Abstract
Background and objectives: Despite the high prevalence of cardiovascular disease among hemodialysis patients, the relationship between age and blood pressure (BP) is not well understood. It was postulated that the relationship of BP to age differs among hemodialysis patients versus the general population and that there is significant variability in dialysis unit BP measurements.
Design, setting, participants, & measurements: To explore this hypothesis, the patterns of systolic, diastolic, mean arterial, and pulse pressures in the general population using data from National Health and Nutrition Examination Survey participants (n = 9242) were compared with those in a cohort of hemodialysis patients (n = 9849).
Results: In contrast to the increase in systolic BP with age in the general population, systolic BP was elevated in young hemodialysis patients and declined slightly among the elderly. The inverted “U”-shape relationship between age and diastolic BP in the general population was absent in hemodialysis patients. Diastolic BP was elevated among hemodialysis patients <50 yr of age and declined with advancing age. Mean arterial and pulse pressures were elevated among young hemodialysis patients and exhibited less age dependency than in the general population. Variability in BP within patients was similar to that between patients.
Conclusions: The relationship of BP to age differed from that in the general population. The variability in dialysis unit BP measurements may limit their use in managing hypertension and predicting outcomes. Nevertheless, dialysis unit BP measurements are necessary to minimize acute complications during the dialysis procedure.
The National Health and Nutritional Examination Survey (NHANES) (1) and the Framingham Study (2) have contributed significantly to our understanding of the relationships between aging, blood pressure (BP), and cardiovascular disease (CVD). Unfortunately, these relationships have been less well characterized among hemodialysis (HD) patients despite their high prevalence of CVD (3). Although hypertension is a modifiable risk factor for CVD mortality in the general population, epidemiologic studies have failed to confirm similar relationships among HD patients. Observational data have suggested that HD patients with systolic BP (SBP) between 150 and 159 mmHg experience lower mortality than their normotensive peers, and there has been no evidence of increased mortality until SBP ≥180 mmHg (4–7). Speculation about the seemingly paradoxical relationship between BP and mortality has centered on the high prevalence of left ventricular systolic dysfunction; however, definitive prospective studies are lacking (3,8–11).
The NHANES and Framingham Studies demonstrated that steady increases in SBP and gradual declines in diastolic BP (DBP) accompany aging in the general population. Hypertensive subjects <50 yr of age in the general population usually exhibit systolic and diastolic hypertension and a narrowed pulse pressure (PP), reflecting increased peripheral vascular resistance. Additionally, older individuals tend to exhibit isolated systolic hypertension and an elevated PP, reflecting reduced elastic artery compliance. Among HD patients, limited observational data indicate that the relationships between BP and age may differ from that of the general population (12–14). However, a rigorous analysis analogous to the NHANES survey investigating these relationships has not been previously published. The high prevalence of accelerated vascular disease and left ventricular hypertrophy among young HD patients is consistent with the hypothesis that BP patterns may be similar to those found in older members of the general population.
Several recent studies have suggested that routine measurement of BP within the dialysis unit may have limited utility in the management of hypertension and in predicting clinical outcomes (15–17). These findings are consistent with the hypothesis that there may be significant variability in routine BP measurements made in the dialysis unit.
The present study explored age-dependent BP parameters among HD patients as compared with the general population by addressing the following questions: 1) Do the relationships between age and BP measurements differ and if so how? 2) How variable are the values for different BP parameters within individual HD patients and across HD patients?
Materials and Methods
Study Sample
We studied all incident patients ≥20 yr of age who began HD at a facility operated by Dialysis Clinic Inc (DCI), a national, not-for-profit dialysis provider between January 1, 1999 and December 31, 2004 and had survived ≥150 d. Patients were excluded if they had previously been on peritoneal dialysis or received a kidney transplant, if they began maintenance HD outside of DCI, or if data on gender, age, or date of diagnosis of end-stage renal disease were missing.
Blood Pressure Measurements
BP measurements were collected during days 31 to 120 of HD as described previously (18). Therefore, patients included in the primary analysis survived 30 d beyond the last day of the 90-d period in which BP measurements were collected. This 30-d lag reduced potential bias associated with changes in BP secondary to a fatal illness. To assess potential selection bias that may have occurred by excluding patients who died between days 120 and 150, we conducted a secondary analysis of all incident patients who survived ≥120 d. Measurements were taken immediately before and after each HD session using routine methods that did not follow a standardized BP measurement technique. SBP and DBP values outside the ranges of 50 to 300 mm and 10 to 150 mmHg, respectively, were excluded. PP was calculated as SBP − DBP, and mean arterial pressure (MAP) as ⅓SBP + ⅔DBP. Measurements on each patient were averaged to create a cross-sectional dataset for comparison with the general population using NHANES public access data files for 1999 to 2004 (19). We included data from non-Hispanic black and non-Hispanic white NHANES participants ≥20 yr of age in the 1999 to 2004 annual surveys. NHANES measurements were collected at Mobile Examination Centers using American Heart Association procedures (20). Three measurements are usually taken, with the first measurement being dropped before averaging. The NHANES quality control procedures include initial extensive training, quarterly recertification, a procedural checklist, and continuous review of the data for systematic error (21).
Statistical Analyses
NHANES uses a nation-wide weighted, nested sampling design; therefore, a weighted analysis was used to compute means and confidence intervals (CIs). Sampling weights for our 6-yr analysis period were calculated as two-thirds the 4-yr weights provided in the 1999 to 2000 and 2001 to 2002 datasets and as one-third the 2-yr weights provided in the 2003 to 2004 dataset (21). Masked Variance Units at the cluster and strata level were used to estimate sampling errors by the Taylor series method (21). We calculated means and 95% CI for race, gender, and age groups for the NHANES data (weighted) and DCI cross-sectional dataset (unweighted) using the SURVEYMEANS procedure in SAS, version 9.1 (22). Weighted percent composition of the sample for demographic characteristics was calculated using the SAS SURVEYFREQ procedure. Comparison of BP parameters among NHANES participants and DCI patients was achieved by examining overlap of 95% CI for the means and by examining 95% CI for the differences between DCI and NHANES means. Linear and quadratic polynomial regression analyses were used to assess whether mean BP parameters showed trends over age groups.
Using BP measurements obtained from day 31 to day 120, we fitted separate hierarchical linear models to each BP parameter to assess the variation in BP within and across patients over a 90-d period. Models included a random intercept representing between patient variation and fixed effects for race/ethnicity, gender, disease, and day of the week, i.e., Monday/Tuesday versus Wednesday/Thursday or versus Friday/Saturday measurements accounting for potential differences in interdialytic weight gains and postdialysis weights. We assumed that the variation in respective BP parameters across patients and the residual within-patient variation were normally and independently distributed with mean equal to zero and variance equal to σ2b and σ2e, respectively. Intraclass correlation coefficients (ICCs) were calculated to summarize the correlation of measurements within patients [ICC = σ2b /(σ2b + σ2e)]. We also fitted three heterogeneous variance models for each BP parameter where both the between- and within-patient variance components were allowed to be different for each category of 1) race, 2) gender, or 3) age group. Likelihood ratio tests were used to determine whether variance components were different with respect to race, gender, or age. The MIXED procedure of SAS, version 9.1, was used to estimate variance components based on restricted maximum likelihood (22).
Results
Study Sample
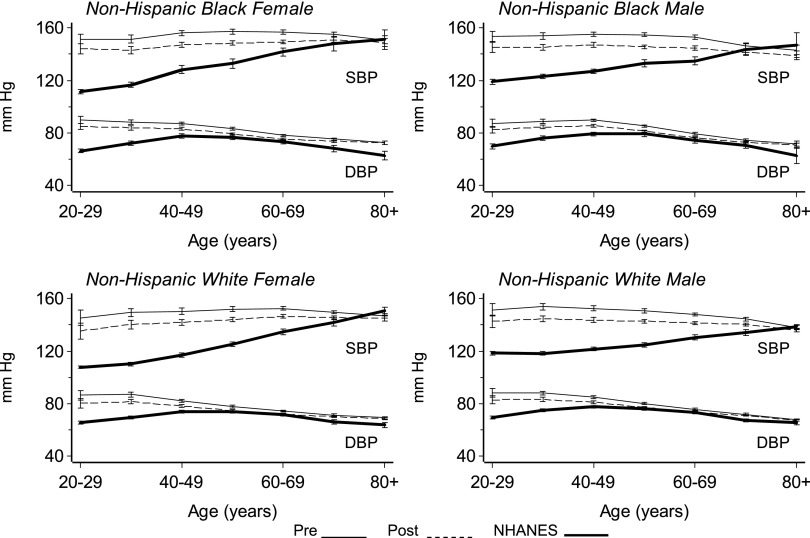
All of the results presented below refer to the primary analysis where we included all incident patients ≥20 yr of age who survived ≥150 d. The results of the secondary analysis of the incident cohort who survived ≥120 d were similar in all respects (data not shown). A comparison of demographic characteristics of DCI study participants, U.S. Renal Data System (USRDS) incident HD patients during the study period and the 1999 to 2004 NHANES survey participants is shown (Table 1). The percentage of blacks was higher among DCI patients (39.0%) versus USRDS (29.6%) and NHANES (12.8%). The 95% CI for the relative frequencies among NHANES participants did not overlap the DCI study sample or the USRDS population with respect to the distributions of age, gender, and race. HD patients were older and more likely to be black males compared with the general population. Average SBP in the general population differed from those of HD patients except among the elderly (Figure 1). The age-dependent linear increase of average SBP seen in the general population was largely absent among HD patients. Even young HD patients have high BPs with little change until the oldest age groups (P < 0.01, quadratic age effect). The SBP of a 20-yr-old, non-Hispanic, white (NHW) female HD patient, for example, was similar to the SBP of a ≥70-yr-old NHW female in the general population. The average SBP among HD patients was 150 mmHg predialysis and 8 to 10 mmHg lower postdialysis. There were fewer differences between predialysis and postdialysis SBPs among patients ≥70 yr of age.
Table 1.
Demographic and clinical characteristics of the DCI study sample (n = 9849), of the USRDS incident cohort (n = 531,945) and of the NHANES sample (n = 9242)
| DCI (%) | USRDS (%)a | NHANES (%)b | |
|---|---|---|---|
| Age (yr) | |||
| 20-44 | 14.5 | 12.6 | 48.2 |
| 45-64 | 37.2 | 35.4 | 33.6 |
| 65-74 | 25.1 | 25.5 | 10.1 |
| 75+ | 23.2 | 26.4 | 8.1 |
| Gender | |||
| Female | 46.6 | 45.9 | 51.8 |
| Race | |||
| Non-Hispanic white | 61.0 | 64.0 | 87.2 |
| Non-Hispanic black | 39.0 | 29.6 | 12.8 |
| Cause of ESRD | |||
| Diabetes | 45.5 | 45.1 | — |
| Hypertension | 25.4 | 27.9 | — |
| Glomerulonephritis | 9.9 | 8.1 | — |
| Other/missing | 19.2 | 18.9 | — |
Percentage is based on the entire 1999 to 2004 USRDS incident hemodialysis population, except for age, which is restricted to those 20 yr of age or older.
Weighted percent calculated using NHANES sampling weights.
Figure 1.
Systolic and diastolic BP versus age in the DCI and NHANES populations.
DBP among NHANES participants increased progressively from the third through the fifth decade and then slowly decreased among the elderly. In contrast, DBP was elevated among young HD patients and decreased with advancing age (P < 0.001). Among those between the ages of 50 and 69, DBP was similar in NHANES participants and HD patients. Predialysis values of DBP were higher than the postdialysis values (P < 0.05). However, the reductions in DBP with HD were less than the corresponding changes in SBP.
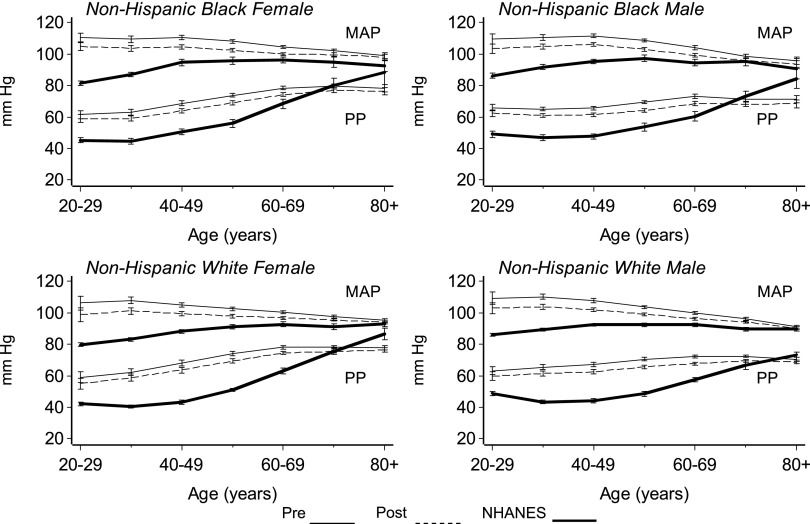
Changes in MAP and PP by age are shown in Figure 2. Among the general population, MAP steadily increased from the age of 20 to 50 or 60 yr and then tended to stabilize. Conversely, MAP values were highest among young HD patients and then declined slightly with advancing age (P < 0.001, quadratic age effect). Among those ≥60 yr of age, MAP was similar in both groups.
Figure 2.
MAP and PP versus age in the DCI and NHANES populations.
PP, a marker of arterial stiffness influenced in part by reflected waves, exhibited a unique pattern among HD patients (23). PP was markedly elevated even among young HD patients but tended to remain stable until age 50 yr. Thereafter, PP tended to increase because of the progressive increase in SBP and the decrease in DBP. The PP of a 20-yr-old NHW female on dialysis was equivalent to that of a 60-yr-old NHW female in the general population. PP continued to increase to age 60 yr, after which it tended to stabilize. In contrast, among NHANES participants, PP remained stable through the third and fourth decades and then increased with advancing age. The curves of PP versus age in HD patients and NHANES participants crossed between the ages of 60 and 79 yr. Among NHANES participants ≥80 yr of age, PP values exceeded those observed in HD patients. This may reflect increased arterial stiffness among HD patients. Among NHANES participants, the increase in PP with age was attributable to increasing SBP and a stable DBP. In contrast, among HD patients ≥50 yr of age, the increase in PP predominately reflected a decrease in DBP.
Blood Pressure Variability among HD Patients
We assessed variability in BP values within and between patients. Within-patient variation refers to visit-to-visit variations about a patient's average BP, whereas between-patient variability refers to how much each patient's average BP differs from the population mean.
Standard deviation (SD) of predialysis and postdialysis SBP, DBP, PP, MAP, and the respective ICCs is shown (Table 2).
Table 2.
Between- and within-patient variance components (SD, intraclass correlations [ICC], and means) of predialysis and postdialysis blood pressure parameters for DCI hemodialysis patients in mmHg measured during days 31 to 120 of hemodialysis
| SBP |
DBP |
MAP |
PP |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Estimate | Lower | Upper | Estimate | Lower | Upper | Estimate | Lower | Upper | Estimate | Lower | Upper | |||
| Dialysis | Measure | |||||||||||||
| Pre | SD | Between | 17.88 | 17.62 | 18.14 | 9.37 | 9.24 | 9.51 | 11.42 | 11.25 | 11.58 | 12.50 | 12.32 | 12.68 |
| Within | 18.03 | 17.99 | 18.07 | 11.03 | 11.00 | 11.05 | 12.04 | 12.01 | 12.07 | 14.15 | 14.12 | 14.18 | ||
| ICC | 0.50 | 0.49 | 0.50 | 0.42 | 0.41 | 0.43 | 0.47 | 0.47 | 0.48 | 0.44 | 0.43 | 0.45 | ||
| Mean | 150.6 | 150.2 | 151.0 | 78.1 | 77.9 | 78.4 | 102.3 | 102.0 | 102.5 | 72.3 | 72.0 | 72.6 | ||
| Post | SD | Between | 17.35 | 17.10 | 17.60 | 8.81 | 8.68 | 8.93 | 10.86 | 10.70 | 11.01 | 12.40 | 12.22 | 12.58 |
| Within | 18.43 | 18.39 | 18.47 | 11.04 | 11.01 | 11.06 | 12.06 | 12.04 | 12.09 | 14.83 | 14.79 | 14.86 | ||
| ICC | 0.47 | 0.46 | 0.48 | 0.39 | 0.38 | 0.40 | 0.45 | 0.44 | 0.46 | 0.41 | 0.41 | 0.42 | ||
| Mean | 144.3 | 143.9 | 143.6 | 75.5 | 75.3 | 75.7 | 98.4 | 98.2 | 98.6 | 68.6 | 68.4 | 68.9 | ||
SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure. Lower and upper 95% confidence interval bounds are also included.
The magnitude of the within-patient SD exceeded the between-patient SD, indicating that BP values for a given patient may vary as much or more than those observed across patients. The ICCs, the proportion of total variance that is between-patient variability, for predialysis BP parameters were 0.50 for SBP, 0.42 for DBP, 0.47 for MAP, and 0.44 for PP. Postdialysis ICCs were 0.02 to 0.03 units smaller as within-patient SD values increased and between-patient SD values decreased. Average BP parameter values were 3% to 5% lower postdialysis. Variance components were affected by race, gender, and age (each P < 0.001). However, differences were small and likely of little clinical significance. Estimated SDs for each race did not differ from those in Table 2 by more than ± 1.0 mmHg, and separate SDs for genders were within ± 0.9 mmHg of the Table 2 values. Age effects were slightly larger with estimates for different age groups within ± 2.4 mmHg of the Table 2 values. Predialysis SBP and DBP varied by the day of the week. Predialysis SBP and DBP were each 1 to 3 mmHg lower on both Wednesday/Thursday and Friday/Saturday compared with Monday/Tuesday (each P < 0.05). There were no significant differences between predialysis SBP and DBP, respectively, on Wednesday/Thursday versus Friday/Saturday. Postdialysis SBP and DBP did not vary significantly by the day of the week. In supplemental analyses using a 120-d follow-up inclusion rule, the SDs for all predialysis and postdialysis BP parameters were always with ± 0.03 mmHg, and ICCs were always within ± 0.005 units of those in Table 2.
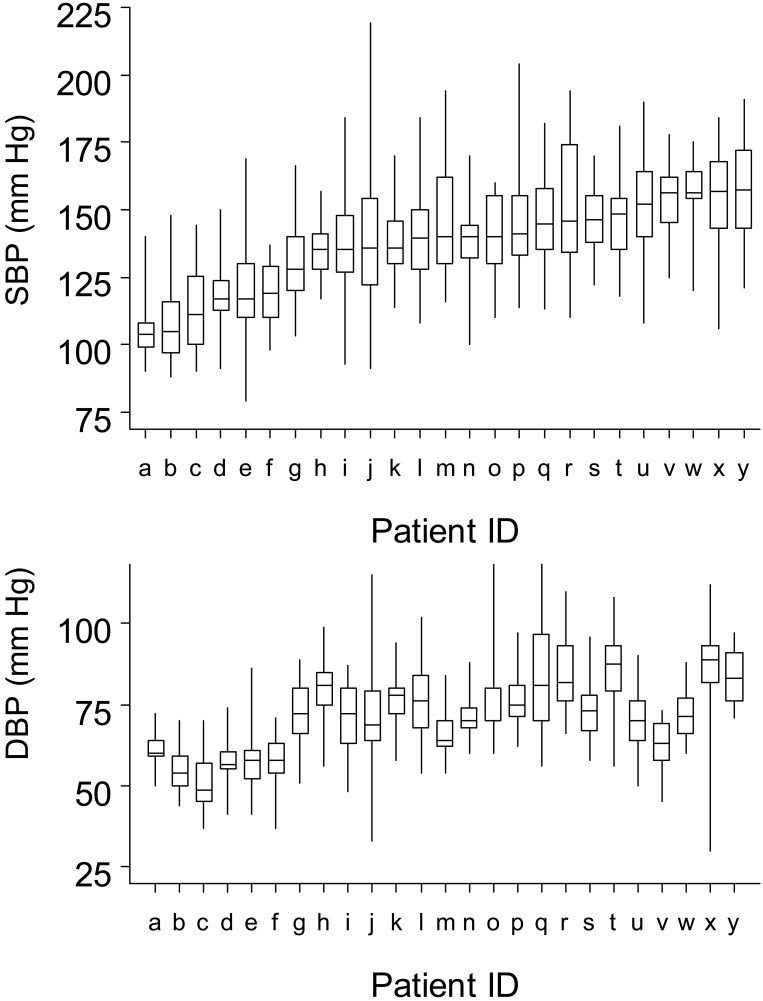
A graphic representation of the variability in predialysis SBP and DBP observed between and within patients in a random sample of 25 HD patients is shown (Figure 3). Approximately 12% of HD patients had increasing or decreasing trends in SBP measurements during days 31 to 120 of HD.
Figure 3.
Range of individual predialysis systolic (SBP) and diastolic (DBP) BP variability during days 31 to 120 of hemodialysis for 25 randomly selected patients. Midline bar represents the median SBP; upper and lower box limits, 75th and 25th percentiles; whiskers, the range of remaining SBP measurements. Patients were ordered by increasing median SBP.
The use of antihypertensive medications among HD patients in this study stratified by age is shown (Table 3). The use of angiotensin converting enzyme inhibitors, angiotensin receptor blocking agents, calcium channel blockers, clonidine, vasodilators (hydralazine and minoxidil), and the number of antihypertensive medications decreased with age (each P < 0.05). This is in concert with the observed decline in SBP and DBP among elderly HD patients.
Table 3.
Percentage of participants on antihypertensive medications
| Age (yr) | N | ACEi/ARB | β blockers | Clonidine | Calcium channel blockers | Vasodilators |
|---|---|---|---|---|---|---|
| 20-39 | 309 | 55.7% (49.9-61.3) | 57.9% (52.2-63.5) | 34.6% (29.3-40.2) | 57.9% (52.2-63.5) | 15.95% (12.0-20.4) |
| 40-59 | 1518 | 54.9% (52.4-57.5) | 55.3% (52.7-57.8) | 26.5% (24.3-28.8) | 48.7% (46.1-51.2) | 15.0% (13.3-16.9) |
| ≥60 | 2900 | 46.6% (44.8-48.5) | 51.6% (49.7-53.4) | 16.6% (15.2-18.0) | 43.2% (41.4-45.0) | 8.1% (7.2-9.2) |
| All | 4727 | 49.9% (48.4-51.3) | 53.2% (51.7-54.6) | 20.9% (19.8-22.1) | 45.9% (44.5-47.3) | 10.9% (10.0-11.8) |
Discussion
The present study demonstrates that the relationships between BP and age differ between HD patients and the general population. In contrast to the increase in SBP with age in the general population, SBP was elevated in all but the oldest HD patients. The inverse “U”-shaped relationship between age and DBP in the general population was absent in HD patients. DBP, MAP, and PP were elevated among young HD patients and demonstrated less age dependency than the general population. In contrast to the general population, there were only small differences in BP among HD patients, stratified by race and gender (7).
The flattening of the relationship between BP and age among HD patients may reflect acceleration of CVD in young patients. Studies using aplanar tonometry support the contention that central artery stiffness at any age is greater among HD patients versus the general population. Among young HD patients, other correlated but less direct measures of arterial stiffness, such as medial arterial calcification and left ventricular hypertrophy, are also highly prevalent (11,29,30).
Elderly HD patients have lower SBPs and MAPs than young HD patients. PP is stable or unchanged because of decreased DBP. This is consistent with persistence of low elastic compliance among older HD patients. The decline in MAP among elderly HD patients is similar to that observed in the oldest hypertensive groups in the Framingham study (31). The observed reduction in MAP likely represents a fall in cardiac output. However, the standard equation for estimating MAP noninvasively may lead to a significant underestimation (32).
We observed a tight correlation between predialysis and postdialysis BP values, regardless of age. However, many dialysis patients experience highly variable BP values. The average individual predialysis SBP variation was similar to the variance across the study group. These findings are in concert with reports that there is a large amount of noise in routine dialysis unit BP measurements (15,16,33). Not surprisingly, dialysis unit BP measurements are imprecise estimates of ABP (15). Predialysis SBP values overestimate ABP (15). Although postdialysis BPs are less biased, they agree poorly with ABP, precluding their use in precisely predicting ABP (15,16) Peixoto et al. reported better reproducibility of BP with ABP monitoring versus routine predialysis and postdialysis BPs (17). Given the greater reproducibility of ABP monitoring, its use may lead to a reduction in errors classifying HD patients as normotensive or hypertensive. Although ABP monitoring is the most accurate method for studying BP in HD patients, variability remains significant and there is poor reproducibility of the nocturnal decline in BP. The variability of BP among HD patients appears to be significantly greater than that observed in the general population. The variability within HD patients was as great as that between HD patients. The large amount of within patient variation is depicted in Figure 3.
Given the large variation in routine dialysis unit BP measurements, it is not surprising that they are unable to predict the presence of left ventricular hypertrophy (LVH) (15). Standardized dialysis unit BP measurements methods also exhibit weak performance at predicting LVH (16). Standardized dialysis unit BP measurements were associated with 14.3/7 mmHg lower predialysis and 13.6/4.4 mmHg lower postdialysis BP compared with routine measurement of BP in the dialysis unit (34). Overall, SBP measured outside the dialysis unit are a more powerful predictor of LVH (16) and mortality (35).
Because of the large variability, averaging multiple isolated predialysis BP measurements has limited utility in managing antihypertensive therapy. Isolated postdialysis BP measurements have greater reproducibility and are more useful in guiding therapy (17). Reduction of classification errors may require 24- to 44-h monitoring or home BP monitoring. Two to three weeks of averaged conventional dialysis unit BP measurements unit may improve the correlation with 24-h ABP monitoring (36,37). Home BP monitoring outperforms routine dialysis unit BP measurements, even when averaged over a 2-wk period (38).
Individual patient and population SDs approached the magnitude of the Joint National Commission hypertension categories (39). SDs among essential hypertensive patients undergoing 24-h ABP monitoring were lower than we observed, ranging from 9.3 to 14.5 mmHg (40,41) and were dominated by diurnal variation. Nonrandom daytime to nighttime variation was not represented in the present study. The large variation in routine dialysis unit BP measurements makes it difficult to distinguish random effects from interventions, including assessing the effects of antihypertensive medications. ABP monitoring and home BP monitoring will likely prove to be useful additions in assessing the response to antihypertensive therapy among HD patients.
The general population exhibits significant within-patient variability in clinic BP measurements. Klungel et al. observed within-patient SD of SBP (6.3) and DBP (5.4) annually between visits (24). Rosner and Polk observed within-patient SD of SBP (7.1 mmHg) and DBP (5.1 mmHg) between visits 1 to 7 d apart (25). ABP monitoring has been widely advocated for assessing BP in patients with chronic kidney disease (26) and the general population (27,28).
The present study has several unique strengths. It is one of the largest studies of BP among HD patients. Although size alone does not confer superiority, the demographic diversity and the fact that the large patients were cared for by academic and private nephrologists throughout the nation increased the study's generalizability. DCI's proprietary database (DARWIN) contains range checks ensuring high-quality patient data characteristics.
The present study also has several limitations. Unidentified confounders may have influenced the relationship between BP and age. In addition, we did not have a formal assessment of comorbidity. Although the study sample was similar to that of the USRDS HD population, blacks were overrepresented. We did not include any home or ABP measurements. In contrast to NHANES, dialysis unit BP measurements were not standardized which may impact comparisons between the general population and HD patients. Antihypertensive therapy may influence BP measurements and the relationship between age and BP. Lastly, oscillometric measurement of BP can overestimate diastolic pressure, which is proportional to the degree of arterial stiffness (42).
In summary, compared with the general population, HD patients exhibited a reduction in the age dependency of SBP, DBP, MAP, and PP. Young dialysis patients demonstrated a relationship between SBP and DBP consistent with a combination of increased peripheral vascular resistance and decreased vascular compliance, whereas the pattern in older patients suggested additional decreases in vascular compliance and cardiac output. BP variation was high and individual SBP variability was equivalent to the overall population variability and approaches Joint National Committee VII categorical ranges in magnitude.
Conclusion
Optimal BP management in HD patients aimed at reducing CVD mortality will require rigorous, randomized trials with well-defined BP goals and attention to the methods, location of BP measurements, and techniques for following participants. Routine predialysis and postdialysis BP measurements are so variable that they have a limited ability to predict CVD risk and are poor measures of titrating drug and nonpharmacologic therapy. A recent meta-analysis has demonstrated that these measurements are imprecise estimates of ABPs (15) and may have limited utility for assessing CVD risk and guiding therapy. Standardization of predialysis and postdialysis BP measurements may improve their utility for assessing CVD risk and guiding therapy. CVD risk stratification and individualization of BP goals and treatment modalities may also have to rely on home blood and ABP measurements and assessment of arterial stiffness. Nevertheless, BP must be measured in the dialysis unit to minimize the risk of acute complications related to hypertension and hypotension during the dialysis procedure.
Disclosures
None.
Acknowledgments
The authors thank Kelly Utterback and Serena Cumber for their expert technical assistance. The manuscript was improved by the reviews of Candice Welhausen and two anonymous reviewers. This research was supported by Dialysis Clinic, Inc.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Burt VL, Whelton P, Roccella EJ, Brown C, Cutler JA, Higgins M, Horan MJ, Labarthe D: Prevalence of hypertension in the US adult population: results from the Third National Health and Nutrition Examination Survey, 1988–1991. Hypertension 25 :305 –313,1995 [DOI] [PubMed] [Google Scholar]
- 2.Klassen PS, Lowrie EG, Reddan DN, DeLong ER, Coladonato JA, Szczech LA, Lazarus JM, Owen WF Jr: Association between pulse pressure and mortality in patients undergoing maintenance hemodialysis. JAMA 287 :1548 –1555,2002 [DOI] [PubMed] [Google Scholar]
- 3.Foley RN, Parfrey PS, Sarnak MJ: Clinical epidemiology of cardiovascular disease in chronic renal disease. Am J Kidney Dis 32 [Suppl]:S112 –S119,1998 [DOI] [PubMed] [Google Scholar]
- 4.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD: Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th Annual Fall Conference and Scientific Sessions. Hypertension 45 :811 –817,2005 [DOI] [PubMed] [Google Scholar]
- 5.Li Z, Lacson E Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW: The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis 48 :606 –615,2006 [DOI] [PubMed] [Google Scholar]
- 6.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW: Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis 33 :507 –517,1999 [DOI] [PubMed] [Google Scholar]
- 7.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P: “U” curve association of blood pressure and mortality in hemodialysis patients. Medical Directors of Dialysis Clinic, Inc. Kidney Int 54 :561 –569,1998 [DOI] [PubMed] [Google Scholar]
- 8.Covic A, Gusbeth-Tatomir P, Goldsmith DJ: Arterial stiffness in renal patients: an update. Am J Kidney Dis 45 :965 –977,2005 [DOI] [PubMed] [Google Scholar]
- 9.Foley RN, Parfrey PS, Harnett JD, Kent GM, Murray DC, Barre PE: Impact of hypertension on cardiomyopathy, morbidity and mortality in end-stage renal disease. Kidney Int 49 :1379 –1385,1996 [DOI] [PubMed] [Google Scholar]
- 10.Kutlay S, Dincer I, Sengul S, Nergizoglu G, Duman N, Erturk S: The long-term behavior and predictors of left ventricular hypertrophy in hemodialysis patients. Am J Kidney Dis 47 :485 –492,2006 [DOI] [PubMed] [Google Scholar]
- 11.Virga G, Stomaci B, Munaro A, Mastrosimone S, Cara M, Artuso E, Piovesana P: Systolic and diastolic function in renal replacement therapy: a cross-sectional study. J Nephrol 19 :155 –160,2006 [PubMed] [Google Scholar]
- 12.Agarwal R, Nissenson AR, Batlle D, Coyne DW, Trout JR, Warnock DG: Prevalence, treatment, and control of hypertension in chronic hemodialysis patients in the United States. Am J Med 115 :291 –297,2003 [DOI] [PubMed] [Google Scholar]
- 13.Mittal SK, Kowalski E, Trenkle J, McDonough B, Halinski D, Devlin K, Boylan E, Flaster E, Maesaka JK: Prevalence of hypertension in a hemodialysis population. Clin Nephrol 51 :77 –82,1999 [PubMed] [Google Scholar]
- 14.Rocco MV, Yan G, Heyka RJ, Benz R, Cheung AK: Risk factors for hypertension in chronic hemodialysis patients: baseline data from the HEMO study. Am J Nephrol 21 :280 –288,2001 [DOI] [PubMed] [Google Scholar]
- 15.Agarwal R, Peixoto AJ, Santos SF, Zoccali C: Pre- and postdialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol 1 :389 –398,2006 [DOI] [PubMed] [Google Scholar]
- 16.Agarwal R, Brim NJ, Mahenthiran J, Andersen MJ, Saha C: Out-of-hemodialysis-unit blood pressure is a superior determinant of left ventricular hypertrophy. Hypertension 47 :62 –68,2006 [DOI] [PubMed] [Google Scholar]
- 17.Peixoto AJ, Santos SF, Mendes RB, Crowley ST, Maldonado R, Orias M, Mansoor GA, White WB: Reproducibility of ambulatory blood pressure monitoring in hemodialysis patients. Am J Kidney Dis 36 :983 –990,2000 [DOI] [PubMed] [Google Scholar]
- 18.Stidley CA, Hunt WC, Tentori F, Schmidt D, Rohrscheib M, Paine S, Bedrick EJ, Meyer KB, Johnson HK, Zager PG: Changing relationship of blood pressure with mortality over time among hemodialysis patients. J Am Soc Nephrol 17 :513 –520,2006 [DOI] [PubMed] [Google Scholar]
- 19.National Center for Health Statistics 1999–2004. National Health and Nutrition Examination Survey Questionnaire, Hyattsville, MD,2007
- 20.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ: Human blood pressure determination by sphygmomanometry. Circulation 88 :2460 –2470,1993 [DOI] [PubMed] [Google Scholar]
- 21.National Center for Health Statistics 2006. National Health and Nutrition Examination Survey (NHANES) Centers for Disease Control and Prevention, Hyattsville, MD, December 1,2006
- 22.SAS 2003. Statistical analysis system, Cary, NC, SAS Institute,2007. .
- 23.Dart AM, Kingwell BA: Pulse pressure: a review of mechanisms and clinical relevance. J Am Coll Cardiol 37 :975 –984,2001 [DOI] [PubMed] [Google Scholar]
- 24.Klungel OH, de Boer A, Paes AH, Nagelkerke NJ, Seidell JC, Bakker A: Estimating the prevalence of hypertension corrected for the effect of within-person variability in blood pressure. J Clin Epidemiol 53 :1158 –1163,2000 [DOI] [PubMed] [Google Scholar]
- 25.Rosner B, Polk BF: Predictive values of routine blood pressure measurements in screening for hypertension. Am J Epidemiol 117 :429 –442,1983 [DOI] [PubMed] [Google Scholar]
- 26.Thompson AM, Pickering TG: The role of ambulatory blood pressure monitoring in chronic and end-stage renal disease. Kidney Int 70 :1000 –1007,2006 [DOI] [PubMed] [Google Scholar]
- 27.White WB, Walsh SJ: Ambulatory monitoring of the blood pressure in multicenter clinical trials. Blood Press Monit 1 :227 –229,1996 [PubMed] [Google Scholar]
- 28.Elijovich F, Laffer CL: Bayesian analysis supports use of ambulatory blood pressure monitors for screening. Hypertension 19 :II268 –II272,1992 [DOI] [PubMed] [Google Scholar]
- 29.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342 :1478 –1483,2000 [DOI] [PubMed] [Google Scholar]
- 30.Mitsnefes MM, Daniels SR, Schwartz SM, Khoury P, Strife CF: Changes in left ventricular mass in children and adolescents during chronic dialysis. Pediatr Nephrol 16 :318 –323,2001 [DOI] [PubMed] [Google Scholar]
- 31.Franklin SS, Gustin W, Wong ND, Larson MG, Weber MA, Kannel WB, Levy D: Hemodynamic patterns of age-related changes in blood pressure: the Framingham Heart Study. Circulation 96 :308 –315,1997 [DOI] [PubMed] [Google Scholar]
- 32.McDonald's Blood Flow in Arteries, edited by Nichola WW, O'Rourke MF, Philadelphia, Lea & Febiger,2005
- 33.Agarwal R, Andersen MJ, Light RP: Location not quantity of blood pressure measurements predicts mortality in hemodialysis patients. Am J Nephrol 28 :210 –217,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rahman M, Griffin V, Kumar A, Manzoor F, Wright JT Jr, Smith MC: A comparison of standardized versus “usual” blood pressure measurements in hemodialysis patients. Am J Kidney Dis 39 :1226 –1230,2002 [DOI] [PubMed] [Google Scholar]
- 35.Peixoto AJ, White WB: Ambulatory blood pressure monitoring in chronic renal disease: technical aspects and clinical relevance. Curr Opin Nephrol Hypertens 11 :507 –516,2002 [DOI] [PubMed] [Google Scholar]
- 36.Agarwal R, Lewis RR: Prediction of hypertension in chronic hemodialysis patients. Kidney Int 60 :1982 –1989,2001 [DOI] [PubMed] [Google Scholar]
- 37.Conion PJ, Walshe JJ, Heinle SK, Minda S, Krucoff M, Schwab SJ: Predialysis systolic blood pressure correlates strongly with mean 24-hour systolic blood pressure and left ventricular mass in stable hemodialysis patients. J Am Soc Nephrol 7 :2658 –2663,1996 [DOI] [PubMed] [Google Scholar]
- 38.Agarwal R, Andersen MJ, Bishu K, Saha C: Home blood pressure monitoring improves the diagnosis of hypertension in hemodialysis patients. Kidney Int 69 :900 –906,2006 [DOI] [PubMed] [Google Scholar]
- 39.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr, Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ: The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA 289 :2560 –2572,2003 [DOI] [PubMed] [Google Scholar]
- 40.Pierdomenico SD, Lapenna D, Bucci A, Manente BM, Mancini M, Cuccurullo F, Mezzetti A: Blood pressure variability and prognosis in uncomplicated mild hypertension. Am Heart J 149 :934 –938,2005 [DOI] [PubMed] [Google Scholar]
- 41.Verdecchia P, Angeli F, Gattobigio R, Rapicetta C, Reboldi G: Impact of blood pressure variability on cardiac and cerebrovascular complications in hypertension. Am J Hypertens 20 :154 –161,2007 [DOI] [PubMed] [Google Scholar]
- 42.Agarwal R, Saha C: Dialysis dose and the diagnosis of hypertension in hemodialysis patients. Blood Press Monit 12 :281 –287,2007 [DOI] [PubMed] [Google Scholar]