Abstract
Selenocysteine is cotranslationally inserted into proteins by recoding the stop codon UGA from termination to selenocysteine insertion. The nucleophilic selenol group of selenocysteine endows this rare amino acid with unique chemical reactivity that allows regiospecific covalent conjugation in the presence of the other natural amino acids. Using a mammalian expression system, we generated an IgG1-derived Fc fragment with a C-terminal selenocysteine in yields comparable to conventional monoclonal antibodies and conjugated it to an electrophilic derivative of a peptidomimetic that binds with high affinity and specificity to integrin α4β1. Through this conjugation, both the biological and chemical components are endowed with pharmacological advantages. We demonstrate that whereas the Fc protein increases the circulatory half-life from minutes to days and mediates transcytosis through binding to the neonatal Fc receptor, the peptidomimetic introduces cross-species binding to cell surface integrin α4β1 and blocks its interaction with vascular cell adhesion molecule-1. Compared with conventional monoclonal antibodies, our technology benefits economically from combining a generic biological component with a variable chemical component.
Keywords: antibody engineering, Fc fragment, neonatal Fc receptor, small synthetic molecules, integrin α4β1
Despite advances in antibody engineering (1), the unlimited chemical diversity of structural space that becomes increasingly accessible for small synthetic molecules through combinatorial chemistry remains out of reach for monoclonal antibodies (mAbs) (2). Thus, notwithstanding the current clinical and commercial success of therapeutic mAbs (3), small synthetic molecules from chemical libraries have the potential to eventually outperform mAbs in terms of both affinity and specificity. In particular, buried and conserved sites that are often implicated in protein/protein interactions are prime targets for small synthetic molecules. Despite gaining on mAbs, because of superior thermodynamic and kinetic binding properties and lower manufacturing costs, the attrition rates of chemical entities in preclinical and clinical development are considerably higher than the attrition rates of mAbs (4). Inferior pharmacokinetic profiles that result in rapid blood clearance, because of absorption, distribution, metabolism, and excretion (ADME), are thought to account for a substantial portion of these failures (5). Methodologies designed to prolong the circulatory half-life of small synthetic molecules by covalent attachment to larger carrier entities, such as polyethylene glycol (6), albumin (7), and antibody molecules (8), have been shown to overcome inferior ADME characteristics. The antibody molecule IgG in general, and its Fc domain in particular, are of exceptional interest in this regard because of their binding to the neonatal Fc receptor (FcRn). FcRn (9) mediates IgG recycling as well as transcytosis across endothelial and epithelial barriers, therefore prolonging circulatory half-life (10–12) and permitting aerosol delivery of IgG and Fc proteins through the lung (13), respectively. Thus, methodologies designed to facilitate defined covalent conjugations of small synthetic molecules to Fc proteins could provide drug discovery and development platforms that merge pharmacological advantages of mAbs and small synthetic molecules.
In this article, we describe the development of a prototype and platform for a distinctive class of pharmaceuticals that employs selenocysteine (Sec) as an engineered interface between a generic Fc protein and a variable small synthetic molecule. Sec, the 21st natural amino acid, is cotranslationally inserted into proteins by recoding the stop codon UGA from termination to Sec insertion. In eukaryotes, this recoding requires the presence of a specific mRNA secondary structure, termed a Sec insertion sequence (SECIS) element, located in the 3′-untranslated region (3′-UTR) (14). The nucleophilic selenol group of Sec (pKa 5.2) endows this rare amino acid with unique chemical reactivity, allowing regiospecific covalent conjugation with electrophilic moieties in the presence of the other natural amino acids, including the thiol group of Cys (pKa 8.3) (15). We demonstrate that a recombinant Fc protein displaying this unique chemical reactivity through an engineered Sec provides a generic mechanism for the defined conjugation of small synthetic molecules.
Results and Discussion
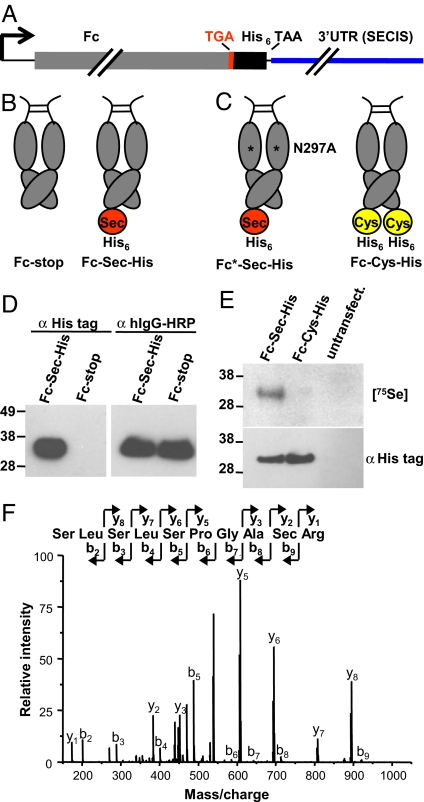
A mammalian expression system was used to generate a recombinant Fc protein with a C-terminal Sec. For this, an exon/intron gene sequence encoding the human IgG1-derived Fc fragment was fused to a TGA codon followed by a (His)6-encoding sequence, a TAA stop codon, and a 3′UTR fragment of the gene of human selenoprotein thioredoxin reductase 1, an enzyme with a natural C-terminal Sec (16) (Fig. 1A). The efficiency of recoding the UGA codon from termination to Sec insertion is influenced by several cis and trans factors, including the position of the SECIS element, the availability of a selenium source, and the abundance of specialized cytoplasmic proteins that are required for Sec synthesis and insertion (14). Because of limitations of these cis and trans factors, termination at the UGA codon typically dominates Sec insertion and read-through, despite the presence of a SECIS element (14). Therefore, we expected to express Fc protein both with Sec and His tags (termed Fc-Sec-His) and without (termed Fc-stop). As a consequence of Fc dimerization and the fact that termination typically dominates Sec insertion, Fc-Sec-His is more likely to dimerize with Fc-stop, resulting in the display of one rather than two Sec-(His)6 C-termini (see Fig. 1B). For controls, we generated two additional Fc proteins, Fc-Cys-His and Fc*-Sec-His (see Fig. 1C). In Fc-Cys-His, the C-terminal Sec was replaced by a Cys. In Fc*-Sec-His, the mutation Asn297Ala eliminated the unique N-glycosylation site in the CH2 domain of Fc to reduce Fcγ receptor binding but not, or only marginally, FcRn binding (17).
Fig. 1.
Molecular configuration and analysis of engineered Fc proteins. (A) A mammalian expression vector, pCEP4-Fc-Sec-His, encoding a human Fc protein (gray) with a C-terminal Sec was genetically engineered by combining a TGA codon (red) with a 3′UTR fragment (blue) that contains a SECIS element derived from the gene of human thioredoxin reductase 1. Downstream from the TGA codon, a (His)6-encoding sequence was added, followed by stop codon TAA. (B) Termination at the UGA codon typically dominates Sec insertion (14), resulting in the two Fc proteins shown. The His tag is only expressed after Sec insertion, facilitating detection and purification of the Fc-Sec-His protein. (C) The same expression cassette as above was used to generate Fc proteins with an Asn297Ala (N297A) mutation that reduces Fcγ receptor binding (Fc*-Sec-His) and with a Cys at the position of Sec (Fc-Cys-His). (D) Confirmation of His tag expression by using Western blot analysis after reducing SDS/PAGE. The molecular mass standard in this and all subsequent figures is given on the left in kDa. (E) Confirmation of Sec incorporation by autoradiography (Upper) and Western blot analysis (Lower) after reducing SDS/PAGE. (F) Confirmation of Sec incorporation by mass spectrometry.
The mammalian expression vector containing the Fc-Sec-His sequence under the control of a CMV promoter, pCEP4-Fc-Sec-His, was transiently transfected into HEK 293F cells that were maintained in suspension in serum-free medium. Fc-Sec-His protein was subsequently purified from the supernatant by a tandem column chromatography process. Protein G affinity chromatography was used to purify the total Fc protein, whereas immobilized metal affinity chromatography (IMAC) was used to subsequently separate Fc-Sec-His from the Fc-stop protein. Given that the His tag was encoded immediately downstream of the UGA codon, detection of the His tag would indicate read-through of the UGA codon, implying recoding from termination to Sec insertion. As shown by using Western blot analysis (see Fig. 1D), IMAC revealed a specific separation of a His tag-containing an Fc protein (eluate) from an Fc protein without His tag (flow-through). The initial yield of IMAC-purified Fc-Sec-His protein was ≈300 μg/liter, representing 3% of total Fc protein (10 mg/liter), after transient transfection. However, addition of 1 μM sodium selenite (Na2SeO3) to the serum-free medium increased the Fc-Sec-His protein yield to 2 mg/liter or 20% of total Fc protein. This yield is well within the 0.5 to 5.0 mg/liter range that we have obtained for conventional mAbs under the same conditions (data not shown). Thus, production of Fc-Sec-His on a larger scale is feasible and may be further increased by optimizing the Sec insertion machinery through identifying and compensating the limiting influence of both cis and trans factors (18).
Although the increased yield of Fc-Sec-His in the presence of 1 μM sodium selenite suggested successful Sec incorporation, a direct confirmation was needed. For this purpose, HEK 293F cells were transiently transfected with pCEP4-Fc-Sec-His and pCEP4-Fc-Cys-His and incubated with 50 μCi of 75Se as selenate (SeO42−) in HNO3. Whereas the supernatant of both HEK 293F/pCEP4-Fc-Sec-His cells and HEK 293F/pCEP4-Fc-Cys-His cells revealed the expression of Fc protein with a His tag, [75Se]-labeled Fc protein was only detected in the supernatant of HEK 293F/pCEP4-Fc-Sec-His cells (see Fig. 1E), indicating selective incorporation of 75Se into Fc-Sec-His protein. To confirm the position of Sec incorporation, Fc-Sec-His protein was analyzed by using mass spectrometry. Because His tags can distort mass spectrometric analyses by forming stable complexes with trace amounts of heavy metal ions, the Fc-Sec-His expression cassette was modified by introducing an Arg codon between Sec and His tag codons. The additional Arg in the resulting Fc-Sec-Arg-His protein mediated the separation of Sec-containing peptide and His tag peptide by trypsin digestion. The amino acid sequence of the Sec-containing peptide was subsequently verified by tandem mass spectrometry (LC-MS/MS) (see Fig. 1F).
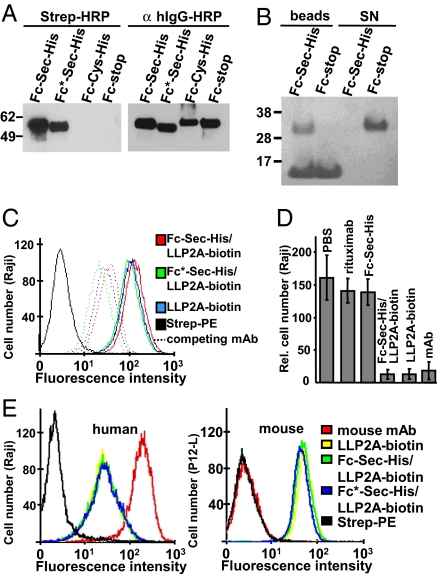
With the molecular composition of the generic Fc protein confirmed, the next step was its selective conjugation to a small synthetic molecule with pharmacological activity. For this conjugation we chose LLP2A, a peptidomimetic that was recently developed by Peng et al. (19) by using combinatorial chemistry. LLP2A was shown to bind with picomolar affinity and high specificity to cell surface integrin α4β1, expressed on lymphocytes. Thus, LLP2A represents a prototype for a growing number of small synthetic molecules that have been derived from chemical libraries to challenge mAbs in terms of both binding properties and manufacturing costs. We synthesized a trifunctional LLP2A derivative with the integrin α4β1-binding core linked to a biotin group for detection and an electrophilic maleimide moiety for conjugation [supporting information (SI) Fig. S1]. We first established conditions for selective conjugation at the Sec interface of Fc-Sec-His (Fig. S2 a and b). Incubation of purified Fc-Sec-His, Fc*-Sec-His, Fc-stop, and Fc-Cys-His proteins with LLP2A-biotin-maleimide for 50 min at pH 5.2 in the presence of 0.1 mM DTT led to selective biotinylation of Fc-Sec-His and Fc*-Sec-His proteins, as shown by using Western blot analysis (Fig. 2A). Whereas all four proteins were detected with anti-human IgG, streptavidin reacted only with Fc-Sec-His and Fc*-Sec-His protein, demonstrating that Sec-dependent conjugation is highly specific. To evaluate the efficacy of biotinylation, Fc-Sec-His and Fc-stop were incubated with LLP2A-biotin-maleimide as before and, after adjustment to pH 7.4, separately incubated with magnetic streptavidin-coated beads. The supernatant and the extensively washed beads were analyzed by reducing SDS/PAGE (Fig. 2B). In contrast to Fc-stop protein, the vast majority of Fc-Sec-His protein bound to beads, suggesting virtually complete conjugation. These experiments demonstrated that the Fc-Sec-His protein, by virtue of its encoded unique chemical reactivity, can be selectively and quantitatively conjugated to a small synthetic molecule by using near physiological reaction conditions.
Fig. 2.
Analysis of Fc-Sec-His/LLP2A-biotin in vitro. (A) Demonstration of selective conjugation of Fc-Sec-His and Fc*-Sec-His to LLP2A-biotin-maleimide by using Western blot analysis after nonreducing SDS/PAGE. (B) Demonstration of near quantitative conjugation of Fc-Sec-His by incubation with magnetic streptavidin-coated beads followed by reducing SDS/PAGE. The additional band seen in the bead samples represents the polypeptide chain monomer of homotetrameric streptavidin (55 kDa). SN, supernatant. (C) Flow cytometry analysis revealing that conjugated and free LLP2A-biotin bind equally well to human Raji cells expressing cell surface integrin α4β1 (solid lines). The binding was competed equally by preincubation of Raji cells with a mouse anti-human integrin α4β1 mAb (dotted lines). (D) Demonstration that Fc-Sec-His/LLP2A-biotin and free LLP2A-biotin interfere with the interaction of integrin α4β1 and VCAM-1 as potently as a mouse anti-human integrin α4β1 mAb. The number of cells adhering to coated VCAM-1 was determined by flow cytometry. Shown are mean ±SD of triplicates. (E) Flow cytometry analysis revealing that LLP2A mediates cross-species binding to human (Left, Raji) and mouse (Right, P12-L) cells expressing cell surface integrin α4β1. A conventionally biotinylated mouse anti-human integrin α4β1 mAb (red), that likely carries more than one biotin, was tested for comparison.
The resulting Fc-Sec-His/LLP2A-biotin conjugate was subsequently analyzed for preserved pharmacological activity of both of its components, the small synthetic molecule, and the generic Fc protein. By using flow cytometry, Fc-Sec-His/LLP2A-biotin was first analyzed for binding to the human B cell line Raji, which is known to express integrin α4β1. As shown in Fig. 2C, Fc-Sec-His/LLP2A-biotin strongly bound to Raji cells. Verifying that the interaction was mediated by integrin α4β1 rather than Fcγ receptors, the corresponding Fc*-Sec-His/LLP2A-biotin conjugate and an equimolar concentration of free LLP2A-biotin revealed the same affinity as Fc-Sec-His/LLP2A-biotin in this assay. This finding was further supported by demonstrating that preincubation of Raji cells with a competing mouse anti-human integrin α4β1 mAb resulted in a very similar reduction of the binding of Fc-Sec-His/LLP2A-biotin, Fc*-Sec-His/LLP2A-biotin, and free LLP2A-biotin (see Fig. 2C). Thus, the high affinity and specificity of LLP2A for integrin α4β1 was preserved after conjugation to the generic Fc protein.
LLP2A was shown to interfere with the interaction of integrin α4β1 and human vascular cell adhesion molecule 1 (VCAM-1) (19). Using a cell adhesion assay, we found that Fc-Sec-His/LLP2A-biotin and free LLP2A-biotin, but neither Fc-Sec-His alone nor rituximab (a chimeric mouse/human IgG1 directed to human CD20), blocked the binding of Raji cells to immobilized VCAM-1 as potently as a mouse anti-human integrin α4β1 mAb (Fig. 2D).
When tested over a concentration range from 0.02 to 200 nM, Fc-Sec-His/LLP2A-biotin was found to be as potent as free LLP2A-biotin (Fig. S2c), suggesting that conjugation to the generic Fc protein did not weaken the pharmacological activity. Similar results (data not shown) were obtained for the binding of Raji cells to TNFα-activated human umbilical vein endothelial cells.
An additional advantage of small synthetic molecules is cross-species reactivity. Limited cross-species reactivity of mAbs complicates the transition from preclinical experiments to the initiation of human clinical trials, in particular when using mouse models of human diseases. Most humanized and human mAbs that are derived from immune mice have undergone negative selection against epitopes displayed by the mouse antigen and consequently lack cross-species reactivity. Although human mAbs can be selected for cross-species reactivity from naïve human antibody libraries by phage display, other in vitro technologies, such as combinatorial chemistry, are also not limited by in vivo selection processes. Thus, small synthetic molecules are preferential reagents for targeting epitopes that are conserved between species. As shown in Fig. 2E, flow cytometry revealed that Fc-Sec-His/LLP2A-biotin, Fc*-Sec-His/LLP2A-biotin, and free LLP2A-biotin strongly bound to both human and mouse cells known to express integrin α4β1, whereas a commercial mouse anti-human integrin α4β1 mAb lacked cross-species reactivity.
A key incentive in the development of a generic Fc protein that can be conjugated to a small synthetic molecule of choice is the utilization of FcRn binding. Therefore, we investigated the pH-dependent binding of Fc-Sec-His/LLP2A-biotin and Fc*-Sec-His/LLP2A-biotin to human FcRn. For this, we first cloned, expressed, and purified recombinant human FcRn consisting of α chain and β2 microglobulin (Fig. S3a) based on the reported generation and crystallization of recombinant rat FcRn (20). Fc-Sec-His/LLP2A-biotin and Fc*-Sec-His/LLP2A-biotin were then analyzed by ELISA for binding to recombinant human FcRn at pH 6.0 and at pH 7.4. As shown in Fig. S3b, both Fc conjugates were found to bind to FcRn at pH 6.0 but not at pH 7.4. By using surface plasmon resonance, this pH-dependent interaction was confirmed (data not shown). In addition to and agreement with published data for human IgG1 (21), very similar association and dissociation kinetics were observed for rituximab, Fc-stop, Fc-Sec-His, Fc-Sec-His/LLP2A-biotin, and Fc*-Sec-His/LLP2A-biotin (Fig. S3c and data not shown), demonstrating that FcRn binding is not influenced by conjugation at the Sec interface. Taken together, our Fc conjugates revealed the characteristic and physiologically relevant pH-dependent interaction with FcRn through which both IgG recycling and transcytosis are mediated (9–13).
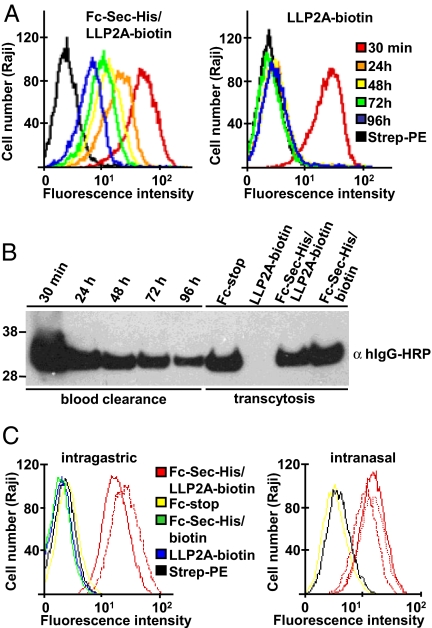
Collectively, these experiments demonstrated that both the small synthetic molecule and the Fc protein maintain their respective binding properties after conjugation at the Sec interface. We next investigated whether these findings translated into an extended circulatory half-life of the small synthetic molecule. For this purpose, 1 mg of Fc-Sec-His/LLP2A-biotin and an equimolar amount of free LLP2A-biotin were injected intravenously into mice. Sera prepared from retro-orbital bleeds were collected after 30 min and every 24 h for 4 days and analyzed for binding to Raji cells by flow cytometry (Fig. 3A) as well as by using Western blotting (Fig. 3B). Whereas free LLP2A-biotin was undetectable 24 h after injection, Fc-Sec-His/LLP2A-biotin was still present after 4 days, clearly demonstrating the power of the Fc protein in protecting the small synthetic molecule from blood clearance. However, based on the observed circulatory half-life of Fc-Sec-His/LLP2A-biotin in the range of 1–2 days (see Fig. 3 A and B), serum retention in this experiment may have been primarily mediated by the molecular weight of the human Fc protein rather than by the likely overloaded mouse FcRn recycling machinery. Overloading the FcRn recycling machinery with exogenous IgG is known to result in a shortening of the circulatory half-life of both endogenous and exogenous IgG (9). The experiment also proved that the conjugation at the Sec interface was stable for at least 4 days in vivo, matching or approaching drug administration intervals of therapeutic mAbs (22). Depending on the therapeutic application, the circulatory half-life of the Fc conjugate can be modulated by switching the Ig isotype of the Fc protein or by engineering mutations in its FcRn binding site (9, 23).
Fig. 3.
Analysis of Fc-Sec-His/LLP2A-biotin in vivo. (A) Comparison of the circulatory half-life of Fc-Sec-His/LLP2A-biotin and free LLP2A-biotin in mice. Sera from mice injected intravenously with Fc-Sec-His/LLP2A-biotin (Left) or an equimolar amount of free LLP2A-biotin (Right) were prepared from retro-orbital bleeds taken 30 min (red), 24 h (orange), 48 h (yellow), 72 h (green), and 96 h (blue) after injection. After diluting 10-fold, the sera were incubated with Raji cells and analyzed for the presence of LLP2A-biotin by using PE-coupled streptavidin for detection. Typical results based on three individual mice in each treatment group are shown. (B) Lanes 1–5 show confirmation of the blood clearance of Fc-Sec-His/LLP2A-biotin by using Western blot analysis after reducing SDS/PAGE. Lanes 6–9 show demonstration of transcytosis of Fc-Sec-His/LLP2A-biotin from neonatal intestine to blood. (C) Flow cytometry analysis of the efficacy of transcytosis after intragastric and intranasal application. (Left) Fc-stop, Fc-Sec-His/LLP2A-biotin, and Fc-Sec-His/biotin, as well as an equimolar amount of free LLP2A-biotin were administered intragastrically to neonatal mice. Typical results based on two individual mice in each treatment group are shown for Fc-stop, Fc-Sec-His/biotin, and free LLP2A-biotin (all negative). The results of both mice (solid and dotted red line) are shown for Fc-Sec-His/LLP2A-biotin. (Right) Fc-stop and Fc-Sec-His/LLP2A-biotin were administered intranasally to adult mice. A typical result based on three individual mice in each treatment group is shown for Fc-stop (negative). The results of all three mice (solid and dotted red lines) are shown for Fc-Sec-His/LLP2A-biotin.
To demonstrate a physiologically relevant interaction of Fc-Sec-His/LLP2A-biotin with FcRn in vivo, we next analyzed the transcytosis of our Fc conjugate from neonatal intestine to blood (9). For this analysis, 0.5 mg of Fc-stop, Fc-Sec-His/LLP2A-biotin, and Fc-Sec-His conjugated to commercially available biotin-iodoacetamide, and an equimolar amount of free LLP2A-biotin were administered intragastrically to 10-day-old mice. Sera prepared after 24 h from cardiac puncture bleeds were analyzed with flow cytometry by using Raji cells and Western blotting. These analyses revealed that transcytosis of Fc-Sec-His/LLP2A-biotin was highly efficient, resulting in serum concentrations that were very similar to those found at the corresponding time point in the previous intravenous injection experiment (see Fig. 3 B and C). In addition, transcytosis of Fc-Sec-His/LLP2A-biotin was as efficient as transcytosis of Fc-stop and Fc-Sec-His/biotin (Fig. S3d). In an additional in vivo experiment, we administered Fc-Sec-His/LLP2A-biotin intranasally to adult mice. Sera prepared after 24 h from cardiac puncture bleeds and analyzed with flow cytometry by using Raji cells demonstrated transcytosis of Fc-Sec-His/LLP2A-biotin from the lung to the blood (see Fig. 3C). With its preserved ability to enter the blood stream through FcRn-mediated transcytosis, the generic Fc protein provides a vehicle for alternative administration routes of small synthetic molecules across epithelial or endothelial cell barriers. In agreement with our finding, the expression of FcRn in human upper airway epithelial cells has been shown to mediate the transport of aerosolized IgG and Fc fusion proteins from the lung to the blood with an efficacy as high as intravenous injection (9, 13).
A central element of our technology is regiospecific covalent conjugation to a Sec interface without interfering with FcRn binding. Conventional covalent conjugations, which usually involve amine or thiol groups, are not regiospecific because of the abundance of Lys, Cys, and other reactive amino acids, and can cause substantial batch-to-batch variability that is a concern in drug discovery and development. Compared with other technologies that use unique chemical reactivities of natural or unnatural amino acids in proteins for regiospecific covalent conjugation of small synthetic molecules, our technology does not require antibody variable domains (8, 24), enzymatic modification (25), or artificial translational machinery (26). In addition, because of the distinctive display of a single Sec interface in an otherwise dimeric protein, our technology permits the generation of monomeric Fc conjugates, which recently have been found to have superior pharmacokinetic profiles compared with conventional dimeric Fc conjugates (27).
Materials and Methods
Fc Protein Engineering.
pCEP4-Fc.
A genomic exon/intron sequence encoding Fcγ1, including the hinge region, was amplified with PCR by using primer pair I-5′/I-3′. The 5′ primer fuses a human heavy-chain signal sequence to the Fcγ1 sequence enabling secretion of the Fc protein. The N-terminal Cys-5 in the γ1 hinge (1EPKSCDKTHTCPPCP15), that normally forms a disulfide bridge with a C-terminal Cys in the constant domain of the light chain, was mutated to Ser as described (28). A silent HindIII site was introduced through the 3′ primer, replacing the codons of Leu-121, Ser-122, and Leu-123 at the C terminus of Fcγ1 upstream of the natural stop codon without changing the amino acid sequence. The PCR fragment was cloned into mammalian expression vector pCEP4 (Invitrogen) by KpnI/HindIII ligation, therefore deleting the last four codons of Fcγ1, including the natural stop codon. This construct, termed pCEP4-Fc, served as the parental template for all further Fc constructs.
pCEP4-Fc-Sec-His.
A PCR fragment containing the last four codons of Fc with a Lys130Ala mutation for proteolytic stability (29), followed by a TGA codon, six His codons, a TAA codon, and a fragment of the 3′-UTR of the thioredoxin reductase 1 gene (30) was amplified by using human genomic DNA as a template. An internal HindIII site in the 3′-UTR of the human thioredoxin reductase 1 gene was deleted with overlap extension PCR by using primer pairs II-5′/II-3′ and III-5′/III-3′. The resulting PCR fragment was cloned into pCEP4-Fc by HindIII/XhoI ligation.
pCEP4-Fc-Cys-His.
This Fc construct was cloned analogous to Fc-Sec-His by using primer IV-5′ that was designed to replace the TGA codon with Cys codon TGC.
pCEP4-Fc-Sec-Arg-His.
For the analysis of Sec incorporation by mass spectrometry, an additional Arg codon was inserted between the Sec and His tag. By using primer V-5′, this Fc construct was cloned analogous to Fc-Sec-His and Fc-Cys-His.
pCEP4-Fc*-Sec-His.
This Fc construct carried mutation Asn297Ala, which removes the only N-glycosylation site in Fcγ1. By using template pCEP4-Fc-Sec-His, PCR fragments amplified with primer pairs VI-5′/VI-3′ and VII-5′/VII-3′ were fused by overlap extension PCR and cloned into pCEP4 by KpnI/XhoI ligation. All constructs were verified by DNA sequencing.
Primer sequences.
I-5′: gggtaccatggactggacctggaggatcctcttcttggtggcagcagccacaggagctcactccgagcccaaatcttctgacaaaactcacaca; I-3′: cggagacaagcttaggctcttctgcgtgtagtggttgtgcag; II-5′: gcctaagcttgtctccgggtgcctgacatcaccatcaccatcactaagccccagtgtggatgctgttg; II-3′: agaagctccaagaactgctggcag; III-5′: cctgccagcagttcttggagcttct; III-3′: agctctcgaggccaaatgagatgaggacgtgag; IV-5′: gcctaagcttgtctccgggtgcctgccatcaccatcaccatcactaagccccagtgtggatgctgttg; V-5′: gcctaagcttgtctccgggtgcctgacggcatcaccatcaccatcactaagccccagtgtggatgctgttg; VI-5′: aggagcagtacgccagcacgtaccgtgtggt; VI-3′: gtggtttgtccaaactcatc; VII-5′: agcagagctcgtttagtgaaccg; and VII-3′: accacacggtacgtgctggcgtactgctcct.
Fc Protein Expression.
The mammalian expression vectors described above were transiently transfected into HEK 293F cells (Invitrogen) with 293fectin (Invitrogen) by using conditions detailed in the manufacturer's protocol. Transfected HEK 293F cells were cultured in FreeStyle serum-free medium (Invitrogen), supplemented with 1 μM Na2SeO3 (Sigma), in spin flasks (Integra Biosciences) under constant rotation at 75 rpm (Integra Biosciences Cellspin stirring platform), in a humidified atmosphere containing 8% CO2 at 37°C. Three days after transfection, the medium was collected after centrifugation, replaced for three additional days, and collected again. The combined supernatants were filtered through a 0.45-μm membrane and concentrated 10-fold by using an ultrafiltration device with a 10-kDa cutoff membrane (Millipore). The concentrate was 1:1 diluted with PBS and loaded on a 1-ml recombinant Protein G column (HisTrap; GE Healthcare). PBS was used for column equilibration and washing, 0.5-M acetic acid (pH 3.0) for elution, and 1 M Tris·HCl (pH 8.0) for immediate neutralization. The neutralized eluate was dialyzed at 4°C overnight against PBS by using Slide-A-Lyzer cassettes with 10-kDa cutoff (Pierce) and concentrated with 10-kDa cutoff centrifugal filter devices (Millipore). To separate Fc-Sec-His protein from Fc-stop protein, the purified Fc protein mixture was diluted 10-fold in loading/washing buffer (500 mM NaCl and 25 mM imidazol in PBS) and loaded on a 1-ml IMAC column (HisTrap; GE Healthcare). The flow-through of the column containing the Fc-stop protein was collected. Subsequently, the column was washed with 50-ml loading/washing buffer and the bound Fc-Sec-His protein was eluted with elution buffer (500 mM NaCl and 500 mM imidazol in PBS). Both eluate and flow-through were dialyzed and concentrated as before.
Western Blot Analysis.
IMAC-purified Fc-Sec-His protein and Fc-stop protein (1 μg each) were electrophoresed on a NuPage 4% to12% gradient gel (Invitrogen), blotted on a nitrocellulose membrane (GE Healthcare), blocked with Western Blocking reagent (Roche), incubated with mouse mAb Pentahis (Qiagen) diluted to 1 μg/ml in Western Blocking reagent, and then followed by polyclonal HRP-coupled goat anti-mouse polyclonal antibodies (Jackson ImmunoResearch Laboratories) diluted 1:10,000 in Western Blocking reagent. HRP-coupled donkey anti-human IgG polyclonal antibodies (Jackson ImmunoResearch Laboratories) were used as a positive control. Immunoreactive bands were developed by using SuperSignal West Pico Chemoluminescent Substrate (Pierce) and visualized by using BioMax MR autoradiography film (Kodak). For the detection of Sec-biotinylated Fc proteins, HRP-coupled streptavidin (BD Biosciences) was used at 1:10,000 dilution in Western Blocking reagent. For the detection of Fc proteins in mouse sera, 8 μl of serum per lane was loaded and analyzed as described above.
Autoradiography.
HEK 293F cells were transiently transfected with either pCEP4-Fc-Sec-His or pCEP4-Fc-Cys-His. Transfected and untransfected cells were incubated in the presence or absence of 50 μCi of 75Se as selenate (SeO42−) in HNO3 (University of Missouri Research Reactor, Columbia, MO), replacing the 1-μM Na2SeO3 supplement. After 24 h, the supernatant was harvested, concentrated 10-fold as before, and 50 μl were electrophoresed under reducing conditions on a NuPage 4% to 12% gradient gel. After drying, the gel was exposed to a BioMax MR autoradiography film.
Mass Spectrometry.
IMAC-purified Fc-Sec-Arg-His protein (5 μg) was electrophoresed under nonreducing conditions on a NuPage 4–12% gradient gel and stained with Simply Blue SafeStain (Invitrogen). The single band was isolated, incubated with trypsin, and custom analyzed by tandem mass spectrometry (LC-MS/MS) (CTL Bio Services).
Selective Conjugation.
To establish optimal pH conditions for selective conjugation at the Sec interface, Fc-Sec-His and Fc-stop were diluted in 15 ml of either 100 mM sodium phosphate buffer (pH 6, pH 7, or pH 8), 100-mM sodium acetate buffer (pH 4 or pH 5.2), or 100 mM glycine-HCl buffer (pH 2.5) and concentrated to 4 μM (200 μg/ml) by using a 10-kDa cutoff centrifugal filter device. DTT at 0.1 mM followed by (+)-biotinyl-iodoacetamidyl-3,6-dioxaoctanediamine (Pierce) at 40-μM final concentration were added to the proteins and incubated for 50 min at room temperature in the dark. The Fc conjugates were subsequently diluted in 15 ml of the corresponding conjugation buffer and concentrated to 250 μl, as described above. This step was repeated once and subsequently twice with 15-ml PBS to remove unconjugated compounds. All subsequent conjugation reactions were performed as described above by using 100-mM sodium acetate buffer (pH 5.2) as the conjugation buffer. The synthesis of the trifunctional LLP2A-biotin-maleimide compound (see Fig. S1), which is based on the reported compound LLP2A (19), will be reported separately (J.D.T., T.H., C.R., and T.R.B., unpublished work). To prove the selective conjugation at the Sec interface with LLP2A-biotin-maleimide, Fc-Sec-His protein and negative controls (Fc-stop protein and Fc-Cys-His protein) were subjected to conjugation as described above. To evaluate the efficacy of selective conjugation, Fc-Sec-His protein and a negative control (Fc-stop protein) were biotinylated with LLP2A-biotin-maleimide as described above and incubated separately with an excess of magnetic streptavidin-coated MyOne beads (Invitrogen) for 40 min at room temperature. After removal of the supernatant, the beads were washed extensively with PBS. Supernatant and beads were subsequently electrophoresed under reducing conditions on a NuPage 4% to 12% gradient gel followed by staining with Simply Blue SafeStain.
Flow Cytometry.
Human Burkitt's lymphoma cell line Raji was purchased from the American Type Culture Collection. Mouse B cell chronic lymphocytic leukemia cell line P12-L (31) was a gift from Elizabeth S. Raveche (University of Medicine and Dentistry of New Jersey, Newark). All incubations were for 1 h on ice. After incubation in 10% (vol/vol) FCS/PBS, cells were centrifuged, resuspended in 1% (vol/vol) FCS/PBS, and aliquots of 50 μl containing 5 × 105 cells were distributed into a V-bottom 96-well plate (Corning). The cells were then incubated with Fc-Sec-His/LLP2A-biotin, Fc*-Sec-His/LLP2A-biotin, and a conventionally biotinylated mouse anti-human integrin α4β1 mAb (R&D Systems) at a final concentration of 5 μg/ml and an equimolar concentration of free LLP2A-biotin. After washing twice with 1% (vol/vol) FCS/PBS, the cells were incubated with a 1:25 dilution of phycoerythrin (PE)-coupled streptavidin (BD Biosciences). After washing twice as before, the cells were resuspended in 400-μl 1% (vol/vol) FCS/PBS and analyzed by using a FACScan instrument (Becton-Dickinson). For the competition experiment, the cells were first incubated with a mouse anti-human integrin α4β1 mAb (Serotec) at a final concentration of 10 μg/ml.
Cell Adhesion Assay.
All incubations were for 1 h at 37°C. A 96-well Costar 3690 plate (Corning) was coated with 1 μg of recombinant human VCAM-1 (R&D Systems) in 25 μl of PBS and blocked with 3% (wt/vol) BSA/PBS. Raji cells (1 × 105 cells in 50 μl of PBS) were incubated with 30-μg/ml rituximab (Genentech), 10 μg/ml Fc-Sec-His, 10 μg/ml Fc-Sec-His/LLP2A-biotin, 30 μg/ml of a mouse anti-human integrin α4β1 mAb (R&D Systems), or an equimolar concentration of free LLP2A-biotin, and added to the prepared plate. Nonadherent cells were removed by washing twice with PBS. Adherent cells were subsequently detached by vigorous pipetting, and their number was determined with flow cytometry by using AccuCount blank particles (Spherotech) for normalization.
In Vivo Studies.
Both mouse studies were carried out by Biocon in accordance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health.
Blood clearance.
Two groups of three adult C57BL/6 mice each were injected intravenously (tail vein) with 100 μl of 10-mg/ml (200 μM) Fc-Sec-His/LLP2A-biotin in PBS or with 100 μl of 360 μg/ml (200 μM) free LLP2A-biotin in DMSO. Sera from retro-orbital bleeds were prepared 30 min, 24 h, 48 h, 72 h, and 96 h after injection. Sera were diluted 10-fold in 1% (vol/vol) FCS/PBS and analyzed with flow cytometry by using Raji cells, as described above.
Transcytosis after intragastric administration.
Four groups of two 10-day-old C57BL/6 mice each received Fc-stop, Fc-Sec-His/LLP2A-biotin, Fc-Sec-His/biotin, or free LLP2A-biotin; 0.5 mg of protein or an equimolar amount of free LLP2A-biotin were combined with 80-μg soybean trypsin inhibitor in a total volume of 50 μl of PBS and administered intragastrically by using a 1-inch straight gavage needle with a 1.25-mm diameter ball. No toxicity was noted. After 24 h, the mice were anesthetized with ketamine xylazine anesthesia mixture and bled out via cardiac puncture. Sera were diluted 10-fold in 1% (vol/vol) FCS/PBS and analyzed with flow cytometry by using Raji cells, as described above.
Transcytosis after intranasal administration.
Two groups of three adult C57BL/6 mice each received Fc-Sec-His/LLP2A-biotin or Fc-stop by instilling 50 μl of 3 mg/ml protein in PBS into each nostril. This step was repeated once after 30 min. Sera were obtained, diluted, and analyzed, as described above.
See SI Text for cloning, expression, and purification of recombinant human FcRn and ELISA, and surface plasmon resonance with recombinant human FcRn.
Supplementary Material
Acknowledgments.
We thank Dr. Dolph L. Hatfield and Bradley A. Carlson for assisting in the autoradiography study, Dr. Kristy J. Brown for carrying out the mass spectrometry study, Dr. Frank Klotz for supervising the in vivo studies, and Dr. Dimiter S. Dimitrov for reading the manuscript. This work was supported by the Intramural Research Program of the Center for Cancer Research, the National Cancer Institute, the National Institutes of Health (C.R. and T.R.B.), and by the National Cancer Institute's Director's Intramural Innovation Award (C.R.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. D.R.B. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/cgi/content/full/0800800105/DCSupplemental.
References
- 1.Carter PJ. Potent antibody therapeutics by design. Nat Rev Immunol. 2006;6:343–357. doi: 10.1038/nri1837. [DOI] [PubMed] [Google Scholar]
- 2.Lipinski C, Hopkins A. Navigating chemical space for biology and medicine. Nature. 2004;432:855–861. doi: 10.1038/nature03193. [DOI] [PubMed] [Google Scholar]
- 3.Baker M. Upping the ante on antibodies. Nat Biotechnol. 2005;23:1065–1072. doi: 10.1038/nbt0905-1065. [DOI] [PubMed] [Google Scholar]
- 4.Reichert JM, Rosensweig CJ, Faden LB, Dewitz MC. Monoclonal antibody successes in the clinic. Nat Biotechnol. 2005;23:1073–1078. doi: 10.1038/nbt0905-1073. [DOI] [PubMed] [Google Scholar]
- 5.Roberts SA. Drug metabolism and pharmacokinetics in drug discovery. Curr Opin Drug Discov Devel. 2003;6:66–80. [PubMed] [Google Scholar]
- 6.Greenwald RB. PEG drugs: An overview. J Control Release. 2001;74:159–171. doi: 10.1016/s0168-3659(01)00331-5. [DOI] [PubMed] [Google Scholar]
- 7.Bertucci C, Domenici E. Reversible and covalent binding of drugs to human serum albumin: Methodological approaches and physiological relevance. Curr Med Chem. 2002;9:1463–1481. doi: 10.2174/0929867023369673. [DOI] [PubMed] [Google Scholar]
- 8.Rader C, Sinha SC, Popkov M, Lerner RA, Barbas CF., III Chemically programmed monoclonal antibodies for cancer therapy: Adaptor immunotherapy based on a covalent antibody catalyst. Proc Natl Acad Sci USA. 2003;100:5396–5400. doi: 10.1073/pnas.0931308100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- 10.Ghetie V, et al. Abnormally short serum half-lives of IgG in beta 2-microglobulin-deficient mice. Eur J Immunol. 1996;26:690–696. doi: 10.1002/eji.1830260327. [DOI] [PubMed] [Google Scholar]
- 11.Junghans RP, Anderson CL. The protection receptor for IgG catabolism is the beta2-microglobulin-containing neonatal intestinal transport receptor. Proc Natl Acad Sci USA. 1996;93:5512–5516. doi: 10.1073/pnas.93.11.5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roopenian DC, et al. The MHC class I-like IgG receptor controls perinatal IgG transport, IgG homeostasis, and fate of IgG-Fc-coupled drugs. J Immunol. 2003;170:3528–3533. doi: 10.4049/jimmunol.170.7.3528. [DOI] [PubMed] [Google Scholar]
- 13.Spiekermann GM, et al. Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: Functional expression of FcRn in the mammalian lung. J Exp Med. 2002;196:303–310. doi: 10.1084/jem.20020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hatfield DL, Gladyshev VN. How selenium has altered our understanding of the genetic code. Mol Cell Biol. 2002;22:3565–3576. doi: 10.1128/MCB.22.11.3565-3576.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson L, et al. Exploiting the 21st amino acid-purifying and labeling proteins by selenolate targeting. Nat Methods. 2004;1:61–66. doi: 10.1038/nmeth707. [DOI] [PubMed] [Google Scholar]
- 16.Sun QA, et al. Redox regulation of cell signaling by selenocysteine in mammalian thioredoxin reductases. J Biol Chem. 1999;274:24522–24530. doi: 10.1074/jbc.274.35.24522. [DOI] [PubMed] [Google Scholar]
- 17.Shields RL, et al. High resolution mapping of the binding site on human IgG1 for Fc gamma RI, Fc gamma RII, Fc gamma RIII, and FcRn and design of IgG1 variants with improved binding to the Fc gamma R. J Biol Chem. 2001;276:6591–6604. doi: 10.1074/jbc.M009483200. [DOI] [PubMed] [Google Scholar]
- 18.Novoselov SV, et al. A highly efficient form of the selenocysteine insertion sequence element in protozoan parasites and its use in mammalian cells. Proc Natl Acad Sci USA. 2007;104:7857–7862. doi: 10.1073/pnas.0610683104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng L, et al. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha4beta1 integrin for in vivo tumor imaging. Nat Chem Biol. 2006;2:381–389. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- 20.Gastinel LN, Simister NE, Bjorkman PJ. Expression and crystallization of a soluble and functional form of an Fc receptor related to class I histocompatibility molecules. Proc Natl Acad Sci USA. 1992;89:638–642. doi: 10.1073/pnas.89.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firan MSD, et al. The MHC class I-related receptor, FcRn, plays an essential role in the maternofetal transfer of gamma-globulin in humans. Int Immunol. 2001;13:993–1002. doi: 10.1093/intimm/13.8.993. [DOI] [PubMed] [Google Scholar]
- 22.O'Mahony D, Bishop MR. Monoclonal antibody therapy. Front Biosci. 2006;11:1620–1635. doi: 10.2741/1909. [DOI] [PubMed] [Google Scholar]
- 23.Vaccaro C, Bawdon R, Wanjie S, Ober RJ, Ward ES. Divergent activities of an engineered antibody in murine and human systems have implications for therapeutic antibodies. Proc Natl Acad Sci USA. 2006;103:18709–18714. doi: 10.1073/pnas.0606304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chmura AJ, Orton MS, Meares CF. Antibodies with infinite affinity. Proc Natl Acad Sci USA. 2001;98:8480–8484. doi: 10.1073/pnas.151260298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrico IS, Carlson BL, Bertozzi CR. Introducing genetically encoded aldehydes into proteins. Nat Chem Biol. 2007;3:321–322. doi: 10.1038/nchembio878. [DOI] [PubMed] [Google Scholar]
- 26.Liu W, Brock A, Chen S, Chen S, Schultz PG. Genetic incorporation of unnatural amino acids into proteins in mammalian cells. Nat Methods. 2007;4:239–244. doi: 10.1038/nmeth1016. [DOI] [PubMed] [Google Scholar]
- 27.Dumont JA, Low SC, Peters RT, Bitonti AJ. Monomeric Fc fusions: Impact on pharmacokinetic and biological activity of protein therapeutics. BioDrugs. 2006;20:151–160. doi: 10.2165/00063030-200620030-00002. [DOI] [PubMed] [Google Scholar]
- 28.Lo KM, et al. High level expression and secretion of Fc-X fusion proteins in mammalian cells. Protein Eng. 1998;11:495–500. doi: 10.1093/protein/11.6.495. [DOI] [PubMed] [Google Scholar]
- 29.Gillies SD, et al. Improved circulating half-life and efficacy of an antibody-interleukin 2 immunocytokine based on reduced intracellular proteolysis. Clin Cancer Res. 2002;8:210–216. [PubMed] [Google Scholar]
- 30.Nalvarte I, et al. Overexpression of enzymatically active human cytosolic and mitochondrial thioredoxin reductase in HEK-293 cells. Effect on cell growth and differentiation. J Biol Chem. 2004;279:54510–54517. doi: 10.1074/jbc.M408494200. [DOI] [PubMed] [Google Scholar]
- 31.Peng B, et al. A cultured malignant B-1 line serves as a model for Richter's syndrome. J Immunol. 1994;153:1869–1880. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.