Abstract
Background and objectives: All glomerular filtration rate (GFR) estimating equations have been developed from cross-sectional data. The aims of this study were to examine the concordance between use of measured GFR (mGFR) and estimated GFR (eGFR) in tracking changes in kidney function over time among patients with moderately severe chronic kidney disease.
Design, setting, participants, & measurements: A retrospective cohort study of subjects who had been enrolled in the MDRD Study A and who had two or more contemporaneous assessments of mGFR and eGFR (n = 542; mGFR range, 25 to 55 ml/min per 1.73 m2) during the chronic phase (month 4 and afterwards). mGFR was based on urinary iothalamate clearance; eGFR was based on the 4-variable MDRD Study equation. Temporal changes in GFR were assessed by within-subject linear regression of time on GFR.
Results: Median follow-up time for all subjects was 2.6 yr; median number of GFR measurements was six. The eGFR slope tended to underestimate measured decrements in GFR. The absolute value of the difference in mGFR and eGFR slopes was ≤2 ml/min per 1.73 m2 per yr among 58.3% of subjects; the remainder of subjects had larger absolute differences. Among the 22 variables studied, none predicted a systematic difference between mGFR slope and eGFR slope.
Conclusions: Although eGFR and mGFR exhibited similar relationships to 22 baseline variables, the overall bias seen in the full cohort suggests that clinicians and researchers should exercise caution when interpreting eGFR slope as a marker of progression of kidney disease.
The glomerular filtration rate (GFR) is considered the best available index of kidney function in health and disease (1). GFR can be measured by clearance techniques involving endogenous (e.g., creatinine and urea) or exogenous (e.g., inulin, iohexol, and iothalamate) filtration markers, with the latter considered to be the gold-standard approach (2). Unfortunately, clearance measurements are both cumbersome and costly; thus, in clinical practice, GFR is often estimated based upon the serum creatinine concentration. Numerous GFR estimating equations have been developed, the most widely used of which was derived from the Modification of Diet in Renal Disease (MDRD) Study (3,4).
Essentially all GFR estimating equations have been developed from cross-sectional data and perform well when used to classify individuals at single points in time, particularly for levels of GFR less than 60 ml/min per 1.73 m2 (3,5–9). Ideally, these equations could also be used to monitor GFR changes over time in research and clinical practice. However, the validity of these equations for longitudinal application has not been sufficiently examined. Temporal changes in factors influencing creatinine production (e.g., muscle mass and dietary intake) and renal creatinine handling (e.g., tubular secretion), or extra-renal elimination (e.g., antibiotics) potentially further limit the accuracy of estimating equations or serum creatinine used alone as a filtration marker when applied over time (10). Understanding this potential limitation is of particular importance for two reasons: 1) the movement toward standardized reporting of estimated GFR (which may lead clinicians to draw longitudinal comparisons), and 2) the use of estimated GFR as an outcome measure in studies of preservation of kidney function.
The MDRD Study is one of only a few trials that tracked decline in kidney function longitudinally in a population with chronic kidney disease (CKD) with measurement of GFR. Thus, data from this study provide a context in which to assess the longitudinal performance of GFR estimating equations. We undertook the following study: 1) to examine and characterize the correspondence between changes over time in measured GFR (mGFR) and estimated GFR (eGFR) and 2) to identify factors that may influence the relationship between longitudinal changes in mGFR and eGFR.
Materials and Methods
The MDRD Study was a randomized, multicenter, 2 × 2 factorial design clinical trial of protein intake and blood pressure control among patients with moderate to severe nondiabetic CKD. Two separate studies were conducted: Study A included subjects with initial mGFR 25 to 55 ml/min per 1.73 m2, and Study B included subjects with initial mGFR of 13 to 24 ml/min per 1.73 m2. We report on Study A participants whose kidney function levels represent a broad segment of the population with CKD. The MDRD Study was conducted between 1989 and 1994. Specifically, this analysis considers data from the 542 MDRD Study A participants who had two or more contemporaneous assessments of mGFR and eGFR. The recruitment lasted 21 mo at the end of which subjects had 1.5 to 4 yr of anticipated follow-up. Details on the design, baseline characteristics of study participants, and the primary results have been published previously (11).
Kidney function was determined by urinary clearance of 125I-iothalamate (mGFR) at months 0, 2, 4, postrandomization, and every 4 mo thereafter. The mGFR was adjusted for the body surface area (which was updated with each GFR measurement) as follows:
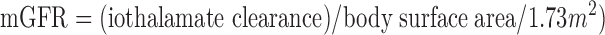 |
Other serum and urine biochemical markers were collected each time mGFR was measured. For the purposes of this study, eGFR was calculated using the 4-variable MDRD Study equation:
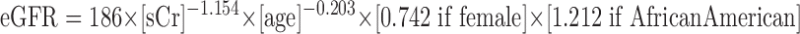 |
Statistical Analysis
Definition of GFR slopes.
Earlier research from the MDRD Study suggested that the mGFR slope differed between the 4-mo period immediately following randomization and subsequent follow-up (attributed to an acute effect of study treatment regimens) (11,12). We therefore chose to model the slope of mGFR and eGFR from 4 mo on (chronic phase) in these analyses, given that long-term changes in GFR bear greater clinical relevance to the majority of patients with CKD.
Analysis was restricted to the 3555 contemporaneous assessments of mGFR and eGFR during the chronic phase; measurements of either mGFR or eGFR not accompanied by the other were not included. For each subject, a linear regression model of time on mGFR was created, and the slope of the regression line was used to estimate that subject's change in mGFR over time. An analogous procedure was used to define each subject's eGFR slope.
Comparison of eGFR and mGFR slopes.
Estimated GFR slope was compared with mGFR slope both with respect to bias and precision. Bias (i.e., systematic differences in estimates) was assessed by comparing average slopes at the group level (e.g., mean mGFR slope versus mean eGFR slope across subjects). Given the potential interplay among levels of dietary protein, protein metabolism, creatinine production, and serum creatinine, analyses were stratified based on protein intake group assignment.
Precision of the difference between eGFR slope and mGFR slope was analyzed in two ways. First, the correlation between mGFR slope and eGFR slope was examined graphically (by scatter plot) and by calculation of Pearson correlation coefficients. Given that the intrasubject accuracy and precision of slope estimation are dependent, in part, on the time interval over which measurements were available, these analyses were further stratified according to subjects’ follow-up time (<2 yr and >2 yr). Second, the intrasubject difference between mGFR slope and eGFR was calculated, and the distribution of this difference over the cohort was examined graphically and via summary statistics.
Analysis of predictors of the difference between eGFR and mGFR slopes.
We explored how various clinical factors were related to differences in mGFR and eGFR slope. Twenty-two baseline candidate predictors were considered, including: demographic factors (age, race, gender), comorbid disease states (hypertension, edema, tobacco use, vascular disease), treatment group assignment (protein intake, blood pressure target), anthropometric data (body mass index, body surface area, triceps skin fold thickness, subscapular skin fold thickness, percent body fat), and biochemical data (albumin, hemoglobin, serum creatinine, cholesterol, triglyceride, urine urea nitrogen, urine protein, urine creatinine).
Estimation of the predictors of the average difference between mGFR and eGFR slopes for each subject is made more challenging by complexities in the variance structure. For example, slope estimates are more precise for subjects with a greater number of measurements taken over a longer period of time. To address this problem, we used Generalized Estimating Equations (13) in which we used exchangeable working correlation structure in the marginal models. This method takes into account the varying amount of information provided by each subject as well as the correlation of measurements within a subject. Measured GFR slopes were modeled as a function of predictors using a series of models, which included a term for the linear effects of time, a main effects term for the covariate of interest, and a two-way interaction term for that variable and time. For example, in the case of gender:
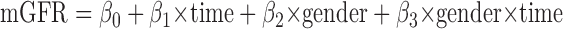 |
In this case, β3 represents the difference in observed mGFR slope (over time) in male versus female subjects. An analogous series of models was fit for eGFR. Extending the example of gender:
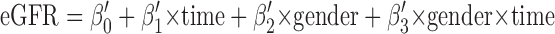 |
β3′ represents the difference in eGFR slope over time observed between male and female subjects. We defined delta (δ) as the difference between β3 and β3′, which in essence represents the differential effect of a given predictor variable (in this case gender) on mGFR versus eGFR trend. In the case of gender, a negative value of delta would indicate that male subjects exhibited a more negative slope for mGFR than eGFR. For continuous variables (e.g., BMI), delta represents the relative difference in mGFR versus eGFR slope per unit change (e.g., per 1 kg/m2). To calculate variance estimates and confidence intervals for delta, we used bootstrapping with replacement (minimum, 1000 replications) (14).
All analyses were performed using STATA 8.0 (College Station, TX).
Results
Summary of Study Subjects
For all subjects, the median follow-up time was 2.6 yr. During the chronic phase (month 4 and after), there were 3555 contemporaneous measurements of mGFR and eGFR among 542 subjects. The median number of GFR measurements per subject was six. Selected demographic and clinical factors measured at baseline are shown in Table 1. Sixty-two percent of the patients were male; 85% were white; mean (SD) age was 52.1 (12.0) years.
Table 1.
The baseline characteristics of the cohort
| Total | n (%) | |
|---|---|---|
| Dichotomous factors | ||
| nutrition allocation group | 542 | |
| low protein/phosphate | 270 (49.8) | |
| usual protein/phosphate | 272 (50.2) | |
| blood pressure allocation group | 542 | |
| moderate | 264 (48.7) | |
| intense | 278 (51.3) | |
| gender | 542 | |
| male | 334 (61.8) | |
| female | 207 (38.2) | |
| race | 542 | |
| white | 462 (85.2) | |
| black | 47 (8.7) | |
| other | 33 (6.1) | |
| vascular disease history | 542 | 70 (12.9) |
| edema | 537 | 49 (9.1) |
| hypertension history | 542 | 448 (82.7) |
| diabetes | 542 | 26 (4.8) |
| tobacco use | 541 | 75 (13.9) |
| Continuous factors | Mean (SD) |
|
| age (yr) | 542 | 52.1 (12.0) |
| albumin (g/dl) | 542 | 4.0 (0.3) |
| body mass index (kg/m2) | 541 | 27.7 (4.5) |
| body surface area (m2) | 542 | 1.9 (0.2) |
| hemoglobin (g/dl) | 526 | 13.5 (1.6) |
| serum creatinine (mg/dl) | 541 | 1.9 (0.5) |
| serum total cholesterol (mg/dl) | 539 | 217.8 (46.2) |
| triglycerides (mg/dl) | 518 | 173.4 (138.9) |
| urine urea nitrogen (g/day) | 476 | 10.7 (2.5) |
| urine protein (g/day) | 476 | 0.9 (1.5) |
| triceps skin fold (mm) | 515 | 17.5 (7.7) |
| subscapular skin fold (mm) | 494 | 19.9 (7.1) |
| percent body fat | 485 | 30.7 (7.1) |
| baseline urine creatinine (mg/day) | 476 | 1495.9 (410.5) |
SD, standard deviation.
Distribution of mGFR and eGFR Slopes over Time
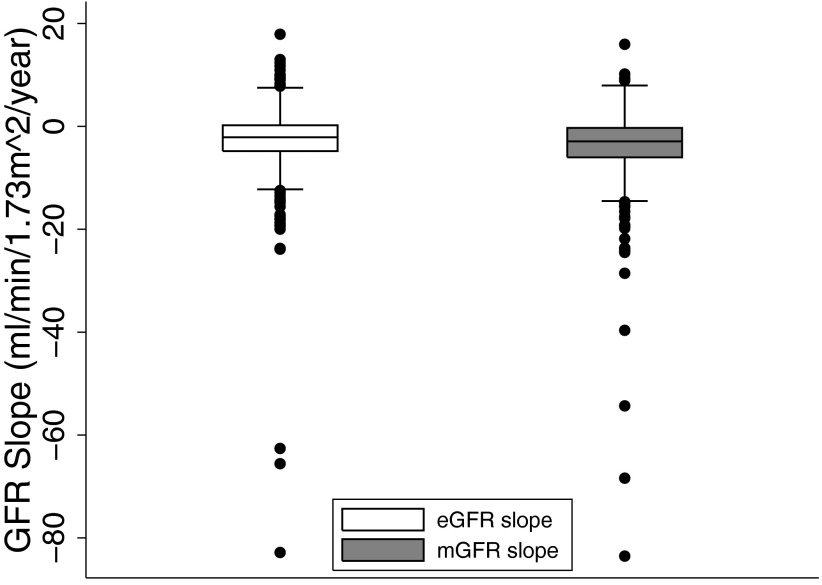
The distributions of mGFR slope and eGFR slope are shown in Figure 1. Overall, eGFR tended to underestimate the (negative) magnitude of mGFR slope. The mean (SD) of measured GFR slope was −3.9 (7.2) ml/min per 1.73 m2 per yr; the mean (SD) eGFR slope was −2.8 (7.1) ml/min per 1.73 m2 per yr, representing, on average, an underestimation of 28%. Neither stratification by assignment to usual versus low protein group nor the presence of polycystic kidney disease significantly altered these distributions (data not shown).
Figure 1.
Intersubject distribution of eGFR slope (left) and mGFR slope (right). All values are expressed as ml/min per 1.73 m2 per yr. Boxes span from 25th to 75th percentile; median is indicated by line within box; whiskers span from 25th percentile minus 1.5 times the interquartile range to the 75th percentile plus 1.5 times the interquartile range. Estimated GFR slope was distributed with mean (SD), 25th percentile, median, 75th percentile of −2.8 (7.1), −4.8, −2.1, 0.2 ml/min/1.73 m2 per yr. Measured GFR slope was distributed with mean (SD), 25th percentile, median, 75th percentile of −3.9 (7.2), −6.0, −2.9, −0.3 ml/min/1.73 m2 per yr.
Correlation between mGFR and eGFR
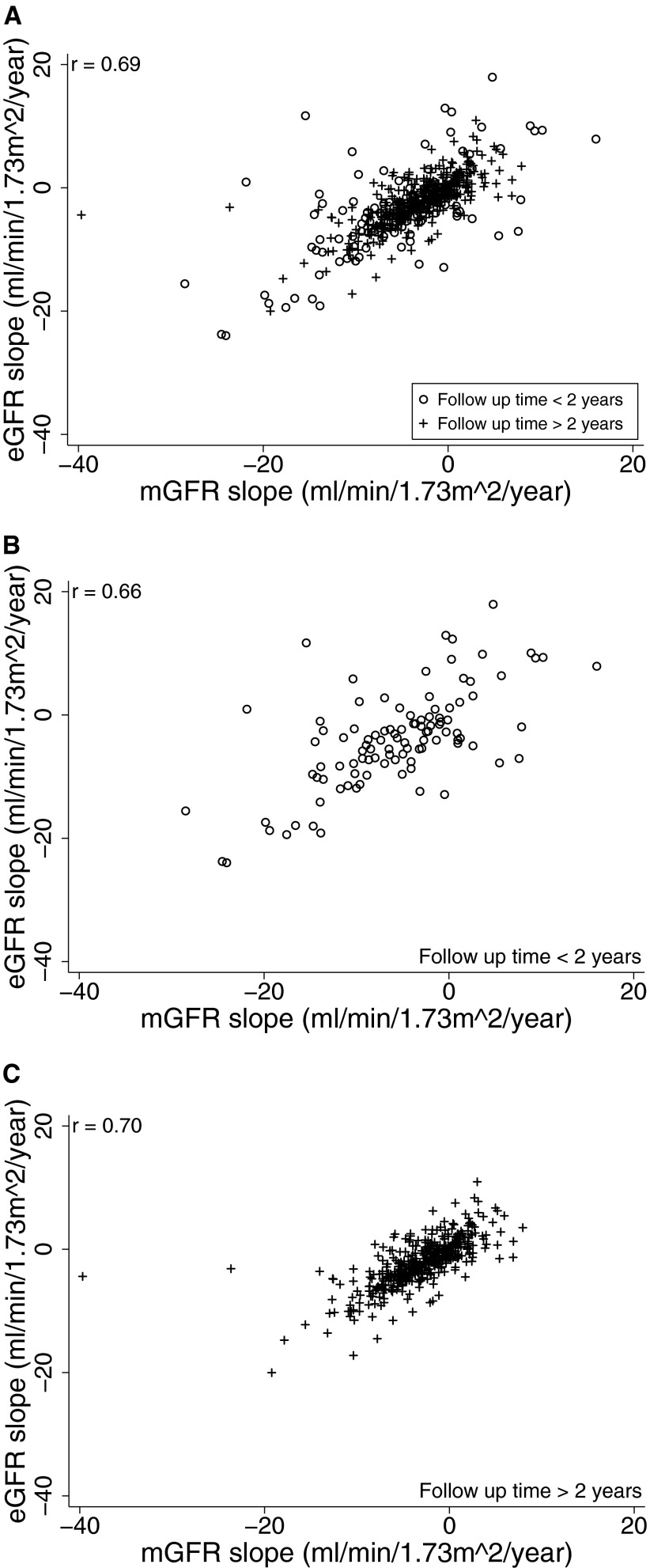
Scatter plots of mGFR slope versus eGFR slope are shown in Figure 2. Overall, the Pearson correlation coefficient was 0.83. However, excluding three outliers with implausible values of GFR slope (absolute value of GFR slope > 50 ml/min per 1.73 m2 per yr), the coefficient was reduced to 0.69; thus, 48% (0.692) of the variance in observed eGFR slope could be explained by a linear relationship to mGFR slope. No differences in correlations were observed between subjects with less than and greater than 2 yr of follow-up: r = 0.66 and 0.70, respectively. However, reflecting greater precision when slope was estimated over a longer time period, the SD of the difference between eGFR slope and iGFR was 6.9 ml/min per 1.73 m2 in those with less than two years of follow-up and was 3.2 ml/min/1.72m2 in those with more than 2 yr of follow-up.
Figure 2.
Scatter plot of eGFR slope versus mGFR slope for: (A) all subjects, (B) subjects with less than 2 yr of follow-up, and (C) subjects with greater than two years of follow-up. Panels A and B exclude three outlier points: (−54.3 ml/min per 1.73 m2 per yr, −62.7 ml/min per 1.73 m2 per yr), (−68.4 ml/min per 1.73 m2 per yr, −82.8 ml/min per 1.73 m2 per yr), and (−83.5 ml/min per 1.73 m2 per yr, −65.6 ml/min per 1.73 m2 per yr).
Difference between mGFR and eGFR
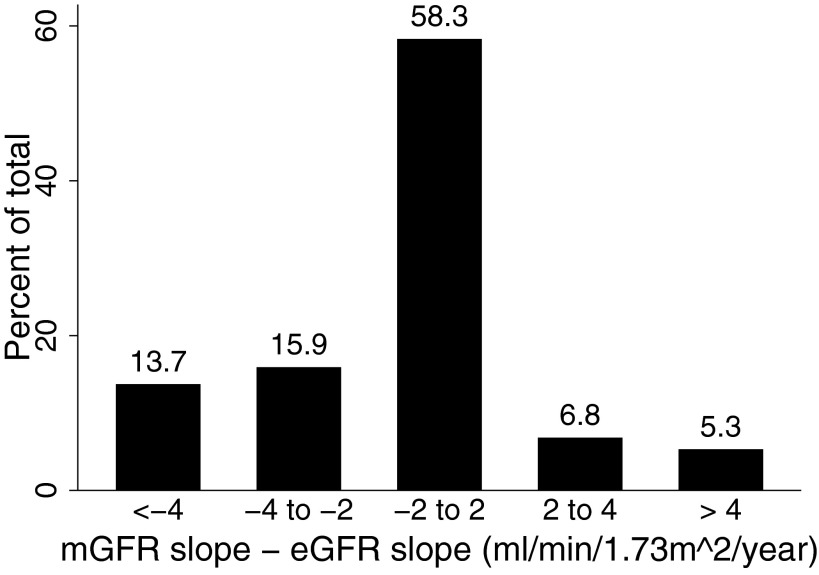
The distribution of the within-subject difference between mGFR slope and eGFR slope is shown in Figure 3. Overall, mGFR slope and eGFR slope were within 2 ml/min per 1.73 m2 per yr of one another in 58.3% of subjects. The two differed by between 2 and 4 ml/min per 1.73 m2 per yr in 22.7% of subjects, and by 4 ml/min per 1.73 m2 per yr or more in 19.1%. Estimated GFR slope underestimated the mGFR decrement in 29.6% of subjects (i.e., difference < −2 ml/min per 1.73 m2 per yr), whereas it overestimated mGFR decrement (i.e., difference >2 ml/min per 1.73 m2 per yr) in 12.2% of subjects. Assignment to nutritional group and polycystic kidney disease did not materially alter these distributions (data not shown).
Figure 3.
Distribution of the intrasubject difference between mGFR slope and eGFR slope.
Predictors of the Difference between mGFR and eGFR Slopes
Generalized Estimating Equations with bootstrap replication were used to identify clinical factors associated with the difference mGFR and eGFR slope (Table 2). Among 22 candidate predictors, none predicted a systematic difference between eGFR slope and mGFR slope at conventional levels of statistical significance.
Table 2.
Estimates of δ for chronic slopes
| Mean | SE | 95% CI | |
|---|---|---|---|
| Dichotomous factors | |||
| allocation to usual protein/phosphate | −0.18 | 0.21 | (−0.55, 0.24) |
| allocation to moderate blood pressure target | −0.12 | 0.21 | (−0.52, 0.30) |
| male gender | −0.19 | 0.22 | (−0.59, 0.24) |
| black race | −0.89 | 0.54 | (−1.99, 0.16) |
| hypertension history | 0.04 | 0.25 | (−0.46, 0.50) |
| vascular disease history | −0.20 | 0.38 | (−0.93, 0.61) |
| edema | 0.12 | 0.38 | (−0.59, 0.91) |
| tobacco use | −0.11 | 0.26 | (−0.63, 0.39) |
| Continuous factors | |||
| age (per year) | −0.01 | 0.01 | (−0.03, 0.01) |
| albumin (per g/dl) | 0.07 | 0.30 | (−0.50, 0.67) |
| baseline body mass index (per kg/m2) | −0.01 | 0.02 | (−0.06, 0.04) |
| body surface area (per m2) | 0.23 | 0.43 | (−0.64, 1.07) |
| hemoglobin (per g/dl) | 0.02 | 0.07 | (−0.10, 0.16) |
| serum creatinine (per mg/dl) | 0.30 | 0.17 | (−0.01, 0.67) |
| serum total cholesterol (per 100 mg/dl) | 0.01 | 0.22 | (−0.41, 0.45) |
| triglycerides (per 100 mg/dl) | 0.02 | 0.12 | (−0.15, 0.28) |
| urine urea nitrogen (per g/day) | 0.01 | 0.05 | (−0.08, 0.10) |
| urine protein (per g/day) | 0.03 | 0.06 | (−0.09, 0.16) |
| triceps skin fold (per mm) | 0.01 | 0.01 | (−0.01, 0.03) |
| subscapular skin fold (per mm) | −0.02 | 0.01 | (−0.05, 0.01) |
| percent body fat (per ) | −0.004 | 0.014 | (−0.031, 0.023) |
| baseline urine creatinine (per 100 mg/day) | 0.01 | 0.03 | (−0.04, 0.06) |
SE, standard error; CI, confidence interval.
Discussion
Use of estimating equations to track longitudinal changes in GFR is desirable for both clinical and research purposes provided that eGFR change offers a valid estimate of true (measured) GFR change. Data from the MDRD Study A permitted examination of the concordance between longitudinal mGFR and eGFR changes. On average, eGFR slope underestimated mGFR slope by 28%, and there was a wide distribution of the within-subject difference between eGFR and mGFR slopes. More than 40% of subjects had an mGFR slope that differed from the eGFR slope by ≥2 ml/min per 1.73 m2 per yr (more than half of the average mGFR decrement of −3.9 ml/min per 1.73 m2 per yr). We were unable to identify any variables that predicted a systematic difference between average mGFR slope and eGFR slope.
To illustrate the clinical importance of the bias we detected in the eGFR slope, we offer the hypothetical example of a patient with a true GFR of 40 ml/min per 1.73 m2 and constant annual decrement of 3 ml/min per 1.73 m2. This patient would be expected to reach a GFR of 10 ml/min per 1.72 m2 (corresponding to kidney failure) in 10 yr. However, if eGFR slope underestimated the decrement in true GFR by 1.1 ml/min per 1.73 m2 per yr, the predicted time horizon would erroneously be 60% longer, or 16 yr.
Our findings that the eGFR slope provides a biased estimate for mGFR slope on the group level confirm and extend prior findings from other studies. Data from the African American Study of Kidney Disease and Hypertension (AASK), which enrolled African American subjects with hypertensive nephropathy and baseline mGFR between 20 and 65 ml/min per 1.73 m2, demonstrate that eGFR slope (based on an eGFR equation derived from AASK data) underestimated the mean decrement in mGFR by 0.28 ml/min per 1.73 m2 per yr (−1.64 versus −1.92 ml/min per 1.73 m2 per yr), an underestimation of approximately 15% (15). Studies in persons with diabetes have also demonstrated that eGFR slope modestly underestimates observed decrements in mGFR. Among Pima Indians with diabetes, proteinuria and hyperfiltration (baseline mGFR >120 ml/min), eGFR slope underestimated mGFR decrement by 1.6% per year (−2.8 versus −4.4% per year) (16). Among diabetic subjects with relatively preserved kidney function and micro- and macro-albuminuria, eGFR slope underestimated mGFR decrement by 1.2 ml/min per 1.73 m2 per yr (−2.9 versus −4.1 ml/min per 1.73 m2 per yr) and 1.0 ml/min per 1.73 m2 per yr (−4.2 versus −5.2 ml/min per 1.73 m2 per yr), respectively (17).
In contrast, data from the Consortium for Radiologic Imaging Studies of Polycystic Kidney Disease study (which enrolled patients with polycystic kidney disease and normal baseline GFR) suggested that eGFR slope overestimated the decline in mGFR by 1.5% per year (−3.0 versus −1.5% per year) (10). It is unknown whether the discrepancy (i.e., eGFR overestimating rather than underestimating the change in kidney function) resulted from biologic differences among patients with polycystic kidney disease, was dependent on the higher baseline kidney function (where estimating equations are less precise), or resulted from other factors. In the present analyses, the differences among mGFR and eGFR slopes were similar among subjects with and without polycystic disease (although these analyses may have been underpowered).
In addition to the bias, we observed substantial deviations between eGFR slope and mGFR slope. These deviations reflect a combination of 1) variability in mGFR slope resulting from measurement error in the GFR assay, 2) variability in eGFR slope due to measurement error in serum creatinine, and 3) variation between true GFR slope and the “true” eGFR slope that would have resulted if serum creatinine were measured without error. We did not attempt to formally decompose the total variation of the differences between eGFR slope and mGFR slope into these three components, in part, because of the unavailability of repeat measurements of mGFR within a narrow time interval (e.g., within several days) that would have been required for a definitive assessment of the measurement error in mGFR. Reports of substantial within-patient error variances in longitudinal analyses of suggest that a substantial portion of the deviations between eGFR slope and mGFR slope may indeed be due to measurement error in mGFR (5,15). Nonetheless, we do find the large deviations between eGFR and mGFR slope to be particularly notable given that the MDRD Study equation was developed in this population, and these findings probably represent an upper limit of its performance abilities. Measured and estimated GFR slopes differed by more than 2 ml/min per 1.73 m2 per yr in 41.8% of subjects studied. This degree of imprecision is very large compared with the expected annual decrement among patients with stage 3 CKD, which is expected to be in the range of 1.4 to 3.9 ml/min per 1.73 m2 based on this and other published studies (18,19). The imprecision of estimated slope should also be viewed relative to the expected magnitude of strategies that aim to preserve kidney function (20–22), which may be substantially lower.
Among the 22 variables studied, none predicted a systematic difference between mGFR slope and eGFR slope. On the one hand, this indicates that despite of an overall systematic bias of eGFR slope, both eGFR slope and mGFR slope exhibited similar relationships with many baseline variables, consistent with a similar finding reported in the AASK Study (19). On the other hand, our data do not permit identification of subjects for with a smaller bias in eGFR slope relative to mGFR slope.
Several important limitations in the current study should be noted. First, the MDRD Study A population was preponderantly white and contained very few subjects with diabetes compared with the overall population with CKD. Moreover, all subjects included in this analysis had baseline mGFRs between 25 and 55 ml/min per 1.73 m2. Thus, caution should be taken in generalizing results to other populations, including those with greater or lesser degrees of kidney function. Second, data were collected in the context of an ongoing clinical trial, and all measurements were made at a centralized laboratory. Although this avoided any concerns related to the calibration of creatinine measurements, our findings probably represent an upper limit of the performance of eGFR (and mGFR) measured longitudinally. Third, this analysis considered only creatinine-based GFR estimating equations. Some (23), but not all (24), recent cross-sectional data suggest that equations based on other filtration markers (i.e., cystatin C) may yield more accurate GFR estimates than those based on creatinine. One small, preliminary study suggests that longitudinal assessments of eGFR using cystatin C may be superior to creatinine-based estimates (16). Additional study is needed to confirm and validate these findings. Fourth, the relative health of participants in the MDRD Study constrained variability among many of the candidate predictors of mGFR-eGFR difference and limited our ability to fully explore them as candidate predictors.
In conclusion, on average, eGFR slope systematically underestimated mGFR slope by approximately 28%. Although eGFR and mGFR exhibited similar relationships to 22 baseline variables, the overall bias seen in the full cohort suggests that clinicians and researchers should exercise caution when interpreting eGFR slope as a marker of progression of kidney disease.
Disclosures
None.
Acknowledgments
The authors thank the MDRD Study investigators for providing the data used in these analyses and A. Russell Localio for assistance with statistical programming. Data from this analysis were presented in abstract form at the American Society of Nephrology Annual Meeting, San Francisco California, November 2007.
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.K/DOQI: Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Part 3. Chronic kidney disease as a public health problem. http://www.kidney.org/professionals/KDOQI/guidelines_ckd_p3_pubhealth.htm. Accessed April 18,2007
- 2.K/DOQI: Clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Part 5. Evaluation of laboratory measurements for clinical assessment of kidney disease. http://www.kidney.org/professionals/KDOQI/guidelines_ckd_p5_lab_g4.htm. Accessed April 18,2007
- 3.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of diet in renal disease study group. Ann Intern Med 130 :461 –470,1999 [DOI] [PubMed] [Google Scholar]
- 4.Levey AS, Greene T, Kusek JW, Beck GJ, MDRD Study Group: A simplified equation to predict glomerular filtration rate from serum creatinine [abstract]. J Am Soc Nephrol 11 :155A ,2000 [Google Scholar]
- 5.Coresh J, Toto RD, Kirk KA, Whelton PK, Massry S, Jones C, Agodoa L, Van Lente F: Creatinine clearance as a measure of GFR in screenees for the African-American study of kidney disease and hypertension pilot study. Am J Kidney Dis 32 :32 –42,1998 [DOI] [PubMed] [Google Scholar]
- 6.Thomas L, Huber AR: Renal function: estimation of glomerular filtration rate. Clin Chem Lab Med 44 :1295 –1302,2006 [DOI] [PubMed] [Google Scholar]
- 7.Zuo L, Ma YC, Zhou YH, Wang M, Xu GB, Wang HY: Application of GFR-estimating equations in Chinese patients with chronic kidney disease. Am J Kidney Dis 45 :463 –472,2005 [DOI] [PubMed] [Google Scholar]
- 8.Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 141 :929 –937,2004 [DOI] [PubMed] [Google Scholar]
- 9.Bertolatus JA, Goddard L: Evaluation of renal function in potential living kidney donors. Transplantation 71 :256 –260,2001 [DOI] [PubMed] [Google Scholar]
- 10.Rule AD, Torres VE, Chapman AB, Grantham JJ, Guay-Woodford LM, Baw KT, Klahr S, Bennett WM, Meyers CM, Thompson DA, Miller JP, CRISP Consortium: Comparison of methods for determining renal function decline in early autosomal dominant polycystic kidney disease: the consortium of radiologic imaging studies of polycystic kidney disease cohort. J Am Soc Nephrol 17 :854 –862,2006 [DOI] [PubMed] [Google Scholar]
- 11.MDRD Study Group: Effects of dietary protein restriction on the progression of moderate renal disease in the modification of diet in renal disease study. J Am Soc Nephrol 7 :2616 –2626,1996 [DOI] [PubMed] [Google Scholar]
- 12.Klahr S, Levey AS, Beck GJ, Caggiula AN, Hunsicker L, Kusek JW, Striker G: The effects of dietary protein restriction and blood-pressure control on the progression of chronic renal disease: modification of diet in renal disease study group. N Engl J Med 330 :877 –884,1994 [DOI] [PubMed] [Google Scholar]
- 13.Liang KY, Zeger SL: Longitudinal data analysis using generalized linear models. Biometrika 73 :13 –22,1986 [Google Scholar]
- 14.Effron B, Tibshirani R: An Introduction to the Bootstrap, Boca Raton, FL; Chapman-Hall,1993
- 15.Lewis J, Greene T, Appel L, Contreras G, Douglass J, Lash J, Toto R, Van Lente F, Wang X, Wright JT, AASK Study Group: A comparison of iothalamate-GFR and serum creatinine-based outcomes: acceleration in the rate of GFR decline in the African-American study of kidney disease and hypertension. J Am Soc Nephrol 15 :3175 –3183,2004 [DOI] [PubMed] [Google Scholar]
- 16.Perkins BA, Nelson RG, Ostrander BEP, Blouch KL, Krolewski AS, Myers BD, Warram JH: Detection of renal function decline in patients with diabetes and normal or elevated GFR by serial measurements of serum cystatin C concentration: results of a 4-year follow-up study. J Am Soc Nephrol 16 :1404 –1412,2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossing P, Rossing K, Gaede P, Pedersen O, Parving HH: Monitoring kidney function in type 2 diabetic patients with incipient and overt diabetic nephropathy. Diabetes Care 29 :1024 –1030,2006 [DOI] [PubMed] [Google Scholar]
- 18.Fontsere N, Salinas I, Bonal J, Bayes B, Riba J, Torres F, Rios J, Sanmarti A, Romero R: Are prediction equations for glomerular filtrate rate useful for the long-term monitoring of type 2 diabetic patients? Nephrol Dial Transplant 21 :2152 –2158,2006 [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Lewis J, Appel L, Cheek A, Contreras G, Faulkner M, Feldman H, Gassman J, Lea J, Kopple J, Sike M, Toto R, Greene T, AASK Investigators: Validation of creatinine-based estimates of GFR when evaluating risk factors in longitudinal studies of kidney disease. J Am Soc Nephrol 17 :2900 –2909,2006 [DOI] [PubMed] [Google Scholar]
- 20.Brenner BM, Cooper ME, de Zeeuw D, Keane WF, Mitch WE, Parving H, Remuzzi G, Snapinn SM, Zhang Z, Shahinfar S, RENAAL Study Investigators: Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med 345 :861 –869,2001 [DOI] [PubMed] [Google Scholar]
- 21.Barnett AH, Bain SC, Bouter P, Karlberg B, Madsbad S, Jervell J, Mustonen J: Diabetics Exposed to Telmisartan and Enalapril Study Group: angiotensin-receptor blockade versus converting-enzyme inhibition in type 2 diabetes and nephropathy. N Engl J Med 351 :1952 –1961,2004 [DOI] [PubMed] [Google Scholar]
- 22.Bakris GL, Copley JB, Vicknair N, Sadler R, Leurgans S: Calcium channel blockers versus other antihypertensive therapies on progression of NIDDM associated nephropathy. Kidney Int 50 :1641 –1650,1996 [DOI] [PubMed] [Google Scholar]
- 23.Grubb A, Nyman U, Bjork J, Lindstrom V, Rippe B, Sterner G, Christensson A: Simple cystatin C-based prediction equations for glomerular filtration rate compared with the modification of diet in renal disease prediction equation for adults and the Schwartz and the Counahan-Barratt prediction equations for children. Clin Chem 51 :1420 –1431,2005 [DOI] [PubMed] [Google Scholar]
- 24.Stevens LA, Coresh J, Schmid CH, Feldman HI, Froissart M, Kusek J, Rossert J, Van Lente F, Bruce RD, Zhang Y, Greene T, Levey AS: Estimating GFR using serum cystatin C alone and in combination with serum creatinine: a pooled analysis of 3,418 individuals with CKD. Am J Kidney Dis 51 :395 –406,2008 [DOI] [PMC free article] [PubMed] [Google Scholar]