Abstract
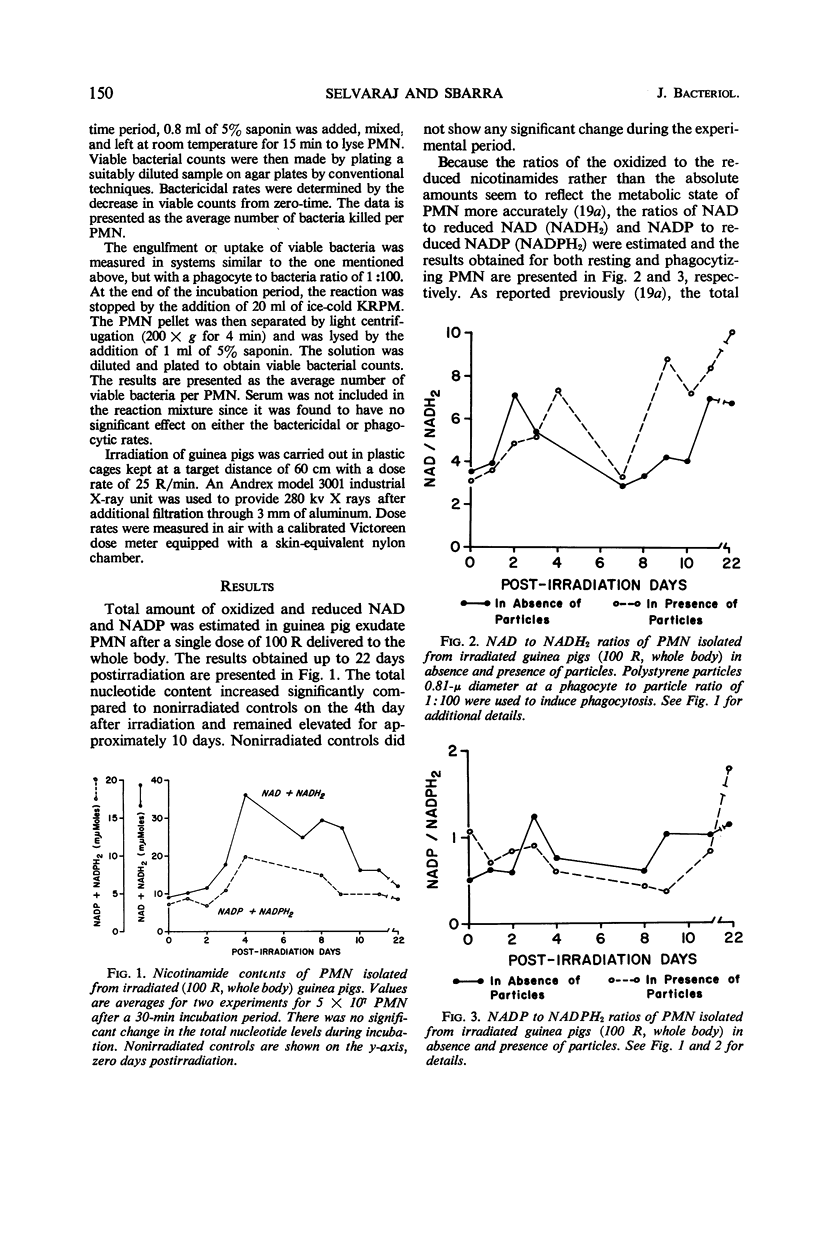
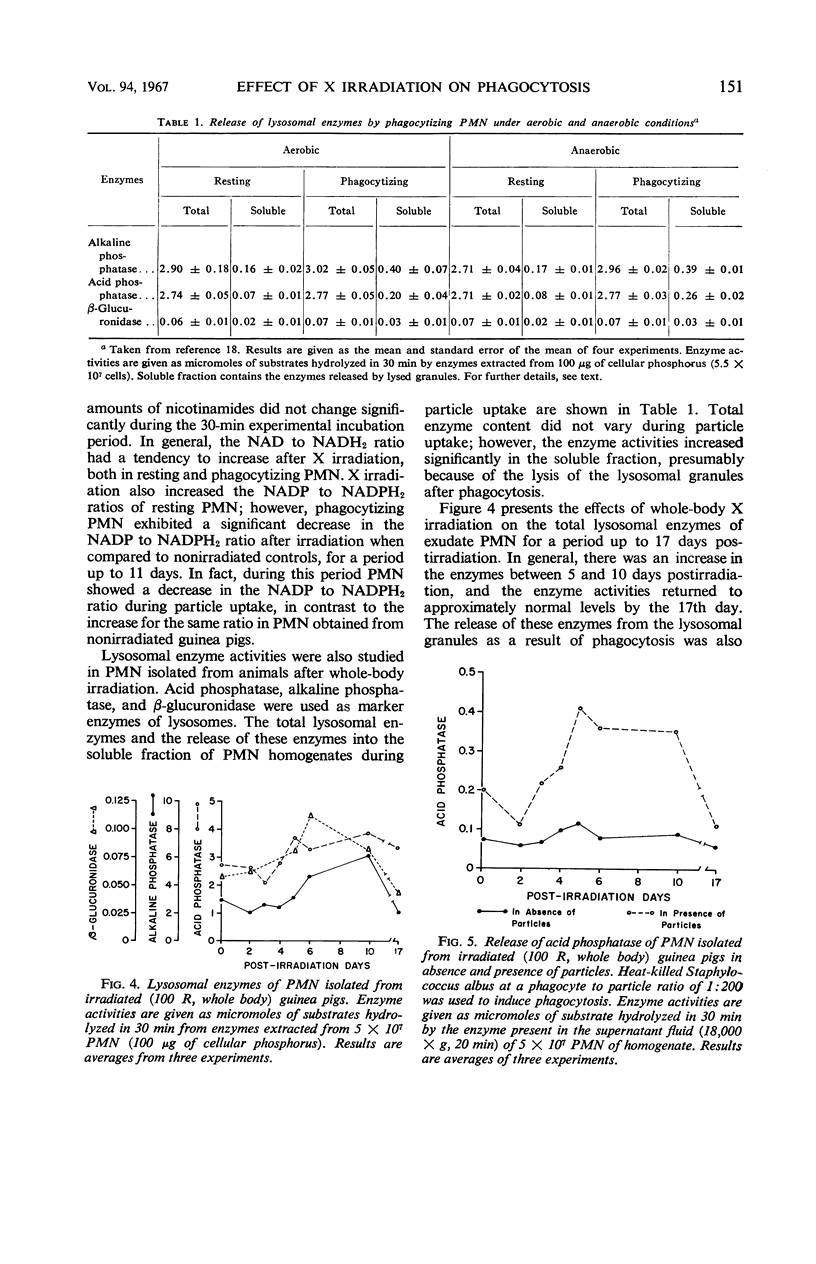
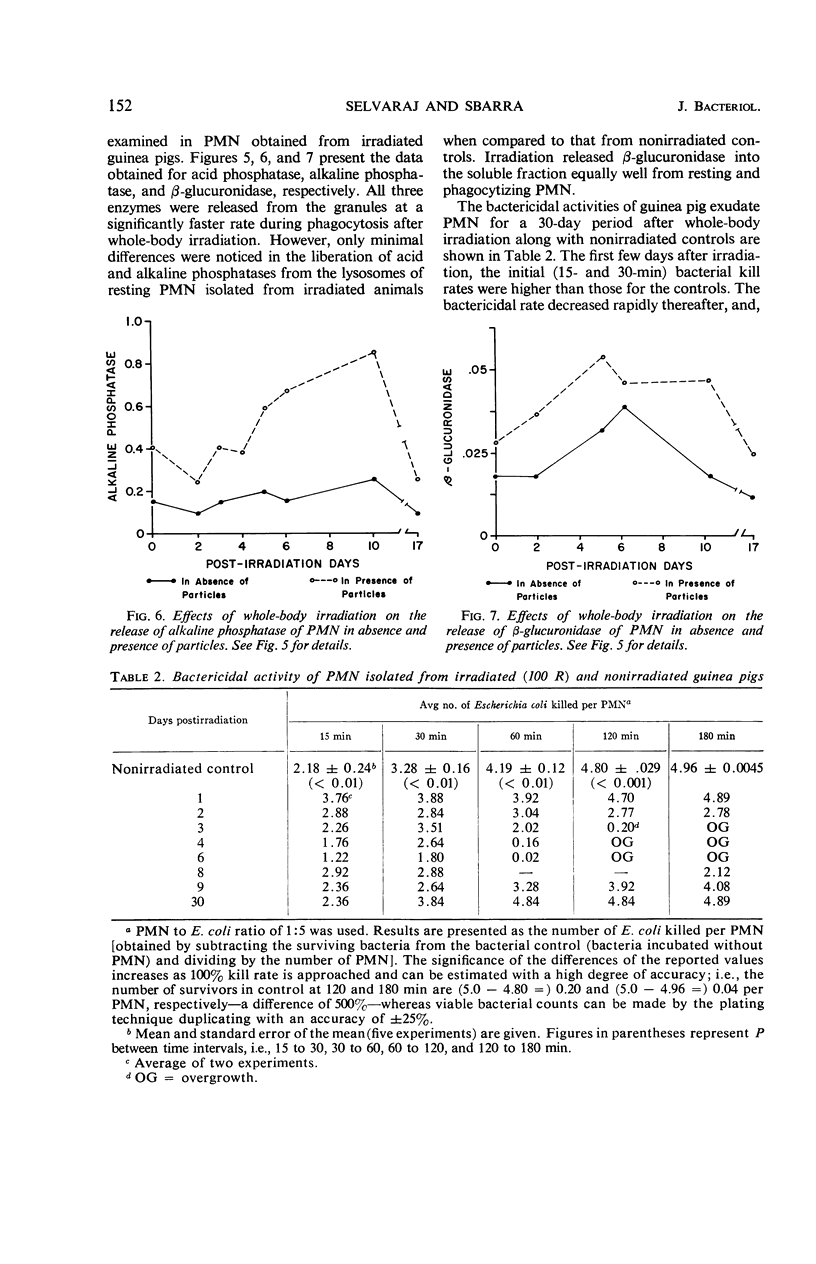
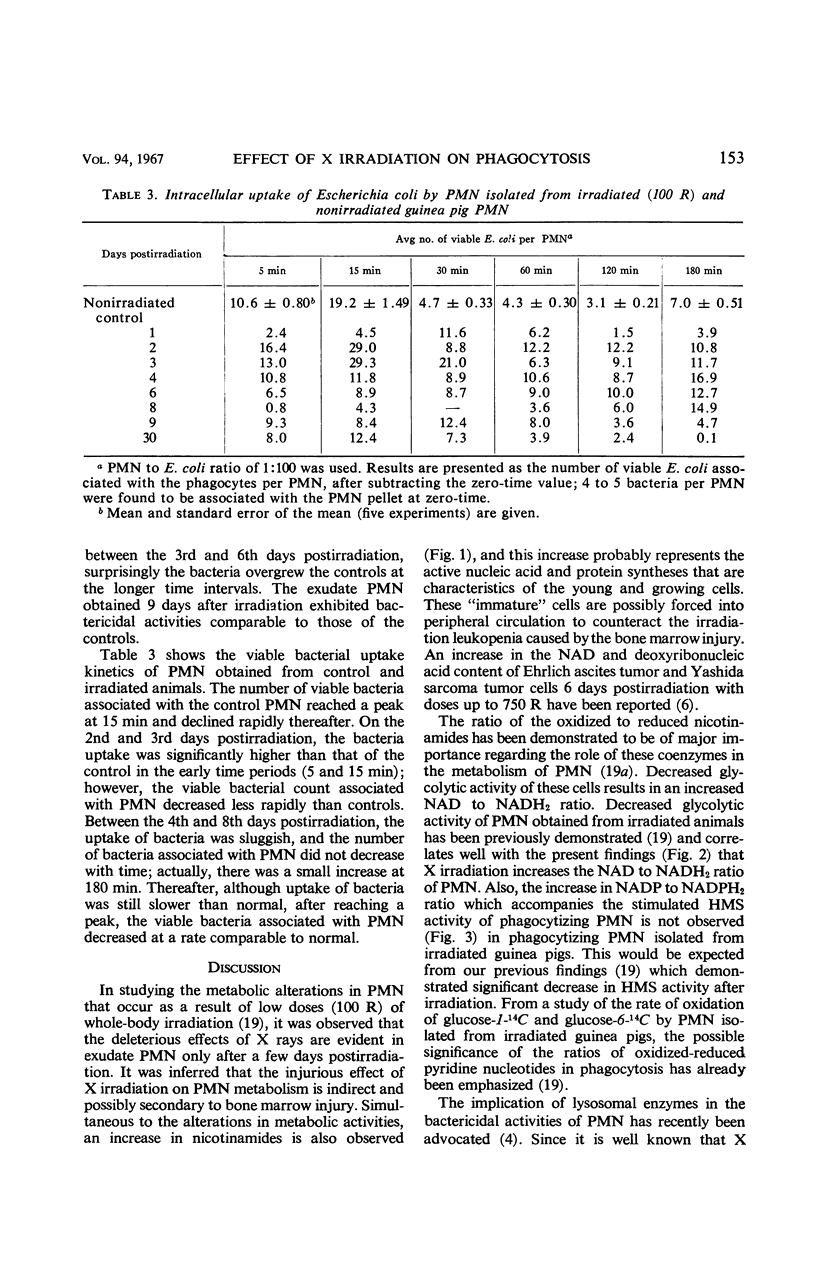
By studying the effects of whole-body X irradiation on phagocytosis, a correlation between the metabolic and bactericidal activities of leukocytes following X irradiation was demonstrated. The total nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) content of polymorphonuclear neutrolphils (PMN) isolated from irradiated guinea pigs increased significantly when compared to nonirradiated controls. The ratio of unreduced to reduced (NAD) generally increased in PMN isolated from irradiated animals. This occurred with both resting and phagocytizing cells. The ratio of unreduced to reduced NADP of resting PMN isolated from irradiated animals had a tendency to increase. However, in phagocytizing cells a significant decrease in the ratio was noted. The total acid and alkaline phosphatase and β-glucuronidase increased up to about 10 days postirradiation. These lysosomal enzymes returned to approximately normal by the 17th day postirradiation. All three lysosomal enzymes (acid and alkaline phosphatases and β-glucuronidase) were released from the granules at a significantly faster rate during phagocytosis after irradiation. The bactericidal activities of PMN isolated from irradiated animals gradually decreased, and in some cases increased growth of the organisms was observed. The uptake or association of bacteria with PMN isolated from irradiated animals varied with the postirradiation time. Generally, a correlation with bactericidal activities could be made. The data indicate that the bactericidal system in phagocytes consists of at least two agents, H2O2 and myeloperoxidase.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AGNER K. Studies on peroxidative detoxification of purified diphtheria toxin. J Exp Med. 1950 Oct 1;92(4):337–347. doi: 10.1084/jem.92.4.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CANELLAKIS E. S., TUTTLE A. L., COHEN P. P. A comparative study of the end-products of uric acid oxidation by peroxidases. J Biol Chem. 1955 Mar;213(1):397–404. [PubMed] [Google Scholar]
- COHN Z. A., HIRSCH J. G. The influence of phagocytosis on the intracellular distribution of granule-associated components of polymorphonuclear leucocytes. J Exp Med. 1960 Dec 1;112:1015–1022. doi: 10.1084/jem.112.6.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DONALDSON D. M., MARCUS S., GYI K. K., PERKINS E. H. The influence of immunization and total body x-irradiation on intracellular digestion by peritoneal phagocytes. J Immunol. 1956 Mar;76(3):192–199. [PubMed] [Google Scholar]
- HILZ H., RUETER J., OLDEKOP M., WUEPPEN I. STIMULATION OF DPN TURNOVER IN ASCITES TUMOR CELLS BY LOW X-RAY DOSES. Life Sci. 1965 Apr;4:765–769. doi: 10.1016/0024-3205(65)90308-5. [DOI] [PubMed] [Google Scholar]
- IYER G. Y., QUESTEL J. H. NADPH and NADH oxidation by guinea pig polymorphonuclear leucocytes. Can J Biochem Physiol. 1963 Feb;41:427–434. [PubMed] [Google Scholar]
- KAGAN E. H., BROWNSON R. H., SUTER D. B. Radiation-caused cytochemical changes in neurons. Arch Pathol. 1962 Sep;74:195–203. [PubMed] [Google Scholar]
- Klebanoff S. J., Clem W. H., Luebke R. G. The peroxidase-thiocyanate-hydrogen peroxide antimicrobial system. Biochim Biophys Acta. 1966 Mar 28;117(1):63–72. doi: 10.1016/0304-4165(66)90152-8. [DOI] [PubMed] [Google Scholar]
- MOLONEY W. C. Leukocyte alkaline phosphatase activity in the rat. Ann N Y Acad Sci. 1958 Oct 13;75(1):31–36. doi: 10.1111/j.1749-6632.1958.tb36848.x. [DOI] [PubMed] [Google Scholar]
- RAHMAN Y. E. Acid phosphatase and beta-glucuronidase activities of thymus and spleen of rats after whole-body x-irradiation. Proc Soc Exp Biol Med. 1962 Feb;109:378–381. doi: 10.3181/00379727-109-27209. [DOI] [PubMed] [Google Scholar]
- RECHCIGL M., Jr, EVANS W. H. ROLE OF CATALASE AND PEROXIDASE IN THE METABOLISM OF LEUCOCYTES. Nature. 1963 Sep 7;199:1001–1002. doi: 10.1038/1991001b0. [DOI] [PubMed] [Google Scholar]
- ROTH J. S., BUKOVSKY J., EICHEL H. J. The effect of whole-body x-irradiation on the activity of some acid hydrolases in homogenates and subcellular fractions of rat spleen. Radiat Res. 1962 Jan;16:27–36. [PubMed] [Google Scholar]
- ROTH J. S., EICHEL H. J. The effect of total-body x-irradiation on the distribution of ribonuclease activity in subcellular fractions of rat spleen. Radiat Res. 1959 Oct;11:572–581. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SCHULTZ J., KAMINKER K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch Biochem Biophys. 1962 Mar;96:465–467. doi: 10.1016/0003-9861(62)90321-1. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Effects of x-irradiation on the metabolic changes accompanying phagocytosis. Nature. 1966 Apr 9;210(5032):158–161. doi: 10.1038/210158a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. The role of the phagocyte in host-parasite interactions. VII. Di- and triphosphopyridine nucleotide kinetics during phagocytosis. Biochim Biophys Acta. 1967 Jul 25;141(2):243–249. doi: 10.1016/0304-4165(67)90097-9. [DOI] [PubMed] [Google Scholar]
- VERCAUTEREN R. E., GILLIS-VAN MAELE A. The oxidoreductases of leucocytes. I. Ultramicroassays for peroxidase, catalase, succinate dehydrogenase and protein in small amounts of leucocyte homogenates. Preparation of particulate hydroperoxidases. Enzymologia. 1962 Jan 2;24:25–36. [PubMed] [Google Scholar]
- WILKINSON M. Changes in the phagocytic activity of polymorphonuclear leukocytes following total body x-irradiation in the rat. Blood. 1954 Aug;9(8):810–816. [PubMed] [Google Scholar]
- Zatti M., Rossi F. Early changes of hexose monophosphate pathway activity and of NADPH oxidation in phagocytizing leucocytes. Biochim Biophys Acta. 1965 Jun 22;99(3):557–561. doi: 10.1016/s0926-6593(65)80213-2. [DOI] [PubMed] [Google Scholar]



