Abstract
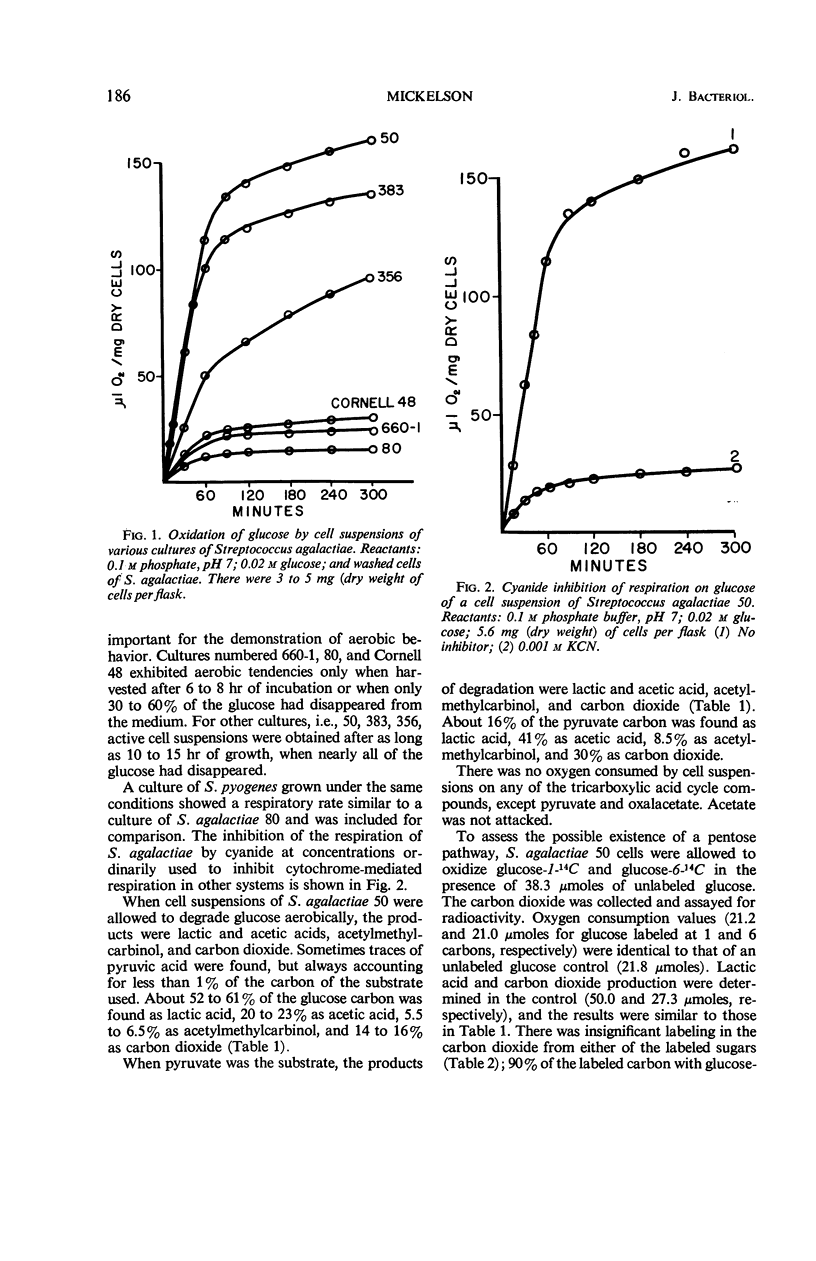
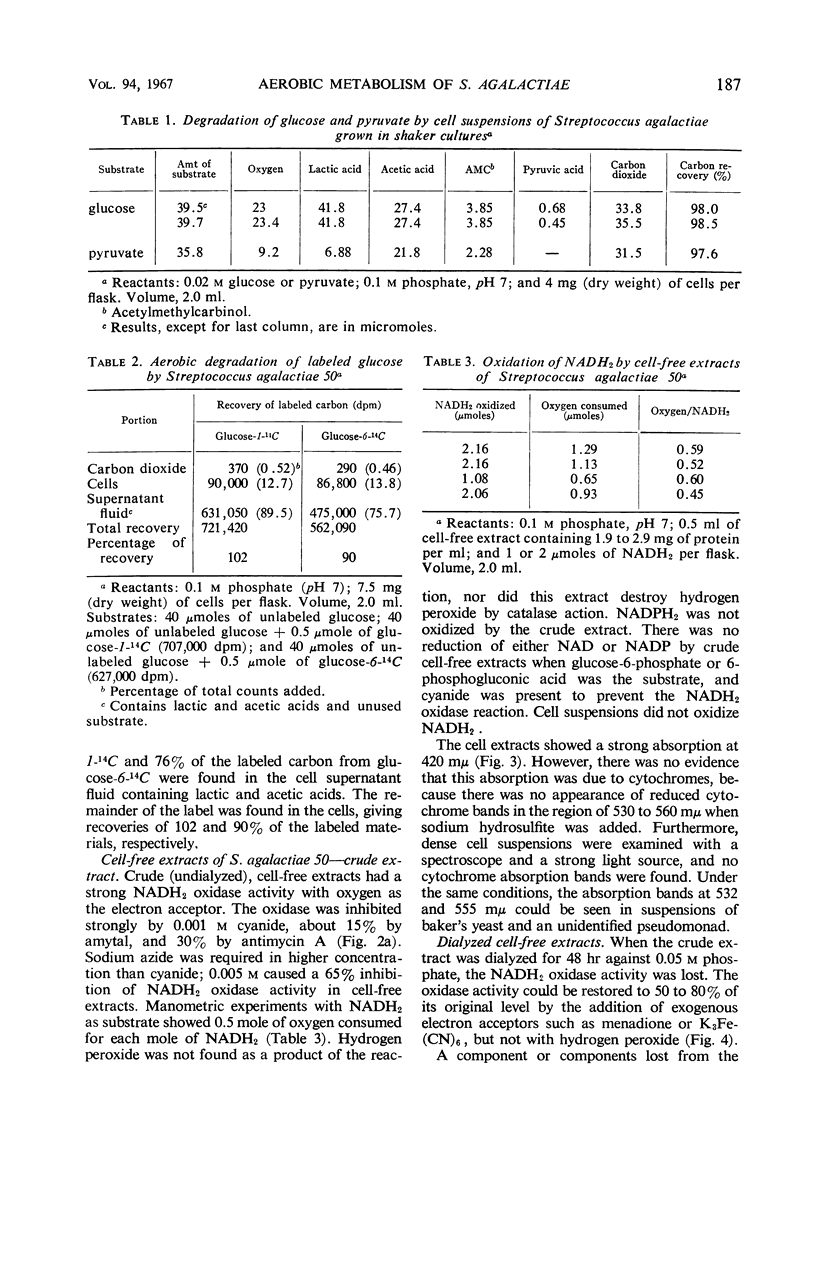
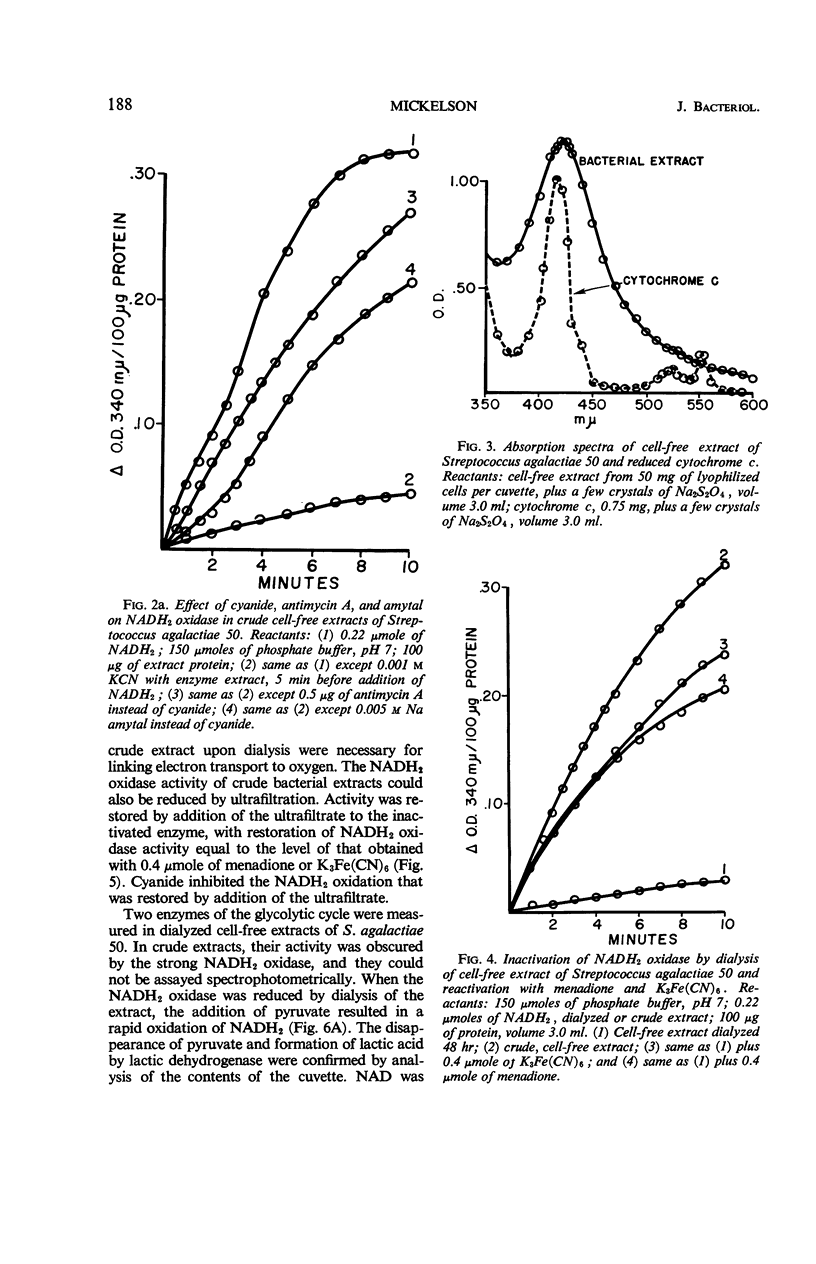
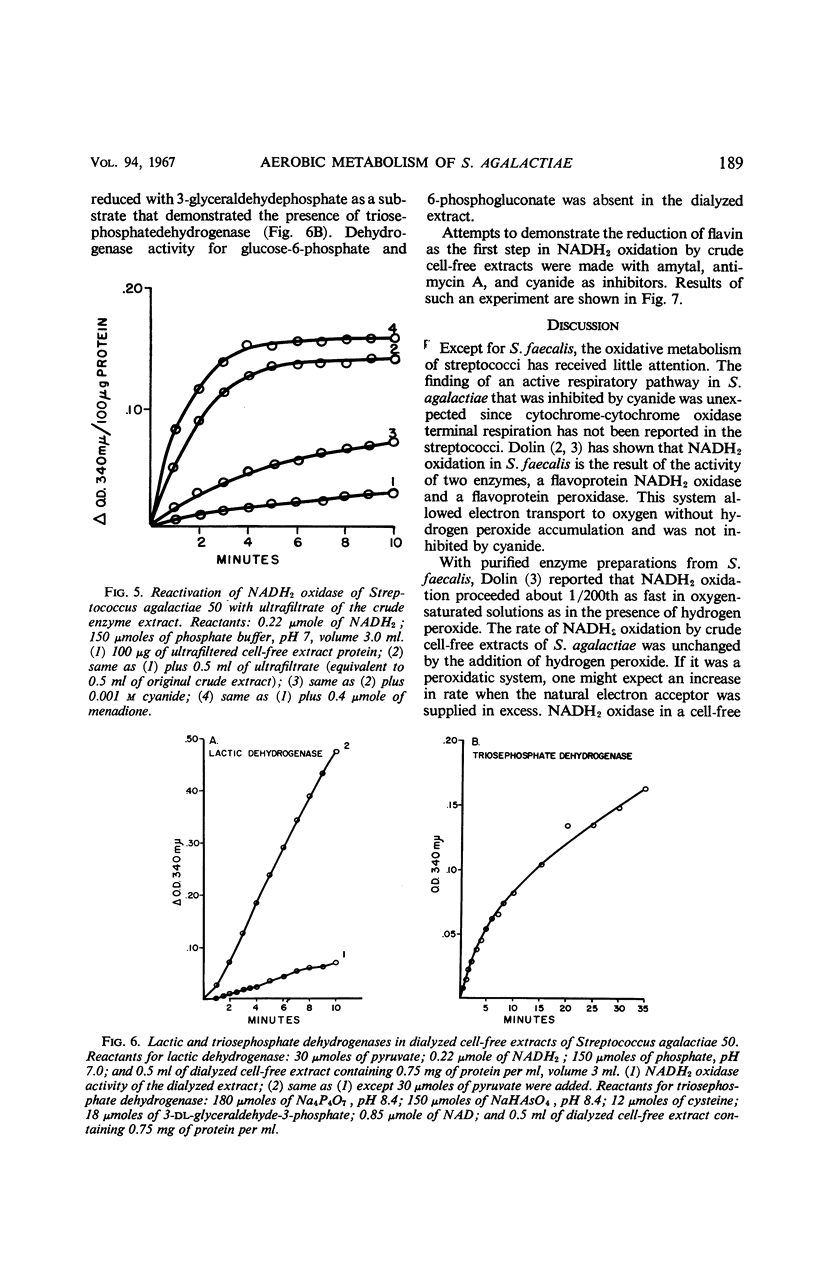
Streptococcus agalactiae cultures possess an aerobic pathway for glucose oxidation that is strongly inhibited by cyanide. The products of glucose oxidation by aerobically grown cells of S. agalactiae 50 are lactic and acetic acids, acetylmethylcarbinol, and carbon dioxide. Glucose degradation products by aerobically grown cells, as percentage of glucose carbon, were 52 to 61% lactic acid, 20 to 23% acetic acid, 5.5 to 6.5% acetylmethylcarbinol, and 14 to 16% carbon dioxide. There was no evidence for a pentose cycle or a tricarboxylic acid cycle. Crude cell-free extracts of S. agalactiae 50 possessed a strong reduced nicotinamide adenine dinucleotide (NADH2) oxidase that is also cyanide-sensitive. Dialysis or ultrafiltration of the crude, cell-free extract resulted in loss of NADH2 oxidase activity. Oxidase activity was restored to the inactive extract by addition of the ultrafiltrate or by addition of menadione or K3Fe(CN)6. Noncytochrome iron-containing pigments were present in cell-free extracts of S. agalactiae. The possible participation of these pigments in the respiration of S. agalactiae is presently being studied.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DOLIN M. I. The Streptococcus faecalis oxidases for reduced diphosphopyridine nucleotide. III. Isolation and properties of a flavin peroxidase for reduced diphosphopyridine nucleotide. J Biol Chem. 1957 Mar;225(1):557–573. [PubMed] [Google Scholar]
- Greisen E. C., Gunsalus I. C. An Alcohol Oxidation System in Streptococci Which Functions without Hydrogen Peroxide Accumulation. J Bacteriol. 1944 Nov;48(5):515–525. doi: 10.1128/jb.48.5.515-525.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunsalus I. C., Wood A. J. The Dehydrogenation of Alcohols by Streptococci of Group B. J Bacteriol. 1942 Nov;44(5):523–528. doi: 10.1128/jb.44.5.523-528.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KOEPSELL H. J., SHARPE E. S. Micro-determination of pyruvic and alpha-keto-glutaric acids. Arch Biochem Biophys. 1952 Jul;38:443–449. doi: 10.1016/0003-9861(52)90050-7. [DOI] [PubMed] [Google Scholar]
- Kurup C. K., Vaidyanathan C. S., Ramasarma T. NADH oxidase system of Agrobacterium tumefaciens. Arch Biochem Biophys. 1966 Mar;113(3):548–553. doi: 10.1016/0003-9861(66)90232-3. [DOI] [PubMed] [Google Scholar]
- Mickelson M. N. Effect of lactoperoxidase and thiocyanate on the growth of Streptococcus pyogenes and Streptococcus agalactiae in a chemically defined culture medium. J Gen Microbiol. 1966 Apr;43(1):31–43. doi: 10.1099/00221287-43-1-31. [DOI] [PubMed] [Google Scholar]
- O'KANE D. J. Influence of the pyruvate oxidation factor on the oxidative metabolism of glucose by Streptococcus faecalis. J Bacteriol. 1950 Oct;60(4):449–458. doi: 10.1128/jb.60.4.449-458.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAMASARMA T., LESTER R. L. Studies on the electron transport system. XXIV. The reduction and oxidation of exogenous coenzyme Q. J Biol Chem. 1960 Nov;235:3309–3314. [PubMed] [Google Scholar]
- SEELEY H. W., VANDEMARK P. J. An adaptive peroxidation by Streptococcus faecalis. J Bacteriol. 1951 Jan;61(1):27–35. doi: 10.1128/jb.61.1.27-35.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]


