Abstract
Background and objectives: In the 2002 dengue outbreak in Taiwan, some fatal cases had the underlying disease of renal failure (RF). Physicians faced difficulty in diagnosis and treatment of these patients; however, the impacts of RF on the clinical presentations and outcomes of dengue infection have not been reported previously.
Design, setting, participants, & measurements: A retrospective review was conducted of medical records, clinical presentations, laboratory findings, and underlying diseases for all cases of dengue infection in a medical center. Characteristics and outcomes of dengue-infected patients with and without RF were compared.
Results: From January 2002 through January 2003, 519 dengue-infected patients were enrolled, including 412 patients with classical dengue fever (DF) and 107 patients with dengue hemorrhagic fever (DHF)/dengue shock syndrome (DSS). Twelve patients died in this outbreak, and all had DHF/DSS. Twenty-one (4.0%) patients were defined as being in the RF group. The RF group had a higher mortality rate than non-RF group (28.6 versus 1.2%; P < 0.001). The severity of GFR impairment was associated with higher percentages of DHF/DSS (P = 0.029) and mortality (P < 0.001). Differences in symptoms/signs and laboratory abnormalities between DF and DHF/DSS were significant in the non-RF group but not apparent in the RF group.
Conclusions: The diagnosis and management of dengue infection among patients with RF must be cautious, because complicated clinical courses with a higher mortality rate were well observed.
Dengue infection, one of the most important mosquito-transmitted diseases, is prevalent in >100 tropical and subtropical countries (1). Despite the advancements in public health and clinical medicine, the incident rate of dengue viral infection is increasing with an annual estimate of 50 to 100 million cases worldwide (2–4). The clinical presentations of dengue infections range from asymptomatic infection or a flu-like illness in classical dengue fever (DF) to the severe forms of dengue hemorrhagic fever (DHF) and dengue shock syndrome (DSS), which may cause fatalities (3). In Southeast Asia, DHF is primarily a disease of children who are younger than 15 yr (2) and a leading cause of hospitalization and mortality in children (5), with a mortality rate varying from 0.5 to 3.5% (6); however, in the adult populations, the existence of various comorbid systemic diseases may affect the severity of dengue viral infections, which has not been comprehensively studied (7,8).
With the rapid growth of population, urbanization, and modernization, countries that are located in the tropical and subtropical areas, both developing and developed, may have the problems of increasing prevalence of chronic kidney disease (CKD)/ESRD (9) and be at risk from dengue endemics (10,11). In the 2002 outbreak of dengue fever in southern Taiwan, most of these patients were adults and several patients who died had the concomitant underlying disease of chronic renal failure (CRF). The influence of CRF on the mortality of patients with DHF/DSS despite intensive care was observed with case reports (12). To our knowledge, no published research has focused on the presentations and outcomes of dengue viral infections in patients with impaired renal function. The aims of this study were to elucidate whether dengue viral infections in patients with renal failure have different clinical presentations and disease outcomes.
Materials and Methods
Participants and Definitions
From January 1, 2002, through January 31, 2003, patients who received a diagnosis of having dengue infections, either as DF or DHF/DSS, at Kaohsiung Medical University Hospital, a medical center located in southern Taiwan, were enrolled for this study. The diagnostic criteria for dengue infection were febrile illnesses associated with one of the following laboratory confirmation tests: (1) Isolation of dengue virus, (2) positive result on reverse transcriptase–PCR, (3) detection of dengue-specific IgM capture antibody, or (4) a four-fold or greater increase of dengue-specific IgG capture antibody by ELISA and hemoagglutination inhibition assay in paired serum samples. All of the tests for confirmation of dengue infection were done by the Center of Disease Control, Taiwan. Cases with only a single positive dengue IgG result or without detailed history were excluded. According to the criteria from the World Health Organization (WHO), patients who have DF and hemorrhagic manifestations, low platelet count (≤100 × 103/μl), and objective evidence of leaky capillaries (≥20% elevation in hematocrit, lower serum albumin, and pleural or other effusions) are classified as having DHF (WHO classification, DHF grades I/II). Those with evidence of circulatory failure (pulse pressure ≤20 mmHg, hypotension, or frank shock) are classified as having DSS (WHO classification, DHF grades III/IV) (1,13). To study the severity of renal function impairment in relation to the development of DHF/DSS and mortality, we calculated the estimated GFR (eGFR) for each patient by the simplified equation of the Modification of Diet in Renal Disease study (MDRD) (14). The definition of renal failure (RF) included patients who had an eGFR <60 ml/min per 1.73 m2 at admission and a concomitant history of CRF or eGFR <60 ml/min per 1.73 m2 before this episode of infection. Patients with no serum creatinine data at admission or with incomplete demographic data were excluded. Patients with acute kidney injury (AKI) were classified according to the RIFLE (risk, injury, failure, loss, ESRD) criteria into “risk for renal failure” (eGFR decrement >25% from the baseline), “injury to the kidney” (eGFR decrement > 50% from the baseline), and “failure of kidney function” (eGFR decrement >75% from the baseline) (15). Because there were patients with preexisting RF and decreased eGFR level, when the acute decrement in eGFR was to a level to receive dialysis therapy, these were classified as “failure of kidney function” regardless of their percentage change of eGFR.
Design
Through a retrospective review of medical charts, demographic data of age, gender, medical history of any underlying diseases in association with the severity of dengue infection, and mortality of each patient were recorded. All of the laboratory results at first hospital visit were collected for statistical analyses. The nadir or peak of the levels of liver enzymes and complete blood counts during hospitalization were also recorded. The recording of underlying diseases was based on the medical charts. Pulmonary diseases included chronic pulmonary obstructive disease, asthma, and bronchiectasis; cardiac diseases included arrhythmia, congestive heart failure, and ischemic heart disease; chronic liver diseases included chronic viral hepatitis and liver cirrhosis; and rheumatologic diseases included systemic lupus erythematosus, rheumatoid arthritis, Behçet disease, gouty arthritis, and osteoarthritis. Proteinuria was defined as urinary protein appearing ≥1+ (30 mg/dl) by dipstick test (Hema-Combistix; Bayer Diagnostics, Wales, UK), and the same criterion was used for urinary occult blood as for microscopic hematuria. Parameters that possibly influenced the clinical presentations and outcomes for development of classical DF or DHF/DSS in patients with or without RF were analyzed and compared. Biochemistries of blood and urine were measured by autoanalyzer (COBAS Integra 400; Roche Diagnostics, Mannheim, Germany).
Statistical Analyses
Categorical variables were compared by χ2 test. Fisher exact test was used when the expected count was less than five. Continuous data were expressed as means ± SD. Continuous variables with non-normal distribution were compared by Mann-Whitney U test between groups. We analyzed the risk factors for development of DHF/DSS and mortality by multiple logistic regression models. The Kaplan-Meier analysis was applied for probability of survival among groups on the basis of DF and DHF and with or without RF. These observations started from the day of hospital visit and ended on the day of patient discharge. Event of interest was considered as patient died during hospitalization. Patient discharge was considered as censored. A two-sided P < 0.05 was defined as significant. Analyses were performed by SPSS 10.0 (SPSS, Chicago, IL) and GraphPad Prism 4.0 (GraphPad Software, San Diego CA).
Results
Among 547 patients with confirmed dengue infection, 28 patients without complete information or lack of serum creatinine data were excluded. The 519 study patients included 265 (51.1%) men and 254 (48.9%) women with a mean age of 48 ± 18 yr. A total of 412 (79.4%) patients had classical DF, and 107 (20.6%) patients had DHF/DSS. Twenty-one patients had eGFR <60 ml/min per 1.73 m2 at admission and a concomitant history of CRF or had eGFR < 60 ml/min per 1.73 m2 before admission; these were defined as the RF group. Eighteen of these 21 patients had previous creatinine records, and all showed eGFR <60 ml/min per 1.73 m2, which could support the diagnosis of CRF. The other three patients, although without second creatinine data available, were suggested by self-statement of CRF at history taking. The demographics, past history of DF, and underlying diseases of patients with and without RF are shown in Table 1. Univariate analysis revealed that the RF group had a significantly higher percentage of hypertension, diabetes, and rheumatologic diseases and older age compared with the non-RF group, but there was no difference in the past dengue infection history. The time from the onset of fever to the detection of DF in RF and non-RF groups did not show a significant difference (2.8 ± 3 versus 3.6 ± 1.9 d).
Table 1.
Demographics, past history of DF and underlying diseases in all patients and in patients with and without RFa
| Variable | All Patients(n = 519) | RR Group(n = 21) | Non-RF Group(n = 498) | P |
|---|---|---|---|---|
| Age (yr; mean ± SD) | 48.9 ± 17.6 | 62.4 ± 10.7 | 48.4 ± 17.7 | <0.01 |
| Male gender (n [%]) | 265 (51.1) | 9 (42.9) | 256 (51.4) | 0.44 |
| Past history of DF (n [%]) | 79 (15.2) | 2 (9.5) | 77 (15.5) | 0.76 |
| Underlying diseases (n [%]) | ||||
| RF | 17 (3.3) | 14 (66.7) | 3 (0.6) | <0.01 |
| cancers | 23 (4.4) | 2 (9.5) | 21 (4.2) | 0.24 |
| diabetes | 46 (8.9) | 5 (23.8) | 41 (8.2) | 0.03 |
| hypertension | 126 (24.3) | 11 (52.4) | 115 (23.1) | <0.01 |
| cardiovascular diseases | 50 (9.6) | 4 (19.0) | 46 (9.0) | 0.13 |
| pulmonary diseases | 16 (3.1) | 0 (0.0) | 16 (3.2) | 1.00 |
| rheumatologic diseases | 29 (5.6) | 6 (28.6) | 23 (4.6) | <0.01 |
| gastrointestinal diseases | 121 (23.3) | 4 (19.0) | 117 (23.5) | 0.80 |
Statistic comparisons are done by Fisher exact test for categorical variables and Mann-Whitney U test for continuous variables between RF and non-RF groups. Statistical significance is considered at P < 0.05. DF, dengue fever; RF, renal failure.
Among the 519 patients, 273 had a second serum creatinine examination before or after recovery from dengue virus infection. According to RIFLE criteria, the frequencies of “risk for renal failure.” “injury to the kidney,” and “failure of kidney function” among these patients were 21.6% (59 of 273), 2.9% (eight of 273), and 2.6% (seven of 273), respectively. The patients with DHF/DSS were more susceptible to developing “injury to the kidney” and “failure of kidney function” with nearly five times the percentage (nine in DHF/DSS group and six in DF group, 15 versus 2.9%; P = 0.001). Patients in the RF group also had significantly higher percentages of “injury to the kidney” and “failure of kidney function” (seven in RF group and eight in non-RF group, 36.8 versus 3.1%; P < 0.001).
Twelve patients died during this outbreak, and all had DHF/DSS (11.2% of DHF/DSS; 2.3% of all patients), including six patients in the RF group. The RF group had a higher mortality rate (28.6%) than the non-RF group (1.2%; P < 0.001). Significant differences in risks for DHF/DSS and mortality between RF and non-RF groups are shown in Table 2. Among patients who died, only one patient died of secondary infection. The other 11 patients died of shock as a result of either intractable bleeding or severe DHF/DSS-induced multiple-organ failure. No one died as a result of the complication of RF. Seven patients received renal replacement therapy during hospitalization, either hemodialysis or continuous venovenous hemodialysis, for removal of excessive fluid or uremic toxins. Six of 97 patients with DHF/DSS in the non-RF group died, and four of six patients died within 5 d after admission. On the contrary, among 21 patients with RF, 10 developed DHF/DSS and six died. Most (five of six patients) died >5 d after hospitalization (Figure 1).
Table 2.
Serum creatinine, type of dengue infection, and outcomes in all patients and in patients with and without RFa
| Parameter | All Patients(n = 519) | RF Group(n = 21) | Non-RF Group(n = 498) |
|---|---|---|---|
| Serum creatinine (mg/dl; mean ± SD) | 1.1 ± 1.0 | 3.8 ± 3.6 | 1.0 ± 0.4b |
| Type of dengue infection | |||
| classical DF (n [%]) | 412 (79.4) | 11 (52.4) | 401 (80.5) |
| DHF/DSS (n [%]) | 107 (20.6) | 10 (47.6) | 97 (19.5)c |
| Mortality cases (n [%]) | 12 (2.3) | 6 (28.6) | 6 (1.2)b |
Statistic comparisons are done by Fisher exact test for categorical variables and Mann-Whitney U test for continuous variables between RF and non-RF groups. Statistical significance is considered at P < 0.05.
P < 0.001.
P < 0.01.
Figure 1.
The Kaplan-Meier survival analysis is based on the total survival days in the period from hospitalization to discharge among patients with dengue fever (DF) or dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS) in the renal failure (RF) group and in the non-RF group. Event of interest was defined as patient death during hospitalization and patient discharge as censored. There is overlapping of the curve in RF and non-RF groups of DF, but the patients with DHF/DSS in the RF group have significantly higher mortality than the others by log rank test (P < 0.001).
By multiple logistic regression analyses, after adjustment for age, gender, past history of DF, and other underlying diseases, the risk for developing DHF/DSS in the RF group was 3.0 times (95% confidence interval [CI] 1.1 to 7.6) that of the non-RF group, and in the diabetes group it was 2.1 times (95% CI 1.1 to 4.2) that of the nondiabetes group (Table 3). The mortality risk in the RF group was also significantly higher with an odds ratio of 33.9 (95% CI 7.0 to 164.1), and it was 15.1 (95% CI 2.2 to 102.1) in patients with pulmonary diseases. Each model was deemed effective, and the goodness of fit was evaluated by Hosmer-Lemeshow test (χ2 values were 7.47 [P = 0.49] and 6.0 [P = 0.65], respectively). The Kaplan-Meier survival analysis also demonstrated significantly that patients in the RF group with DHF/DSS had the lowest probability of survival (P < 0.001; Figure 1).
Table 3.
Multiple logistic regression analysis for factors associated with DHF/DSS and mortalitya
| Parameter | DHF/DSS |
Mortality |
||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| Age (yr) | 1.0 | 1.0 to 1.0 | 1.0 | 1.0 to 1.1 |
| Gender (male) | 1.1 | 0.7 to 1.7 | 1.1 | 0.3 to 4.3 |
| Past history of DF | 1.4 | 0.8 to 2.5 | b | b |
| Underlying diseases | ||||
| RF | 3.0 | 1.1 to 7.6 | 33.9 | 7.0 to 164.1 |
| cancers | 0.9 | 0.3 to 2.6 | 1.8 | 0.2 to 18.5 |
| diabetes | 2.1 | 1.1 to 4.2 | 2.1 | 0.1 to 11.6 |
| hypertension | 1.0 | 0.6 to 1.7 | 0.6 | 0.1 to 3.0 |
| cardiovascular diseases | 1.2 | 0.6 to 2.6 | 0.8 | 0.1 to 6.1 |
| pulmonary diseases | 1.3 | 0.4 to 4.1 | 15.1 | 2.2 to 102.1 |
| rheumatologic diseases | 1.9 | 0.8 to 4.5 | 4.2 | 0.8 to 22.2 |
| gastrointestinal diseases | 1.3 | 0.8 to 2.1 | 2.2 | 0.5 to 9.8 |
CI, confidence interval; DHF/DSS, dengue hemorrhagic fever/dengue shock syndrome; OR, odds ratio.
OR and 95% CI not calculated because no case with past history of DF had mortality outcome.
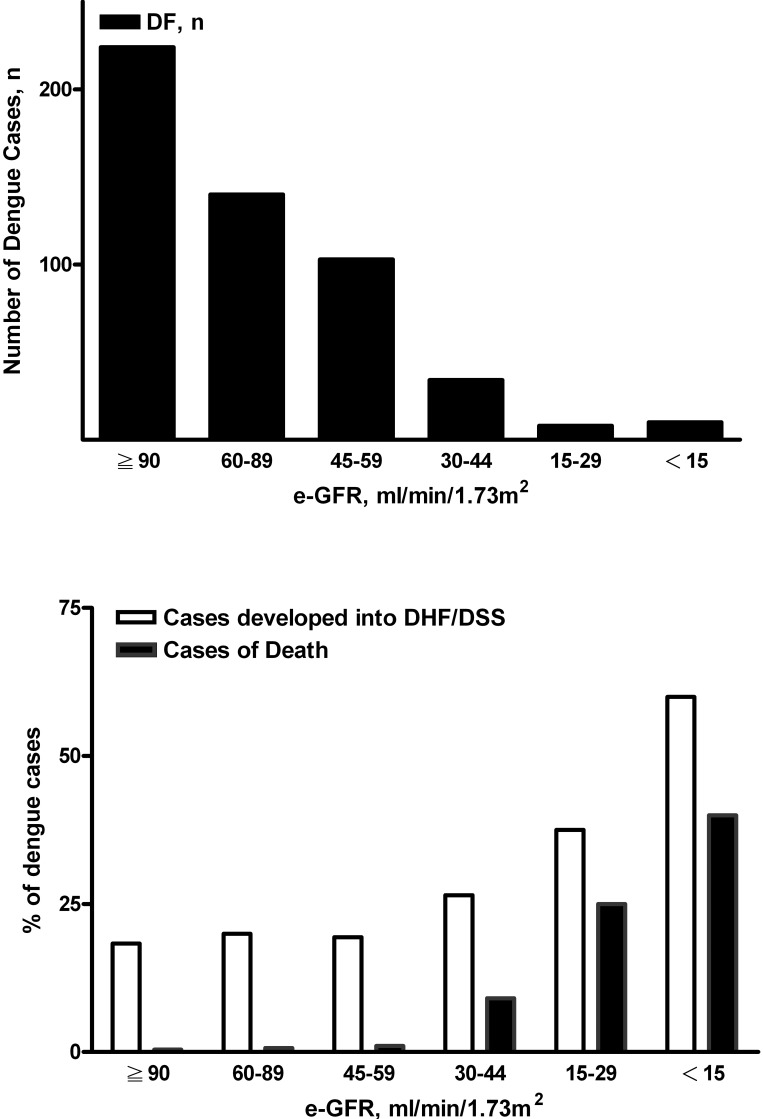
On the basis of the levels of eGFR, the distribution of eGFR in all of the cases of dengue infection is shown Figure 2, top. The percentages of DHF/DSS and mortality in each eGFR category are expressed in Figure 2, bottom. The increased percentages of DHF/DSS and mortality, respectively, followed the decrease in eGFR (Figure 2, bottom). Patients with more advanced renal function impairment had a higher percentage of DHF/DSS (P = 0.029) and also a higher percentage of mortality (P < 0.001, χ2 test for linear trend).
Figure 2.
Case distributions in different grades of estimated GFR (eGFR; top). The percentages of DHF/DSS and mortality in groups on the basis of different grades of eGFR are parallel to the severity of RF (bottom). Patients with more severe renal function impairment had higher percentages of DHF/DSS (P = 0.029) and mortality (P < 0.001) by χ2 test for linear trend. eGFR calculated by simplified Modification of Diet in Renal Disease (MDRD) equation.
The differences in clinical presentations of DF and DHF/DSS between patients with and without RF are shown in Table 4. There were significantly higher frequencies of ecchymosis/petechia, bleeding, proteinuria, hyponatremia, higher serum aspartate aminotransferase (AST), lower serum albumin, and lower nadir platelet count in the DHF/DSS group compared with DF in the patients without RF and similar to the situation usually expected in general populations; however, only the level of higher serum AST was significantly different between DF and DHF/DSS in the patients with RF, and there was no significant difference in frequency of other mentioned symptoms/signs and parameters between DF and DHF/DSS in the patients with RF.
Table 4.
Comparisons on the frequencies of symptoms/signs, laboratory data at admission between classical DF and DHF/DSS in the RF and non-RF groupsa
| Symptoms/Signs | RF Group (n = 21) |
Non-RF Group (n = 498) |
||||
|---|---|---|---|---|---|---|
| DF(n = 11) | DHF/DSS(n = 10) | P | DF(n = 401) | DHF/DSS(n = 97) | P | |
| Skin rash (%) | 45.5 | 20.0 | 0.361 | 44.1 | 35.1 | 0.100 |
| Ecchymosis/petechia (%) | 72.7 | 70.0 | 1.000 | 58.1 | 75.3 | 0.002 |
| Bleeding (%) | 81.8 | 100.0 | 0.476 | 72.8 | 92.8 | <0.001 |
| Seizure (%) | 0.0 | 0.0 | – | 0.5 | 3.1 | 0.053 |
| Proteinuria (%) | 72.7 | 60.0 | 0.659 | 24.9 | 47.4 | <0.001 |
| Hematuria (%) | 45.5 | 70.0 | 0.387 | 29.4 | 34.0 | 0.377 |
| Hyponatremia (%) | 36.4 | 40.0 | 1.000 | 23.4 | 36.1 | 0.014 |
| Hypokalemia (%) | 9.1 | 10.0 | 1.000 | 24.2 | 20.6 | 0.457 |
| Serum AST (U/L; mean ± SD) | 81.80 ± 44.50 | 4201.60 ± 7638.70 | 0.024 | 121.00 ± 138.60 | 512.80 ± 1593.90 | <0.001 |
| Serum albumin (g/dl; mean ± SD) | 3.60 ± 0.30 | 3.60 ± 0.20 | 0.600 | 3.80 ± 0.30 | 3.50 ± 0.50 | <0.001 |
| Platelet count (103/μl; mean ± SD) | 32.80 ± 30.50 | 20.30 ± 21.40 | 0.250 | 49.90 ± 44.50 | 29.20 ± 32.00 | <0.001 |
| Serum creatinine (mg/dl; mean ± SD) | 2.90 ± 2.60 | 4.80 ± 4.40 | 0.220 | 0.95 ± 0.30 | 1.02 ± 0.54 | 0.311 |
Statistic comparisons are done by χ2 or Fisher exact test for categorical variables and Mann-Whitney U test for continuous variables. Statistical significance is considered at P < 0.05.
Discussion
Our case series study demonstrates that patients with RF and dengue viral infections have significantly higher risks for DHF/DSS and mortality. Poor outcome, including both DHF/DSS and mortality, correlated with poor renal function.
Besides the viral virulence factor, it has been shown that factors of previous history of dengue infection, age, and underlying diseases are risk factors for the development of severe forms of dengue viral infections (2). Adult DHF/DSS was reported to be more frequent in patients with asthma, diabetes, and sickle cell anemia in Cuba (7). In the 1997 outbreak in Brazil, hypertension, diabetes, and bronchial asthma all were identified as individual risk factors for the development of DHF/DSS (8). Aside from these reports, there has been no further study for the relationship between CKD and DHF/DSS. This could be because DHF/DSS occurs predominantly in pediatric patients whose second infection with a dengue virus serotype is different from the first episode, which is explained by the antibody-dependent enhancement hypothesis (16,17). In our study, the history of previous dengue infection by patients’ self-reporting was not a significant risk factor for development of DHF as in another report (2); however, bias may have existed regarding this parameter in this study because of the lack of awareness of previous dengue infection by patients themselves when only minor or asymptomatic illnesses appeared during the previous infection. Adults were the main population of infection in the 2002 dengue outbreak in Taiwan, which is different from other countries’ situation, in which the majority of mortalities were children who had DHF (5). At the same time, there were increasing numbers of patients with CKD/ESRD in Taiwan (18,19). Both the adult population of dengue infection in this outbreak and the relatively high percentage of patients with CKD/ESRD made it possible for this study to evaluate the impact of renal insufficiency and RF on dengue infection. In our study, the risk for developing DHF/DSS was significantly higher in patients with diabetes and RF (Table 3). Diabetes was identified as a risk factor for development of DHF in previous studies (7,8). Our results indicate that patients who have RF and dengue infection have a higher risk for DHF/DSS.
The mechanism and pathogenesis of DHF/DSS have not been studied in patients with RF. The possible mechanism on the basis of some lines of evidence could be proposed. The increased risk for DHF/DSS in patients who had a second dengue infection of different serotype from a previous infection has been explained by the antibody-dependent enhancement hypothesis for the mechanism of DHF/DSS development (20). The residual antibody from a previous infection may bind to the virus of the second episode and construct the virus-antibody complexes. These complexes are taken up more easily than uncoated virus by cells expressing Fcr receptors, such as monocytes and macrophages. A series of cytokine cascades will follow with subsequent complement activation and increasing endothelium and vascular permeability (21). IFN-γ, TNF-α, IL-2, IL-6, IL-1β, and IL-8 are the cytokines involved in the pathogenesis of DHF/DSS (22). Among these cytokines, TNF-α and IL-6 are reported to be increased in patients with CRF (23). Thus, when the patients with have dengue viral infection, the augmented levels of cytokines might increase the vascular permeability markedly and put patients with RF at a higher risk for developing DHF/DSS. In addition to cytokine cascade, endothelial cells play a crucial role in DHF/DSS. In the uremic milieu, it can cause endothelial cell dysfunction by decreasing the attachment of endothelial cells and reducing thrombogenicity (24). This phenomenon might also contribute to the pathogenesis of DHF/DSS in patients with RF. Aside from these two mechanisms, abnormal von Willebrand factor (vWF) multimers are reported in patients with DHF, which indicates that vWF is also an important factor in the pathogenesis of DHF (25). In patients with RF, platelet dysfunction is well known and vWF is the most important factor responsible for this problem. All of these increase the risk for DHF/DSS development in patients with CRF.
The Kaplan-Meier survival analysis revealed that the lowest survival probability existed in patients with RF and DHF/DSS (Figure 1). Although the average age in the RF group was greater than in the non-RF group (Table 1), in multiple logistic regression analysis, age was not a significant factor for the difference in mortality. In addition to RF, pulmonary diseases were associated with significantly higher risks for mortality, which was also noted in patients with asthma (7). The rapid clinical course to mortality in four of sex deceased patients from the 97 non-RF patients with DHF/DSS indicates that they were the most severe cases in this group; however, most of the patients who died (five of six) from the 21 patients with RF and DHF/DSS died >5 d after hospitalization, suggesting that obscure symptoms/signs of DHF/DSS and CRF upon admission might have resulted in delayed diagnosis and management. That is why the mortality cases in RF group seemed to have had a less severe condition on admission but progressed quickly to cause death.
In treating patients with DF, there are certain signs that may raise physicians’ suspicion of the development of DHF/DSS, such as extensive petechia/ecchymosis, overt bleeding, proteinuria, hyponatremia, elevated AST/alanine aminotransferase, hypoalbuminemia, and marked thrombocytopenia; however, the difference of clinical presentations, including ecchymosis/petechia, bleeding, hypoalbuminemia, and nadir platelet count between DF and DHF/DSS in patients with RF is not obvious, which is different from the situation in patients without RF (Table 4). Furthermore, the low baseline hematocrit in patients with RF and low baseline platelet count in dialysis patients as a result of uremia and the use of heparin in dialysis could be other obstacles to early recognition of hemoconcentration and thrombocytopenia in dengue viral infection. These may lead to ignorance of clinical symptoms/signs and misinterpretation of the laboratory data and thereby increase the risk for delayed diagnosis of DHF/DSS in patients with RF and dengue viral infection. Another problem that may affect the outcomes in patients with RF and DHF/DSS is the difficulty in treating patients with severe RF, especially in proper fluid management and control of bleeding, as in our previous case report (12).
This study is one hospital-based design with the disadvantage of limited case number and the advantage of reducing bias on the factor of difference in medical care quality. Compared with the percentage distributions of DF and DHF/DSS in the whole of Taiwan (a total of 5388 classical DF and 242 DHF/DSS cases), the higher percentage of patients who had DHF/DSS and were admitted to our hospital than that of patients who had DF should be due to the nature of the medical center. It was reasonable that patients with more severe infection condition should be sent to the medical center, and there could be a significant number of patients not hospitalized or just admitted to local hospitals because of self-limited or mild clinical symptoms/signs of infection. There was a higher percentage of stage 3 CKD in our study compared with that in a national survey on hypertension, hyperglycemia, and hyperlipidemia (18). This higher percentage of patients with stage 3 CKD was explained by the severity of the condition of study patients as mentioned already. This is the first report to demonstrate the association of RF and mortality in patients with dengue infection on the basis of epidemiologic analysis, although the mechanisms of how decreased GFR causes the development of DHF/DSS and high mortality were not studied. The results demonstrate that not only the history of CRF but also the current status of RF is significantly associated with the outcomes in patients with dengue viral infection.
Dengue infection is a problem even in a developed country with a tropical climate (26,27). Taiwan has a well-established public health system and mandatory national health insurance system, but it is not able to escape the threat of a dengue outbreak. The increasing numbers of patients with CKD/ESRD in Taiwan (18,19) constitute a vulnerable group for severe dengue infection. Our experiences would be most helpful for areas where the dengue infections were not primarily in children and where a good health care system brings up the prevalence of CKD/ESRD.
Conclusions
Patients with dengue infection and more severe renal function impairment have a higher possibility of having DHF/DSS and a higher risk for mortality. For this complicated clinical course and high mortality, the diagnosis and treatment for such patients must be cautious.
Disclosures
None.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Gibbons RV, Vaughn DW: Dengue: An escalating problem. BMJ 324 :1563 –1566,2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ: Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 11 :480 –496,1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kautner I, Robinson MJ, Kuhnle U: Dengue virus infection: Epidemiology, pathogenesis, clinical presentation, diagnosis, and prevention. J Pediatr 131 :516 –524,1997 [DOI] [PubMed] [Google Scholar]
- 4.Malavige GN, Fernando S, Fernando DJ, Seneviratne SL: Dengue viral infections. Postgrad Med J 80 :588 –601,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinheiro FP, Corber SJ: Global situation of dengue and dengue haemorrhagic fever, and its emergence in the Americas. World Health Stat Q 50 :161 –169,1997 [PubMed] [Google Scholar]
- 6.Halstead SB: Is there an inapparent dengue explosion? Lancet 353 :1100 –1101,1999 [DOI] [PubMed] [Google Scholar]
- 7.Bravo JR, Guzman MG, Kouri GP: Why dengue haemorrhagic fever in Cuba? 1. Individual risk factors for dengue haemorrhagic fever/dengue shock syndrome (DHF/DSS). Trans R Soc Trop Med Hyg 81 :816 –820,1987 [DOI] [PubMed] [Google Scholar]
- 8.Cunha RV, Schatzmayr HG, Miagostovich MP, Barbosa AM, Paiva FG, Miranda RM, Ramos CC, Coelho JC, dos Santos FB, Nogueira RM: Dengue epidemic in the State of Rio Grande Do Norte, Brazil, in 1997. Trans R Soc Trop Med Hyg 93 :247 –249,1999 [DOI] [PubMed] [Google Scholar]
- 9.Eknoyan G, Lameire N, Barsoum R, Eckardt KU, Levin A, Levin N, Locatelli F, MacLeod A, Vanholder R, Walker R, Wang H: The burden of kidney disease: Improving global outcomes. Kidney Int 66 :1310 –1314,2004 [DOI] [PubMed] [Google Scholar]
- 10.da Fonseca BA, Fonseca SN: Dengue virus infections. Curr Opin Pediatr 14 :67 –71,2002 [DOI] [PubMed] [Google Scholar]
- 11.Hales S, de Wet N, Maindonald J, Woodward A: Potential effect of population and climate changes on global distribution of dengue fever: an empirical model. Lancet 360 :830 –834,2002 [DOI] [PubMed] [Google Scholar]
- 12.Kuo MC, Chang JM, Lu PL, Chiu YW, Chen HC, Hwang SJ: Difficulty in diagnosis and treatment of dengue hemorrhagic fever in patients with chronic renal failure: Report of three cases of mortality. Am J Trop Med Hyg 76 :752 –756,2007 [PubMed] [Google Scholar]
- 13.Rigau-Perez JG, Clark GG, Gubler DJ, Reiter P, Sanders EJ, Vorndam AV: Dengue and dengue haemorrhagic fever. Lancet 352 :971 –977,1998 [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130 :461 –470,1999 [DOI] [PubMed] [Google Scholar]
- 15.Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P: Acute renal failure: Definition, outcome measures, animal models, fluid therapy and information technology needs—The second international consensus conference of the Acute Dialysis Quality Initiative (ADQI) group. Crit Care 8 :R204 –R212,2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Halstead SB: Antibody, macrophages, dengue virus infection, shock, and hemorrhage: A pathogenetic cascade. Rev Infect Dis 11 [Suppl 4]:S830 –S839,1989 [DOI] [PubMed] [Google Scholar]
- 17.Morens DM: Antibody-dependent enhancement of infection and the pathogenesis of viral disease. Clin Infect Dis 19 :500 –512,1994 [DOI] [PubMed] [Google Scholar]
- 18.Hsu CC, Hwang SJ, Wen CP, Chang HY, Chen T, Shiu RS, Horng SS, Chang YK, Yang WC: High prevalence and low awareness of CKD in Taiwan: A study on the relationship between serum creatinine and awareness from a nationally representative survey. Am J Kidney Dis 48 :727 –738,2006 [DOI] [PubMed] [Google Scholar]
- 19.Kuo HW, Tsai SS, Tiao MM, Yang CY: Epidemiological features of CKD in Taiwan. Am J Kidney Dis 49 :46 –55,2007 [DOI] [PubMed] [Google Scholar]
- 20.Rothman AL, Ennis FA: Immunopathogenesis of dengue hemorrhagic fever. Virology 257 :1 –6,1999 [DOI] [PubMed] [Google Scholar]
- 21.Mairuhu AT, Mac Gillavry MR, Setiati TE, Soemantri A, ten Cate H, Brandjes DP, van Gorp EC: Is clinical outcome of dengue-virus infections influenced by coagulation and fibrinolysis? A critical review of the evidence. Lancet Infect Dis 3 :33 –41,2003 [DOI] [PubMed] [Google Scholar]
- 22.Pang T, Cardosa MJ, Guzman MG: Of cascades and perfect storms: The immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS). Immunol Cell Biol 85 :43 –45,2007 [DOI] [PubMed] [Google Scholar]
- 23.Pecoits-Filho R, Heimburger O, Barany P, Suliman M, Fehrman-Ekholm I, Lindholm B, Stenvinkel P: Associations between circulating inflammatory markers and residual renal function in CRF patients. Am J Kidney Dis 41 :1212 –1218,2003 [DOI] [PubMed] [Google Scholar]
- 24.Aznar-Salatti J, Escolar G, Cases A, Gomez-Ortiz G, Anton P, Castillo R, Revert L, Ordinas A: Uraemic medium causes endothelial cell dysfunction characterized by an alteration of the properties of its subendothelial matrix. Nephrol Dial Transplant 10 :2199 –2204,1995 [DOI] [PubMed] [Google Scholar]
- 25.Sosothikul D, Seksarn P, Pongsewalak S, Thisyakorn U, Lusher J: Activation of endothelial Cells, coagulation and fibrinolysis in children with dengue virus infection. Thromb Haemost 97 :627 –634,2007 [PubMed] [Google Scholar]
- 26.Goh KT: Changing epidemiology of dengue in Singapore. Lancet 346 :1098 ,1995 [DOI] [PubMed] [Google Scholar]
- 27.Ooi EE, Hart TJ, Tan HC, Chan SH: Dengue seroepidemiology in Singapore. Lancet 357 :685 –686,2001 [DOI] [PubMed] [Google Scholar]