Abstract
Chronic stalling of DNA replication forks caused by DNA damage can lead to genomic instability. Cells have evolved lesion bypass pathways such as postreplication repair (PRR) to resolve these arrested forks. In yeast, one branch of PRR involves proliferating cell nuclear antigen (PCNA) polyubiquitination mediated by the Rad5-Ubc13-Mms2 complex that allows bypass of DNA lesion by a template-switching mechanism. Previously, we identified human SHPRH as a functional homologue of yeast Rad5 and revealed the existence of RAD5-like pathway in human cells. Here we report the identification of HLTF as a second RAD5 homologue in human cells. HLTF, like SHPRH, shares a unique domain architecture with Rad5 and promotes lysine 63-linked polyubiquitination of PCNA. Similar to yeast Rad5, HLTF is able to interact with UBC13 and PCNA, as well as SHPRH; and the reduction of either SHPRH or HLTF expression enhances spontaneous mutagenesis. Moreover, Hltf-deficient mouse embryonic fibroblasts show elevated chromosome breaks and fusions after methyl methane sulfonate treatment. Our results suggest that HLTF and SHPRH are functional homologues of yeast Rad5 that cooperatively mediate PCNA polyubiquitination and maintain genomic stability.
Chronic stalling of DNA replication forks by DNA damage such as UV irradiation, ionizing irradiation, chemicals, and reactive cellular metabolites impedes the progression of the cell cycle and eventually causes cell death. To circumvent such situations, cells have evolved the postreplication repair (PRR) pathway that bypasses DNA lesions to resolve stalled forks without removing the actual damage (1). In budding yeast Saccharomyces cerevisiae, PRR is carried out by 2 distinct pathways: translesion synthesis (TLS) and template switching (TS) (Fig. 1A). TLS uses multiple low-fidelity TLS polymerases to incorporate nucleotides across DNA lesions (2, 3). Switching from replicative polymerases δ or ε to TLS polymerases is promoted through the interaction between monoubiquitinated PCNA at lysine 164 (K164) and a ubiquitin-binding motif in TLS polymerases—a mechanism conserved from the budding yeast to human. The monoubiquitination of PCNA at K164 requires the RING-type ubiquitin ligase Rad18 (E3) and the ubiquitin-conjugating enzyme Rad6 (E2).
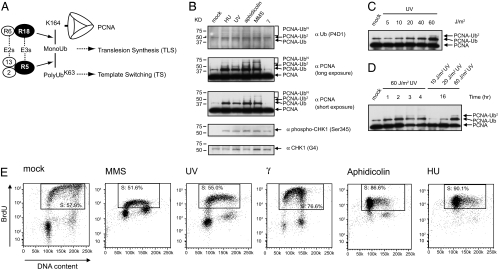
Fig. 1.
PCNA polyubiquitination is enhanced by DNA damage that stalls DNA replication forks. (A) Distinct patterns of PCNA ubiquitination result in activation of either TS or TLS of postreplication repair. R18, RAD18; R5, RAD5; R6, RAD6; 13, UBC13; 2, MMS2. (B) Human HEK293T cells were treated with DNA-damaging agents, including 2 mM hydroxyurea (HU), 60 J/m2 of UV irradiation, 0.01% MMS, 10 μg/ml aphidicolin, or 20 Gy of γ irradiation, and the chromatin-bound form of PCNA was immunoprecipitated with a monoclonal anti-PCNA antibody (PC10). The PCNA ubiquitination was detected by HRP-conjugated anti-PCNA and anti-Ub (P4D1) antibodies. Asterisks indicate nonspecific bands. PCNA polyubiquitination was enhanced in a dose-dependent (C) and time-dependent (D) manner. Ub, Ub2, and UbN indicate mono-, di-, and polyubiquitinated species of PCNA, respectively. (E) The BrdU-incorporated flow cytometric analysis of HEK293T cell population to monitor the progression of DNA replication. HEK293T cells were treated with various DNA-damaging agents, labeled with BrdU for 1 h, and BrdU incorporation was measured on a FACS LSRII. The x and y axes represent DNA content as measured by 7-amino-actinomycin D staining and cells undergoing DNA replication as measured by BrdU incorporation, respectively. S in the rectangles represents the S phase of the cell cycle.
The TS pathway bypasses DNA damage by switching a stalled replicating end to the nascent daughter strand of the sister chromatid (1, 4). This pathway involves a lysine 63 (K63)-linked polyubiquitin chain that is further added onto the monoubiquitinated PCNA by Rad5 (E3) along with the Ubc13-Mms2 (E2 and E2 variant, respectively) heterodimer complex (Fig. 1A). Distinct from the K48-linked polyubiquitination leading to protein degradation, the K63-linked polyubiquitination of PCNA is thought to promote TS in a proteasome-independent manner (5).
The importance of the TLS pathway in the suppression of mammalian tumorigenesis emerged with the identification of a mutation in TLS polymerase η in patients with the variant form of xeroderma pigmentosum and from studies with mouse models (6, 7). Despite the presence of UBC13 and MMS2 homologues in humans, the importance of the TS pathway is less clear in mammals because K63-linked polyubiquitination of PCNA, a hallmark event for the TS pathway, had not been observed until recently (8–10). We recently identified human SHPRH, which possesses SWI2/SNF2 and RING domains with similar architecture to the yeast Rad5 as a functional homologue of yeast Rad5 (9). Specifically, we demonstrated the in vivo activity of SHPRH in promoting a K63-linked polyubiquitination of PCNA as well as physical interactions of SHPRH with PCNA, RAD18, and UBC13. Depletion of SHPRH increases genomic instability after genotoxic stress. Consistent with our work, another study also demonstrated that SHPRH could polyubiquitinate PCNA in vitro (11).
In the present study, we demonstrated that ectopic expression of HLTF/SMARCA3/RUSH/HIP116/Zbu1 (hereafter, HLTF) enhanced PCNA polyubiquitination in vivo. Depletion of SHPRH or HLTF significantly reduced polyubiquitination of chromatin-bound PCNA upon treatment of cells with DNA-damaging agents that cause stalled DNA replication forks. Furthermore, Hltf-deficient mouse embryonic fibroblasts (MEFs) showed elevated chromosome breaks and fusions after MMS treatment. Our results suggest that HLTF and SHPRH are functional homologues of yeast Rad5 that cooperatively mediate PCNA polyubiquitination in response to DNA damage and maintain genomic stability.
Results
PCNA Polyubiquitination Is Induced by DNA Damage Causing Stalling of DNA Replication Fork.
Exposure of human cells to MMS or UV irradiation induces K63-linked polyubiquitination of PCNA at K164 (8–10). However, it was not clear how and what types of DNA damage cause PCNA polyubiquitination. In addition, previous studies did not distinguish whether the non-chromatin-bound or the chromatin-bound fraction of PCNA is polyubiquitinated in response to DNA damage. To gain more insights into nature of PCNA polyubiquitination after DNA damage, we treated HEK293T cells with various DNA-damaging agents and immunoprecipitated the chromatin-bound form of PCNA with monoclonal anti-PCNA antibodies from nuclear extracts (Fig. 1B). Hydroxyurea, MMS, aphidicolin, and UV irradiation induced chromatin-bound PCNA ubiquitinations as suggested by the appearance of novel bands with ≈8 kDa repetitive increases above PCNA that reflects the mono-, di-, and polyubiquitinated PCNAs. These PCNA ubiquitination bands were recognized by either anti-PCNA or anti-Ub monoclonal antibodies. The intensity of the PCNA ubiquitination was increased in a dose- and time-dependent manner by UV irradiation. The di-ubiquitinated PCNA was detected 1 h after UV irradiation at 60 J/m2, and its intensity persisted up to 16 h (Fig. 1 C and D). USP1, a deubiquitinating enzyme, is responsible for removing the monoubiquitin adduct of PCNA (12). Depletion of USP1 by siRNA resulted in enhancement of both mono- and polyubiquitin of PCNA, suggesting that USP1 might remove both mono- and polyubiquitin chains from PCNA [supporting information (SI) Fig. S1]. Intriguingly, γ irradiation did not induce PCNA ubiquitination (Fig. 1B), although clear CHK1 activation was detected (α phospho-CHK1 (Ser-345) panel in Fig. 1B). Therefore, the chromatin-bound form of PCNA is polyubiquitinated in response to several types of DNA-damaging agents, except γ irradiation.
The chromatin-bound form of PCNA associates with the DNA replication forks (13). To examine a correlation between DNA damage-induced PCNA polyubiquitination and DNA replication, we monitored the progression of DNA replication by measuring BrdU incorporation after treatments with different DNA-damaging agents. Treatment of cells with MMS strongly reduced the level of BrdU incorporation (y axis) in the entire S phase (gated by the rectangle), noted by the change of the diagonal pattern in mock treated to the flat pattern in MMS-treated, suggesting that MMS-induced damage rapidly and strongly stalls replication forks (Fig. 1E). UV irradiation had similar but milder effects on BrdU incorporation (Fig. 1E). By contrast, γ irradiation, which mainly induces DNA double-strand breaks (DSBs), did not show apparent effects on ongoing DNA replication (Fig. 1E). Depletion of dNTP pools by hydroxyurea or inhibition of DNA polymerase activities by aphidicolin also resulted in the accumulation of low BrdU population in the early S phase (Fig. 1E). The strong correlation between the levels of PCNA polyubiquitination and the reduction of BrdU incorporation suggests that PCNA ubiquitination is induced by the stalling of DNA replication forks.
Human HLTF Is a Second Functional Homologue of Yeast Rad5.
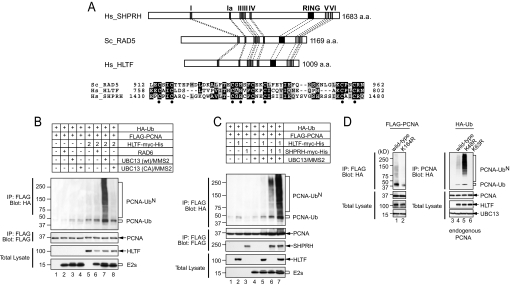
Yeast Rad5, human SHPRH, and HLTF share a unique structural feature whereby the RING domains are embedded between the conserved motifs IV and V of the SWI2/SNF2 domain (Fig. 2A). The SWI2/SNF2 domains of HLTF and SHPRH have 59.8% and 45.5% identities with that of yeast Rad5, respectively. The RING domains of HLTF and SHPRH share 35.5% and 36.4% identities to that of yeast Rad5, respectively, and all 3 proteins show high conservation in the C3HC4 motifs of the RING domain (Fig. 2A and Table S1). Similar to SHPRH, the expression of HLTF did not complement the UV sensitivity of rad5 null yeast strains (Fig. S2). To determine whether HLTF is another functional homologue of yeast Rad5, we expressed HLTF together with UBC13/MMS2 in HEK293T cells. Similar to SHPRH, ectopically expressed HLTF was able to polyubiquitinate PCNA (Fig. 2B). The PCNA polyubiquitination was enhanced only when UBC13/MMS2, but not catalytic inactive UBC13 (C87A)/MMS2, or RAD6, were expressed together with HLTF (Fig. 2B). Moreover, coexpression of SHPRH and HLTF showed strong synergistic enhancement of PCNA polyubiquitination (Fig. 2C), suggesting a cooperative relationship between these E3 activities. Arginine mutations of HA-Ub at lysine 63 (K63R), but not at lysine 48 (K48R), abolished PCNA polyubiquitination by HLTF, suggesting that the polyubiquitin chain is assembled through K63 of ubiquitin (Fig. 2D). The polyubiquitination of PCNA seems to occur at K164 because mutation of this residue of PCNA abrogated ubiquitination (Fig. 2D).
Fig. 2.
Ectopic expression of human HLTF and SHPRH promotes polyubiquitination of PCNA at K164 with K63-linked polyubiquitin chains. (A) Schematic representation of human (Hs) HLTF, SHPRH, and yeast (Sc) Rad5. SWI2/SNF2 (subdomains I, Ia, II, III, IV, V, and VI) and RING domains are indicated. An alignment of the RING finger domains is shown below. Conserved cysteines and histidine (C3HC4) are indicated with dots. (B) HLTF promotes PCNA polyubiquitination. HEK293T cells were transfected with HA-ubiquitin (HA-Ub, 0.5 μg), FLAG-PCNA (0.5 μg), HLTF-myc-His (2.0 μg), RAD6-HA (100 ng), UBC13-HA (50 ng), UBC13(C87A)-HA (50 ng), and MMS2-HA (50 ng) in the indicated combinations. PCNA (anti-FLAG) immunoprecipitates were blotted with an anti-HA antibody. Ub and UbN indicate mono- and polyubiquitinated species of PCNA, respectively. Expression of transfected constructs was confirmed by blotting total lysates with respective antiepitope tag antibodies. (C) HLTF and SHPRH cooperatively promote PCNA polyubiquitination. HLTF-myc-His (1.0 μg), SHPRH-myc-His (1.0 μg), or both were expressed along with HA-Ub, UBC13, and RAD6 as indicated. (D) Specificity of ubiquitin ligase activity in HLTF. Similar to (A), HEK293T cells were transfected with a PCNA-K164R mutant (left panels), or HA-ubiquitin mutants (right panels), and ubiquitinated species of PCNA were detected with an anti-HA antibody. Endogenous PCNA was immunoprecipitated in the right panel. + and − in boxes represent presence and absence of DNA listed in the right panel in transfection. 2 and 1 in boxes indicate the fold difference of DNA amount used for transfection.
Reduction of HLTF Expression Reduces PCNA Polyubiquitination.
To determine whether chromatin-bound PCNA polyubiquitination upon DNA damage could be catalyzed by HLTF and SHPRH, we examined MMS-induced PCNA polyubiquitination after depletion of HLTF and SHPRH by siRNA. Depletion of SHPRH or HLTF expression significantly reduced PCNA polyubiquitination after treatment with 0.01% MMS (Fig. 3A). A double knockdown of HLTF and SHPRH also showed a similar reduction of PCNA polyubiquitination (Fig. S3).
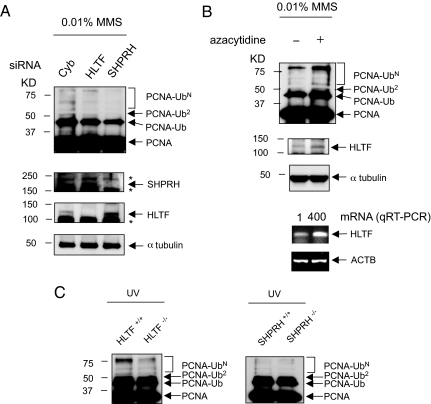
Fig. 3.
SHPRH and HLTF catalyze endogenous PCNA ubiquitination. (A) The reduction of HLTF or SHPRH expression by siRNA reduced PCNA polyubiquitination. Depletion of HLTF and SHPRH was achieved by siRNA in HEK293T cells. Seventy-two hours after transfection, cells were either mock treated or treated with 0.01% MMS. Cells were harvested 1.5 h later. Anti-PCNA immunoprecipitations were performed as described in Fig. 1. Cyb is a control siRNA transfection targeting cyclophilin B that does not have any known function in DNA metabolism. The efficiency of siRNA knockdown of SHPRH or HLTF was checked by Western blot with specific antibodies. Asterisks indicate nonspecific bands. (B) Reduced PCNA polyubiquitination in SW480 cells was enhanced when HLTF expression was restored. − and + represent the absence and presence of 5-azacytidine treatment. The restoration of HLTF expression was confirmed by both Western blots and quantitative RT-PCR (qRT-PCR). β-actin (ACTB) was used as a loading control. The number indicates fold induction, measured by qRT-PCR. (C) PCNA ubiquitination is significantly reduced in the Shprh−/− and Hltf−/− MEF cells. MEFs were treated with mock or 60 J/m2 of UV irradiation as indicated. Ub, Ub2, and UbN indicate mono-, di-, and polyubiquitinated species of PCNA, respectively.
The expression of HLTF is significantly reduced in a human colorectal cancer cell line, SW480, owing to the hypermethylation of its promoter (14). Next, we asked whether the restoration of HLTF gene expression in SW480 cells could promote PCNA polyubiquitination. The treatment of a demethylating agent, 5-azacytidine, successfully restored HLTF expression in SW480 cells (Fig. 3B). Consistently, MMS-induced PCNA polyubiquitination was enhanced when HLTF expression was restored (Fig. 3C). By contrast, 5-azacytidine treatment of HEK293T cells did not show significant differences in PCNA polyubiquitination (data not shown).
Because siRNA-mediated gene knockdown cannot completely suppress gene expression, the reduced but substantial level of PCNA polyubiquitination in HEK293T cells might come from untransfected or less-silenced cells. We therefore generated Shprh- and Hltf-deficient MEFs from Hltf−/− and Shprh−/− mice. PCNA ubiquitination upon DNA damage was significantly reduced, but not completely eliminated, in both Hltf- and Shprh-deficient MEFs compared with wild-type MEFs (Fig. 3C). These results suggest that HLTF and SHPRH function cooperatively in PCNA polyubiquitination.
Physical Interactions Between HLTF and Proteins Participating in PCNA Ubiquitination.
SHPRH directly interacts with PCNA, RAD18, and UBC13, but not with RAD6 (9). To examine whether HLTF has similar binding properties, 3XFLAG-HLTF-containing cell extract was incubated with GST-fused PCNA, UBC13, or RAD6 (Fig. S4 A and B). 3XFLAG-HLTF was retrieved by GST-PCNA and GST-UBC13, but not by GST-RAD6 or GST alone (Fig. S4 A and B). Similarly, 3XFLAG-HLTF was coimmunoprecipitated with UBC13 (Fig. S4C). To examine the interactions between HLTF, SHPRH, and RAD18, either SHPRH-myc-His or RAD18-myc-His were coexpressed with 3XFLAG-HLTF, and HLTF was detected in anti-myc immunoprecipitates. SHPRH and RAD18 both coimmunoprecipitate HLTF (Fig. S4D). Thus, HLTF interacts with PCNA, SHPRH, RAD18, and UBC13.
Reduction of SHPRH or HLTF Expression Increases Mutagenesis in Burkit's Lymphoma B Cells.
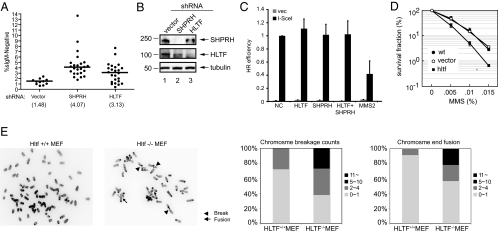
The disruption of TS by mutations in yeast MMS2 or RAD5 significantly increases TLS-mediated spontaneous mutations (15, 16). In addition, the loss of human MMS2 expression significantly increases the frequency of mutations induced by UV irradiation in human fibroblasts (17). To determine whether reduced expression of SHPRH or HLTF similarly affects mutagenesis, we knocked down the expression of SHPRH or HLTF in the Burkit's lymphoma B cell line, RAMOS, using shRNA, and the frequency of mutation was measured by the loss of surface IgM (sIgM) by flow cytometry (18). In this established assay, the loss of sIgM is attributable to mutations, which generate stop codons in the Ig VH domain, and these mutations are dependent on the error-prone DNA polymerases, Polη, Polι, Polκ, and REV1. The reduced expression of SHPRH and HLTF increased the mutation frequency 3- and 2-fold respectively, when compared with vector control, suggesting an increased usage of TLS in the absence of the SHPRH- or the HLTF-dependent pathway (Fig. 4 A and B).
Fig. 4.
Reduced expression of SHPRH or HLTF enhances genomic instability and mutagenesis but not homologous recombination (HR). (A) Ramos cells expressing low levels of HLTF or SHPRH increased mutagenesis as measured by the loss of expressing surface IgM. (B) Expression of HLTF and SHPRH were reduced by shRNA (C) HLTF- and SHPRH-depleted cells showed no significant change in the frequency of HR according to FACS analysis of GFP-positive cells relative to the red fluorescent cells (transfection control). Depletion of each gene was achieved by siRNA-mediated gene knockdown. Vec and I-SceI represent transfections of vector (no DSB) and I-SceI (DSB). HR frequency was normalized to NC (nontargeting siRNA). (D) Cells expressing low levels of HLTF by shRNA showed higher sensitivity to MMS. (E) HLTF −/− MEF cells showed elevated levels of abnormal chromosomes after MMS treatment. MEFs were treated with 0.01% MMS for 1 h, and the metaphase spreads were analyzed 24 h after treatment. Examples of metaphase spreads from wild-type (+/+) and homozygous null (−/−) cells are shown in the left panel. The percentage of chromosome breakages and fusions from 100 metaphases are presented as graphs in the right panel.
SHPRH and HLTF Are Not Directly Involved in Homologous Recombination.
On the basis of yeast genetic studies, it has been suggested that the Rad5 and homologous recombination (HR) pathways are reciprocally recruited to DNA damage during replication (19–21). Defects in the Rad5 pathway could channel the repair intermediates from disabled PRR into HR, thus enhancing rates of damage-induced and spontaneous recombination (16, 21, 22). Yeast Ubc13 and Mms2 are exclusively involved in the Rad5 pathway, but not in HR. In contrast, in higher eukaryotes, UBC13 and MMS2 seem to function in HR in addition to their roles in PRR (23, 24). To determine whether SHPRH and HLTF are involved in HR, we used a recombination reporter assay (DR-GFP reporter assay), which can measure the frequency of HR between tandem GFP repeats with inactivating mutations (25). Transient expression of the I-SceI endonuclease in these cells generates a single DSB that is repaired by HR to produce a functional GFP. Transfection of a U2OS human cell line harboring the DR-GFP reporter (U2OS/DR-GFP) with plasmids carrying I-SceI did not enhance PCNA ubiquitination, suggesting that a DSB does not induce PCNA ubiquitination (Fig. S5B). We transfected the U2OS/DR-GFP cells with siRNA against SHPRH, HLTF, RAD18, or MMS2 to knockdown gene expression. The knockdown efficiency was measured by quantitative RT-PCR. The expression of SHPRH, HLTF, and MMS2 were reduced to approximately 24%, 12%, and 15% of the nontargeting siRNA control, respectively (Fig. S5A). Similar to a previous report, the depletion of MMS2 expression resulted in a 3-fold reduction in the HR frequency (Fig. 4C and Fig. S5C). In contrast, depletion of SHPRH or HLTF resulted in no significant differences in the HR frequency compared with the control (Fig. 4C and Fig. S5C). Therefore, unlike UBC13/MMS2, SHPRH and HLTF are not involved in HR. However, we cannot rule out the possibility that residual amounts of SHPRH or HLTF could still mediate HR efficiently in this assay.
Inactivation of HLTF Elevates the Sensitivity of Cells to MMS and Elevates the Level of Chromosome Abnormalities upon DNA Damage.
The reduced expression of SHPRH enhances sensitivity to MMS (9). Similarly, when HLTF expression was reduced by shRNA in HCT116 cells, the cells became more sensitive to MMS compared with wild type (Fig. 4D). In yeast, a rad5 mutation elevates the rate of gross chromosomal rearrangements (19, 26). Given that HLTF and SHPRH have functions in promoting PCNA polyubiquitination similar to Rad5, it is possible that HLTF and SHPRH may also function to maintain genomic stability. To test whether HLTF functions in the maintenance of genomic stability, we analyzed metaphase chromosomes from Hltf-deficient MEFs. More than 70% of wild-type MEFs showed no (or at most 1) chromosome breaks or fusions after the treatment with 0.01% MMS (Fig. 4E). In contrast, elevated levels of chromosome breaks and fusions were observed in Hltf-deficient MEFs. Approximately 60% and 40% of metaphase spreads had >2 chromosome breaks or fusions, respectively. Because the reduced expression of SHPRH also enhanced MMS-induced chromosome breaks compared with wild type (9), SHPRH and HLTF seem to function to suppress genomic instability upon DNA damage.
Discussion
Several lines of evidence have shown that PCNA monoubiquitination occurs both in yeast and higher eukaryotes, primarily in response to the DNA damage-induced stalling at DNA replication forks (13, 27–29). In the present study we have demonstrated that in addition to the monoubiquitination of PCNA, the chromatin-bound PCNA polyubiquitination is enhanced by DNA damage that stalls DNA replication forks, but not by γ-irradiation (Fig. 1). Similar to our BrdU-incorporation analysis (Fig. 1E), DNA fiber labeling, which can also detect replication progression, showed stalling of DNA replication by UV irradiation, MMS, hydroxyurea, and aphidicolin but not by γ-irradiation (30). Although all these DNA-damaging agents can prohibit origin firing by activating checkpoints, there is no evidence to suggest that the replication checkpoint serves as a signal for PCNA ubiquitination (Liaw and Myung unpublished data; and 31, 32). Depletion of ATM or ATR does not abolish the mono- and polyubiquitination of PCNA upon MMS treatment (Fig. S1). Experiments using the Xenopus laevis cell free system suggested that accumulation of ssDNA coated with RPA is a signal for PCNA ubiquitination (31). It will be of interest to determine whether elongated ssDNA coated with RPA is a signal for PCNA ubiquitination in mammalian cells. However, depletion of RPA1 (a 70-kD subunit of the RPA heterotrimer complex) or CDC45L (a DNA helicase required for DNA replication) in human cells had no apparent effects on PCNA polyubiquitination (Fig. S1). More studies may be required to determine whether RPA-coated ssDNA is a signal for PCNA ubuquitination in mammalian cells.
Previously, we identified SHPRH as a functional human homologue of yeast Rad5. In this report we have demonstrated that PCNA modification with a K63-linked polyubiquitin chain is mediated by the ubiquitin ligases SHPRH and HLTF in mammalian cells. We provided 4 pieces of evidence to support this conclusion. First, ectopic expressions of SHPRH or HLTF along with UBC13/MMS2 robustly enhanced the PCNA polyubiquitination in HEK293T cells (Fig. 2 B and C) (9). Second, silencing of endogenous SHPRH or HTLF expression by siRNA significantly reduced the MMS-induced PCNA polyubiquitination (Fig. 3A). Third, Shprh- and Hltf-deficient MEFs showed reduced levels of UV-induced PCNA polyubiquitination (Fig. 3C). Fourth, restoration of HLTF expression in SW480 cells by 5-azacytidine, a DNA demethylating agent, enhanced PCNA polyubiquitination (Fig. 3B). Importantly, SHPRH and HLTF synergistically promote PCNA polyubiquitination (Fig. 2C) and physically interact with each other (Fig. S4D). These results suggest that SHPRH and HLTF redundantly or cooperatively function on the same DNA lesion. Nevertheless, the presence of additional domains, which are not shared by SHPRH and HLTF, such as the Linker Histone and PHD domains in SHPRH and the HIRAN domain in HLTF, may provide additional functions for these 2 proteins. Extensive sequence comparison of SWI2/SNF2 proteins from various species showed that HLTF and SHPRH are evolutionarily related to yeast RAD5 and IRC20 (Increased Recombination Center 20), respectively (33). Interestingly, the irc20 mutant showed increased levels of spontaneous Rad52 foci (www.yeastgenome.org).
The inactivation of the TS pathway by mutations in yeast RAD5, UBC13, or MMS2 significantly increased the rate of CAN1 forward mutations (16), suggesting that inactivation of TS facilitates TLS for mutagenesis. Consistent with the yeast data, depletion of human MMS2 also significantly increased the mutation rate induced by UV irradiation in human fibroblasts (17). Our results showed that the knockdown of SHPRH or HLTF also increased the frequency of mutation in Ramos cells (Fig. 4A), suggesting that a reduction of SHPRH/HLTF can channel cells into the TLS pathway and lead to mutagenesis.
rad5 or rad18 mutations in yeast elevate spontaneous gene conversion and recombination between direct repeats (16). Unlike yeast, UBC13- or MMS2-deficient chicken DT40 and UBC13- or MMS2-depleted HeLa cells are defective in HR, suggesting that UBC13 and MMS2 function in both PRR and HR in vertebrates (23, 24, 34). Consistent with these results, we also observed that the depletion of MMS2 in human U2OS/DRGFP cells reduced HR efficiency (Fig. 4C and Fig. S5C). In contrast, depletion of either SHPRH or HLTF expression by siRNA did not affect HR frequencies (Fig. 4C and Fig. S5C). Therefore, PCNA polyubiquitination by SHPRH or HLTF does not seem to be required for DSB-induced HR. However, we cannot rule out the possibility that the apparent lack of HR defect in SHPRH/HLTF-silenced U2OS/DR-GFP cells might be simply due to the insufficient reduction or redundancy of SHPRH and HLTF.
What could be the biologic and clinical consequences of PCNA polyubiquitination by SHPRH and HLTF? Because the K63-linked polyubiquitin chain is added over the monoubiquitin of PCNA, this polyubiquitination modification could be a molecular switch mechanism to either remove proteins functioning in the TLS pathway, such as error-prone polymerases, or recruit proteins for the error-free TS pathway. Recent studies showed that RAP80, the ubiquitin-interacting motif-containing protein, is preferentially recruited to the K63-linked polyubiquitin chain, where it promotes the assembly of the multiprotein repair complex at DSBs (35). An analogous mechanism might operate to promote the assembly of the protein complex specifically required for the TS pathway. Alternatively, PCNA polyubiquitination could simply signal for the removal of all of the DNA replication machinery and mark the DNA damage until the necessary DNA repair machinery comes to fix the DNA damage.
Despite the established clinical importance of TLS in preventing human carcinogenesis (6, 7), little is known about the TS pathway in carcinogenesis. Silencing of the HLTF gene expression (14) and point mutations of the SHPRH gene has been observed in human ovarian and colorectal cancer cells (36). Based on our evidence that mutagenesis, damage sensitivity, and damage-induced chromosomal aberrations are increased when the expression of HLTF and SHPRH are decreased, the inactivation of TS due to reduced PCNA polyubiquitination may contribute to the carcinogenesis of cancers with HLTF or SHPRH inactivation.
Materials and Methods
Detection of Chromatin-Bound PCNA Polyubiquitination.
To make nuclear extracts, ≈1 × 107 cultured cells were resuspended in buffer A (10 mM Hepes [pH 7.9], 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol, 1 mM PMSF, 5 μg/ml aprotinin, and 20 μg/ml leupeptin). Triton X-100 was added (to a final concentration of 0.1%), and the nuclear fraction was precipitated by centrifugation at 1300 × g for 5 min at 4°C. The nuclear fraction was resuspended in TSE500 buffer (20 mM Tris [pH 8.1], 2 mM EDTA, 500 mM NaCl, 0.1% SDS, 1% Triton X-100, and protease inhibitor mixture [Roche]) and sonicated. After centrifugation at 17 000 × g for 5 min, the supernatant containing the released chromatin-bound proteins was used for immunoprecipitation by incubating with 1 μg of anti-PCNA monoclonal antibodies (PC10, Santa Cruz Biotechnology) overnight at 4°C. The anti-PCNA immunoprecipitates were separated by SDS/PAGE and transferred onto PVDF membrane and detected by anti-PCNA (PC10) or anti-Ub (P4D1) monoclonal antibodies. The identification of polyubiquitinated species of PCNA after transfection with various plasmids expressing different proteins was performed as described in ref. 9.
Methods.
Methods for general molecular biology, plasmid construction, protein interaction, chromosome analysis, recombination assay, and mutagenesis assay are described in SI Methods.
Supplementary Material
Acknowledgments.
We thank D. Bodine (National Human Genome Research Institute [NHGRI]), P. Liu (NHGRI), M. Lichten (National Cancer Institute), P. Meltzer (NHGRI), and Y. Shiloh (Tel Aviv University, Israel) for helpful discussions; R. Harris (University of Minnesota), M. Jasin (Memorial Sloan-Kettering Cancer Center [MSKCC]), K. Nakanishi (MSKCC), and R. Sood (NHGRI) for cells, plasmids, and antibody; S. Anderson (NHGRI) and M. Kirby (NHGRI) for FACS analysis; and A. Dutra (NHGRI) and E. Pak (NHGRI) for metaphase chromosome analysis. We also thank E. Hendrickson (University of Minnesota), the National Institutes of Health (NIH) Fellows Editorial Board, and members of the Myung laboratory for comments on the manuscript and J. Fekecs (NHGRI) for figure preparation. K.M. especially thanks E. Cho. This work was supported by the Canada Research Chair program and the National Cancer Institute of Canada (to H.D.), the Dutch Cancer Society (to J.H. and H.R.), the Japan Society for the Promotion of Science (to A.M.), and the intramural research program of the NHGRI, NIH (to K.M.). H.D. is the holder of a Canada Research Chair, and S.D.M. is an investigator of Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0805685105/DCSupplemental.
References
- 1.Ulrich HD. The RAD6 pathway: Control of DNA damage bypass and mutagenesis by ubiquitin and SUMO. Chembiochem. 2005;6:1735–1743. doi: 10.1002/cbic.200500139. [DOI] [PubMed] [Google Scholar]
- 2.Lehmann AR, et al. Translesion synthesis: Y-family polymerases and the polymerase switch. DNA Repair (Amst) 2007;6:891–899. doi: 10.1016/j.dnarep.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Yang W, Woodgate R. What a difference a decade makes: Insights into translesion DNA synthesis. Proc Natl Acad Sci USA. 2007;104:15591–15598. doi: 10.1073/pnas.0704219104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence CW. Following the RAD6 pathway. DNA Repair (Amst) 2007;6:676–686. doi: 10.1016/j.dnarep.2006.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Sun L, Chen ZJ. The novel functions of ubiquitination in signaling. Curr Opin Cell Biol. 2004;16:119–126. doi: 10.1016/j.ceb.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Cordonnier AM, Lehmann AR, Fuchs RP. Impaired translesion synthesis in xeroderma pigmentosum variant extracts. Mol Cell Biol. 1999;19:2206–2211. doi: 10.1128/mcb.19.3.2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masutani C, et al. The XPV (xeroderma pigmentosum variant) gene encodes human DNA polymerase eta. Nature. 1999;399:700–704. doi: 10.1038/21447. [DOI] [PubMed] [Google Scholar]
- 8.Chiu RK, et al. Lysine 63-polyubiquitination guards against translesion synthesis-induced mutations. PLoS Genet. 2006;2:e116. doi: 10.1371/journal.pgen.0020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motegi A, et al. Human SHPRH suppresses genomic instability through proliferating cell nuclear antigen polyubiquitination. J Cell Biol. 2006;175:703–708. doi: 10.1083/jcb.200606145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brun J, et al. hMMS2 serves a redundant role in human PCNA polyubiquitination. BMC Mol Biol. 2008;9:24. doi: 10.1186/1471-2199-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unk I, et al. Human SHPRH is a ubiquitin ligase for Mms2-Ubc13-dependent polyubiquitylation of proliferating cell nuclear antigen. Proc Natl Acad Sci USA. 2006;103:18107–18112. doi: 10.1073/pnas.0608595103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang TT, et al. Regulation of monoubiquitinated PCNA by DUB autocleavage. Nat Cell Biol. 2006;8:339–347. doi: 10.1038/ncb1378. [DOI] [PubMed] [Google Scholar]
- 13.Moldovan GL, Pfander B, Jentsch S. PCNA, the maestro of the replication fork. Cell. 2007;129:665–679. doi: 10.1016/j.cell.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 14.Moinova HR, et al. HLTF gene silencing in human colon cancer. Proc Natl Acad Sci USA. 2002;99:4562–4567. doi: 10.1073/pnas.062459899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broomfield S, Chow BL, Xiao W. MMS2, encoding a ubiquitin-conjugating-enzyme-like protein, is a member of the yeast error-free postreplication repair pathway. Proc Natl Acad Sci USA. 1998;95:5678–5683. doi: 10.1073/pnas.95.10.5678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liefshitz B, Steinlauf R, Friedl A, Eckardt-Schupp F, Kupiec M. Genetic interactions between mutants of the ‘error-prone’ repair group of Saccharomyces cerevisiae and their effect on recombination and mutagenesis. Mutat Res. 1998;407:135–145. doi: 10.1016/s0921-8777(97)00070-0. [DOI] [PubMed] [Google Scholar]
- 17.Li Z, Xiao W, McCormick JJ, Maher VM. Identification of a protein essential for a major pathway used by human cells to avoid UV- induced DNA damage. Proc Natl Acad Sci USA. 2002;99:4459–4464. doi: 10.1073/pnas.062047799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris RS, Sale JE, Petersen-Mahrt SK, Neuberger MS. AID is essential for immunoglobulin V gene conversion in a cultured B cell line. Curr Biol. 2002;12:435–438. doi: 10.1016/s0960-9822(02)00717-0. [DOI] [PubMed] [Google Scholar]
- 19.Motegi A, Kuntz K, Majeed A, Smith S, Myung K. Regulation of gross chromosomal rearrangements by ubiquitin and SUMO ligases in Saccharomyces cerevisiae. Mol Cell Biol. 2006;26:1424–1433. doi: 10.1128/MCB.26.4.1424-1433.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pfander B, Moldovan GL, Sacher M, Hoege C, Jentsch S. SUMO-modified PCNA recruits Srs2 to prevent recombination during S phase. Nature. 2005;436:428–433. doi: 10.1038/nature03665. [DOI] [PubMed] [Google Scholar]
- 21.Smirnova M, Klein HL. Role of the error-free damage bypass postreplication repair pathway in the maintenance of genomic stability. Mutat Res. 2003;532:117–135. doi: 10.1016/j.mrfmmm.2003.08.026. [DOI] [PubMed] [Google Scholar]
- 22.Stelter P, Ulrich HD. Control of spontaneous and damage-induced mutagenesis by SUMO and ubiquitin conjugation. Nature. 2003;425:188–191. doi: 10.1038/nature01965. [DOI] [PubMed] [Google Scholar]
- 23.Zhao GY, et al. A critical role for the ubiquitin-conjugating enzyme Ubc13 in initiating homologous recombination. Mol Cell. 2007;25:663–675. doi: 10.1016/j.molcel.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Simpson LJ, Sale JE. UBE2V2 (MMS2) is not required for effective immunoglobulin gene conversion or DNA damage tolerance in DT40. DNA Repair (Amst) 2005;4:503–510. doi: 10.1016/j.dnarep.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi K, et al. Human Fanconi anemia monoubiquitination pathway promotes homologous DNA repair. Proc Natl Acad Sci USA. 2005;102:1110–1115. doi: 10.1073/pnas.0407796102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith S, et al. Mutator genes for suppression of gross chromosomal rearrangements identified by a genome-wide screening in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 2004;101:9039–9044. doi: 10.1073/pnas.0403093101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lehmann AR. Translesion synthesis in mammalian cells. Exp Cell Res. 2006;312:2673–2676. doi: 10.1016/j.yexcr.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 28.Kannouche PL, Lehmann AR. Ubiquitination of PCNA and the polymerase switch in human cells. Cell Cycle. 2004;3:1011–1013. [PubMed] [Google Scholar]
- 29.Kannouche PL, Wing J, Lehmann AR. Interaction of human DNA polymerase eta with monoubiquitinated PCNA: A possible mechanism for the polymerase switch in response to DNA damage. Mol Cell. 2004;14:491–500. doi: 10.1016/s1097-2765(04)00259-x. [DOI] [PubMed] [Google Scholar]
- 30.Merrick CJ, Jackson D, Diffley JF. Visualization of altered replication dynamics after DNA damage in human cells. J Biol Chem. 2004;279:20067–20075. doi: 10.1074/jbc.M400022200. [DOI] [PubMed] [Google Scholar]
- 31.Chang DJ, Lupardus PJ, Cimprich KA. Monoubiquitination of proliferating cell nuclear antigen induced by stalled replication requires uncoupling of DNA polymerase and mini-chromosome maintenance helicase activities. J Biol Chem. 2006;281:32081–32088. doi: 10.1074/jbc.M606799200. [DOI] [PubMed] [Google Scholar]
- 32.Frampton J, et al. Postreplication repair and PCNA modification in Schizosaccharomyces pombe. Mol Biol Cell. 2006;17:2976–2985. doi: 10.1091/mbc.E05-11-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Flaus A, Martin DM, Barton GJ, Owen-Hughes T. Identification of multiple distinct Snf2 subfamilies with conserved structural motifs. Nucleic Acids Res. 2006;34:2887–2905. doi: 10.1093/nar/gkl295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Szüts D, Simpson LJ, Kabani S, Yamazoe M, Sale JE. Role for RAD18 in homologous recombination in DT40 cells. Mol Cell Biol. 2006;26:8032–8041. doi: 10.1128/MCB.01291-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim H, Chen J. New players in the BRCA1-mediated DNA damage responsive pathway. Mol Cells. 2008;25:457–461. [PMC free article] [PubMed] [Google Scholar]
- 36.Sood R, et al. Cloning and characterization of a novel gene, SHPRH, encoding a conserved putative protein with SNF2/helicase and PHD-finger domains from the 6q24 region. Genomics. 2003;82:153–161. doi: 10.1016/s0888-7543(03)00121-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.