Abstract
Background and objectives: The relationship between aspirin use and arteriovenous fistula (AVF) survival has been lacking. The aim of this study was to evaluate the association between AVF survival and aspirin use.
Design, setting, participants, & measurements: Data on 2815 incident hemodialysis patients (on dialysis ≤ 30 d) using an AVF at enrollment into the Dialysis Outcomes and Practice Patterns Study between 1996 and 2004 were analyzed. Cox regression was used to examine the association between aspirin use and the risk of final AVF failure, first AVF failure, and a gastrointestinal bleeding event. Aspirin use was determined at baseline and one year later. Patients using aspirin at baseline and one year later were considered consistent aspirin users. All models accounted for facility clustering effects and were adjusted for age, race, gender, body mass index, prior permanent access failure, prior placement of a catheter, 10 comorbid conditions, laboratory data, and other medications, and stratified by regions.
Results: Consistent aspirin use was significantly related to a lower risk of final AVF failure. Facility-level analysis, which may reduce confounding by indication, also showed a nearly significant trend of reduced risk of final AVF failure with greater prevalence of consistent aspirin use within dialysis facilities (P for trend = 0.07). The occurrence of a new gastrointestinal bleeding event during the study period was not associated with aspirin use.
Conclusions: These results suggest that consistent aspirin use may be beneficial for AVF survival among incident hemodialysis patients.
Recently, both the costs and the morbidity related to the management of vascular access (VA) failure among hemodialysis (HD) patients have been increasing (1). Although it has been demonstrated that arteriovenous fistulas (AVFs) have better outcomes than arteriovenous grafts (AVGs) and catheters (2), practice patterns of VA type selection are very different among countries. In Japan, more than 90% of HD patients have an AVF (3), whereas in the United States, AVG has been the most common VA and only a fourth of HD patients were using AVF in 1996 to 2000 (4). However, the Center for Medicare and Medicaid Services promoted the “Fistula First” initiative in 2003; consequently, the prevalence of AVF has been increasing in the United States (5). AVF has been recommended as the VA of first choice by guideline committees across many countries in the world.
The main cause of VA failure is outflow venous stenosis as a result of vascular intimal hyperplasia and thrombosis. This formation of neointima hyperplasia and thrombosis may be initiated by activated platelet function, endothelial cell injury, vascular smooth muscle cell proliferation, etc., and these may occur because of unphysiologic vascular anastomosis of VA (6,7). In view of these postulated mechanisms of VA failure, possible clinical benefit of antiplatelet therapy has been proposed for prevention of VA failure (8). In particular, aspirin, the most widespread antiplatelet drug, has been evaluated as prophylaxis for VA failure in several studies, mainly among AVG. In a small-sized randomized controlled trial (RCT), aspirin use did not improve AVG survival (9). Another RCT that examined the efficacy of combination therapy of aspirin and clopidogrel for prevention of AVG failure was carried out (10), but this study was stopped before completion because the prevalence of hemorrhagic complication was significantly higher in the intervention group. Meanwhile, in an exploratory observational study, Saran et al. (11) recently described aspirin use associated with significantly better AVG survival among HD patients in the United States. Consequently, evidence for the effectiveness of aspirin for prevention of VA failure has been controversial for AVG and largely lacking for AVF.
Furthermore, few studies addressed the safety of aspirin use for HD patients. Generally speaking, HD patients are assumed to be more likely to develop hemorrhagic complications such as gastrointestinal (GI) bleeding than the general population because HD patients are more likely to have platelet dysfunction (12) and they receive intermittent anticoagulant therapy during HD sessions. However, two recent observational studies reported that the occurrence of a GI bleeding event was not associated with aspirin use: U.S. Renal Data System (13) and the Dialysis Outcomes and Practice Patterns Study (DOPPS) (14).
Because large studies on the clinical benefits of aspirin use for AVF function are lacking for HD patients, we investigated the association between aspirin use and AVF survival and the risk of GI bleeding among HD patients in the present study using data from DOPPS.
Materials and Methods
Data Sources
DOPPS is an international prospective observational cohort study of maintenance HD patients being carried out in four regions (Europe, Australia and New Zealand, Japan, and North America). DOPPS has prospectively investigated various practice patterns and HD patient outcomes in detail using the same study protocol and questionnaire across countries. Details of the subjects and methods have been reported elsewhere (15,16). The analysis used data from DOPPS phase 1 (1996 through 2001) and phase 2 (2002 through 2004). Briefly, in DOPPS, nationally representative samples of dialysis facilities were enrolled using stratified random selection from 7 countries (France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States) for phase 1 and from 12 countries (phase 1 countries plus Australia, Belgium, Canada, New Zealand, and Sweden) for phase 2. Data were then collected from random samples of 20 to 40 patients within each facility dependent on facility size.
Data Collection
Data for the present study were restricted to adult incident HD patients (on dialysis ≤ 30 d, age ≥ 18 yr), who were using an AVF at enrollment into DOPPS. The main predictor in the current study was aspirin use or not. The medication information was collected at both baseline and one year later. Patients using aspirin at baseline and one year later were considered consistent aspirin users. The primary outcome measure was final AVF failure (i.e., complete AVF failure requiring creation of a new VA) and the two secondary outcome measures were 1) first AVF intervention for AVF thrombosis or AVF salvage procedure and 2) a new GI bleeding event during the study period. Survival time was defined as the time from first AVF use to individual outcomes, data for which were collected longitudinally during the course of study follow-up for each sampled patient. We also looked at time to the different events with consistent aspirin use versus no use at all. Consistent use was defined as a patient having used aspirin at baseline and one year later. No use was defined as a patient not using aspirin at baseline and one year later. Patients using aspirin at baseline but not one year later or vice versa were not included in this analysis. If a patient using aspirin at baseline had an AVF failure before one year and was also reported to have been using aspirin one year later, he or she was considered to have been using aspirin the entire year and was included in the analysis. So, taking aspirin at baseline and one year later was a proxy for consistent aspirin use.
Statistical Analysis
Descriptive analyses were calculated to describe the variables such as patient characteristics by baseline aspirin use and the distribution of aspirin use at the baseline by regions. Cox regression models were used to assess whether aspirin use was associated with the risk of final AVF failure, first AVF failure, and a GI bleeding event. Furthermore, facility-level analysis was performed to reduce confounding by indication based on the percentage of facility HD patients prescribed aspirin. Survival models were adjusted for baseline values of the following factors or potential confounders; age, race, gender, body mass index, prior permanent VA failure, prior placement of catheter, 10 summary comorbid conditions (coronary artery disease, congestive heart failure, other cardiac disease, hypertension, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, lung disease, cancer [other than skin], and GI bleeding), laboratory data (hemoglobin, serum albumin, serum creatinine), and other medications (warfarin, other antiplatelet agent, angiotensin converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, statin). All models accounted for facility clustering effects and stratified by regions of Europe/Australia and New Zealand, Japan, and North America. For the Cox regression model, the robust sandwich estimator was used to account for clustering at the facility level (17). All statistical analyses were performed with SAS, version 8.2 (SAS Institute, Cary, NC).
Ethical Considerations
Although the present study was an observational study, institutional review boards approved the study in each country and facility as required. Informed consent was obtained for each sampled patient in accordance with the requirement of each country, review board, and facility. Furthermore, data collection was performed in a manner that maintained patient anonymity.
Results
Data from 2815 incident HD patients on dialysis ≤ 30 d and who were using an AVF at study entry were analyzed. Table 1 compares the patient characteristics by baseline aspirin use. Patients who had aspirin prescribed at baseline were more likely to be older and male. They also were more likely to have comorbidities, such as coronary artery disease, congestive heart failure, other cardiac disease, hypertension, cerebrovascular disease, peripheral vascular disease, and diabetes mellitus. Furthermore, patients given aspirin at study entry had slightly higher mean hemoglobin but lower mean serum creatinine than patients who were not prescribed aspirin. Patients who received aspirin at baseline also were less likely to be prescribed a warfarin but more likely to be prescribed a statin
Table 1.
Patient characteristics by baseline aspirin use
| Characteristic | Aspirin (n = 612) | No aspirin (n = 2203) |
|---|---|---|
| Age, years (mean ± SD) | 65.1 ± 12.7 | 60.6 ± 14.9 |
| Black race, % | 4.9 | 6.4 |
| Male, % | 74.0 | 67.0 |
| BMI, kg/m2 (mean ± SD) | 25.4 ± 4.9 | 24.6 ± 5.1 |
| Prior permanent VA failure, % | 14.1 | 12.3 |
| Prior placement of catheter, % | 8.0 | 7.5 |
| Comorbidities, % | ||
| Coronary artery disease | 56.5 | 28.4 |
| Congestive heart failure | 31.7 | 23.1 |
| Other cardiac disease | 31.1 | 22.3 |
| Hypertension | 86.9 | 80.8 |
| Cerebrovascular disease | 20.4 | 10.8 |
| Peripheral vascular disease | 29.4 | 16.4 |
| Diabetes mellitus | 49.5 | 33.2 |
| Lung disease | 11.4 | 8.6 |
| Cancer (other than skin) | 11.4 | 9.9 |
| Cerebrovascular disease | 18.9 | 18.2 |
| Gastrointestinal bleeding | 6.2 | 4.8 |
| Laboratory data | ||
| Hemoglobin, g/dl (mean ± SD) | 10.4 ± 1.7 | 9.9 ± 5.1 |
| Albumin, g/dl (mean ± SD) | 3.5 ± 0.5 | 3.6 ± 0.6 |
| Creatinine, mg/dl (mean ± SD) | 7.3 ± 2.6 | 8.1 ± 3.0 |
| Other medications, % | ||
| Warfarin | 1.0 | 2.8 |
| Other antiplatelet agent | 8.2 | 9.4 |
| Angiotensin converting enzyme inhibitor | 28.1 | 22.3 |
| Angiotensin receptor blocker | 11.1 | 14.1 |
| Calcium channel blocker | 53.6 | 54.2 |
| Statin | 33.0 | 14.8 |
As shown in Figure 1, there was substantial variation across regions in the baseline percent aspirin use. The total baseline percent aspirin use was 21.7% and ranged from 11.6% in Japan to 24.5% in North America.
Figure 1.
Percentage of aspirin use at baseline by region. Based on a point prevalent cross section at study entry of HD patients on dialysis ≤ 30 d participating in DOPPS: n = 707 in North America, n = 1590 in Europe/Australia and New Zealand, n = 518 in Japan, and n = 2815 in total.
Table 2 indicates the adjusted hazard ratio (AHR) of individual events by baseline aspirin use. The mean (SD) years of study follow-up time for final AVF failure, first AVF failure, and GI bleeding were 0.91 (0.68), 0.78 (0.65), and 1.02 (0.69), respectively. The occurrence of a GI bleeding event was found not to be associated with baseline aspirin use (AHR = 1.01; 95% confidence interval [CI], 0.58 to 1.77, P = 0.97). There was not a significant reduced risk of first AVF failure (AHR = 0.93; 95% CI, 0.79 to 1.10, P = 0.42) and final AVF failure (AHR = 0.89; 95% CI, 0.69 to 1.15, P = 0.38) with baseline aspirin use.
Table 2.
Adjusted hazard ratio (AHR) of events by baseline and consistent aspirin use
| Model description | Individual outcomes | No. of events | AHRa | 95% CI | P |
|---|---|---|---|---|---|
| Baseline aspirin use (yes versus no, n = 2815) | |||||
| Final AVF failure | 361 | 0.89 | 0.69-1.15 | 0.38 | |
| First AVF failure | 740 | 0.93 | 0.79-1.10 | 0.42 | |
| GI bleeding | 84 | 1.01 | 0.58-1.77 | 0.97 | |
| Consistent aspirin useb (yes versus no, n = 1411) | |||||
| Final AVF failure | 203 | 0.63 | 0.42-0.95 | 0.03 | |
| First AVF failure | 413 | 0.86 | 0.67-1.10 | 0.24 | |
| GI bleeding | 46 | 0.68 | 0.28-1.66 | 0.40 |
CI, confidence interval.
AHR for individual events (final AVF failure, first AVF failure, and gastrointestinal bleeding) by baseline and consistent aspirin use based upon a multivariate analysis. The multivariate analysis was adjusted for age, race, gender, BMI, 10 comorbid conditions, prior permanent VA failure, prior placement of catheter, laboratory data (hemoglobin, serum albumin, serum creatinine), medications (warfarin, other antiplatelet agent, angiotensin converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, statin), accounted for facility clustering effects, and was stratified by region.
Consistent aspirin use was defined as a patient having used aspirin at baseline and one year later, and consistent no aspirin use was defined as a patient not using aspirin at baseline and one year later.
We then looked at time to the different events with consistent aspirin use versus no use at all. Consistent use was defined as a patient having used aspirin at baseline and one year later (17.4% of patients). No use was defined as a patient not using aspirin at baseline and one year later (69.5% of patients). Patients using aspirin at baseline but not one year later (9.1% of patients) or vice versa (4.1% of patients) were not included in this analysis.
Table 2 also shows the AHR of individual events by consistent aspirin use. The mean (SD) years of study follow-up time for final AVF failure, first AVF failure, and GI bleeding were 1.26 (0.65), 1.09 (0.67), and 1.41 (0.59), respectively. There was no significant relationship between consistent aspirin use and the risk of GI bleeding (AHR = 0.68; 95% CI, 0.28 to 1.66, P = 0.40) or first AVF intervention (AHR = 0.86; 95% CI, 0.67 to 1.10, P = 0.24). However, patients who were prescribed aspirin consistently over the one-year period displayed a 37% lower risk (P = 0.03) of final AVF failure (AHR = 0.63; 95% CI, 0.42 to 0.95).
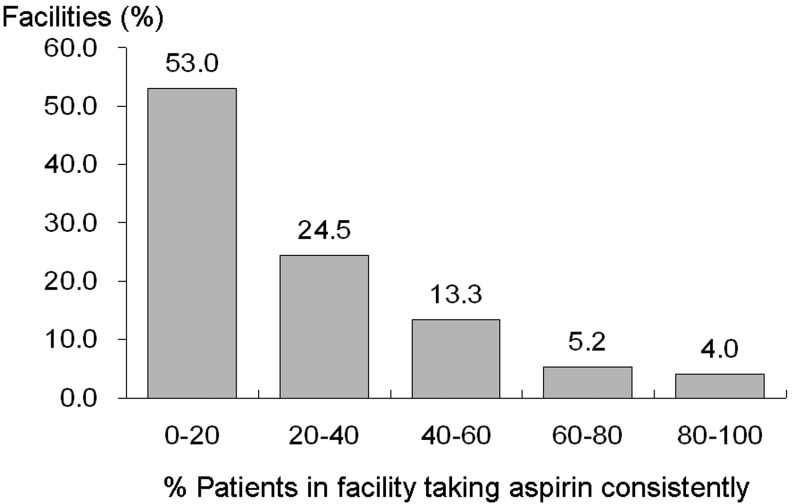
Figure 2 illustrates the distribution of the facility practice by the percent of patients with consistent aspirin use. Wide variation was seen across dialysis units regarding the percent of patients in a facility taking aspirin consistently. More than half of dialysis facilities prescribed aspirin consistently to <20% of facility patients the entire year. Mean and median percent consistent facility aspirin use were 21.9% and 14.3%, respectively.
Figure 2.
Distribution of percent consistent facility aspirin use. The percent of patients in a facility taking aspirin consistently for a year was determined using the medication information at both baseline and one year later (n = 260 facilities). *Restricting to facilities having at least 5 patients with aspirin prescription information at both baseline and one year later.
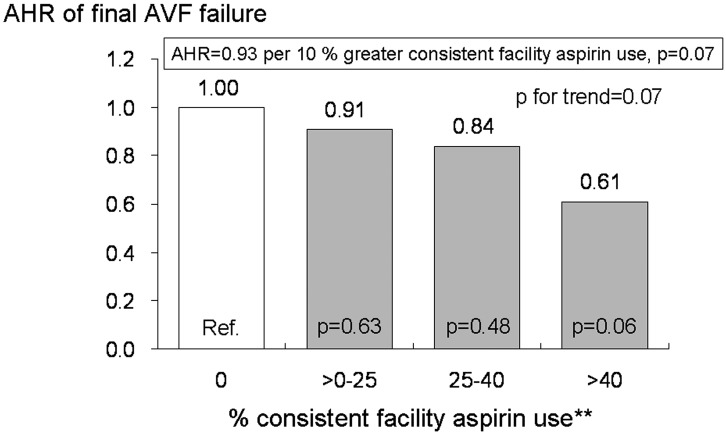
The AHR of final AVF failure by percent consistent facility aspirin use is shown in Figure 3. Facility-level analysis, which tends to reduce confounding by indication for aspirin prescription, also showed a nearly significant lower risk of final AVF failure with greater prevalence of consistent facility aspirin use in both a continuous model (AHR = 0.93, per 10% greater consistent facility aspirin use; 95% CI, 0.86 to 1.01, P = 0.07) and a categorical model (AHR = 0.61, facility with >40% of patients taking aspirin consistently; 95% CI, 0.36 to 1.02, P = 0.06). There was an almost significant trend between percent consistent aspirin use and the risk of final AVF failure as well (P for trend = 0.07).
Figure 3.
Adjusted hazard ratio (AHR) of final AVF failure by percent consistent facility aspirin use. AHR for final AVF failure by percent consistent facility aspirin use based on a multivariate analysis. Results are provided for percent consistent facility aspirin use as a continuous variable or as categories of percent consistent aspirin use. The multivariate analysis was adjusted for age, race, gender, body mass index, 10 comorbid conditions, prior permanent VA failure, prior placement of catheter, laboratory data (hemoglobin, serum albumin, serum creatinine), medications (warfarin, other antiplatelet agent, angiotensin converting enzyme inhibitor, angiotensin receptor blocker, calcium channel blocker, statin), accounted for facility clustering effects, and was stratified by region (n = 1082). *Restricting to facilities having at least 5 patients with aspirin prescription information at both baseline and one year later. **The 80th percentile of consistent facility aspirin use was 40% of facility patients taking aspirin consistently for 1 yr.
Discussion
Aspirin is a widely used antiplatelet drug and one of the most efficient prophylaxes for cardiovascular disease (CVD). The effectiveness of aspirin for primary and secondary prevention of CVD has been established by many clinical studies and a subsequent meta-analysis (18). Therefore, it is strongly recommended to prescribe aspirin for groups at high risk of CVD in the general population. Although end-stage renal disease (ESRD) is strongly associated with CVD (19), evidence regarding the effectiveness and safety of aspirin use among ESRD patients is limited (20). Nevertheless, National Kidney Foundation–Kidney Disease Outcomes Quality Initiative clinical practice guidelines (21) provide the opinion that aspirin may be useful for prevention of CVD among ESRD patients with careful monitoring for adverse effects such as GI bleeding.
Atherosclerotic disease may increase the risk of VA failure (4), but the relation between aspirin use and VA survival has been controversial and the relevant studies focused on AVF are few and limited. Consequently, in the present study, we investigated the effectiveness of aspirin use for AVF survival, practice patterns for aspirin prescription, and the relationship to odds of a GI bleeding among HD patients using data from DOPPS.
There was large variation among regions in the prevalence of aspirin use, and Japanese HD patients were less likely to have used aspirin in the current study. This variation may be partly explained by the differences in CVD morbidity among regions. Additionally, the lower prevalence of aspirin use in Japan may also be explained in part by the fact that Japanese medical insurance did not cover aspirin as a prophylaxis for CVD until 2002.
In the present study, consistent aspirin use was related to a significantly lower risk of final AVF failure, which suggests its beneficial effect for AVF survival among incident HD patients. However, there are several previous studies that concluded to the absence of clinical benefit of aspirin use for AVG survival, in contrast to the present study. Sreedhara et al. (9) reported that aspirin use was associated with a tendency toward an increased risk of thrombosis in AVG. Even although this was an RCT, the number of patients on the intervention was very low (n = 20). Another RCT examined the efficacy of the combination therapy of aspirin and clopidogrel for prevention of AVG failure (10), in which there was a trend toward a reduced risk of AVG failure, but that study was discontinued because of more frequent hemorrhagic complications in the intervention group. Conversely, the observational study of Saran et al. (11) showed that aspirin use was associated with better AVG survival among HD patients in the United States. However, there was no significant clinical benefit of aspirin use for AVF survival in that study, but it must be considered that those results were only obtained from the United States where the practice patterns concerning VA practice patterns are very different from other countries, i.e., only one fourth of HD patients have AVF in the United States versus more than 80% in the others. Furthermore, the risk of AVF failure has been shown to be twofold higher in the United States compared with Europe, suggesting that factors or practices affecting AVF survival may differ substantially between the United States and Europe (4).
Recently, it has been proposed that the existence of oxidative stress and inflammation accelerates the formation of neointima and thrombosis, one of the main causes of VA failure (22). It also has been reported that aspirin not only works as an antiplatelet agent but also reduces oxidative stress (23) and inflammation (24), which is considered to be the proper mechanism of aspirin for prevention of atherosclerotic diseases and VA failure.
The biggest reason that physicians are reluctant to prescribe aspirin for HD patients is the potential increased risk of hemorrhagic complication such as GI bleeding. However, in the present study, the occurrence of a new GI bleeding event during the study period was found not to be associated with baseline aspirin use, and this finding is in agreement with the observational studies from the U.S. Renal Data System (13) and DOPPS (14). Our consistent aspirin use model is biased toward not finding an association with GI bleeding because, if a patient developed a new GI bleeding event during the first year of the study period, it is likely that the patients would not have been prescribed aspirin continuously. However, our baseline, intent-to-treat aspirin use model does not have this bias, and there was no significant relationship seen in this model between aspirin use and the risk of a GI bleeding event.
The strength of this study is using the data from the international DOPPS, in which divergent data from a large number of randomly sampled patients were prospectively collected in great detail. These divergent data, including numerous confounding factors, medications, and laboratory measurements, provided for extensive adjustment for estimating the risks associated with aspirin use. A limitation of an observational study is that confounding by indication is inevitable. In this study, we carried out not only patient-level analysis adjusted for many factors but also facility-level analysis to reduce confounding by indication concerning prescription of aspirin. It is necessary to consider that misclassification may occur in intent-to-treat models based on aspirin use only at study entry since baseline information of drug prescription is not able to warrant its consistent use. To address this limitation, we used a consistent aspirin use model taking advantage of longitudinal medication data, and it was by this approach that the benefit of aspirin use could be seen in regards to enhanced AVF survival.
Conclusion
These results suggest that consistent aspirin use may be beneficial for AVF survival among new HD patients at the start of ESRD. It is expected that these findings may facilitate advances in prolonging AVF survival and thereby improve practice patterns in this area for better HD patient outcomes. These findings also should stimulate more research in this important issue and an incentive for RCT to resolve the uncertainties in benefit of aspirin therapy for AVF survival.
Disclosures
The authors have no conflict of interest with the subject matter of this study.
Acknowledgments
This study is supported by research grants from Amgen Inc and Kirin Pharma without restrictions on publications.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Hakim R, Himmelfarb J: Hemodialysis access failure: a call to action. Kidney Int 54 :1029 –1040,1998 [DOI] [PubMed] [Google Scholar]
- 2.Rodriguez JA, Armadans L, Ferrer E, Olmos A, Codina S, Bartolome J, Borrellas J, Piera L: The function of permanent vascular access. Nephrol Dial Transplant 15 :402 –408,2000 [DOI] [PubMed] [Google Scholar]
- 3.Akiba T, Akizawa T, Fukuhara S, Saito A, Ohira S, Sekino H, Yamazaki C, Kishimoto T, Osawa G, Fujimi S, Marumo F, Kurokawa K, Bragg-Gresham JL, Pisoni RL, Port FK, Held PJ: Results of the international DOPPS hemodialysis study in Japan. J Jpn Soc Dial Ther 37 :1865 –1873,2004 [Google Scholar]
- 4.Pisoni RL, Young EW, Dykstra DM, Greenwood RN, Hecking E, Gillespie B, Wolfe RA, Goodkin DA, Held PJ: Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int 61 :305 –316,2002 [DOI] [PubMed] [Google Scholar]
- 5.Sands JJ: Increasing AV fistulae and decreasing dialysis catheters: two aspects of improving patient outcomes. Blood Purif 25 :99 –102,2007 [DOI] [PubMed] [Google Scholar]
- 6.Stracke S, Konner K, Kostlin I, Friedl R, Jehle PM, Hombach V, Keller F, Waltenberger J: Increased expression of TGF-beta1 and IGF-I in inflammatory stenotic lesions of hemodialysis fistulas. Kidney Int 61 :1011 –1019,2002 [DOI] [PubMed] [Google Scholar]
- 7.Sterpetti AV, Cucina A, Santoro L, Cardillo B, Cavallaro A: Modulation of arterial smooth muscle cell growth by haemodynamic forces. Eur J Vasc Surg 6 :16 –20,1992 [DOI] [PubMed] [Google Scholar]
- 8.Himmelfarb J: Pharmacologic prevention of vascular access stenosis. Curr Opin Nephrol Hypertens 8 :569 –572,1999 [DOI] [PubMed] [Google Scholar]
- 9.Sreedhara R, Himmelfarb J, Lazarus JM, Hakim RM: Anti-platelet therapy in graft thrombosis: results of a prospective, randomized, double-blind study. Kidney Int 45 :1477 –1483,1994 [DOI] [PubMed] [Google Scholar]
- 10.Kaufman JS, O’Connor TZ, Zhang JH, Cronin RE, Fiore LD, Ganz MB, Goldfarb DS, Peduzzi PN: Randomized controlled trial of clopidogrel plus aspirin to prevent hemodialysis access graft thrombosis. J Am Soc Nephrol 14 :2313 –2321,2003 [DOI] [PubMed] [Google Scholar]
- 11.Saran R, Dykstra DM, Wolfe RA, Gillespie B, Held PJ, Young EW: Association between vascular access failure and the use of specific drugs: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 40 :1255 –1263,2002 [DOI] [PubMed] [Google Scholar]
- 12.Carvalho AC: Acquired platelet dysfunction in patients with uremia. Hematol Oncol Clin North Am 4 :129 –143,1990 [PubMed] [Google Scholar]
- 13.Wasse H, Gillen DL, Ball AM, Kestenbaum BR, Seliger SL, Sherrard D, Stehman-Breen CO: Risk factors for upper gastrointestinal bleeding among end-stage renal disease patients. Kidney Int 64 :1455 –1461,2003 [DOI] [PubMed] [Google Scholar]
- 14.Ethier J, Bragg-Gresham JL, Piera L, Akizawa T, Asano Y, Mason N, Gillespie BW, Young EW: Aspirin prescription and outcomes in hemosialysis patients: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 50 :602 –611,2007 [DOI] [PubMed] [Google Scholar]
- 15.Young EW, Goodkin DA, Mapes DL, Port FK, Keen ML, Chen K, Maroni BL, Wolfe RA, Held PJ: The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int 57 [Suppl]:74 –81,2000 [Google Scholar]
- 16.Pisoni RL, Gillespie BW, Dickinson DM, Chen K, Kutner MH, Wolfe RA: The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis 44 :7 –15,2004 [DOI] [PubMed] [Google Scholar]
- 17.Klein J, Moeschberger M: Survival Analysis Techniques for Censored and Truncated Data, New York, Springer,1997. , pp416 –418
- 18.Antithrombotic Trialists’ Collaboration: Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ 324 :71 –86,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wright RS, Reeder GS, Herzog CA, Albright RC, Williams BA, Dvorak DL, Miller WL, Murphy JG, Kopecky SL, Jaffe AS: Acute myocardial infarction and renal dysfunction: a high-risk combination. Ann Intern Med 137 :563 –570,2002 [DOI] [PubMed] [Google Scholar]
- 20.Reddan DN: Therapy for cardiovascular disease in patients with chronic kidney disease: appropriate caution or the absence of data. Am Heart J 144 :206 –207,2002 [DOI] [PubMed] [Google Scholar]
- 21.K/DOQI Workgroup: K/DOQI clinical practice guidelines for cardiovascular disease in dialysis patients. Am J Kidney Dis 45 [Suppl]:1 –153.2005 [PubMed] [Google Scholar]
- 22.Weiss MF, Scivittaro V, Anderson JM: Oxidative stress and increased expression of growth factors in lesions of failed hemodialysis access. Am J Kidney Dis 37 :970 –980,2001 [DOI] [PubMed] [Google Scholar]
- 23.Grosser N, Schroder H: Aspirin protects endothelial cells from oxidant damage via the nitric oxide-cGMP pathway. Arterioscler Thromb Vasc Biol 23 :1345 –1351,2003 [DOI] [PubMed] [Google Scholar]
- 24.Ikonomidis I, Andreotti F, Economou E, Stefanadis C, Toutouzas P, Nihoyannopoulos P: Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation 100 :793 –798,1999 [DOI] [PubMed] [Google Scholar]