Abstract
Endothelial inflammation with chemokine involvement contributes to acute coronary syndromes (ACS). We tested the hypothesis that variation in the chemokine gene CXCL5, which encodes epithelial neutrophil-activating peptide (ENA-78), is associated with ACS prognosis. We also investigated whether statin use, a potent modulator of inflammation, modifies CXCL5's association with outcomes and characterized the in vitro effect of atorvastatin on endothelial ENA-78 production. Using a prospective cohort of ACS patients (n = 704) the association of the CXCL5 −156 G>C polymorphism (rs352046) with 3-year all-cause mortality was estimated with hazard ratios (HR). Models were stratified by genotype and race. To characterize the influence of statins on this association, a statin*genotype interaction was tested. To validate ENA-78 as a statin target in inflammation typical of ACS, endothelial cells (HUVECs) were treated with IL-1β and atorvastatin with subsequent quantification of CXCL5 expression and ENA-78 protein concentrations. C/C genotype was associated with a 2.7-fold increase in 3-year all-cause mortality compared to G/G+G/C (95%CI 1.19–5.87; p = 0.017). Statins significantly reduced mortality in G/G individuals only (58% relative risk reduction; p = 0.0009). In HUVECs, atorvastatin dose-dependently decreased IL-1β-stimulated ENA-78 concentrations (p<0.0001). Drug effects persisted over 48 hours (p<0.01). CXCL5 genotype is associated with outcomes after ACS with potential statin modification of this effect. Atorvastatin lowered endothelial ENA-78 production during inflammation typical of ACS. These findings implicate CXCL5/ENA-78 in ACS and the statin response.
Introduction
Endothelial inflammation plays a major role in the development and perpetuation of cardiovascular disease (CVD). The inflammatory process is complex, with various cellular mediators known to contribute [1], [2]. Multifunctional cytokines such as interleukin-1 (IL-1) play central roles in vascular inflammation. IL-1 produced from monocytes, macrophages, and other cells can initiate inflammation by inducing the production of chemokines from endothelial cells [3], [4]. Chemokines, in turn, propagate the process through recruitment and activation of additional cellular mediators of inflammation including neutrophils. This process is involved in many cardiovascular conditions including acute coronary syndrome (ACS). Moreover, previous work has established that monocyte chemokines such as monocyte chemoattractant protein-1 (MCP-1) are prognostic in ACS [5]. However, the role of neutrophil chemokines in ACS has not been well described.
CXCL5 (MIM *600324 a.k.a. epithelial neutrophil-activating peptide 78 or ENA-78) is a C-X-C chemokine that attracts and activates neutrophils. Furthermore, CXCL5 expression has been shown to be highly inducible in endothelial and vascular smooth muscle cells by IL-1β [6]–[9]. Recent data have implicated CXCL5 and/or its receptors in congestive heart failure and ischemic stroke, making CXCL5 a candidate gene for other manifestations of CVD including ACS [10]–[12]. We previously identified a −156G>C (rs352046) single nucleotide polymorphism (SNP) in the CXCL5 promoter region to occur with high minor allele frequency in the general population and associate with both elevated plasma ENA-78 concentrations and leukocyte production of ENA-78 [13]. We therefore tested the primary hypothesis that this SNP is associated with 3-year all-cause mortality in a prospectively enrolled cohort of ACS patients.
Beyond testing this association, we also sought to describe potential associations with ACS therapies, namely statins, plausibly capable of influencing inflammation and outcome as a function of CXCL5 genotype. Statins improve outcomes in ACS in part through anti-inflammatory properties. [14]. We previously reported that basal endothelial ENA-78 production is modulated by atorvastatin [15]. Therefore, we also tested the hypothesis that statin treatment may modify the association between CXCL5 genotypes and outcomes in our ACS cohort. Finally, to offer insight into our epidemiological findings and validate CXCL5 as a potential candidate in statin pharmacogenetics, we tested whether atorvastatin treatment modulates CXCL5 expression and ENA-78 production from endothelial cells exposed to IL-1β, a model of cardiovascular inflammation typical of ACS.
Results
Clinical Characteristics
The mean patient age was 61±12 years, and the cohort was comprised of 36% women and 79% Caucasians. Complete clinical characteristics are displayed in Table 1. The minor allele frequency for −156C was 17%. Genotype frequencies did not deviate from Hardy-Weinberg expectations. The patients were similar when compared by CXCL5 −156G>C genotype with the following exceptions: compared with G/G homozygotes, those with the C/C genotype were slightly younger, less likely to be Caucasian, had a greater prevalence of unstable angina as their ACS type, had higher admission DBP, had higher discharge HDL, and were less likely to be discharged on a statin (Table 1). G/C heterozygotes generally exhibited the above phenotypes in a fashion intermediate between G/G and C/C individuals. When baseline characteristics were compared by genotype in Caucasians alone, patients with the C/C genotype were older, but otherwise had no significantly different characteristics from the other genotype groups (Table 2).
Table 1. Baseline Characteristics.
| Overall | G/G | G/C | C/C | P value | |
| (n = 704) | (n = 498) | (n = 175) | (n = 31) | ||
| Age | 60.6±12.4 | 61.4±12.2 | 58.3±12.5 | 60.0±14.1 | 0.019 |
| White | 555 (79%) | 437 (88%) | 107 (61%) | 11 (35%) | <0.0001 |
| Women | 253 (36%) | 179 (36%) | 60 (34%) | 14 (45%) | 0.51 |
| ACS Type | |||||
| Unstable angina | 284 (40%) | 188 (38%) | 75 (43%) | 21 (68%) | |
| ST elevation MI | 202 (29%) | 154 (31%) | 45 (26%) | 3 (10%) | |
| Non ST elevation MI | 215 (31%) | 154 (31%) | 54 (31%) | 7 (23%) | 0.042 |
| Old LBB/Unknown | 3 (0.4%) | 2 (0.4%) | 1 (0.6%) | 0 (0%) | |
| History/risk factors: | |||||
| Dyslipidemia | 403 (57) | 297 (60%) | 91 (52%) | 15 (48%) | 0.113 |
| Diabetes | 197 (28) | 136 (27%) | 50 (29%) | 11 (35%) | 0.604 |
| Heart failure | 55 (8) | 32 (6%) | 19 (11%) | 4 (13%) | 0.0955 |
| MI | 238 (34) | 168 (34%) | 58 (33%) | 12 (39%) | 0.832 |
| HTN | 459 (65) | 323 (65%) | 114 (65%) | 22 (71%) | 0.787 |
| BMI (kg/m2) | 29.6±6.3 | 29.4±6.0 | 30.3±7.1 | 29.4±7.2 | 0.215 |
| Smoking | |||||
| Current | 248 (35%) | 168 (34%) | 69 (40%) | 11 (36%) | 0.622 |
| Former | 258 (37%) | 190 (38%) | 56 (32%) | 12 (39%) | |
| Never | 197 (28%) | 140 (28%) | 49 (28%) | 8 (26%) | |
| Disease severity | |||||
| EF (n = 670) | 47.2±12.8 | 46.8±12.6 | 48.5±13.4 | 47.0±13.6 | 0.345 |
| Admission SBP | 136.6±27.0 | 136.4±26.8 | 135.9±26.7 | 144.4±31.0 | 0.257 |
| Admission DBP | 74.3±16.7 | 72.8±16.0 | 77.1±17.5 | 81.2±20.2 | 0.0009 |
| Discharge total cholesterol* | 178.7±42.7 | 176.4±40.6 | 184.7±47.8 | 181.0±43.0 | 0.118 |
| Discharge HDL† | 41.9±15.4 | 40.8±12.7 | 44.3±20.0 | 47.7±22.1 | 0.0097 |
| Discharge LDL‡ | 102.9±37.3 | 100.7±34.6 | 108.3±43.0 | 107.2±41.8 | 0.0957 |
| Discharge triglycerides§ | 176.8±122.7 | 181.3±131.7 | 169.3±99.4 | 141.3±71.5 | 0.228 |
| Treatment strategy | |||||
| Medical management | 259 (37%) | 167 (34%) | 75 (43%) | 17 (55%) | 0.048 |
| PCI | 414 (59%) | 309 (62%) | 92 (53%) | 13 (42%) | |
| CABG | 31 (4%) | 22 (4%) | 8 (5%) | 1 (3%) | |
| Discharge statin | 532 (76%) | 390 (78%) | 125 (71%) | 17 (55%) | 0.0044 |
*n = 603; †n = 594; ‡n = 565; §n = 593; ACS = Acute coronary syndrome; MI = Myocardial infarction; LBB = Left bundle block; HTN = Hypertension; BMI = Body mass index; EF = Ejection fraction; SBP = Systolic blood pressure; DBP = Diastolic blood pressure; HDL = High density lipoprotein; LDL = Low density lipoprotein; PCI = Percutaneous coronary intervention; CABG = Coronary artery bypass graft.
Table 2. Baseline Characteristics in Caucasians.
| All Caucasians | G/G | G/C | C/C | P value | |
| (n = 555) | (n = 437) | (n = 107) | (n = 11) | ||
| Age | 62.0±12.5 | 62.1±12.1 | 60.0±13.1 | 72.1±15.5 | 0.014 |
| Women | 194 (35%) | 155 (35%) | 32 (30%) | 7 (64%) | 0.073 |
| ACS Type | |||||
| Unstable angina | 204 (37%) | 159 (36%) | 40 (37%) | 5 (45%) | |
| ST elevation MI | 171 (31%) | 137 (31%) | 32 (30%) | 2 (18%) | |
| Non ST elevation MI | 178 (32%) | 139 (32%) | 35 (33%) | 4 (36%) | 0.958 |
| LBBB/Unknown | 2 (0.4%) | 2 (0.5%) | 0 (0%) | 0 (0%) | |
| History/risk factors: | |||||
| Dyslipidemia | 324 (58%) | 263 (60%) | 57 (53%) | 4 (36%) | 0.134 |
| Diabetes | 134 (24%) | 110 (25%) | 21 (20%) | 3 (27%) | 0.472 |
| Heart failure | 30 (5%) | 25 (6%) | 4 (4%) | 1 (9%) | 0.619 |
| MI | 179 (32%) | 142 (32%) | 32 (30%) | 5 (45%) | 0.560 |
| HTN | 346 (62%) | 280 (64%) | 59 (55%) | 7 (64%) | 0.231 |
| BMI (kg/m2) | 29.4±5.9 | 29.4±5.8 | 29.8±6.1 | 28.8±5.8 | 0.762 |
| Smoking | |||||
| Current | 180 (32%) | 142 (32%) | 35 (33%) | 3 (27%) | 0.766 |
| Former | 215 (39%) | 174 (40%) | 36 (34%) | 5 (45%) | |
| Never | 159 (29%) | 121 (28%) | 35 (33%) | 3 (27%) | |
| Disease severity: | |||||
| EF (n = 532) | 47.5±12.4 | 47.1±12.3 | 49.7±12.0 | 44.1±15.1 | 0.103 |
| Admission SBP | 134.2±24.6 | 134.6±25.0 | 133.3±23.5 | 129.1±22.3 | 0.695 |
| Admission DBP | 72.3±14.9 | 71.9±15.0 | 74.1±14.6 | 71.0±14.9 | 0.403 |
| Discharge total cholesterol* | 177.2±40.9 | 175.8±40.2 | 183.9±43.2 | 165.8±48.0 | 0.174 |
| Discharge HDL† | 40.6±14.8 | 40.2±12.3 | 42.3±22.6 | 38.8±10.3 | 0.452 |
| Discharge LDL‡ | 101.5±34.9 | 100.1±34.0 | 107.5±36.9 | 100.8±49.0 | 0.224 |
| Discharge triglycerides§ | 185.0±131.2 | 186.7±136.9 | 181.7±110.2 | 135.4±48.5 | 0.574 |
| Treatment strategy | |||||
| Medical management | 157 (28%) | 132 (30%) | 23 (22%) | 2 (18%) | 0.312 |
| PCI | 371 (67%) | 283 (65%) | 80 (75%) | 8 (73%) | |
| CABG | 27 (5%) | 22 (5%) | 4 (4%) | 1 (9%) | |
| Discharge statin | 439 (79%) | 349 (80%) | 82 (77%) | 8 (73%) | 0.665 |
*n = 473; †n = 464; ‡n = 436; §n = 465; ACS = Acute coronary syndrome; MI = Myocardial infarction; LBB = Left bundle block; HTN = Hypertension; BMI = Body mass index; EF = Ejection fraction; SBP = Systolic blood pressure; DBP = Diastolic blood pressure; HDL = High density lipoprotein; LDL = Low density lipoprotein; PCI = Percutaneous coronary intervention; CABG = Coronary artery bypass graft.
Main Effect of CXCL5 Genotype on ACS Outcomes
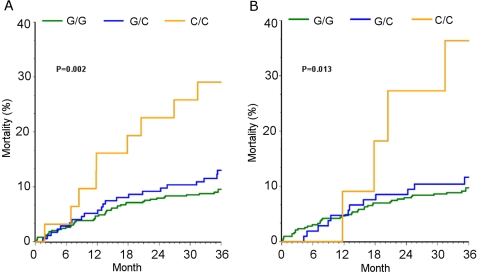
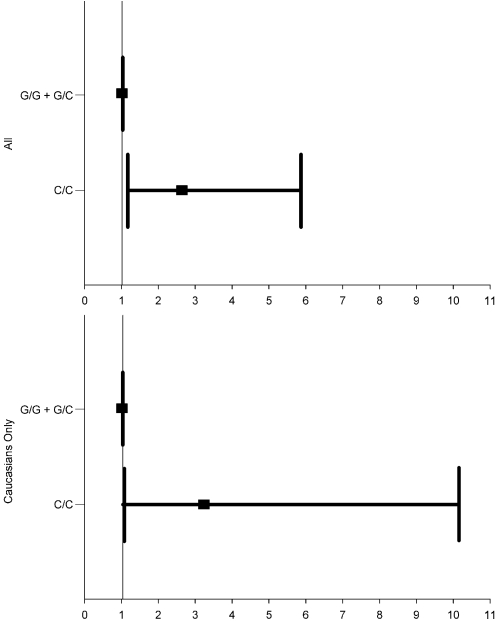
CXCL5 −156 G>C genotype was significantly associated with 3-year all-cause mortality in ACS patients. The death rate was 10% in G/G, 13% in G/C, and 29% in C/C individuals (p = 0.005). C/C genotype was associated with a hazard ratio of 3.09 (95% confidence interval [CI], 1.54–6.19; p = 0.002) compared to G/C+G/G genotypes (Figure 1A). This effect remained significant when Caucasians alone were analyzed (HR 4.0, 95%CI 1.45–11.05; p = 0.008) (Figure 1B). The increased risk of mortality remained significant after adjustment for clinical covariates both in the overall population (HR 2.65, 95%CI 1.19–5.87; p = 0.017) (Figure 2A) and in Caucasians alone (HR 3.25, 95%CI 1.04–10.13; p = 0.043) (Figure 2B).
Figure 1. Kaplan Meier estimates for all-cause mortality by CXCL5 −156 G>C genotype.
Panel A represents the overall population; panel B represents the Caucasians only.
Figure 2. Adjusted hazard ratio and 95% confidence intervals for all-cause mortality by genotype.
Top panel is overall population (p = 0.017) and bottom panel is Caucasians only (p = 0.043). Models adjusted for age, race, sex, ACS type, revascularization strategy, history of diabetes, and history of heart failure.
Statin Interaction
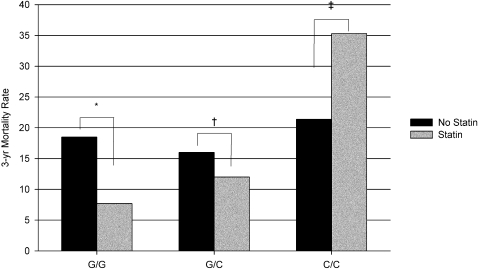
We identified an interaction between discharge statin therapy and CXCL5 −156 G>C genotype (p = 0.09). Individuals with the G/G genotype who were prescribed a statin at hospital discharge had a significantly lower 3-year all-cause mortality rate compared to those who did not receive a statin at hospital discharge (absolute risk reduction 10.8%; relative risk reduction [RRR] 58.4%; p = 0.0009) (Figure 3). Statin benefit was diminished in the G/C and C/C genotype groups. Patients with the G/C genotype experienced a non-significant RRR of 25% when discharged on statins (p = 0.48), while patients with the C/C genotype prescribed statin therapy had a numerically greater, although not significant, mortality rate compared to those not prescribed statin therapy (35.3% versus 21.4%, respectively; p = 0.46) (Figure 3).
Figure 3. All-cause mortality rate by genotype among statin-treated and non-treated patients.
*p = 0.0009., †p = 0.48; ‡p = 0.46.
Effect of Atorvastatin on IL-1β-induced ENA-78 Production from Endothelial Cells
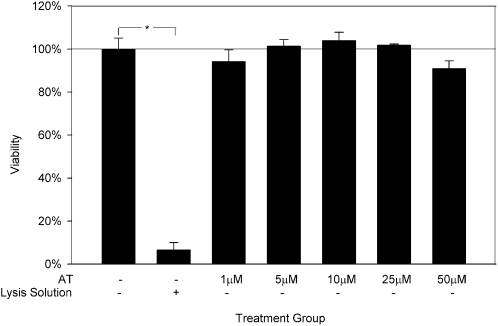
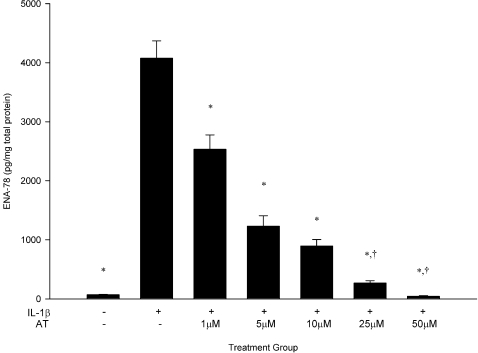
To validate CXCL5/ENA-78 as an endothelial target for statins, we utilized a HUVEC in vitro model of cardiovascular inflammation. There were no significant differences in HUVEC viability between control conditions and any dose of atorvastatin ranging from 1 µM to 50 µM (Figure 4). IL-1β increased ENA-78 production by nearly 60-fold relative to constitutive concentrations (4075±296 pg/mg vs. 69±7 pg/mg; p<0.0001; Figure 5). Treatment with atorvastatin attenuated this effect by 38%, 70%, 78%, 93%, and 99% in the five atorvastatin dose groups (p<0.001 for all comparisons vs. IL-1β).
Figure 4. Cell viability of HUVECs cultured for 24 hours with atorvastatin.
Data are presented as mean±SEM of 3 experiments. *p<0.0001. AT, atorvastatin.
Figure 5. Atorvastatin attenuates IL-1β-induced ENA-78 production in a dose-dependent fashion.
Data are presented as mean±SEM of 4 experiments. * p<0.0001 vs. IL-1β, †p = NS vs. control. AT, atorvastatin.
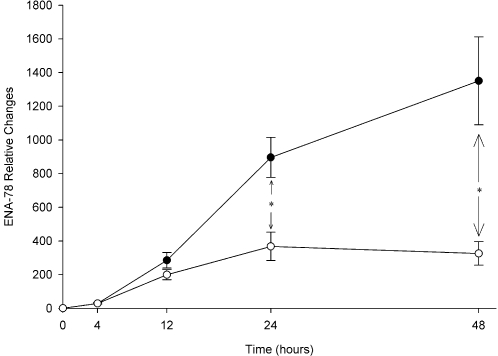
The time-dependent course of ENA-78 changes relative to control in response to IL-1β and atorvastatin/IL-1β co-treatment are shown in Figure 6. Atorvastatin's time effect was significant at 24 hours and most pronounced at 48 hours. Specifically, atorvastatin attenuated IL-1β stimulated production of ENA-78 at 4, 12, 24, and 48 hours by 0%, 30%, 59% (p = 0.01), and 76% (p = 0.009), respectively.
Figure 6. Atorvastatin attenuates ENA-78 production over time.
Levels are relative to baseline (0 hour) for each condition. Data are presented as mean±SEM of 4 experiments. *p≤0.01. —○—, IL-1β stimulation+atorvastatin 10 µM; —•—, IL-1β stimulation alone; AT, atorvastatin.
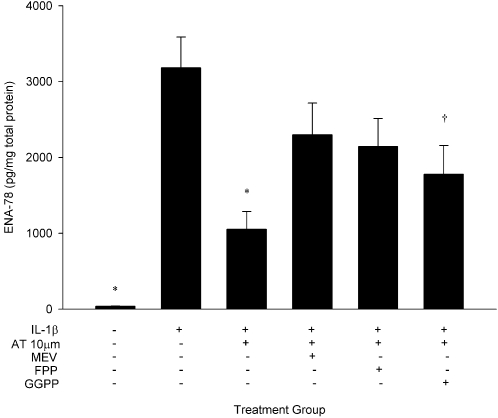
To determine whether atorvastatin effects were dependent on HMG-CoA reductase inhibition, HUVECs were cultured with IL-1β and atorvastatin 10 µM in the presence or absence of mevalonate. To assess whether downstream products of mevalonate biosynthesis are implicated in the drug effect, conditions also included cultures in the presence or absence of FPP and GGPP (Figure 7). Consistent with the previous experiments, atorvastatin significantly attenuated IL-1β-stimulated ENA-78 production (3181±407 pg/mg vs. 1054±234 pg/mg; p<0.001). Co-treatment with mevalonate and FPP reversed the effect of atorvastatin (p = NS vs. IL-1β), and GGPP partially reversed the effect of atorvastatin (p = 0.05 vs. IL-1β).
Figure 7. Atorvastatin effects on ENA-78 are reversed by mevalonate and its downstream metabolites.
Data are presented as mean±SEM of 10 experiments. *p<0.001 and †p = 0.05 compared to IL-1β stimulation alone. AT, atorvastatin; FPP, farnesyl pyrophosphate; GGPP, geranylgeranyl pyrophosphate; MEV, mevalonate.
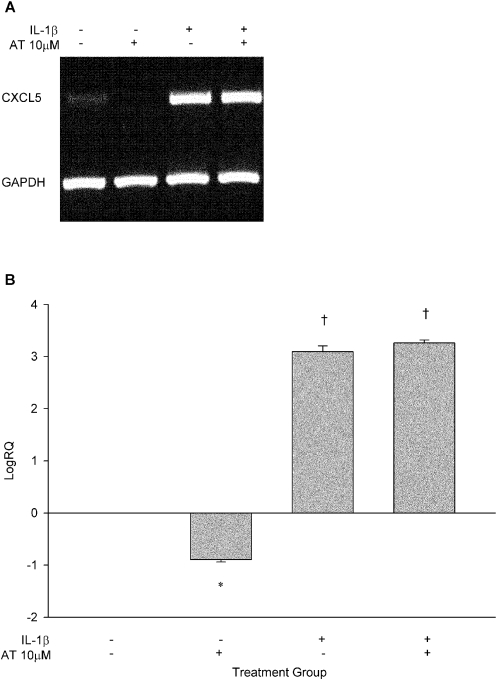
Atorvastatin significantly lowered constitutive expression of CXCL5 (p<0.005), while IL-1β significantly induced CXCL5 expression in HUVECs at 24 hours (p<0.0001). However, pre-treatment of HUVECs with atorvastatin did not blunt IL-1β induction of CXCL5 expression (Figure 8).
Figure 8. CXCL5 expression is modulated by atorvastatin and IL-1β.
(A) Gel electrophoresis of CXCL5 and GAPDH PCR products; (B) Log10 relative quantification of CXCL5 modulated by atorvastatin, IL-1β, and their combination normalized to GAPDH (N = 2 experiments). *P<0.005, †P<0.0001. AT, atorvastatin.
Discussion
In the current analysis, we demonstrate that a common promoter polymorphism in the ENA-78 gene (CXCL5) is associated with 3-year all-cause mortality in ACS patients, adding further support for a role of neutrophil chemoattractants in acute ischemic syndromes. Even after adjustment for age, sex, ACS type, revascularization strategy, heart failure, and diabetes, patients with two copies of the −156C variant allele were at 2.7-fold higher risk for mortality than patients with at least one copy of the −156G wild-type allele. The overall death rates for G/G, G/C, and C/C individuals were 10%, 13%, and 29%, respectively.
Importantly, we also demonstrated a pharmacogenetic relationship between CXCL5 genotypes and discharge statin use. G/G patients experienced a significant RRR of 58% in all-cause mortality; G/C heterozygotes experienced a non-significant reduction of 25%; and C/C homozygotes experienced a non-significant increase of 39%. Our findings of an association between discharge statin use and mortality, as a function of CXCL5 genotype, is an important extension of previous work. Several lines of evidence suggest that part of the cardioprotective effect of statins is related to their ability to modulate neutrophil function by various mechanisms, including reduced production of neutrophil chemokines 16–20. Our findings that the absolute mortality rate was numerically higher in C/C homozygotes discharged on a statin compared with C/C homozygotes not discharged on a statin is congruent with findings regarding the influence of statins on NAP-2, a neutrophil-activating chemokine exhibiting over 50% homology with ENA-78 [21]. Smith and colleagues demonstrated elevated NAP-2 plasma concentrations in patients with unstable angina [21]. However, while aspirin lowered plasma NAP-2, statin treatment stimulated NAP-2 production in patients, peripheral blood mononuclear cells, and platelets. This pro-inflammatory effect has also been observed by others; Kiener and colleagues demonstrated that some, but not all, statins stimulate monocyte production of pro-inflammatory cytokines including IL-8 (22% homology with ENA-78) in vitro and increase influx of leukocyte subtypes (including neutrophils) in vivo [22]. Therefore, while there is a strongly beneficial effect of statin therapy in the general population, some subsets of ACS patients may not derive such benefit. We have previously demonstrated CXCL5 −156C carriers exhibit significantly greater plasma concentrations of ENA-78 and greater ENA-78 production from cultured leukocytes [13]. Therefore, it is conceivable that patients with the −156 C/C genotype may simultaneously represent a population at higher risk for ACS mortality that experiences little, if any, benefit from discharge statin therapy. The exact mechanism of this observation warrants further investigation.
In addition to our epidemiological analyses, we performed in vitro experiments and showed that CXCL5/ENA-78 is an endothelial target for statins. We corroborated previous work which showed ENA-78 is highly inducible by IL-1β in endothelial cells [6], [7], [9]. In the current experiments, atorvastatin modulated IL-1β-mediated ENA-78 production in a dose- and time-specific manner, through pathways involving HMG-CoA reductase and prenylation proteins. The drug effect began as early as 12 hours after stimulation and persisted out to 48 hours with no signs of diminishment. It has been hypothesized that statins exhibit early anti-inflammatory effects that may explain their benefit in acute manifestations of CVD (e.g., ACS). Furthermore, it has been shown that short-term statin treatment in certain cardiovascular settings result in demonstrable improvement in outcomes [23]–[25]. Our data suggest that early and persistent inhibition of IL-1β-induced endothelial ENA-78 production should be considered part of the spectrum of statin pleiotropism, and may represent an important mechanism of statin benefit in ACS and conditions marked by neutrophil involvement.
While postulated to be independent of LDL lowering, the anti-inflammatory effects of statins are thought to result from HMG-CoA reductase inhibition and/or inhibited production of prenylation precursors, such as FPP and GGPP. Our findings support this body of evidence in that atorvastatin's modulatory effect on ENA-78 was reversed by the addition of mevalonate and FPP, and partially reversed by GGPP. Previous studies have explored the effects of FPP and GGPP on statin responses, with the hope of elucidating the contribution of prenylation pathways on drug effects. Reversal of statin effects in vitro by the addition of FPP and GGPP implicate the Ras and Rho signaling pathways. Previous studies have shown that either Ras or Rho signaling may be involved in the statin effect, depending on the specific drug response tested [26]–[29].
While we showed atorvastatin to lower endothelial ENA-78 protein production under pro-inflammatory conditions, we also investigated whether atorvastatin lowered CXCL5 expression. We found a significant reduction in basal CXCL5 expression with atorvastatin treatment that was not observed for conditions with IL-1β stimulation. Nonetheless, we have previously shown basal endothelial ENA-78 protein production to be reduced by atorvastatin in a dose-dependent fashion, and currently extend this observation to a pro-inflammatory state [15]. There are several possible reasons for the disparate impact of atorvastatin on CXCL5 expression in non-stimulated and stimulated conditions. Firstly, in a pro-inflammatory state (such as that induced by IL-1β), the predominant statin effect may be post-transcriptional in nature. Similar findings have been reported for the effect of rosuvastatin on MMP-7 production and expression whereby protein production but not gene expression was lowered [30]. A second explanation can be found from recent studies that implicate geranylgeranylation as an important homeostatic mechanism in IL-1β secretion from macrophages and peripheral blood mononuclear cells [31], [32]. Specifically, it was demonstrated that inhibition of isoprenoid production with statins led to upregulation of IL-1β. This effect was more pronounced upon addition of exogenous IL-1β (as in our experiments) suggesting that depletion of isoprenoids by statins shuts off the IL-1β negative feedback loop. As such, one can speculate that co-treatment of HUVECs with IL-1β and atorvastatin in our experiments may have potentiated IL-1β autocrine activity. Since CXCL5 is highly inducible by IL-1β in HUVECs, the preponderant drug effect manifested as a reduction of ENA-78 protein concentrations (presumable through a post-transcriptional mechanism) with no impact on CXCL5 expression.
There are several limitations to our analysis. Because of the epidemiological nature of the cohort study, data on white blood cell counts and differentials were unavailable. Therefore, the effect of these parameters on clinical endpoints in our population could not be directly tested. Along these lines, blood samples were not available to directly quantify ENA-78 protein concentrations in our patients. Nonetheless, we have previously shown that CXCL5 genotype is a predictor of circulating ENA-78 concentrations and leukocyte production of ENA-78 [13]. Furthermore, the CXCL5 genotype may in fact be a more stable marker of ACS risk than circulating ENA-78 concentrations since protein concentrations are more likely to be influenced by multiple stress-related factors. We have, in fact, shown this to be the case whereby endothelial nitric oxide (NO) synthase (NOS3) genotype was robustly associated with arterial stiffness, while circulating surrogate markers of NO activity (serum superoxide dismutase and nitrite) were not [33], [34]. We noted an interaction between discharge statin use and CXCL5 genotype on outcomes. Because we did not have drug-specific information, we could not determine if this effect was differential influenced by the specific statin used, or the type of statin (i.e., hydrophilic vs. lipophilic); nor would we likely have the power to conduct such an analysis if the data were available. We also do not have information on the persistence of discharge statin use over time in these patients. Additionally, because of the small sample size for this subgroup analysis, there is a possibility of a false positive finding between genotype and discharge statin use. Nonetheless, others have estimated persistence of discharge statin use to be nearly 80% among patients similar in characteristics to our own [35]. In addition, our in vitro endothelial experiments validated ENA-78/CXCL5 as an endothelial target of statin action in an inflammatory environment typical of ACS, adding biological plausibility to our epidemiological finding. Finally, because of the lack of granularity in the Social Security Administration Death Master File, the exact cause of mortality could not be determined. This is a known limitation of epidemiologic data and further analyses in independent cohorts are warranted. Despite these limitations, our data suggest that the ENA-78 gene, CXCL5, is associated with post-ACS all-cause mortality and is an endothelial target of atorvastatin in vitro. Whether ENA-78 modulation by statins is a central mediator of drug benefit in ACS is unknown. However, our findings strongly suggest a role of ENA-78 and CXCL5 genetic variants as determinants of CVD and drug response.
Methods
Acute Coronary Syndrome Cohort and Gene/SNP Selection
The methods of accrual and adjudication for this prospective cohort have been previously described [36]. Briefly, patients with a confirmed ACS were enrolled from two Kansas City hospitals. Myocardial infarction (MI) was defined by elevated troponin level in combination with chest pain symptoms or electrocardiographic findings (ST-segment elevation or non-ST-segement elevation) consistent with MI. Unstable angina was defined by a negative troponin level and any one of the following: new-onset angina (<2 months), prolonged angina (>20 minutes) at rest, recent worsening angina, or angina that occurred within 2 weeks of MI [37]. Demographic information was obtained from patient interviews during hospital admission. Medical history, medication history, laboratory values, and inpatient treatment during the ACS hospitalization were obtained from chart abstractions. The Social Security Administration Death Master File (http://www.ntis.gov/products/ssa-dmf.asp) was queried approximately once yearly to obtain three-year all-cause mortality assessment. The study was approved by the institutional review boards of both institutions and written informed consent was obtained from all participants. Participants were genotyped according to previously published methods [38]. CXCL5 (MIM 600324) was chosen as our a priori candidate gene based on 1) the known role of the neutrophil pathway in cardiovascular diseases including ACS [39]–[41], and 2) our previously published data demonstrating atorvastatin to significantly lower basal production of endothelial ENA-78 [15]. The −156G>C SNP (rs352046) was chosen as our candidate SNP because of our previous work demonstrating this SNP to be associated with variable ENA-78 protein concentration in vivo [13].
In Vitro Endothelial Experiments
Cell Culture and Reagents
Human umbilical vein endothelial cells (HUVECs) (Lonza Walkersville, Inc., Walkersville, MD) were cultured as previously described [15]. Briefly, HUVECs between passes three and four were seeded at a density of 2.5×104 cells per cm2 and cultured to 80% confluence with endothelial cell growth–supplemented medium. Once confluent, media were changed to serum free endothelial basal media for 24 hours prior to treatment after which basal media supplemented to 2% FBS concentration was used.
Treatments consisted of atorvastatin calcium (LKT Laboratories Inc., St. Paul, MN) 1–50 µM dissolved in dimethyl sulfoxide (DMSO). IL-1β, mevalonate, farnesyl pyrophosphate (FPP), and geranylgeranyl pyrophosphate (GGPP; all from Sigma-Aldrich, St. Louis, MO) were all dissolved in molecular grade water and used at concentrations of 2 ng/ml, 250 µM, 5 µM, and 5 µM respectively. All final DMSO concentrations were less then 0.1%.
Treatments
Cell viability was assessed by trypan blue staining. In experiments investigating the impact of atorvastatin dose on ENA-78 production, HUVECs were pre-treated with atorvastatin followed by addition of IL-1β two hours later. After dose-ranging studies were performed, the time-dependent effects of atorvastatin were investigated at 0, 4, 12, 24, and 48 hours. In separate experiments, cells were cultured with IL-1β and atorvastatin in the presence or absence of mevalonate, FPP, and GGPP to determine whether atorvastatin inhibition of ENA-78 production was dependent on HMG-CoA reductase inhibition and subsequent downstream pathways. Cell culture supernates for all experiments were stored at −20°C until analysis, which was performed within seven days.
Protein Quantification and Gene Expression
ENA-78 concentrations were measured and normalized to total protein content using cytometric immunofluorescence as previously described [15]. Ribonucleic acid (RNA) was isolated using a commercial kit (RNeasy mini kit, Qiagen Inc., Valencia, CA). Complementary deoxyribonucleic acid (cDNA) conversion was performed by high capacity cDNA reverse transcription (Applied Biosystems, Foster City, CA) per protocol using roughly 2 µg of total RNA. RNA quality was assessed by absorbance (Nanodrop, Nanodrop Technologies, Wilmington, DE). Real-time reverse-transcription PCR (RT-PCR) was performed using primer and probe sets for CXCL5 normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Taqman Gene Expression Assays, Applied Biosystems Inc.). Relative quantification was verified by gel electrophoresis of PCR products using exonic primers for CXCL5 (5-CGGGAAGGAAATTTGTCTTGA-3; 5-AGCTTAAGCGGCAAACATAGG-3) and GAPDH (5-AAAATCAAGTGGGGCGATGCT-3; 5-GCCAGGGGTGCTAAGCAGTT-3). PCR for both CXCL5 and GAPDH were performed in parallel reactions using the following conditions: 95°C for 2 minutes; 35 cycles of 95°C for 30 seconds, 59°C for 30 seconds, and 72°C for 30 seconds; followed by 7 minutes of elongation at 72°C.
Statistical Analyses
ACS Cohort
Baseline characteristics were compared by ANOVA, student's t-test or chi-square tests as appropriate. Allele frequencies were calculated by allele counting and deviations from Hardy-Weinberg equilibrium were tested by chi-square. Death rates were compared by chi-square or Fisher's exact tests. To minimize multiple testing and maximize statistical power, one mode of inheritance was tested (recessive) only after visual inspection of survival curves for all three genotype groups. We had 80% power to detect a hazard ratio of 1.57 under the tested recessive model of inheritance, given our sample size of 704 participants and a minor allele frequency of 17%.
Cox proportional hazards models were developed that included the following pre-specified covariates: age, sex, ACS type (ST elevation MI, non ST elevation MI, or unstable angina), coronary revascularization strategy (medical management, percutaneous coronary intervention, or coronary artery bypass graft), heart failure, diabetes, and CXCL5 genotype. Additionally, interactions between genotype and discharge statin use were assessed in the models. All models were conducted in the overall population and separately by race to minimize confounding by population stratification. Survival plots were generated by the Kaplan-Meier technique. The statin*genotype interaction term was considered significant if p<0.1 (two-sided) since multiplicative interaction testing is generally not powered to detect significance at an α = 0.05 [42]. In all other analyses, p<0.05 was considered significant. SAS version 9.1 was used for all analyses.
In Vitro Experiments
ENA-78 protein concentrations and CXCL5 gene expression levels were compared for all conditions by one-way ANOVA with Tukey correction for multiple comparisons. An independent t-test was used to compare treatment differences at each time point in the time-course studies. Relative CXCL5 gene expression was determined by the 2−ΔΔCt method [43]. A p-value<0.05 was considered significant. Statistical analyses were performed with SPSS software, version 11.5 (SPSS Inc., Chicago, USA). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Footnotes
Competing Interests: The University of Florida has filed a patent for CXCL5 polymorphisms and ENA-78 concentrations as diagnostic and prognostic tools with Dr. Zineh as a co-inventor. No final decision has been rendered.
Funding: This work was supported by American Heart Association Florida/Puerto Rico Affiliate Scientist Development Grant 0435278B, NIH C06 Grant RR17568, and NIH P50 Grant HL077113. The sponsors did not have a role in the design and conduct of the study, in the collection, analysis, and interpretation of the data, or in the preparation, review, or approval of the manuscript.
References
- 1.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 3.Dinarello CA. Interleukin-1 and interleukin-1 antagonism. Blood. 1991;77:1627–1652. [PubMed] [Google Scholar]
- 4.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87:2095–2147. [PubMed] [Google Scholar]
- 5.de Lemos JA, Morrow DA, Blazing MA, Jarolim P, Wiviott SD, et al. Serial measurement of monocyte chemoattractant protein-1 after acute coronary syndromes: results from the A to Z trial. J Am Coll Cardiol. 2007;50:2117–2124. doi: 10.1016/j.jacc.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 6.Walz A, Burgener R, Car B, Baggiolini M, Kunkel SL, et al. Structure and neutrophil-activating properties of a novel inflammatory peptide (ENA-78) with homology to interleukin 8. J Exp Med. 1991;174:1355–1362. doi: 10.1084/jem.174.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schmouder RL, Streiter RM, Walz A, Kunkel SL. Epithelial-derived neutrophil-activating factor-78 production in human renal tubule epithelial cells and in renal allograft rejection. Transplantation. 1995;59:118–124. doi: 10.1097/00007890-199501150-00021. [DOI] [PubMed] [Google Scholar]
- 8.Imaizumi T, Kumagai M, Hatakeyama M, Tamo W, Yamashita K, et al. Effect of 15-deoxy-delta12,14-prostaglandin J2 on IL-1beta-induced expression of epithelial neutrophil-activating protein-78 in human endothelial cells. Prostaglandins Leukot Essent Fatty Acids. 2003;69:323–327. doi: 10.1016/s0952-3278(03)00145-5. [DOI] [PubMed] [Google Scholar]
- 9.Strieter RM, Kunkel SL, Burdick MD, Lincoln PM, Walz A. The detection of a novel neutrophil-activating peptide (ENA-78) using a sensitive ELISA. Immunol Invest. 1992;21:589–596. doi: 10.3109/08820139209069393. [DOI] [PubMed] [Google Scholar]
- 10.Damas JK, Gullestad L, Ueland T, Solum NO, Simonsen S, et al. CXC-chemokines, a new group of cytokines in congestive heart failure–possible role of platelets and monocytes. Cardiovasc Res. 2000;45:428–436. doi: 10.1016/s0008-6363(99)00262-x. [DOI] [PubMed] [Google Scholar]
- 11.Zaremba J, Skrobanski P, Losy J. The level of chemokine CXCL5 in the cerebrospinal fluid is increased during the first 24 hours of ischaemic stroke and correlates with the size of early brain damage. Folia Morphol (Warsz) 2006;65:1–5. [PubMed] [Google Scholar]
- 12.Damas JK, Eiken HG, Oie E, Bjerkeli V, Yndestad A, et al. Myocardial expression of CC- and CXC-chemokines and their receptors in human end-stage heart failure. Cardiovasc Res. 2000;47:778–787. doi: 10.1016/s0008-6363(00)00142-5. [DOI] [PubMed] [Google Scholar]
- 13.Zineh I, Aquilante CL, Langaee TY, Beitelshees AL, Arant CB, et al. CXCL5 gene polymorphisms are related to systemic concentrations and leukocyte production of epithelial neutrophil-activating peptide (ENA-78). Cytokine. 2006;33:258–263. doi: 10.1016/j.cyto.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GG, Olsson AG. The case for intensive statin therapy after acute coronary syndromes. Am J Cardiol. 2005;96:45F–53F. doi: 10.1016/j.amjcard.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 15.Zineh I, Luo X, Welder GJ, Debella AE, Wessel TR, et al. Modulatory effects of atorvastatin on endothelial cell-derived chemokines, cytokines, and angiogenic factors. Pharmacotherapy. 2006;26:333–340. doi: 10.1592/phco.26.3.333. [DOI] [PubMed] [Google Scholar]
- 16.Chello M, Anselmi A, Spadaccio C, Patti G, Goffredo C, et al. Simvastatin increases neutrophil apoptosis and reduces inflammatory reaction after coronary surgery. Ann Thorac Surg. 2007;83:1374–1380. doi: 10.1016/j.athoracsur.2006.10.065. [DOI] [PubMed] [Google Scholar]
- 17.Cowled PA, Khanna A, Laws PE, Field JB, Varelias A, et al. Statins inhibit neutrophil infiltration in skeletal muscle reperfusion injury. J Surg Res. 2007;141:267–276. doi: 10.1016/j.jss.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Guasti L, Marino F, Cosentino M, Cimpanelli M, Maio RC, et al. Simvastatin treatment modifies polymorphonuclear leukocyte function in high-risk individuals: a longitudinal study. J Hypertens. 2006;24:2423–2430. doi: 10.1097/01.hjh.0000251903.62804.77. [DOI] [PubMed] [Google Scholar]
- 19.Marino F, Guasti L, Cosentino M, Ferrari M, Rasini E, et al. Angiotensin II type 1 receptor expression in polymorphonuclear leukocytes from high-risk subjects: changes after treatment with simvastatin. J Cardiovasc Pharmacol. 2007;49:299–305. doi: 10.1097/FJC.0b013e31803d35ce. [DOI] [PubMed] [Google Scholar]
- 20.Sugano R, Matsuoka H, Haramaki N, Umei H, Murase E, et al. Polymorphonuclear leukocytes may impair endothelial function: results of crossover randomized study of lipid-lowering therapies. Arterioscler Thromb Vasc Biol. 2005;25:1262–1267. doi: 10.1161/01.ATV.0000163842.91226.ba. [DOI] [PubMed] [Google Scholar]
- 21.Smith C, Damas JK, Otterdal K, Oie E, Sandberg WJ, et al. Increased levels of neutrophil-activating peptide-2 in acute coronary syndromes: possible role of platelet-mediated vascular inflammation. J Am Coll Cardiol. 2006;48:1591–1599. doi: 10.1016/j.jacc.2006.06.060. [DOI] [PubMed] [Google Scholar]
- 22.Kiener PA, Davis PM, Murray JL, Youssef S, Rankin BM, et al. Stimulation of inflammatory responses in vitro and in vivo by lipophilic HMG-CoA reductase inhibitors. Int Immunopharmacol. 2001;1:105–118. doi: 10.1016/s0162-3109(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 23.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 24.Schwartz GG, Olsson AG, Ezekowitz MD, Ganz P, Oliver MF, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. Jama. 2001;285:1711–1718. doi: 10.1001/jama.285.13.1711. [DOI] [PubMed] [Google Scholar]
- 25.Patti G, Pasceri V, Colonna G, Miglionico M, Fischetti D, et al. Atorvastatin pretreatment improves outcomes in patients with acute coronary syndromes undergoing early percutaneous coronary intervention: results of the ARMYDA-ACS randomized trial. J Am Coll Cardiol. 2007;49:1272–1278. doi: 10.1016/j.jacc.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 26.Brown JH, Del Re DP, Sussman MA. The Rac and Rho hall of fame: a decade of hypertrophic signaling hits. Circ Res. 2006;98:730–742. doi: 10.1161/01.RES.0000216039.75913.9e. [DOI] [PubMed] [Google Scholar]
- 27.Ota K, Suehiro T, Arii K, Ikeda Y, Kumon Y, et al. Effect of pitavastatin on transactivation of human serum paraoxonase 1 gene. Metabolism. 2005;54:142–150. doi: 10.1016/j.metabol.2004.06.018. [DOI] [PubMed] [Google Scholar]
- 28.Aarons CB, Cohen PA, Gower A, Reed KL, Leeman SE, et al. Statins (HMG-CoA reductase inhibitors) decrease postoperative adhesions by increasing peritoneal fibrinolytic activity. Ann Surg. 2007;245:176–184. doi: 10.1097/01.sla.0000236627.07927.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park HJ, Zhang Y, Georgescu SP, Johnson KL, Kong D, et al. Human umbilical vein endothelial cells and human dermal microvascular endothelial cells offer new insights into the relationship between lipid metabolism and angiogenesis. Stem Cell Rev. 2006;2:93–102. doi: 10.1007/s12015-006-0015-x. [DOI] [PubMed] [Google Scholar]
- 30.Furman C, Copin C, Kandoussi M, Davidson R, Moreau M, et al. Rosuvastatin reduces MMP-7 secretion by human monocyte-derived macrophages: potential relevance to atherosclerotic plaque stability. Atherosclerosis. 2004;174:93–98. doi: 10.1016/j.atherosclerosis.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Lindholm MW, Nilsson J. Simvastatin stimulates macrophage interleukin-1beta secretion through an isoprenylation-dependent mechanism. Vascul Pharmacol. 2007;46:91–96. doi: 10.1016/j.vph.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Mandey SH, Kuijk LM, Frenkel J, Waterham HR. A role for geranylgeranylation in interleukin-1beta secretion. Arthritis Rheum. 2006;54:3690–3695. doi: 10.1002/art.22194. [DOI] [PubMed] [Google Scholar]
- 33.Haller MJ, Pierce GL, Braith RW, Silverstein JH. Serum superoxide dismutase activity and nitric oxide do not correlate with arterial stiffness in children with type 1 diabetes mellitus. J Pediatr Endocrinol Metab. 2006;19:267–269. doi: 10.1515/jpem.2006.19.3.267. [DOI] [PubMed] [Google Scholar]
- 34.Zineh I, Beitelshees AL, Haller MJ. NOS3 polymorphisms are associated with arterial stiffness in children with type 1 diabetes. Diabetes Care. 2007;30:689–693. doi: 10.2337/dc06-1697. [DOI] [PubMed] [Google Scholar]
- 35.Muhlestein JB, Horne BD, Bair TL, Li Q, Madsen TE, et al. Usefulness of in-hospital prescription of statin agents after angiographic diagnosis of coronary artery disease in improving continued compliance and reduced mortality. Am J Cardiol. 2001;87:257–261. doi: 10.1016/s0002-9149(00)01354-0. [DOI] [PubMed] [Google Scholar]
- 36.Lanfear DE, Jones PG, Marsh S, Cresci S, McLeod HL, et al. Beta2-adrenergic receptor genotype and survival among patients receiving beta-blocker therapy after an acute coronary syndrome. Jama. 2005;294:1526–1533. doi: 10.1001/jama.294.12.1526. [DOI] [PubMed] [Google Scholar]
- 37.Braunwald E. Unstable angina. A classification. Circulation. 1989;80:410–414. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- 38.Zineh I, Welder GJ, Langaee TY. Development and cross-validation of sequencing-based assays for genotyping common polymorphisms of the CXCL5 gene. Clin Chim Acta. 2006;370:72–75. doi: 10.1016/j.cca.2006.01.025. [DOI] [PubMed] [Google Scholar]
- 39.Kruk M, Przyluski J, Kalinczuk L, Pregowski J, Deptuch T, et al. Association of Non-Specific Inflammatory Activation With Early Mortality in Patients With ST-Elevation Acute Coronary Syndrome Treated With Primary Angioplasty. Circ J. 2008;72:205–211. doi: 10.1253/circj.72.205. [DOI] [PubMed] [Google Scholar]
- 40.Nijm J, Wikby A, Tompa A, Olsson AG, Jonasson L. Circulating levels of proinflammatory cytokines and neutrophil-platelet aggregates in patients with coronary artery disease. Am J Cardiol. 2005;95:452–456. doi: 10.1016/j.amjcard.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 41.Takeshita S, Isshiki T, Ochiai M, Ishikawa T, Nishiyama Y, et al. Systemic inflammatory responses in acute coronary syndrome: increased activity observed in polymorphonuclear leukocytes but not T lymphocytes. Atherosclerosis. 1997;135:187–192. doi: 10.1016/s0021-9150(97)00160-3. [DOI] [PubMed] [Google Scholar]
- 42.Lee CR, North KE, Bray MS, Fornage M, Seubert JM, et al. Genetic variation in soluble epoxide hydrolase (EPHX2) and risk of coronary heart disease: The Atherosclerosis Risk in Communities (ARIC) study. Hum Mol Genet. 2006;15:1640–1649. doi: 10.1093/hmg/ddl085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]