Abstract
Tumor necrosis factor receptor 1-associated death domain protein (TRADD) is the core adaptor recruited to TNF receptor 1 (TNFR1) upon TNFα stimulation. In cells from TRADD-deficient mice, TNFα-mediated apoptosis and TNFα-stimulated NF-κB, JNK, and ERK activation are defective. TRADD is also important for germinal center formation, DR3-mediated costimulation of T cells, and TNFα-mediated inflammatory responses in vivo. TRADD deficiency does not enhance IFNγ-induced signaling. Importantly, TRADD has a novel role in TLR3 and TLR4 signaling. TRADD participates in the TLR4 complex formed upon LPS stimulation, and TRADD-deficient macrophages show impaired cytokine production in response to TLR ligands in vitro. Thus, TRADD is a multifunctional protein crucial both for TNFR1 signaling and other signaling pathways relevant to immune responses.
Keywords: TNF, innate immunity
Tumor necrosis factor alpha (TNFα) is a pleiotropic cytokine involved in a broad range of biological activities, including inflammation and cell differentiation, survival, and death (1). TNFα mediates these activities by engaging two distinct cell surface receptors: TNFR1 and TNFR2. Activation of TNFR1 leads to the recruitment of the intracellular death domain (DD)-containing adaptor TNFR-associated DD protein (TRADD) through a homotypic interaction with DD of TNFR1. On one hand, the recruitment of TRADD can promote the association of the TNFR1 complex with Fas-associated DD (FADD), which induces caspase activation and cell death. On the other hand, TRADD can also recruit receptor-interacting protein kinase 1 (RIP1) and TNFR-associated factor-2 (TRAF2), which trigger NF-κB activation, leading to cell survival and proinflammatory responses (1).
TRADD was originally identified in a yeast two-hybrid screen performed to identify TNFR1-interacting proteins (2). Intriguingly, TRADD is the first adaptor protein identified that binds directly to the DD of TNFR1 but transduces signals resulting in either apoptosis or NF-κB activation (2–4). Indeed, TRADD overexpression in 293T cells activates both apoptotic and cell-survival signaling pathways (2). In addition to TNFR1, TRADD may mediate signaling downstream of the death receptors (and TNFR superfamily members) DR3 and DR6 (5, 6). As well, recent TRADD knockdown studies have indicated that TRADD may be involved in signaling mediated by receptors unrelated to the TNFR superfamily, such as in IFNγ receptor (7–9). These findings give tantalizing hints of the potential breadth of TRADD functions.
To date, a knockout animal model has yet to be reported for TRADD, even though this important adaptor was identified more than ten years ago. In this study, we generate TRADD knockout mice by using conditional gene targeting and show that not only is TRADD indispensable for TNFα-induced NF-κB activation and apoptosis in vitro and TNFα-induced inflammatory responses in vivo, but also that this molecule is involved in germinal center (GC) formation, T cell costimulation, and TLR signaling. Our TRADD knockout mice represent a very useful tool for extending the ever-increasing list of TRADD functions in vitro and in vivo.
Results
TRADD Is Indispensable for TNFα-Mediated Cell Death.
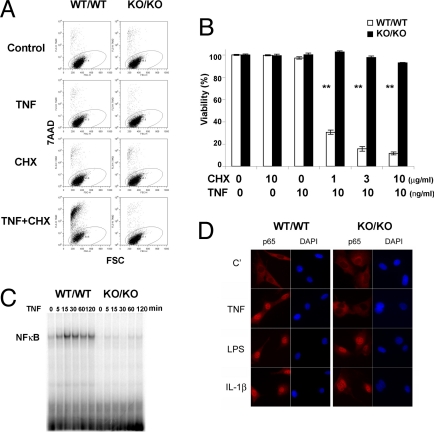
To dissect the role of TRADD in TNFα-induced signaling, we generated E14.5 mouse embryonic fibroblast cells (MEFs) from traddWT/WT and traddKO/KO mice (supporting information (SI) Figs. S1 and S2), treated them with TNFα plus cycloheximide (CHX) for 24 h, and assessed cell death by 7-AAD staining and flow cytometry. More than 70% of traddWT/WT MEFs underwent apoptosis after stimulation with TNFα plus CHX (Fig. 1 A and B). In contrast, traddKO/KO MEFs were completely resistant to TNFα-induced cell death under the same conditions. MEFs of both genotypes showed equivalent sensitivity to other cell-death-inducing agents, including sorbitol, etoposide, doxorubicin, anisomycin, and UV irradiation (data not shown). Similarly, TRADD deficiency did not alter the susceptibility of CD4+ T cells to Fas-mediated activation-induced cell death (10), indicating that TRADD plays no role in Fas-mediated apoptosis (data not shown). Taken together, our results show that TRADD is indispensable for the cell-death arm of TNFR1 signaling but is not involved in the death triggered by many other agents.
Fig. 1.
TRADD is indispensable for TNFα-induced cell death and TNFα-mediated NF-κB activation. Primary MEFs from traddWT/WT and traddKO/KO mice were treated for 24 h with TNFα alone (10 ng/ml), CHX alone (1–10 μg/ml), or TNFα plus CHX. Viability was assessed by 7-AAD staining and flow cytometry. (A) Representative flow cytometric data with 1 μg/ml CHX. (B) Bar graph summary of all flow cytometric data. Results shown are the mean viability ± SD of triplicate determinations. **, P < 0.01, Student's t test. For A and B, data are representative of at least three independent experiments. (C) Gel mobility shift assay. MEFs from traddWT/WT and traddKO/KO mice were stimulated with 10 ng/ml TNFα for the indicated times, and NF-κB activation was evaluated by EMSA. (D) RelA/p65 translocation. The MEFs in A were subjected to immunofluorescent staining to detect RelA/p65 translocation into the nucleus. MEFs were also treated with LPS (500 ng/ml) or IL-1β (10 ng/ml), and RelA/p65 translocation was assessed.
TRADD Is Essential for TNFα-Mediated NF-κB Activation.
NF-κB activation is a key signaling event required for TNFα-induced production of inflammatory cytokines (1–3). We compared NF-κB activation in MEFs and bone-marrow-derived macrophages (BM-Macs) (data not shown) obtained from traddWT/WT and traddKO/KO mice and found that TNFα-induced NF-κB activation could not be triggered in the absence of TRADD (Fig. 1 C and D); meanwhile, LPS or IL-1β-induced NF-κB activation remained intact (Fig. 1D). Importantly, the abrogation of TNFα-induced NF-κB activation observed in traddKO/KO cells was not because of up-regulation of NF-κB inhibitors such as IκB and A20 (11–14) (Fig. S3a, Center and Right). These observations corroborate with cytoplasmic signaling events upstream of NF-κB activation in TNFα-stimulated traddWT/WT and traddKO/KO MEFs and BM-Macs. In contrast to the situation in traddWT/WT cells, TNFα treatment failed to induce significant degradation of IκB in traddKO/KO MEFs (Fig. 2A) or BM-Macs (Fig. 2B). To bolster the above findings, we also evaluated IL-6 production in TNFα-stimulated traddWT/WT and traddKO/KO MEFs by ELISA. We showed that IL-6 was not induced in the absence of TRADD (Fig. S3b), confirming our real-time RT-PCR results (Fig. S3a Left). In contrast, IL-6 production in response to IL-1β and LPS stimulation was unaltered in the absence of TRADD (Fig. 3B).
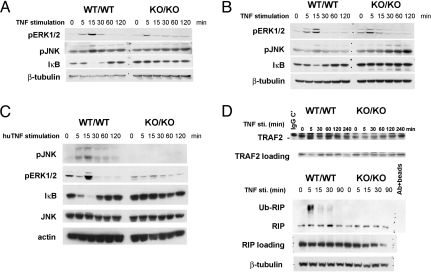
Fig. 2.
Alterations to TNFR1 signaling and complex formation in the absence of TRADD. (A–C) ERK, JNK, and IκB. traddWT/WT and traddKO/KO MEFs (A) and BM-Macs (B) were stimulated with 10 ng/ml murine TNFα (A and B) or 10 ng/ml human TNF (C) for the indicated times, and ERK and JNK phosphorylation and IκB degradation were assessed by Western blotting. β-tubulin and actin were used as loading controls. (D) TNFR1 complex composition. traddWT/WT and traddKO/KO MEFs were stimulated with 10 ng/ml TNFα for the indicated times, and the TNFR1 complex was isolated by IP. TRAF2, RIP, and ubiquitinated RIP (Ub-RIP) within the TNFR1 complex were detected by Western blotting. Data shown are representative of at least three independent experiments.
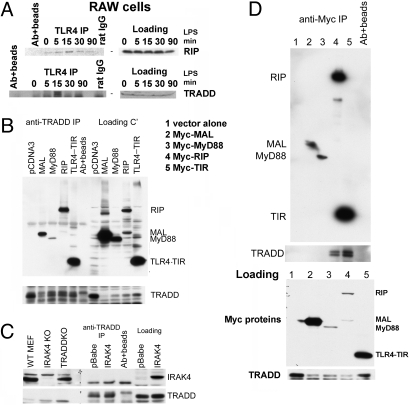
Fig. 3.
TRADD is recruited to the TLR4 signaling complex upon LPS stimulation. (A) TRADD participates in the TLR4 complex. RAW cells were stimulated with LPS (100 ng/ml) for the indicated times, and lysates were subjected to IP with anti-TLR4. TRADD and RIP were identified by Western blotting to be components of the TLR4 complex. (B and C) TRADD associates with TLR4-TIR but not IRAK4. 293T cells were cotransfected with a TRADD-expressing vector plus vectors expressing the indicated Myc-tagged mediators. Proteins associating with TRADD were identified by TRADD-IP and anti-Myc Western blotting. IRAK4KO was used as a negative control; pCDNA3 and pBABE were used as empty vector controls. (D) TLR4-TIR associates with TRADD; reciprocal IP involving anti-Myc IP and anti-TRADD Western blotting. For A–D, data are representative of at least two independent experiments.
TRADD Is Essential for TNFR1-Mediated MAP Kinase Activation.
MAP kinase (MAPK) activation is reportedly involved in transducing TNFα signaling (15). We therefore analyzed the effect of TRADD deficiency on TNFα-induced ERK and JNK activation. Surprisingly, traddKO/KO cells still exhibited some degree of JNK and ERK activation in response to murine TNFα stimulation, albeit with altered kinetics (Fig. 2 A and B). To eliminate potential confounding effects because of MAPK activation downstream of TNFR2, we stimulated traddWT/WT and traddKO/KO cells with human TNFα, which is thought to preferentially activate TNFR1 in murine cells (16). Under these conditions, MAPK activation was completely abolished in the absence of TRADD (Fig. 2C), confirming that TRADD is essential for TNFR1-mediated MAPK activation.
The Composition of the TNFR1 Complex Is Altered in TNFα-Stimulated TRADD-Deficient Cells.
TRADD is believed to be the core adaptor that recruits RIP, TRAF2, and FADD into the TNFR1 complex in response to TNFα stimulation (3, 4). To determine whether deletion of TRADD would affect TNFR1 complex formation, we treated traddWT/WT and traddKO/KO MEFs with TNFα and carried out immunoprecipitation (IP) experiments by using an antibody specific for TNFR1. Peak binding of RIP and TRAF2 to TNFR1 was detected in the WT after 5 min of TNFα stimulation, with RIP exhibiting a ladder-like pattern of ubiquitination (Fig. 2D). Surprisingly, the association of RIP with TNFR1 occurs even without receptor triggering, and RIP (but not its ubiquitinated counterpart) remained associated with TNFR1 in TNFα-stimulated traddKO/KO cells. Recruitment of TRAF2 to the TNFR1 complex after TNFα stimulation was abolished in the absence of TRADD (Fig. 2D). No FADD was detected in the TNFR1 complexes in traddWT/WT and traddKO/KO cells treated with TNFα alone (data not shown). Thus, TRADD deficiency alters the components that associate with TNFR1 upon TNFα stimulation. Most importantly, our data suggest that TRADD is essential for the ubiquitination of RIP1 that occurs upon its recruitment to the TNFR1 complex.
TRADD Deficiency Compromises TNFα-Induced Responses in Vivo.
To verify that the defects we observed in TRADD-deficient cells in vitro were also relevant in vivo, we intravenously injected groups of traddWT/WT, traddKO/KO, and tnfr1−/− (negative control) mice with 3.0 μg of recombinant TNFα and evaluated serum IL-6 at 0 and 6 h after injection. One traddWT/WT mouse was found dead at 4 h after injection. However, all other WT mice, but none of the traddKO/KO or tnfr1−/− mice, responded to the TNFα treatment by exhibiting a dramatic increase in serum IL-6 at 6 h after injection (Fig. S4a). Histological analyses of the TNFα injection sites in the mutant strains confirmed that TRADD and TNFR1 are essential for the local tissue damage associated with TNFα injection (Fig. S4b).
In addition to inflammatory cytokine production, engagement of TNFR1 is also required for the formation of follicular dendritic cell (FDC) clusters in resting animals and splenic GC formation in immunized animals (17). We found that in contrast to WT mice, TRADD-deficient mice and TNFR1-deficient mice showed no organized FDC clusters in the spleen (Fig. S4c Left). As well, upon sheep RBC immunization, explicit GC formation at the junction of the T cell and B cell regions was observed in splenic follicles of traddWT/WT mice but not in traddKO/KO mice (Fig. S4c Right). These results demonstrate that the development of normal FDC clusters and GC formation in the spleen require TRADD.
TRADD Outside TNFR1.
In addition to TNFR1 signaling, TRADD may mediate signaling by the TNF receptor superfamily member DR3 (5, 6), which is thought to provide costimulatory signals during T cell activation (5). We validated this hypothesis by showing that WT CD4+ T cells, but not TRADD-deficient CD4+ T cells, exhibited increased proliferation after anti-CD3 plus TL1a stimulation (Fig. S5). Thus, TRADD is indeed indispensable for DR3-mediated T cell costimulation.
Recently, TRADD was implicated in the cross-talk between TNFR1 signaling and IFNγ signaling. In particular, in RAW cells, TRADD knockdown increases IFNγ-induced STAT1 activation and function (7). To validate these observations in primary TRADD-deficient cells, we examined IFNγ-induced STAT1 phosphorylation in traddWT/WT and traddKO/KO MEFs (Fig. S6a), BM-Macs (Fig. S6b) and CD4+ T cells (Fig. S6c). Although RIP and IκB levels appeared normal in mutant MEFs, no enhancement of IFNγ-induced STAT1 activation was detected in any traddKO/KO cell type. In fact, if anything, the TRADD-deficient cells of a tested pair sometimes showed a slight reduction in STAT1 activation after IFNγ stimulation. Further analysis of IFNγ signaling needs to be performed both in primary and transformed TRADD-deficient cells to resolve this controversy.
TRADD Is Involved in TLR Signaling and Responses.
Several signaling mediators involved in TNFR downstream signaling have also been reported to play roles in modulating TLR signaling (18–20). To determine whether TRADD is one of these mediators, we treated RAW cells with the TLR4 ligand LPS for 5–90 min and carried out IP with anti-TLR4 antibody to isolate the TLR4 receptor complex. We found that both TRADD and RIP coimmunoprecipiated with TLR4 (Fig. 3A). To further assess the interaction between TRADD and components of the TLR4 complex, we cotransfected 293T cells with a TRADD-expressing vector plus vectors expressing Myc-tagged downstream TLR4 signaling mediators, including the TIR domain of TLR4 (TLR4-TIR), MAL, MyD88, IRAK4, and RIP. In a TRADD IP, we were able to pull down RIP as well as TLR4-TIR (Fig. 3B). TRADD also bound weakly to MAL and MyD88 under these overexpression conditions. However, no interaction could be detected between TRADD and IRAK4, a key DD-containing TLR signaling mediator (Fig. 3C). Reciprocal IPs in which complexes associated with tagged TLR4 signaling mediators were isolated and Western blotted to detect TRADD showed that RIP and TLR4-TIR, but not MAL or MyD88, can associate with TRADD (Fig. 3D). Taken together, these data demonstrate that TRADD indeed participates in the TLR4 complex that forms upon LPS stimulation. We speculate that TRADD may do so either directly by interacting with TLR4-TIR and/or indirectly through RIP in the TRIF-dependent pathway (18).
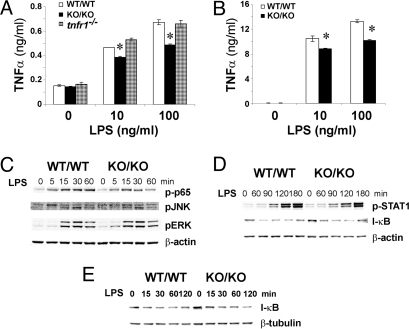
To determine whether TRADD is required for TLR-mediated cellular functions, we examined the effect of TRADD deficiency on inflammatory cytokine production in response to TLR4 engagement. We stimulated WT, TRADD-deficient, and TNFR1-deficient BM-Macs (Fig. 4A), as well as WT and TRADD-deficient peritoneal macrophages (Fig. 4B), with the TLR4 ligand lipopolysaccharide (LPS) and analyzed TNFα production 24 h later. In both cases, induction of TNFα in response to LPS was clearly reduced in traddKO/KO macrophages compared with traddWT/WT macrophages. To further dissect the role of TRADD in TLR4 signaling, we examined the impact of TRADD deficiency on LPS-stimulated NF-κB and MAPK activation in BM-Macs. There were no obvious alterations in MAPK activation (Fig. 4C), STAT1 activation (Fig. 4D), or IκB degradation (Fig. 4 D and E) in the absence of TRADD. However, we did observe a reduction in p65 phosphorylation status in traddKO/KO cells, particularly at later timepoints (Fig. 4C, first row). TLR4 engagement leads to activation of the Myd88-dependent pathway that mediates early NF-κB and MAPK activation, as well as the MyD88-independent, TRIF-dependent pathway that mediates late NF-κB and IRF3 activation (21, 22). Given the reductions in serum TNFα production and late stage p65 phosphorylation in LPS-stimulated TRADD-deficient cells, we suspect that it is the TRIF-dependent pathway that is compromised in the absence of TRADD.
Fig. 4.
TRADD is involved in TLR4 signaling and TLR4 mediated cytokine production. (A and B) Reduced LPS-stimulated TNFα production. traddWT/WT, traddKO/KO, and tnfr1−/− BM-Macs (A) and traddWT/WT and traddKO/KO peritoneal macrophages (B) were stimulated with LPS (10, 100, or 300 ng/ml) for 24 h, and TNFα in the culture supernatants was evaluated by ELISA. *, P < 0.05, Student's t test. (C–E) Reduced LPS-stimulated NF-κB and MAPK activation. traddWT/WT and traddKO/KO BM-Macs were stimulated with 100 ng/ml LPS for the indicated times. The phosphorylation of p65, ERK, JNK, and STAT1, and IκB degradation were assessed by Western blotting. β-actin and β-tubulin were used as loading controls. For A–E, data are representative of at least two independent experiments.
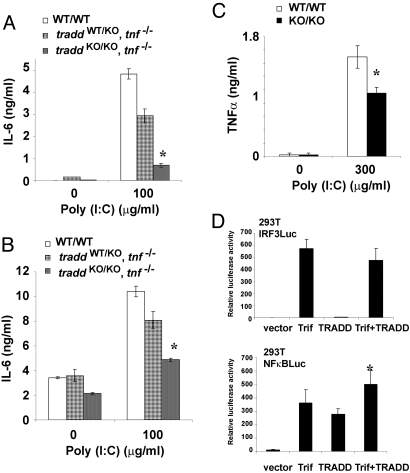
TRIF is crucial for the MyD88-independent signaling pathway activated by engagement of TLR3 or TLR4 (21). To elucidate the role of TRADD in the TRIF-dependent pathway while eliminating any potentially compensatory effects of the Myd88-dependent pathway (23), we examined the role of TRADD in TLR3 signaling. We first examined IL-6 production in response to polyinosine-polycytidylic acid [poly(I:C)], a synthetic TLR3 ligand. We used TRADD/TNF double knockout mice for these experiments to ensure that autocrine TNFα could not indirectly regulate the TLR3 response. We found that BM-Macs and lung fibroblasts from the double mutants produced significantly less IL-6 than did WT cells after 24 h poly(I:C) stimulation (Fig. 5 A and B). Next, we examined TNFα production in response to poly(I:C) and found that peritoneal macrophages from traddKO/KO mice produced significantly less TNFα at 24 h after treatment than did peritoneal macrophages from WT mice (Fig. 5C). These data corroborate the deficit in TLR signaling that we noted in LPS-treated TRADD-deficient cells and implicate TRADD in the TRIF-dependent pathway.
Fig. 5.
TRADD is involved in TLR3 mediated cytokine production. (A and B) Reduced poly(I:C)-stimulated IL6 production. traddWT/WT, traddWT/WTtnf−/− and traddKO/KOtnf−/− BM-Macs (A) and lung fibroblasts (B) were stimulated with 100 μg/ml poly(I:C), and IL-6 in the culture supernatants was evaluated 24 h later by ELISA. (C) Reduced poly(I:C)-stimulated TNFα production. traddWT/WT and traddKO/KO peritoneal macrophages were stimulated with 300 μg/ml poly(I:C), and TNFα in the culture supernatants was evaluated 24 h later by ELISA. (D) TRADD augments TRIF-dependent NF-κB activation. 293T cells were transfected with either an NF-κB or IRF3 luciferase reporter in the presence of TRADD and/or TRIF expression vectors. Results are expressed as luciferase activity relative to β-galactosidase activity ± SD. For A–D, *, P < 0.05, Student's t test. Data are representative of at least two independent experiments.
Signaling through the TRIF pathway results in the activation of NF-κB and IRF3 (22). To further define TRADD's role in TRIF signaling, we carried out luciferase reporter assays in which 293T cells were transfected with either an NF-κB or IRF3 luciferase reporter construct in the presence of TRADD and/or TRIF expression vectors. We found that TRADD overexpression did not augment TRIF-dependent IRF3 activation (Fig. 5D Upper) but appeared to stimulate TRIF-dependent NF-κB activation (Fig. 5D Lower). Although detailed signaling analyses are required to define the exact role of TRADD in the TRIF pathway, our data provide evidence indicating that TRADD plays an important role in TLR signaling via participation in TRIF-dependent NF-κB activation.
Discussion
Although TRADD has been identified for over a decade, the TRADD knockout mouse has been generated only recently. Our analyses of various cells from these mutant animals have confirmed the answers to some questions while raising others. Our results show that TRADD is essential for TNFα-induced apoptosis and TNFα-induced TNFR1-mediated NF-κB activation. TRADD deficiency did not completely abolish murine TNFα-mediated ERK and JNK activation. However, human (TNFR1-specific) TNFα-mediated ERK and JNK activation was completely abolished in the absence of TRADD. Our result indicates that TRADD is necessary for TNFR1 but not TNFR2-mediated MAPK activation.
Our analyses of the TNFR1 signaling complex also revealed an essential role for TRADD in the ubiquitination of RIP that supports NF-κB activation. The most intriguing result emerging from this analysis was that the association of un-ubiquitinated RIP with TNFR1 is TRADD-independent. Given that ubiquitination of RIP has been proposed as an essential step during TNFα-induced NF-κB activation (24), the role of TRADD in mediating TNFα-induced NF-κB activation may occur through the induction of RIP ubiquitination. Nonetheless, our data suggest that the identity and association sequence of the various signaling mediators and adaptors that participate in the TNFR1 receptor complex in response to TNFα may need to be reconsidered.
Several lines of evidence point to roles for TNFR1 downstream signal adaptors (including FADD, RIP, and TRAF2) in lymphopoiesis and immune responses. In the current study, we found TRADD to be crucial for the formation of FDC clusters in splenic primary follicles and for GC formation after immunization. This defect is reminiscent of that observed in TNFR1 KO mice (17). Our histological result served as an in vivo confirmation that TNF-TNFR1-TRADD signaling is essential for FDC and subsequent GC formation in the spleen. Whether TRADD is further involved in TNFR1-independent adaptive responses or in T cell and B cell development and functions remains an open question and mandates further investigation.
Previous reports have suggested that TRADD interacts with STAT1 and down-regulates IFNγ-induced transcription and bioactivity. This hypothesis arises chiefly from work done by using TRADD knockdown in RAW cells, which showed that signaling in response to IFNγ stimulation increased in the absence of TRADD (25). However, our investigation does not support a negative regulatory role for TRADD in IFNγ-induced responses. In fact, we sometimes observed a slight reduction in STAT1 phosphorylation in TRADD-deficient MEFs, BM-Macs, and T cells. TRADD's role in IFNγ signaling thus remains an unresolved controversy. Studies of pathogen infection in vivo and Th1/Th2 differentiation analysis will help to fully characterize the role of TRADD in IFNγ signaling. It will also be interesting to determine whether TRADD is involved in cytokine signaling mediated by other STATs.
The most exciting result of our study is the uncovering of an important role for TRADD in TLR signaling. We found that TRADD participates in the TLR4 signaling complex formed in LPS-stimulated cells. Our biochemical analyses indicate that this involvement of TRADD occurs very far upstream in the signaling pathway and takes the form of a direct association between TRADD and TLR4-TIR and/or an indirect association between TRADD and RIP in the TRIF-dependent pathway. The latter proposition is supported by our studies of the effects of TRADD deficiency on the TLR3 pathway because signaling downstream of TLR3 engagement relies solely on TRIF. Upon TLR3 stimulation by poly(I:C), we noted a significant decrease in cytokine production by TRADD-deficient cells compared with controls. Detailed analyses of TLR3 signaling, as well as assessments of TLR4 signaling in TRADD/MyD88 double-knockout mice, will be crucial for the delineation of the precise involvement of TRADD in the TRIF pathway. Nevertheless, our study is seminal in that it has revealed a new role for TRADD in TLR signaling that is independent of its functions in the TNFR1 pathway. A recent paper by Michallet et al. (26) has revealed TRADD's participation in the RIG-like helicase antiviral pathway. This finding, in combination with our current observations, suggests that TRADD may be a critical player in the host antiviral and antibacterial response. This unearthing of TRADD as a player in antiviral immunity may contribute to the development of new therapeutic strategies against viral diseases.
Methods
Cells and Reagents.
MEFs, bone-marrow-derived macrophages, lung fibroblasts, and T cells were prepared as described (21, 27). Mouse TNFα, human TNFα, mouse IL-1β, mouse IFNγ, mouse IL-2, and human DR6-Fc were all from R&D Systems. Poly(I:C) was from Invivogen. Mouse TL1a was the kind gift of Ping Wei (Human Genome Science, Rockville, Maryland). LPS, LTA, PGN, 7-AAD, CHX, sorbitol, etoposide, doxorubicin, anisomycin, dexamethasone, and staurosporine were all from Sigma. The anti-CD3 and anti-CD28 antibodies used for T cell stimulation were from BD Bioscience.
Western Blotting.
MEFs and BM-Macs were rested in serum-free DMEM or RPMI, respectively, for 2 h before stimulation with 10 ng/ml TNFα, 10 U/ml IFNγ, 100 ng/ml LPS, or 100 μg/ml poly(I:C). Cells were lysed in RIPA buffer [150 mM NaCl, 50 mM Tris-HCl (pH 8.0), 1% Nonidet P-40, 0.5% DOC, 0.1% SDS, and complete proteinase inhibitor mixture from Roche], and protein concentrations were determined by using the BCA protein assay (Thermo Scientific). Samples containing equal amounts of protein were fractionated on a precast 4–20% SDS/PAGE gel (Invitrogen) and transferred onto a PVDF membrane. TRADD levels were determined by incubating the blot with rabbit anti-TRADD polyclonal antibody (Santa Cruz Biotechnology). For signaling studies, rabbit anti-pERK1/2, anti-pp38, anti-pAKT, and anti-pp65 (from Cell Signaling), anti-pJNK (from Santa Cruz Biotechnology), and anti-pSTAT1 (from Upstate) were used as primary antibodies to determine levels of phosphorylated kinases. Anti-IκB (from Cell Signaling) was used to determine IKB degradation. To visualize proteins, blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse secondary antibodies (GE Health), and processed with the ECL Plus system (GE Health).
Immunoprecipitation.
Cells cultured on 15-cm tissue culture plates were treated with TNFα (10 ng/ml) or LPS (100 ng/ml) as indicated in the figures. Cells were washed, harvested, and lysed in IP buffer [20 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.2% Nonidet P-40, 10% glycerol, and a complete proteinase mixture]. Each IP binding sample contained 500–1,000 μg of total lysate protein. Anti-TNFR1 or anti-TLR4 antibody (both from BD Biosciences) was mixed first with protein G beads (GE Health) and then with the lysates and incubated overnight. The beads plus the antibodies and associated proteins were washed five times in IP buffer and resuspended in SDS/PAGE sample buffer. Samples were fractionated by SDS/PAGE, and protein components of receptor complexes were determined by Western blotting.
Cytokine Levels.
Levels of IL-6 and TNFα in serum samples or culture supernatants were measured by using ELISA kits from BD Biosciences, following the manufacturer's instructions.
SI.
Further information, including a list of primers used in this work (Table S1), is available in SI Methods.
Supplementary Material
Acknowledgments.
We thank Drs. S. Suzuki and N. Suzuki for their helpful discussions during the initiation of this investigation, Ms. C. Mirtsos for technical assistance, and Dr. M. Saunders for scientific editing. This work was supported by grants from the Leukemia Lymphoma Society, the Canadian Institute of Health Research, and the Terry Fox Cancer Research Foundation (to T.W.M.); the University of Toronto through the Connaught Scholarship (to I.I.C.C.); and the Development of Top-Level International University Plan 96A-D-P3-02 at National Yang-Ming University (N.-J.C.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0806585105/DCSupplemental.
References
- 1.Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 2.Hsu H, Xiong J, Goeddel DV. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 3.Chen G, Goeddel DV. TNF-R1 signaling: A beautiful pathway. Science. 2002;296:1634–1635. doi: 10.1126/science.1071924. [DOI] [PubMed] [Google Scholar]
- 4.Mak TW, Yeh WC. Signaling for survival and apoptosis in the immune system. Arthritis Res 4 Suppl. 2002;3:S243–252. doi: 10.1186/ar569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chinnaiyan AM, et al. Signal transduction by DR3, a death domain-containing receptor related to TNFR-1 and CD95. Science. 1996;274:990–992. doi: 10.1126/science.274.5289.990. [DOI] [PubMed] [Google Scholar]
- 6.Pan G, et al. Identification and functional characterization of DR6, a novel death domain-containing TNF receptor. FEBS Lett. 1998;431:351–356. doi: 10.1016/s0014-5793(98)00791-1. [DOI] [PubMed] [Google Scholar]
- 7.Wesemann DR, Qin H, Kokorina N, Benveniste EN. TRADD interacts with STAT1-alpha and influences interferon-gamma signaling. Nat Immunol. 2004;5:199–207. doi: 10.1038/ni1025. [DOI] [PubMed] [Google Scholar]
- 8.Zheng L, et al. Competitive control of independent programs of tumor necrosis factor receptor-induced cell death by TRADD and RIP1. Mol Cell Biol. 2006;26:3505–3513. doi: 10.1128/MCB.26.9.3505-3513.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sethi G, Ahn KS, Chaturvedi MM, Aggarwal BB. Epidermal growth factor (EGF) activates nuclear factor-kappaB through IkappaBalpha kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IkappaBalpha. Oncogene. 2007;26:7324–7332. doi: 10.1038/sj.onc.1210544. [DOI] [PubMed] [Google Scholar]
- 10.Ju ST, et al. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1995;373:444–448. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 11.Beg AA, Sha WC, Bronson RT, Baltimore D. Constitutive NF-κ B activation, enhanced granulopoiesis, and neonatal lethality in I κ B α-deficient mice. Genes Dev. 1995;9:2736–2746. doi: 10.1101/gad.9.22.2736. [DOI] [PubMed] [Google Scholar]
- 12.Klement JF, et al. IκBα deficiency results in a sustained NF-κB response and severe widespread dermatitis in mice. Mol Cell Biol. 1996;16:2341–2349. doi: 10.1128/mcb.16.5.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Song HY, Rothe M, Goeddel DV. The tumor necrosis factor-inducible zinc finger protein A20 interacts with TRAF1/TRAF2 and inhibits NF-kappaB activation. Proc Natl Acad Sci USA. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heyninck K, et al. The zinc finger protein A20 inhibits TNF-induced NF-kappaB-dependent gene expression by interfering with an RIP- or TRAF2-mediated transactivation signal and directly binds to a novel NF-kappaB-inhibiting protein. ABIN J Cell Biol. 1999;145:1471–1482. doi: 10.1083/jcb.145.7.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kalb A, Bluethmann H, Moore MW, Lesslauer W. Tumor necrosis factor receptors (Tnfr) in mouse fibroblasts deficient in Tnfr1 or Tnfr2 are signaling competent and activate the mitogen-activated protein kinase pathway with differential kinetics. J Biol Chem. 1996;271:28097–28104. doi: 10.1074/jbc.271.45.28097. [DOI] [PubMed] [Google Scholar]
- 16.Ameloot P, et al. Identification of tumor necrosis factor (TNF) amino acids crucial for binding to the murine p75 TNF receptor and construction of receptor-selective mutants. J Biol Chem. 2001;276:37426–37430. doi: 10.1074/jbc.M102020200. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto M, et al. Role of lymphotoxin and the type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 18.Meylan E, et al. RIP1 is an essential mediator of Toll-like receptor 3-induced NF-κ B activation. Nat Immunol. 2004;5:503–507. doi: 10.1038/ni1061. [DOI] [PubMed] [Google Scholar]
- 19.Bannerman DD, Eiting KT, Winn RK, Harlan JM. FLICE-like inhibitory protein (FLIP) protects against apoptosis and suppresses NF-κB activation induced by bacterial lipopolysaccharide. Am J Pathol. 2004;165:1423–1431. doi: 10.1016/s0002-9440(10)63400-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imtiyaz HZ, et al. The Fas-associated death domain protein is required in apoptosis and TLR-induced proliferative responses in B cells. J Immunol. 2006;176:6852–6861. doi: 10.4049/jimmunol.176.11.6852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto M, et al. Role of adaptor TRIF in the MyD88-independent toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- 22.Sato S, et al. Toll/IL-1 receptor domain-containing adaptor inducing IFN-beta (TRIF) associates with TNF receptor-associated factor 6 and TANK-binding kinase 1, and activates two distinct transcription factors, NF-κ B and IFN-regulatory factor-3, in the Toll-like receptor signaling. J Immunol. 2003;171:4304–4310. doi: 10.4049/jimmunol.171.8.4304. [DOI] [PubMed] [Google Scholar]
- 23.Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science. 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- 24.Li H, Kobayashi M, Blonska M, You Y, Lin X. Ubiquitination of RIP is required for tumor necrosis factor α-induced NF-κB activation. J Biol Chem. 2006;281:13636–13643. doi: 10.1074/jbc.M600620200. [DOI] [PubMed] [Google Scholar]
- 25.Wesemann DR, Benveniste EN. STAT-1 α and IFN-γ as modulators of TNF-α signaling in macrophages: Regulation and functional implications of the TNF receptor 1:STAT-1 alpha complex. J Immunol. 2003;171:5313–5319. doi: 10.4049/jimmunol.171.10.5313. [DOI] [PubMed] [Google Scholar]
- 26.Michallet MC, et al. TRADD protein is an essential component of the RIG-like helicase antiviral pathway. Immunity. 2008;28:651–661. doi: 10.1016/j.immuni.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 27.Chen NJ, et al. C5L2 is critical for the biological activities of the anaphylatoxins C5a and C3a. Nature. 2007;446:203–207. doi: 10.1038/nature05559. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.