Abstract
Netrins are secreted molecules with roles in axon guidance and angiogenesis. We identified Netrin-4 as a gene specifically overexpressed in VEGF-stimulated endothelial cells (EC) in vitro as well as in vivo. Knockdown of Netrin-4 expression in EC increased their ability to form tubular structures on Matrigel. To identify which receptor is involved, we showed by quantitative RT-PCR that EC express three of the six Netrin-1 cognate receptors: neogenin, Unc5B, and Unc5C. In contrast to Netrin-1, Netrin-4 bound only to neogenin but not to Unc5B or Unc5C receptors. Neutralization of Netrin-4 binding to neogenin by blocking antibodies abolished the chemotactic effect of Netrin-4. Furthermore, the silencing of either neogenin or Unc5B abolished Netrin-4 inhibitory effect on EC migration, suggesting that both receptors are essential for its function in vitro. Coimmunoprecipitation experiments demonstrated that Netrin-4 increased the association between Unc5B and neogenin on VEGF- or FGF-2-stimulated EC. Finally, we showed that Netrin-4 significantly reduced pathological angiogenesis in Matrigel and laser-induced choroidal neovascularization models. Interestingly, Netrin-4, neogenin, and Unc5B receptor expression was up-regulated in choroidal neovessel EC after laser injury. Moreover, Netrin-4 overexpression delayed tumor angiogenesis in a model of s.c. xenograft. We propose that Netrin-4 acts as an antiangiogenic factor through binding to neogenin and recruitment of Unc5B.
The formation of new blood vessels is characteristic of various physiological situations including embryonic development or wound healing as well as pathological events such as tumor progression, diabetic retinopathy, and age-related macular degeneration. Developmental and adult neovascularization are fundamentally different processes involving different mechanisms, growth factor receptors, and recruitment of vascular progenitors. Another feature of adult neovascularization is that, unlike developmental angiogenesis, which is hardwired, it is modulated differentially by various environmental stimuli (e.g., estrogens in corpus luteum formation, lipofuschin in age-related macular degeneration, and hypoxia in most pathological situations) and the genetic changes associated with tumors. All of these stimuli lead to overexpression of VEGF. Pathological tumor vessels and retinal vessels depend on VEGF for survival, but it is not clear whether new choroidal vessels are so VEGF-dependent (1). It is therefore possible that VEGF induces different patterns of gene expression in different target cells.
To investigate this possibility, we compared gene expression patterns in endothelial cells (EC) after long-term exposure, or no exposure, to VEGF by using a subtractive hybridization strategy and identified Netrin-4 (N4) as a gene up-regulated in angiogenic EC.
Originally identified as axonal guidance molecules, netrins are laminin-like secreted proteins that have been recently involved in angiogenesis and blood vessels network formation (2–4). The netrin system comprises at least five ligands (netrins 1, 2, 4, G1a, and G1b) and seven receptors (neogenin, DCC, Unc5A, Unc5B, Unc5C, Unc5D, and A2b) (5). Netrins are bifunctional guidance cues, attracting some axons while repelling others. The DCC and neogenin subfamily receptors induce axon attraction toward a Netrin-1 gradient. If commissural neurons also express the Unc5B receptor, the signal is converted into a repulsion signal through association of the conserved P1 domain of DCC and the DB domain of the Unc5 receptor (6). However, the repulsive action of Netrin-1 in some neurons is independent of DCC (7).
As observed in the nervous system, netrins may also act as bifunctional cues in angiogenesis. Thus, different results have been reported concerning the role of netrins in vessel branching in zebrafish (8, 9) and EC functions in vitro (10, 11). Wilson et al. (8) reported that netrins stimulate proliferation, migration, and tube formation of EC, independent of any expression of cognate netrin receptors. They also showed that knockdown of Netrin-1 mRNA in zebrafish inhibits vascular sprouting, suggesting a proangiogenic role of Netrin-1. In contrast, disruption of the Unc5B gene in mice or in zebrafish leads to excessive vessel branching and abnormal navigation, suggesting a negative role for Netrin-1 in vasculogenesis (9). This led to the suggestion that Netrin-1 attenuates developmental angiogenesis through the activation of Unc5B. More recently, Larrivée et al. (12) provided evidence that Netrin-1 also inhibits postnatal sprouting angiogenesis and neovascularization via the activation of the Unc5B receptor. However, the role of N4 in angiogenesis and its mechanism of action have not been elucidated.
In this study we identified N4 as a gene overexpressed in vitro in EC upon VEGF exposure. We investigated the role of N4 in EC both in vitro and in vivo. We observed that N4 inhibits VEGF-mediated migration and tubulogenesis of EC in vitro. We showed that EC express neogenin, Unc5B, and Unc5C. Silencing neogenin and Unc5B expression in vitro demonstrated that both receptors are essential for N4 function. Moreover, expression of N4, neogenin, and Unc5B was up-regulated in the laser-induced choroidal neovascularization. Finally, we provided evidence, in three models of pathological angiogenesis in adult, that N4 exerts an antiangiogenic activity in vivo.
Results
N4 Is Overexpressed in Angiogenic EC.
The phenotype of cultured EC may determine their response to angiogenic modulators. For instance, we had shown that pigment epithelial-derived factor acts as an antiangiogenic factor on retinal EC cultured in the absence of VEGF and as a proangiogenic factor on EC cultured for at least 3 weeks with VEGF (13). Therefore, we set up a strategy to select clones of various bovine EC derived from retina or aorta that make tubular structures in response to VEGF (angiogenic EC) or that do not (nonangiogenic EC). We used retinal EC to search for angiogenesis-induced genes by a cDNA subtractive hybridization procedure. Nonangiogenic retinal EC [supporting information (SI) Fig. S1] were used as the driver, and angiogenic retinal EC were used as the tester. The relative expression of genes was determined in the subtractive bank by dot blotting. This led to the identification of 250 clones that were then sequenced. The expression of 40 genes was compared in aortic EC and retinal pigment epithelial cells, which are autocrine for VEGF (14). Three genes were up-regulated in angiogenic EC but not in retinal pigment epithelial cells exposed to VEGF (Fig. 1): Thrombospondin-1, which has already been described as an antiangiogenic factor (15), and a cholesterol transporter that is mutated in the Nieman–Pick type 2 (NPC-2) disease. The third up-regulated gene, N4, was 12-fold more strongly expressed in angiogenic arterial EC than in their nonangiogenic counterparts but equally expressed in retinal pigment epithelium (RPE) (Fig. 1).
Fig. 1.
Overexpression of N4 in angiogenic EC. Shown are Northern blots of RNA extracted from bovine aortic EC (Fig. S1) or from retinal pigment epithelium (RPE) that have been exposed to VEGF (V) or not (0). Blots were probed for thrombospondin-1 (TSP-1), NPC-2, and N4. Band intensity ratios were normalized to 1 for non-VEGF-exposed cells.
N4 Inhibits VEGF-Mediated EC Migration and Vascular Tube Formation.
The overexpression of a gene in angiogenesis does not predetermine its role. We first investigated whether the phenotype of cultured EC affected their responses to N4, as we previously observed for PEDF (13). We established EC cultures of veins and arteries of the same human umbilical cords. One important aspect of EC functionality is their ability to form new blood vessels. Matrigel tube formation assay was used to analyze human umbilical artery EC (HUAEC) angiogenic activity. A RNA interference strategy showed that N4 expression was effectively and specifically silenced at the RNA (Fig. 2A) and protein (0.21 ng/48 h/106 HUAEC after siRNA treatment compared with 1.4 ng/48 h/106 cells in control; data not shown) levels. N4 silencing increased by 180% the ability of HUAEC to organize into tubular structures on Matrigel (Fig. 2A). Accordingly, addition of N1 or N4 inhibited HUAEC tube formation (Fig. S2). When HUAEC were incubated in the absence of VEGF, a weak migration occurred, which was not affected by N4 (Fig. 2B). In contrast, N1 and N4 inhibited VEGF-driven HUAEC migration, and this inhibition was dose-dependent with an IC50 at 100 ng/ml (Fig. 2B). Focal adhesion kinase (FAK) may mediate axon attraction downstream from DCC activation (16). The finding that N1 and N4 inhibit EC migration and branching prompted us to examine FAK phosphorylation. N1 and N4 inhibited VEGF-induced phosphorylation of FAK by >60% (Fig. 2C).
Fig. 2.
N4 inhibits EC tube formation and migration induced by VEGF. (A) HUAEC were transfected with control siRNA or N4 siRNA before being incubated on top of Matrigel. Silencing the N4 gene in HUAEC increased both the branching and their ability to form tubular structures on Matrigel compared with nontransfected cells (NT) or with cells transfected with the control siRNA. RT-PCR analysis indicates that the N4 gene has been selectively silenced in N4 siRNA-treated HUAEC. (B) N4 does not affect the basal migration of growth-arrested HUAEC (C) but inhibits the migration induced by VEGF (V) as well as N1 (N1). (C) N1 and N4 inhibit the VEGF-induced FAK phosphorylation in HUAEC (Upper). (Lower) The total FAK immunoprecipitated. The ratio of phosphorylated FAK to total FAK is normalized to unstimulated HUAEC. **, P < 0.01; *, P < 0.05.
N4 Activity Is Mediated by Binding to Neogenin and Recruitment of Unc5B.
To ascertain whether or not netrins use cognate neuronal receptors to signal in EC, we used quantitative real-time RT-PCR analysis to detect them in paired HUAEC and HUVEC cultured from the same donors (n = 4) (Fig. 3A). The neogenin mRNA levels in HUAEC and HUVEC were equivalent and <0.1% of that in fetal brain. Unc5C mRNA was 10 times more abundant in HUVEC than in HUAEC. We also found that the Unc5B transcript was 40-fold more abundant in HUAEC than in HUVEC (Fig. 3A), which is consistent with the lack of effect of N4 on HUVEC. In a first attempt to demonstrate whether N1 and N4 could bind to the same receptors, we performed immunoprecipitation experiments. N1 or N4 were mixed with extracellular domains (ECD) of Netrin-1 receptors (or VEGFR-2 receptor as a negative control) fused to an Ig Fc fragment. N4 bound only to neogenin in contrast to N1, which bound to neogenin, Unc5B, and Unc5C (Fig. 3B). Accordingly ECD-Fc of neogenin inhibited the effect of N4 whereas ECD-Fc of neogenin, Unc5B, and Unc5C quenched the activity of N1 (Fig. 3C).
Fig. 3.
N4 is a neogenin ligand. (A) HUAEC and HUVEC express Unc5B (UB), Unc5C (UC), and neogenin (NE). The results are expressed relative to their abundance in human fetal brain and normalized to the β-actin mRNA content. (B) Immunoprecipitation of N1 and N4 by chimera of human Fc IgG fused to neogenin (NE), Unc5B (UB), or Unc5C (UC). VEGFR2 (VR2) is used as a negative control. (C) VEGF-driven HUAEC migration is inhibited by N4 and N1. The activity of N4 is almost totally abolished by addition of the chimera NE but not by chimera UB or UC. In contrast, N1 activity is inhibited by the three chimeras.
Interestingly, neogenin was localized in filipodia of HUAEC (Fig. 4A). Preincubation of N4 with HUAEC led to the disappearance of the filipodia staining and relocalization in the cytoplasm, as would be expected for a ligand–receptor complex internalization. However, these findings did not exclude the possibility that other netrin receptors may be involved in N4 activity. To investigate the contribution of other netrin receptors, we used a RNA interference strategy. The decrease of >80% of Unc5B or Unc5C had no significant effect on the expression of the other receptor (Fig. S3). Invalidation of either neogenin or Unc5B abolished almost totally the functions of N1 and N4 (Fig. 4B), thus suggesting that Unc5B, although not required for N4 binding, was mandatory for its signaling. We confirmed the functional relevance of neogenin by demonstrating that, among neutralizing antibodies for each receptor (validated in Fig. S4), only anti-neogenin antibody inhibited N4 chemotactic activity (Fig. 4C). It has been reported that N1 can promote a direct association between the conserved sequence 1149–1166 of DCC (or 1145–1162 of neogenin) and the DB domain of Unc5A, Unc5B, or Unc5C in transfected COS cells with the two receptors (17). We used a coimmunoprecipitation strategy to examine this possibility. Growth-arrested HUAEC monolayers were incubated or not overnight with FGF-2 or VEGF and further challenged with 400 ng/ml N4 for 20 min. After immunoprecipitation of HUAEC lysates with anti-Unc5B antibody, Western blot probed with an anti-neogenin antibody showed a band migrating at a molecular weight similar to that of lysates of CHO cells transfected with a neogenin-expressing plasmid. Although N4 addition did not modify the amount of neogenin coimmunoprecipitated with Unc5B in quiescent EC (Fig. 4D), the activation by either VEGF or FGF-2 led to an increase of this association (5.2- and 3.7-fold, respectively).
Fig. 4.
Neogenin and Unc5B are required for N1- and N4-induced inhibition of HUAEC migration. (A) Anti-neogenin (red) antibody staining shows that NE is localized in filipodia of HUAEC. Upon incubation with N4, NE staining is relocalized in the perinuclear area. Nuclei are labeled in blue with DAPI. (Scale bars: 10 μm.) (B) Silencing either Unc5B or NE is sufficient to abrogate the chemotactic effect of N1 or N4. (C) The inhibition of N4 binding to NE abolishes its function whereas the inhibition of its binding to Unc5B or Unc5C has no effect. (D) Neogenin is equally detected in N4-unstimulated (0) and Netrin-4-stimulated HUAEC (0N4) or in VEGF-stimulated (V) or FGF-2-stimulated (F) HUAEC, whereas N4 increases the interaction between UNC5B and neogenin in VEGF-stimulated (VN4) or FGF-2-stimulated (FN4) HUAEC. Detection of neogenin in CHO-transfected cells served as a control (far right lane, NEO).
N4 Has an Antiangiogenic Activity in Vivo.
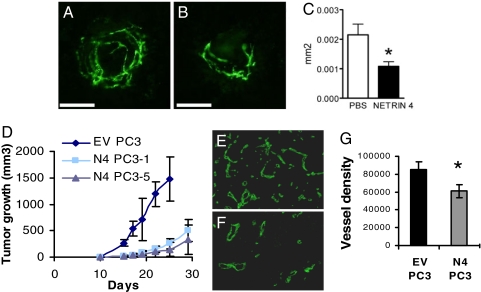
N4 angiogenic activity was evaluated by using the mouse model of laser-induced choroidal neovascularization (18). In the control eye, N4 was slightly expressed (Fig. S5) and neogenin was expressed in the inner retina as described in ref. 19, whereas Unc5B was not detected (data not shown). Quantitative PCR and immunohistochemistry demonstrated an up-regulation of N4, neogenin, and Unc 5B in choroidal neovessels (Fig. S5). We found that intravitreous injections of N4 (1 μg), after the onset of angiogenesis, reduced choroidal neovascularization in mice, as visualized by dextran perfusion (Fig. 5 A–C; n = 10).
Fig. 5.
N4 has an antiangiogenic activity in vivo. (A–C) Antiangiogenic effect of Netrin-4 on choroidal neovascularization. (A and B) Laser-induced choroidal neovascularization is visualized in vehicle-treated PBS (n = 10) (A) or N4-treated (n = 10) (B) mice 7 and 10 days after the onset of angiogenesis. At day 14 the neovascularization was visualized by systemic injection of fluorescent dextran on retinas. (Scale bars: 50 μm.) (C) Quantification of the size of the CNV complex area, scored by morphometric analysis, shows that the choroidal neovascularization is significantly reduced in N4-treated eyes. (D–G) Netrin-4 inhibits PC3 angiogenesis in xenograft tumors. (D) Time course of tumor growth of N4-transfected PC3 (N4 PC3 clones 1 and 5) and empty vector-transfected PC3 cells (EV PC3). N4 PC3 Cl 1 and Cl 5 cells give rise to smaller tumors (n = 8 per group) in nude mice than EV PC3. (E–G) CD31 staining was performed on four distinct tumors of each group [EV PC3 (E) and N4 PC3 (F)]. (G) Five fields were examined by immunofluorescence microscopy, the data were analyzed by Image J software, and results are expressed as mean pixels by square micrometers. Mean vessel density (20 fields per condition) is represented (P < 0.05).
Because dermal-derived angiogenesis elicited by s.c. Matrigel implant is an usual standard for assessing the activity of a given compound in controlled angiogenesis, we also analyzed N4 angiogenic activity in Matrigel implant in vivo. N4 reduced VEGF- and FGF-2-driven angiogenesis. Note that N4 had no proangiogenic effect in the absence of VEGF and FGF-2 (Fig. S6).
To decipher the potential inhibitory activity of N4 on tumor angiogenesis, we transfected the PC3 prostatic cancer cell line with an expression vector encoding N4. We established stable clones by screening the ability of their conditioned medium to inhibit HUAEC proliferation and measurement of N4 by ELISA (Fig. S7). We selected clones that had a doubling time similar to that of empty vector transfected cells (32 ± 5 h), in accordance with the finding that N4 had no effect on PC3 cell proliferation (Fig. S8) or migration (data not shown). Because their secretion of VEGF (4,700 ± 400 pg/106 cells/48 h) was similar to those of parental cells, analyzing tumor growth was informative. These clones were grafted into nude mice (n = 8 per group), and tumor volume was recorded. The growth curve slope was reduced by N4 constitutive expression (Fig. 5D). Immunohistochemistry analysis of tumor sections showed that vessel density (as indicated by CD31 staining) was reduced by N4 overexpression (Fig. 5 E–G).
Discussion
In this study, we identified N4 as a gene up-regulated in angiogenic EC. In vitro data showed that N4 activity in EC is mediated via binding to neogenin and recruitment of Unc5B. N4 and both receptors are up-regulated in choroidal neovessels after laser injury. Furthermore, N4 has an antiangiogenic activity in three mouse models.
N4 was identified by using a subtractive hybridization strategy as a gene up-regulated in VEGF-stimulated EC. These data suggest that VEGF may induce expression of antiangiogenic factors such as thrombospondin, NPC-2 (which exerts an antiangiogenic effect not discussed here), or N4. Therefore, N4 could be a negative feedback regulator of pathological angiogenesis. Thus, altering the balance between proangiogenic and antiangiogenic modulators by increasing the level of endogenous antiangiogenic factor is a plausible approach to fighting tumor angiogenesis. It is not that surprising that angiogenesis stimulators can trigger a chain of events within EC leading to growth inhibition. Negative feedback regulation is one of the most important physiological mechanisms and is the basis of the control of a wide range of phenomena. TSP-1 is a major endogenous negative regulator of angiogenesis (also detected by our screening procedure) that is up-regulated in EC exposed to positive regulators of angiogenesis, and particularly CD95 (15). Vasohibin is another negative feedback regulator of angiogenesis that is up-regulated in HUVEC by exposure to VEGF or FGF-2 (20). Although vasohibin expression is increased in the mouse model of the retinopathy of the premature, its invalidation stimulates retinal angiogenesis, and conversely intravitreal injection of vasohibin decreases retinal angiogenesis (21), suggesting that the balance theory (22) must be revisited in the light of a dynamic equilibrium between increased proangiogenic and antiangiogenic factors. The restricted expression of such negative regulators in EC is not totally unexpected: clinical trials with anti-VEGF therapy have demonstrated that neovessels undergo apoptosis whereas most normal vessels do not. However, some VEGF antagonists can cause regression of normal fenestrated capillaries in some organs, including the thyroid and the trachea (23). It would be of interest to determine whether these EC express these negative regulators in these fenestrated endothelia in adult. On the other hand, the recent demonstration that the increased level of N4 expression in breast cancer might serve as a good independent prognosis outcome (24) favors a role of N4 in human pathology.
Using Matrigel tube formation assay, we showed that N4 is a physiological inhibitor of in vitro angiogenesis ability of human arterial EC to form tubular structures on Matrigel. Thus, silencing N4 by siRNA increased by 180% HUAEC ability to form vascular tube formation on Matrigel (Fig. 2A), and exogenous addition of N4 decreased it (Fig. S2). Using quantitative real-time RT-PCR (Fig. 3A), we found that human arterial EC express three of the six known N1 receptors, namely neogenin, Unc5B, and Unc5C, and that Unc5B was 20 times more expressed in HUAEC than in HUVEC. The low expression of Unc5B in HUVEC (as already reported by Lu in ref. 9) might explain why HUVEC did not respond to N4. In contrast, Wilson et al. (8) found a proangiogenic effect of both N1 and N4 in EC in vitro but did not detect significant expression of netrin receptors in HUAEC or HUVEC, based on a detection threshold of <10% of the expression in fetal brain. Our experimental data demonstrate that such a threshold of detection may miss the detection of neogenin or Unc5C but not that of Unc5B, which was twice as abundant in HUAEC as in fetal brain. However, according to our data, Park et al. (10) detected a faint expression of neogenin and Unc5B in EC using RT-PCR.
Although N1 binds as predicted to neogenin, Unc5B, and Unc5C receptors (Fig. 3A), we found that N4 binds only to neogenin. Ligand–receptor invalidation experiments, using either silencing the receptor gene by siRNA or blocking its binding ability by antibodies, showed that both neogenin and Unc5B were required for N4 activity in HUAEC. Because N4 does not bind to Unc5B, we tested whether it may form a trimeric complex with N4–neogenin. We detected an interaction between neogenin and Unc5B, which was enhanced when HUAEC were treated with VEGF or FGF-2 (Fig. 4D). Interestingly, all of the N4 effects were always observed on VEGF- or FGF-2-stimulated EC but never on quiescent EC, suggesting that N4 is active only on angiogenic EC.
Our findings that neogenin is localized to filipodia in HUAEC and that N4 inhibits VEGF-induced FAK phosphorylation suggest that N4 may function as an EC-repulsive factor. Although neogenin is equally expressed in HUAEC (netrin-responsive) and HUVEC (not netrin-responsive) it may not represent the critical checkpoint for inhibition of in vitro angiogenesis. Recent evidence demonstrated that neogenin is overexpressed in brain EC 14 days after ischemia, whereas DCC is overexpressed in neurons and astrocytes (25), suggesting that neogenin may be involved in pathological angiogenesis. The association of N4–neogenin complex to Unc5B is highly consistent with the fact that neuron repulsion requires the association of DCC or neogenin to an Unc5 receptor (6) to convert an attraction toward a netrin gradient into a repulsive activity.
Netrins have been involved in the navigation of growth cones in the nervous system as well as for tips cells in the vascular system (4). However, the role of netrins in angiogenesis is still unclear. Here we provide evidence that N4 has an antiangiogenic activity in three different in vivo models: the Matrigel plug assay (Fig. S6), the choroidal neovascularization mouse model (Fig. 5 A–C), and the tumor angiogenesis model (Fig. 5 D–G). We also observed an up-regulation of N4, neogenin, and Unc5B during angiogenesis. These data are consistent with those of Larrivée et al. (12), who demonstrated that N1 is an antiangiogenic factor via the activation of the Unc5B receptor, which is restricted to active angiogenesis. However, Park et al. (10) described Netrin-1 as proangiogenic in the corneal angiogenesis assay. This discrepancy might be explained by the fact that netrins have a strong affinity for heparan sulfates and thus may displace endogenous FGF-2 from its storage sites in the corneal stroma. We have previously noticed such a phenomenon for small peptides designed on the sequence of the exon 6 of VEGF 189 (26), which could induce a strong corneal angiogenesis despite binding to VEGF receptors in limbal endothelial cells. Recent evidence demonstrates that corneal vascularization relies on soluble VEGFR-1 expression (27), and it would be interesting to determine whether N1 or N4 could down-regulate VEGFR1 in the cornea. Wilson et al. (8) recently reported that the local production of N1 or N4 stimulated postischemia hindlimb angiogenesis in diabetic mice and that this proangiogenic effect was similar to that displayed by a plasmid encoding VEGF. Furthermore, as recently reported for N1 (12), we observed that constitutive expression of N4 in prostate cancer cells delays tumor growth when grafted s.c. in nude mice (Fig. 5 D–G). Analysis of tumors showed that N4 overexpression reduces vessel density, confirming that N4 is an inhibitor of tumor angiogenesis. However, we cannot exclude that N4 secreted by cancer cells would contribute to decrease angiogenesis by acting on other cells such as pericytes, which are another target for N1 (10) and N4 (our unpublished data).
The intense inhibition of pathological angiogenesis in the adult by N4 suggests that it could be a promising alternative avenue for therapeutic intervention to anti-VEGF therapies (28).
Methods
EC Cultures and Assays.
HUVEC and HUAEC from the same donors were cultured in EBM (Clonetics) supplemented with 10% FCS and 2 ng/ml VEGF every other day. Migration assay was performed as previously described (13, 26) using recombinant VEGF, N1, and N4 (R & D Systems) as modulators. A total of 30,000 HUAEC were applied on the top of the Matrigel (BD Biosciences) on a 24-well plate and incubated at 37°C; 1 h later the modulators were added. Tube formation was assessed the following day. Photographs of 10 representative fields were taken and quantified by using Histolab software (Microvision). Neogenin was detected in HUAEC treated for 2 h or not with N4 after fixation in 4% PFA followed by 0.1% Triton X-100. The antibody against human neogenin (R & D Systems) was detected with 2 μg/ml Alexa Fluor donkey anti-goat secondary antibody (Molecular Probes). Cells were counterstained with DAPI, rinsed, and then observed by using a fluorescence microscope with appropriate filters.
Netrin Receptor Dosage by Real-Time RT-PCR and Silencing by siRNA.
A total of 0.5 μg of total cellular RNA was transcribed by using the Transcriptor first-strand cDNA synthesis kit and a random hexamer primer (Roche Diagnostics). The cDNA product was amplified by using the LightCycler FastStart DNA master SYBR green I mix and a LightCycler 1.5 apparatus. The primers used for PCR are given in Table S1. Standard curves for each mRNA were generated by serial dilution of cDNA synthesized from total RNA isolated from human fetal brain. The mRNA levels were normalized to β-actin mRNA. Subconfluent HUAEC were treated or not with a mixture of 3 μl/ml TKO (Dharmacon) and 50 nM siRNA diluted in EBM supplemented with 2% SVF. After 2 days RNA was extracted with RNeasy kit (Qiagen), and netrin receptors were assayed as above.
Receptor and Coimmunoprecipitation Assays.
A total of 1 μg of each extracellular domain of netrin receptor fused to human IgG Fc (or VEGFR-2/Fc IgG as a negative control) was incubated for 2 h with 400 ng of N1 or N4 in a final volume of 200 μl of PBS containing 0.05% Tween 20. The immune complexes were recovered on G protein Sepharose, and the proteins were separated by gel electrophoresis, transferred to a nitrocellulose membrane, and revealed with anti-polyhistidine antibody. Coimmunoprecipitation studies were performed on subconfluent HUAEC (107 cells) incubated or not overnight with 2 ng/ml VEGF or FGF-2 and preincubated for 20 min with 1 mM orthovanadate to inhibit tyrosine phosphatase before addition of N4 (400 ng/ml) for 40 more minutes. HUAEC were lysed in RIPA buffer supplemented with anti-proteases and orthovanadate and then mixed with 1 μg of anti-UNC5B antibody. Proteins were separated in reducing conditions on 3–8% acrylamide SDS/PAGE and immune complexes revealed by anti-neogenin antibody (R & D Systems). Equal loading was ascertained by reprobing the membranes with anti-Unc5B (R & D Systems). To study FAK phosphorylation, subconfluent HUAEC were stimulated or not with 50 ng/ml VEGF and 400 ng/ml N1 or N4 for 30 min. HUAEC lysates were mixed with 1 μg of anti-FAK antibody and after immunoprecipitation analyzed by Western blot. Equal loading was ascertained by reprobing the membranes with anti-FAK.
Solid Phase Receptor Assay.
The solid phase receptor assay was used to measure the specificity of anti-netrin receptor antibodies. Anti-human Fc antibodies (Jackson ImmunoResearch) were immobilized on 96-well plates overnight at 4°C in 50 mM carbonate buffer (pH 9.6). The plates were saturated with 0.5% BSA diluted in the same buffer and washed three times with PBS/0.05% Tween 20/0.5% BSA. Human chimeric recombinant receptor (R & D Systems), at a concentration of 100 ng/ml, was incubated in the same buffer for 4 h. Antibodies were coincubated with 5 ng of netrin-1 overnight at 4°C and washed three times with PBS/0.05% Tween 20/0.5% BSA, and bound antibodies were revealed by an anti-polyhistidine monoclonal antibody linked to peroxidase.
Matrigel Assay.
Matrigel was mixed with 300 ng/ml FGF-2 and VEGF in the presence or absence of 6 μg/ml N4 (R & D Systems). Aliquots of 0.3 ml of the mixture were injected s.c. into the flanks of C57BL/6 mice. After 7 days, the mice were killed, the Matrigel pellets were excised and frozen, and the water-soluble material was extracted by homogenization in 1 ml of 20 mM Hepes (pH 7.4) at 4°C. After centrifugation, the hemoglobin content of the supernatant was measured (OD at 595 nm).
Choroidal Neovascularization.
Photocoagulation lesions were performed in 6-week-old C57BL/6 mice around the optic nerve with an argon laser photocoagulator (Quantel) set at 532 nm, mounted on a slit lamp and with a cover glass fulfilling the role of contact lens (150 mW, 100 ms, and 100 μm). N4, neogenin, Unc5B, and lectin staining were assessed in 5-μm sections of mouse eyes. One microliter of PBS containing or not 1 μg of N4 (R & D Systems) was injected in the vitreous on days 7 and 10. On day 14, animals were perfused with 1 ml of PBS containing 50 mg/ml FITC-dextran (250,000 Da) and killed. The eyes were fixed in PBS containing 4% paraformaldehyde, and retinas and choroids were dissected into quarters, flat-mounted, and examined under a fluorescence microscope. The incidence and size of the CNV complex were scored by morphometric analysis of the images with NIH Image J Software (version 1.36).
Transfection of PC3 Prostate Carcinoma Cells and Xenograft in Nude Mice.
Female athymic nude mice were randomized (eight per group). Animals were injected s.c. with 2 × 106 either N4 or pcDNA3 (empty vector)-transfected PC3 cells. Tumor volume, recorded by a caliper twice a week, was calculated with this formula: length (mm) × width (mm)2 × π/6. For immunohistochemistry, rat anti-CD31 (BD Biosciences) was used as a primary antibody (EC marker) and revealed with fluorescent-labeled secondary antibody. Sections were counterstained with DAPI. CD31 staining was quantified at ×20 magnification using the Image J software program. Statistical analysis was performed with the Mann–Whitney U test. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments.
We thank Yves Millet for help in the Animal Care Facility and the Department of Obstetrics and Gynecology of Lariboisière Hospital for providing umbilical cords. We acknowledge financial support from the Centre National de la Recherche Scientifique during the early stage of this work and from the Institut National de la Santé et de la Recherche Médicale. J.P. was supported by grants from Retina France, GIS Longévité, the Association de la Recherche pour le Cancer (CL3124), la Ligue Contre le Cancer (Comité de Paris), l'Agence Nationale pour la Recherche (COD-022), and the Institut National du Cancer. S.R. was supported by a fellowship from the Fondation de la Recherche Médicale, E.L. was supported by the Association de la Recherche pour le Cancer, and T.X.J. was supported by the Fondation Del Duca.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0804008105/DCSupplemental.
References
- 1.Campochiaro PA. Ocular versus extraocular neovascularization: Mirror images or vague resemblances. Invest Ophthalmol Vis Sci. 2006;47:462–474. doi: 10.1167/iovs.05-1494. [DOI] [PubMed] [Google Scholar]
- 2.Eichmann A, Makinen T, Alitalo K. Neural guidance molecules regulate vascular remodeling and vessel navigation. Genes Dev. 2005;19:1013–1021. doi: 10.1101/gad.1305405. [DOI] [PubMed] [Google Scholar]
- 3.Eichmann A, Le Noble F, Autiero M, Carmeliet P. Guidance of vascular and neural network formation. Curr Opin Neurobiol. 2005;15:108–115. doi: 10.1016/j.conb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Klagsbrun M, Eichmann A. A role for axon guidance receptors and ligands in blood vessel development and tumor angiogenesis. Cytokine Growth Factor Rev. 2005;16:535–548. doi: 10.1016/j.cytogfr.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 5.Cirulli V, Yebra M. Netrins: Beyond the brain. Nat Rev Mol Cell Biol. 2007;8:296–306. doi: 10.1038/nrm2142. [DOI] [PubMed] [Google Scholar]
- 6.Hong K, et al. A ligand-gated association between cytoplasmic domains of UNC5 and DCC family receptors converts netrin-induced growth cone attraction to repulsion. Cell. 1999;97:927–941. doi: 10.1016/s0092-8674(00)80804-1. [DOI] [PubMed] [Google Scholar]
- 7.Guijarro P, et al. Netrin1 exerts a chemorepulsive effect on migrating cerebellar interneurons in a Dcc-independent way. Mol Cell Neurosci. 2006;33:389–400. doi: 10.1016/j.mcn.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Wilson BD, et al. Netrins promote developmental and therapeutic angiogenesis. Science. 2006;313:640–644. doi: 10.1126/science.1124704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu X, et al. The netrin receptor UNC5B mediates guidance events controlling morphogenesis of the vascular system. Nature. 2004;432:179–186. doi: 10.1038/nature03080. [DOI] [PubMed] [Google Scholar]
- 10.Park KW, et al. The axonal attractant Netrin-1 is an angiogenic factor. Proc Natl Acad Sci USA. 2004;101:16210–16215. doi: 10.1073/pnas.0405984101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci USA. 2006;103:6530–6535. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larrivee B, et al. Activation of the UNC5B receptor by Netrin-1 inhibits sprouting angiogenesis. Genes Dev. 2007;21:2433–2447. doi: 10.1101/gad.437807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchings H, Maitre-Boube M, Tombran-Tink J, Plouet J. Pigment epithelium-derived factor exerts opposite effects on endothelial cells of different phenotypes. Biochem Biophys Res Commun. 2002;294:764–769. doi: 10.1016/S0006-291X(02)00548-X. [DOI] [PubMed] [Google Scholar]
- 14.Guerrin M, et al. Vasculotropin/vascular endothelial growth factor is an autocrine growth factor for human retinal pigment epithelial cells cultured in vitro. J Cell Physiol. 1995;164:385–394. doi: 10.1002/jcp.1041640219. [DOI] [PubMed] [Google Scholar]
- 15.Volpert OV, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nat Med. 2002;8:349–357. doi: 10.1038/nm0402-349. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, et al. Netrin requires focal adhesion kinase and Src family kinases for axon outgrowth and attraction. Nat Neurosci. 2004;7:1222–1232. doi: 10.1038/nn1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koch M, et al. A novel member of the netrin family, beta-netrin, shares homology with the beta chain of laminin: Identification, expression, and functional characterization. J Cell Biol. 2000;151:221–234. doi: 10.1083/jcb.151.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinores SA, et al. Implication of the hypoxia response element of the Vegf promoter in mouse models of retinal and choroidal neovascularization, but not retinal vascular development. J Cell Physiol. 2006;206:749–758. doi: 10.1002/jcp.20525. [DOI] [PubMed] [Google Scholar]
- 19.Rajagopalan S, et al. Neogenin mediates the action of repulsive guidance molecule. Nat Cell Biol. 2004;6:756–762. doi: 10.1038/ncb1156. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe K, et al. Vasohibin as an endothelium-derived negative feedback regulator of angiogenesis. J Clin Invest. 2004;114:898–907. doi: 10.1172/JCI21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen J, et al. Vasohibin is up-regulated by VEGF in the retina and suppresses VEGF receptor 2 and retinal neovascularization. FASEB J. 2006;20:723–725. doi: 10.1096/fj.05-5046fje. [DOI] [PubMed] [Google Scholar]
- 22.Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 23.Baffert F, et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am J Physiol. 2006;290:H547–H559. doi: 10.1152/ajpheart.00616.2005. [DOI] [PubMed] [Google Scholar]
- 24.Esseghir S, et al. Identification of NTN4, TRA1, and STC2 as prognostic markers in breast cancer in a screen for signal sequence encoding proteins. Clin Cancer Res. 2007;13:3164–3173. doi: 10.1158/1078-0432.CCR-07-0224. [DOI] [PubMed] [Google Scholar]
- 25.Tsuchiya A, et al. Expression of netrin-1 and its receptors DCC and neogenin in rat brain after ischemia. Brain Res. 2007;1159:1–7. doi: 10.1016/j.brainres.2006.12.096. [DOI] [PubMed] [Google Scholar]
- 26.Jonca F, Ortega N, Gleizes PE, Bertrand N, Plouet J. Cell release of bioactive fibroblast growth factor 2 by exon 6-encoded sequence of vascular endothelial growth factor. J Biol Chem. 1997;272:24203–24209. doi: 10.1074/jbc.272.39.24203. [DOI] [PubMed] [Google Scholar]
- 27.Ambati BK, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.