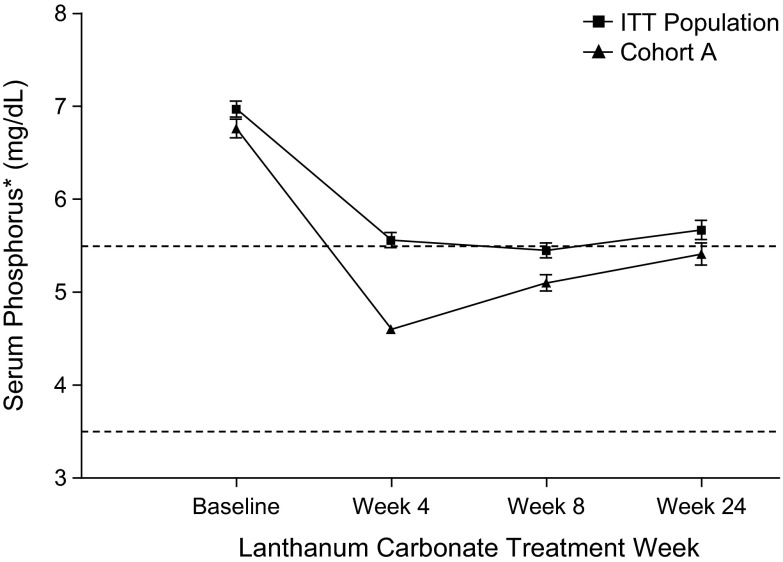
After the initial 4 wk of ≤3000 mg/d lanthanum carbonate, serum phosphorus (mean ± SD) for the ITT population (n = 383) decreased to 5.6 ± 1.6 mg/dl compared with 7.0 ± 1.8 mg/dl at baseline (n = 435; Figure 3). Reductions in serum phosphorus from baseline were statistically significant at all visits (P < 0.0001) through the end of the study (last observation carried forward). Furthermore, a majority of patients in the study maintained serum phosphorus control during part 3 of the study to 24 wk of lanthanum carbonate monotherapy. Overall, 81% of cohort A patients and 76% of cohort B patients were adherent to study drug (consumption of >80% of prescribed study dose).
Figure 3.
Predialysis serum phosphorus levels at baseline and weeks 4, 8, and 24 for intention-to-treat (ITT) population and cohort A. Dotted lines indicate the upper (5.5 mg/dl; 1.78 mmol/L) and lower (3.5 mg/dl; 1.13 mmol/L) ends of the Kidney Disease Outcomes Quality Initiative (KDOQI)-recommended range for serum phosphorus in patients with stage 5 chronic kidney disease (CKD). P < 0.0001 for serum phosphorus levels at weeks 4, 8, and 24 compared with baseline for both populations. *Means ± SD of three separate measurements taken during that week.
Cohort A
As previously noted, 54% of patients achieved the target range for serum phosphorus by the end of week 4 and entered cohort A. In these patients, the serum phosphorus levels decreased from 6.8 ± 0.1 mg/dl at baseline to 4.6 ± 0.1 mg/dl, using ≤3000 mg/d lanthanum carbonate, at the end of 4 wk. This represented a mean change of −2.2 mg/dl (Figure 3). Patients who entered cohort A maintained serum phosphorus control during that phase of the study, with mean serum phosphorus levels remaining ≤5.5 mg/dl (or 1.78 mmol/L).
Other mean laboratory values remained within normal clinical ranges during the 24-wk study, although increases in serum calcium values were observed at several assessments (Table 3). Ca × P levels were significantly reduced compared with baseline at all visits. Measurement of biointact PTH showed slight reductions during the initial 4-wk open-label titration period and slight increases at subsequent visits.
Table 3.
Laboratory values at baseline, weeks 4 and 8, and end of study (ITT population)a
| Parameter | Baseline (Mean ± SD) | End of Week 4 (Mean ± SD) | End of Week 8 (Mean ± SD) | End of Study or LOCF (Mean ± SD) |
|---|---|---|---|---|
| Predialysis serum phosphorus (mg/dl) | 6.97 ± 1.78 (n = 435) | 5.56 ± 1.60 (n = 383)b | 5.45 ± 1.40 (n = 297)b | 5.96 ± 1.85 (n = 413)b |
| Corrected serum calcium (mg/dl) | 9.38 ± 0.73 (n = 431) | 9.56 ± 0.69 (n = 370)b | 9.58 ± 0.71 (n = 285)b | 9.53 ± 0.79 (n = 404)b |
| Ca × P (mg2/dl2) | 66.0 ± 17.5 (n = 352) | 53.3 ± 14.6 (n = 339)b | 51.8 ± 13.3 (n = 254)b | 56.8 ± 16.8 (n = 336)b |
| Biointact PTH (pg/ml) | 266 ± 192 (n = 422) | 248 ± 179 (n = 383)c | 271 ± 197 (n = 296) | 287 ± 239 (n = 410)d |
| Serum albumin (g/dl) | 3.68 ± 0.331 (n = 506)e | 3.66 ± 0.36 (n = 383) | 3.68 ± 0.34 (n = 295) | 3.64 ± 0.366 (n = 424)c |
Ca × P, calcium-phosphorus product; ITT, intention-to-treat; LOCF, last observation carried forward; PTH, parathyroid hormone.
P ≤ 0.0001 for the comparison with baseline.
P < 0.05 for the comparison with screening.
P = 0.0042 for the comparison with baseline.
Serum albumin levels at screening.\
Medication Preference and Satisfaction
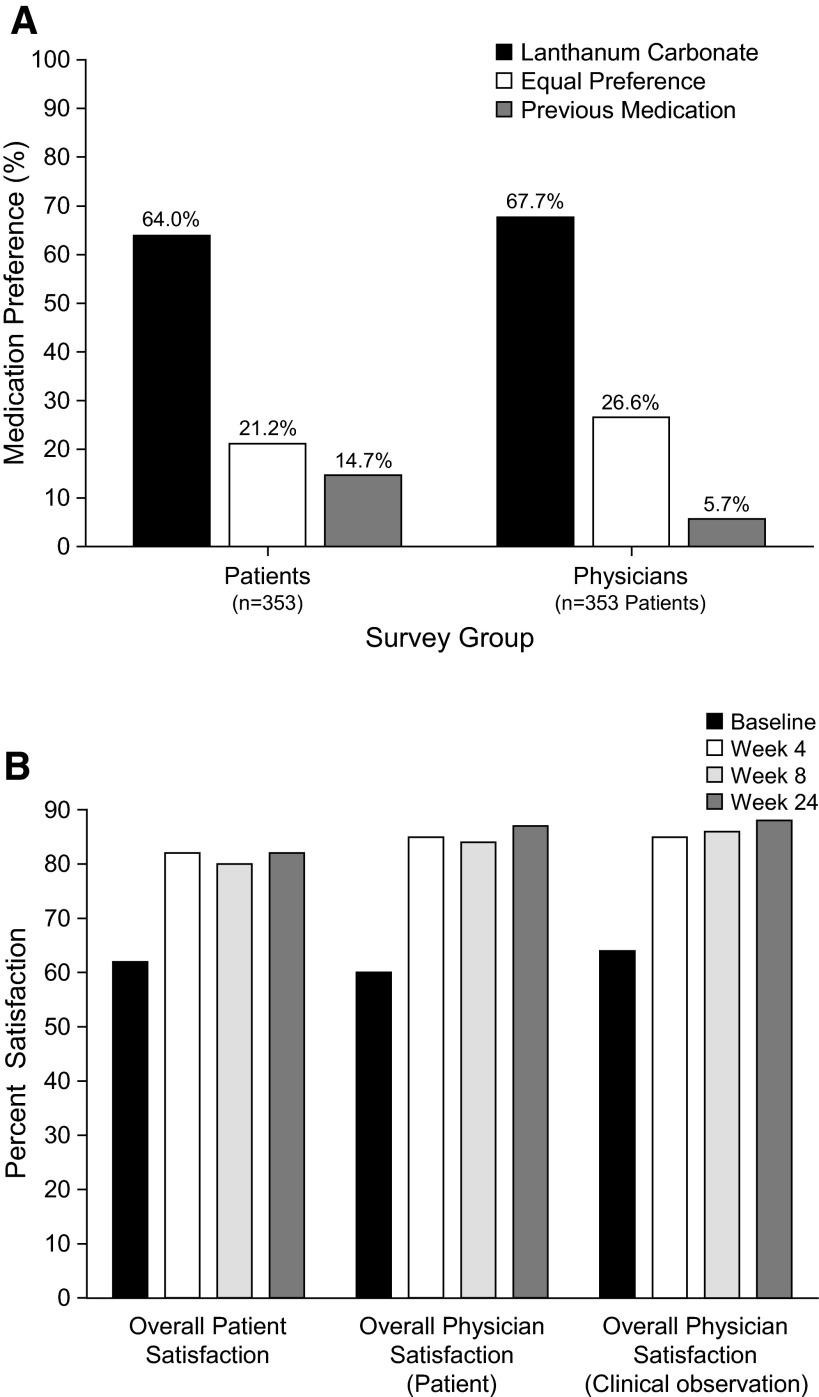
Preference for lanthanum carbonate versus previous phosphate-binder medications was expressed by both patients (ITT population) and physicians after 4 wk of treatment. Patient preference surveys revealed that, overall, 64% of patients preferred lanthanum carbonate, 21% had an equal preference for the study drug and previous therapy, and 15% preferred their previous medication(s) (P < 0.001; Table 4, Figure 4A). “Number of tablets” was the domain in which patients indicated the strongest preference for the study drug. Similarly, 68% of physicians preferred lanthanum carbonate, 27% showed no preference, and only 6% preferred previous medication(s) used by their patients (P < 0.001).
Table 4.
Patient preference for lanthanum carbonate versus previous therapy (ITT population)
| Parameter | Preferred Lanthanum Carbonate (%) | Preferred Previous Medication (%) | Equal Preference (%) | Pa |
|---|---|---|---|---|
| Overall satisfaction | 64.0 (n = 226) | 14.7 (n = 52) | 21.2 (n = 75) | <0.0001 |
| No. of tablets | 62.3 (n = 220) | 15.9 (n = 56) | 21.8 (n = 77) | <0.0001 |
| Easy to take medication | 57.4 (n = 202) | 21.6 (n = 76) | 21.0 (n = 74) | 0.0060 |
| Compliance | 59.7 (n = 210) | 14.5 (n = 51) | 25.9 (n = 91) | 0.0003 |
| Control of symptoms | 53.9 (n = 188) | 14.0 (n = 49) | 32.1 (n = 112) | 0.1500 |
| Adverse effects | 45.3 (n = 159) | 16.8 (n = 59) | 37.9 (n = 133) | 0.0800 |
P values for the comparison of lanthanum carbonate versus previous medication and equal preference combined.
Figure 4.
Preference for and satisfaction with lanthanum carbonate treatment. (A) Patient and physician medication preference (ITT population). P < 0.0001 for binomial test procedure with null hypothesis of proportion = 0.5 for lanthanum carbonate versus equal preference and previous medication combined. (B) Overall patient and physician satisfaction with lanthanum carbonate treatment (ITT population).
After 4 wk of lanthanum carbonate therapy, overall patient satisfaction with lanthanum carbonate was significantly higher than that with previous therapy at baseline (P < 0.0001). Trends for improved satisfaction persisted at weeks 8 and 24. Physician questionnaires revealed that overall satisfaction at week 4 was also significantly (P < 0.0001) improved among investigators conducting the study, which continued at weeks 8 and 24 (Figure 4B).
Safety Evaluation
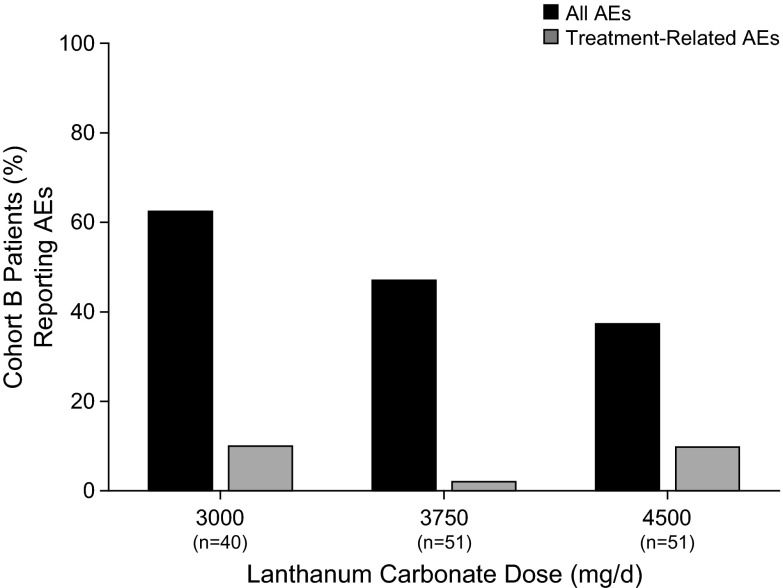
The most common AE were gastrointestinal in nature and included nausea (16.2%), vomiting (15.0%), and diarrhea (11.3%). AE were considered likely related to study medication for 18.3% of enrolled patients. Treatment-emergent AE for the ITT population during part 1 are summarized in Table 5. There was no increased incidence of AE with higher dosages of lanthanum carbonate up to 4500 mg/d in part 2, cohort B (Figure 5). Treatment-related AE in cohort B were observed in 9.8% of patients who were taking 4500 mg/d compared with 10% in patients who were taking 3000 mg/d. A total of 65 (12.7%) patients discontinued treatment because of AE. All deaths (n = 9) and serious AE were considered definitely not related or unlikely related to study medication.
Table 5.
Treatment-related AE that occurred in five or more enrolled patientsa
| AE | Patients Reporting AE (n [%]) |
|||||
|---|---|---|---|---|---|---|
| Enrolled(n = 513) | Part 1(n = 439) | Part 2 |
Part 3(n = 292) | Completed(n = 226) | ||
| Cohort A(n = 215) | Cohort B(n = 142) | |||||
| Any AE | 94 (18.3) | 94 (21.4) | 45 (20.9) | 25 (17.6) | 55 (18.8) | 32 (14.2) |
| Nausea | 33 (6.4) | 33 (7.5) | 14 (6.5) | 8 (5.6) | 19 (6.5) | 5 (2.2) |
| Vomiting | 20 (3.9) | 20 (4.6) | 8 (3.7) | 4 (2.8) | 11 (3.8) | 5 (2.2) |
| Constipation | 10 (1.9) | 10 (2.3) | 5 (2.3) | 3 (2.1) | 6 (2.1) | 5 (2.2) |
| Diarrhea | 10 (1.9) | 10 (2.3) | 3 (1.4) | 2 (1.4) | 5 (1.7) | 3 (1.3) |
| Dyspepsia | 6 (1.2) | 6 (1.4) | 4 (1.9) | 2 (1.4) | 4 (1.4) | 0 (0.0) |
| Flatulence | 6 (1.2) | 6 (1.4) | 3 (1.4) | 2 (1.4) | 4 (1.4) | 4 (1.8) |
| Pruritus | 6 (1.2) | 6 (1.4) | 3 (1.4) | 1 (0.7) | 2 (0.7) | 2 (0.9) |
AE, adverse event.
Figure 5.
AE reported in cohort B.
There were no significant mean changes in alanine aminotransferase or aspartate aminotransferase. Slight increases in γ-glutamyl transpeptidase and alkaline phosphatase were observed (Table 6). There were no changes in electrocardiogram results, BP, or heart rate that were associated with lanthanum carbonate treatment during the study. There was no significant difference in plasma lanthanum levels among the three cohort B dosage groups at week 8.
Table 6.
Liver function tests at baseline, weeks 4 and 8, and end of study
| Parameter (U/L) | Value at Screening (Mean ± SD) | Change at End of Week 4 (Mean ± SD) | Change at End of Week 8 (Mean ± SD) | End of Study or LOCF (Mean ± SD) |
|---|---|---|---|---|
| Alanine aminotransferase | 17.8 ± 11.0 (n = 503) | −0.3 ± 11.2 (n = 373) | 0.2 ± 13.5 (n = 292) | −0.8 ± 12.6 (n = 412) |
| % above normal range | 5.8 | 5.1 | 4.1 | 2.9 |
| Aspartate aminotransferase | 18.3 ± 9.0 (n = 500) | 0.8 ± 14.1 (n = 368) | 0.7 ± 7.8 (n = 292) | 0.2 ± 11.0 (n = 407) |
| % above normal range | 3.4 | 3.5 | 3.4 | 3.9 |
| Alkaline phosphatase | 117.4 ± 72.3 (n = 506) | 4.2 ± 30.0 (n = 374)a | 8.7 ± 33.3 (n = 295)a | 11.7 ± 54.0 (n = 414)a |
| % above normal range | 29.1 | 31.8 | 35.3 | 37.7 |
| γ -Glutamyl transpeptidase | 38.6 ± 54.9 (n = 506) | 2.3 ± 25.6 (n = 374) | 3.4 ± 23.7 (n = 294)a | 4.7 ± 35.1 (n = 414)a |
| % above normal range | 16.8 | 15.5 | 16.0 | 19.6 |
P < 0.05 versus screening.
Discussion
With accumulating evidence linking hyperphosphatemia to high cardiovascular mortality and morbidity, lowering serum phosphorus is an important clinical concern for patients who undergo maintenance dialysis. Options for phosphate-binder therapy have become somewhat limited because emerging data suggest that calcium-containing agents may be associated with an increased risk for vascular calcification and adynamic bone disease (10–12). These issues have led to increased use of calcium-free phosphate binders. Lanthanum carbonate is a calcium-free phosphate binder that is well tolerated and effective for the short- and long-term treatment of patients who are on dialysis and have elevated serum phosphate. Because tablet burden is probably a key factor for nonadherence with phosphate-binder therapy in patients with stage 5 CKD, lanthanum carbonate was reformulated to offer higher strength and reduced tablet sizes, both of which may facilitate reductions in daily tablet burden. This phase IIIb study examined the efficacy of the reformulated preparation of lanthanum carbonate in controlling serum phosphorus.
In the 4-wk, open-label titration phase, 54% of patients who received daily lanthanum carbonate dosages of ≤3000 mg achieved target serum phosphorus. Patients who did not respond to 3000 mg/d lanthanum carbonate during part 1 were randomly assigned to receive one of three fixed dosages. Because 25% of patients who were randomly assigned to receive 3000 mg/d (a continuation of their part 1 treatment) subsequently achieved a serum phosphorus value ≤5.5 mg/dl, the fixed-dosage period may not have been sufficiently long to assess the total response rate in that portion of the study. There was a trend for a larger proportion of patients to achieve the target serum phosphorus at dosages of 3750 or 4500 mg/d compared with those who received 3000 mg/d. These higher dosage groups did not experience any increase in AE compared with the 3000-mg/d group; therefore, there may be a benefit of titrating to dosages up to 4500 mg/d for some patients who do not respond to lower dosages of lanthanum carbonate.
A large proportion of patients and physicians expressed satisfaction with lanthanum carbonate treatment compared with previous binder treatment (sevelamer hydrochloride and calcium-based binders). Slightly lower levels of satisfaction among cohort B patients in part 2 versus cohort A may be related to the higher tablet burden imposed by the study design in this group to maintain blinding among the three separate dosage groups. Higher tablet burden in cohort B is also consistent with a slightly lower level of patient adherence to the medication regimen. A large proportion of both patients and physicians preferred lanthanum carbonate to previous phosphate-binder medications, with 68% of physicians and 64% of patients expressing a clear overall preference for lanthanum carbonate.
The AE profile was consistent with the results of previous studies, with no increased incidence at higher dosages. Most AE were gastrointestinal in nature. Laboratory values largely remained within normal clinical ranges throughout the study. Plasma lanthanum levels did not increase significantly with higher dosages.
The major limitation of this study is that it did not include a comparator arm, in which patients were treated with another phosphate binder. In addition, satisfaction and preference are subjective assessments. Data comparing treatment received during a clinical trial with prestudy treatment may be potentially biased by the quality of care received during a clinical trial.
The data derived from part 2 suggest that higher dosages of lanthanum carbonate may control serum phosphorus effectively in some patients who do not respond to lower dosages. These poor responders may be patients with more complex medical histories, high PTH levels, or higher baseline serum phosphorus levels. These findings form the basis for future studies to address the efficacy and tolerability of both common and elevated dosages of lanthanum carbonate.
This study demonstrates that in many patients with stage 5 CKD, hyperphosphatemia can be managed effectively with daily lanthanum carbonate dosages ≤3000 mg. The 750- and 1000-mg tablets of lanthanum carbonate allow for a prescription of one tablet per meal (three tablets per day) for patients who requiring ≤3000 mg/d of the drug. Poor adherence with phosphate-binder therapy is correlated with high tablet burden (5,13). Compared with the reported tablet burden of seven (800 mg) to 17 (403 mg) pills per day for sevelamer hydrochloride and eight to 12 pills per day for calcium-based agents (14–16), the tablet burden with the reformulated preparation is substantially lower. The lower tablet burden associated with lanthanum carbonate compared with other oral phosphate binder medications (17) may be indicative, at least in part, of the greater relative potency of lanthanum carbonate in in vitro studies (18). Reduction in daily phosphate-binder tablet burden and improved satisfaction with higher strength lanthanum carbonate may help improve patient adherence, with a potentially positive impact on clinical outcomes.
Disclosures
R.M. has received research support from Amgen, Baxter, Shire, and Genzyme; serves as a consultant for Novartis and Shire; and has received honoraria from Baxter and Shire.
Acknowledgments
This study was funded by Shire Pharmaceuticals.
We acknowledge the support of Lauren Gallagher, PhD, at Complete Healthcare Communications, Inc., for editorial assistance in developing this manuscript. We acknowledge the following participating investigators and sites: Horacio Adrogue, Baylor College of Medicine (Houston, TX); Mohammad Akmal, USC Keck School of Medicine (Los Angeles, CA); Frank Apantaku, Illinois Center for Clinical Research (Chicago, IL); Habib Azad, UC Irvine Medical Center (Orange, CA); M. Edwina Barnett, Barnett Research and Communications (Torrance, CA); Yousri M.H. Barri, Dallas Nephrology Associates (Dallas, TX); Istvan Bognar (Greenville, SC); Philip Butera, Coastal Clinical Research Inc. (Mobile, AL); Vito Campese, National Institute of Clinical Research (Los Angeles, CA); Dalila B. Corry, Holy Cross Renal Center (Mission Hills, CA); Earl Dunnigan, Clinical Research Services (Bismarck, ND); Mohamed El-Shahawy, DaVita Monterey Park Dialysis Center (Monterey Park, CA); William F. Finn, UNC School of Medicine (Chapel Hill, NC); Michael Germain, Western New England Renal Transplant Assoc. (Springfield, MA); Robert Geronemus (Ft. Lauderdale, FL); Stuart Handelsman (Atlanta, GA); Cem Harmanci, Lewistown Dialysis (Lewistown, PA); Theodore Herman, Western New York Dialysis, LLC (Orchard Park, NY); Onyekachi Ifudu, Midwood Dialysis (Brooklyn, NY); Talat Alp Ikizler, Vanderbilt University Medical Center (Nashville, TN); Keith Kapatkin, PAB Clinical Research (Brandon, FL); Pran Kar, Central Florida Kidney Center Inc. (Winter Gardens, FL); Kevin J. Kelley (Canal Fulton, OH); Karl Koenig, Richmond Nephrology (Richmond, VA); Robert Kopelman, Bakersfield Dialysis Center (Bakersfield, CA); Michelle Krause, University of Arkansas for Medical Sciences (Little Rock, AR); Barry Lankhorst, Sioux Valley Clinic–Clinical Research Center (Sioux Falls, SD); David Levenson (Pittsburgh, PA); Barton Sherman Levine, VA Greater Los Angeles Healthcare System (Los Angeles, CA); Brian Ling, Mountain Kidney Research, Inc. (Ashville, NC); Harold Locay, Discovery Medical Research GLP (Ocala, FL); Robert Lynn, Bronx Westchester Medical Group (Bronx, NY); Dwight Makoff, Beverly Hills Dialysis Center (Los Angeles, CA); Philip Marin, Altru Health Research Center (Grand Forks, ND); John P. Middleton, Duke University (Durham, NC); Raffi Minasian, Glendale Kidney Center (Glendale, CA); Sharon Martin Moe, Indiana University–Wishard Memorial Hospital (Indianapolis, IN); Saied Murphy, SMO-USA (Canton, GA); Geetha Narayan, St. Elizabeth Medical Center (Boston, MA); Chika Oguagha (Brooklyn, NY); Efrain Reisin; LSU Medical Center (New Orleans, LA); Stephen Rifkin, PAB Clinical Research (Brandon, FL); Michael Roppolo, Renal Associates (Baton Rouge, LA), Ghodrat Siami, VA Medical Center (Nashville, TN); Jonathan Slater, Future Care Studies, Inc. (Springfield, MA); Miroslaw Smogorzewski, USC School of Medicine (Los Angeles, CA); Bruce Spinowitz, Trude Weishaupt Memorial Satellite Dialysis (Fresh Meadows, NY); Leslie Steed, Northwest Renal Clinic (Portland, OR); Harold Szerlip, Medical College of Georgia (Augusta, GA); Edgard Vera (Victorville, CA); Marc Weinberg, Hypertension Nephrology Inc. (Providence, RI); Jay Barry Wish, University Hospitals of Cleveland (Cleveland, OH); Duane Wombolt, Clinical Research Associates of Tidewater (Norfolk, VA); and Ming J. Wu, Suncoast Clinical Research (New Port Richey, FL).
K.J.M. and S.M.S. are consultants for Shire Pharmaceuticals.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Kalpakian MA, Mehrotra R: Vascular calcification and disordered mineral metabolism in dialysis patients. Semin Dial 20 :139 –143,2007 [DOI] [PubMed] [Google Scholar]
- 2.National Kidney Foundation: K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42 :S1 –S201,2003 [PubMed] [Google Scholar]
- 3.US Renal Data System: Medication use among dialysis patients in the Dialysis Morbidity and Mortality Study. United States Renal Data System. Am J Kidney Dis 32 :S60 –S68,1998 [DOI] [PubMed] [Google Scholar]
- 4.Manley HJ, Garvin CG, Drayer DK, Reid GM, Bender WL, Neufeld TK, Hebbar S, Muther RS: Medication prescribing patterns in ambulatory haemodialysis patients: Comparisons of USRDS to a large not-for-profit dialysis provider. Nephrol Dial Transplant 19 :1842 –1848,2004 [DOI] [PubMed] [Google Scholar]
- 5.Tomasello S, Dhupar S, Sherman RA: Phosphate binders, K/DOQI guidelines, and compliance: The unfortunate reality. Dial Transplant 33 :236 –240,2004 [Google Scholar]
- 6.Finn WF, Joy MS, Hladik G: Efficacy and safety of lanthanum carbonate for reduction of serum phosphorus in patients with chronic renal failure receiving hemodialysis. Clin Nephrol 62 :193 –201,2004 [DOI] [PubMed] [Google Scholar]
- 7.Joy MS, Finn WF: Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: A new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis 42 :96 –107,2003 [DOI] [PubMed] [Google Scholar]
- 8.Finn WF: Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: Safety and efficacy in chronic maintenance hemodialysis patients. Clin Nephrol 65 :191 –202,2006 [DOI] [PubMed] [Google Scholar]
- 9.Hutchison AJ, Maes B, Vanwalleghem J, Asmus G, Mohamed E, Schmieder R, Backs W, Jamar R, Vosskuhler A: Long-term efficacy and tolerability of lanthanum carbonate: Results from a 3-year study. Nephron Clin Pract 102 :c61 –c71,2006 [DOI] [PubMed] [Google Scholar]
- 10.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342 :1478 –1483,2000 [DOI] [PubMed] [Google Scholar]
- 11.Hercz G, Pei Y, Greenwood C, Manuel A, Saiphoo C, Goodman WG, Segre GV, Fenton S, Sherrard DJ: Aplastic osteodystrophy without aluminum: The role of “suppressed” parathyroid function. Kidney Int 44 :860 –866,1993 [DOI] [PubMed] [Google Scholar]
- 12.Raggi P, Bommer J, Chertow GM: Valvular calcification in hemodialysis patients randomized to calcium-based phosphorus binders or sevelamer. J Heart Valve Dis 13 :134 –141,2004 [PubMed] [Google Scholar]
- 13.Loghman-Adham M: Medication noncompliance in patients with chronic disease: Issues in dialysis and renal transplantation. Am J Manag Care 9 :155 –171,2003 [PubMed] [Google Scholar]
- 14.PhosLo Calcium Acetate, Boca Raton, FL, Nabi Biopharmaceuticals,2005
- 15.Braun J, Asmus HG, Holzer H, Brunkhorst R, Krause R, Schulz W, Neumayer HH, Raggi P, Bommer J: Long-term comparison of a calcium-free phosphate binder and calcium carbonate: Phosphorus metabolism and cardiovascular calcification. Clin Nephrol 62 :104 –115,2004 [DOI] [PubMed] [Google Scholar]
- 16.Qunibi WY, Hootkins RE, McDowell LL, Meyer MS, Simon M, Garza RO, Pelham RW, Cleveland MV, Muenz LR, He DY, Nolan CR: Treatment of hyperphosphatemia in hemodialysis patients: The Calcium Acetate Renagel Evaluation (CARE Study). Kidney Int 65 :1914 –1926,2004 [DOI] [PubMed] [Google Scholar]
- 17.Vemuri N, Pratt R: Conversion to lanthanum carbonate maintains serum phosphorus levels and lowers tablet burden [Abstract]. J Am Soc Nephrol 17 :805A ,2006. 16421224 [Google Scholar]
- 18.Autissier V, Damment SJP, Henderson RA: Relative in vitro efficacy of the phosphate binders lanthanum carbonate and sevelamer hydrochloride. J Pharm Sci 96 :2818 –2827,2007 [DOI] [PubMed] [Google Scholar]