Abstract
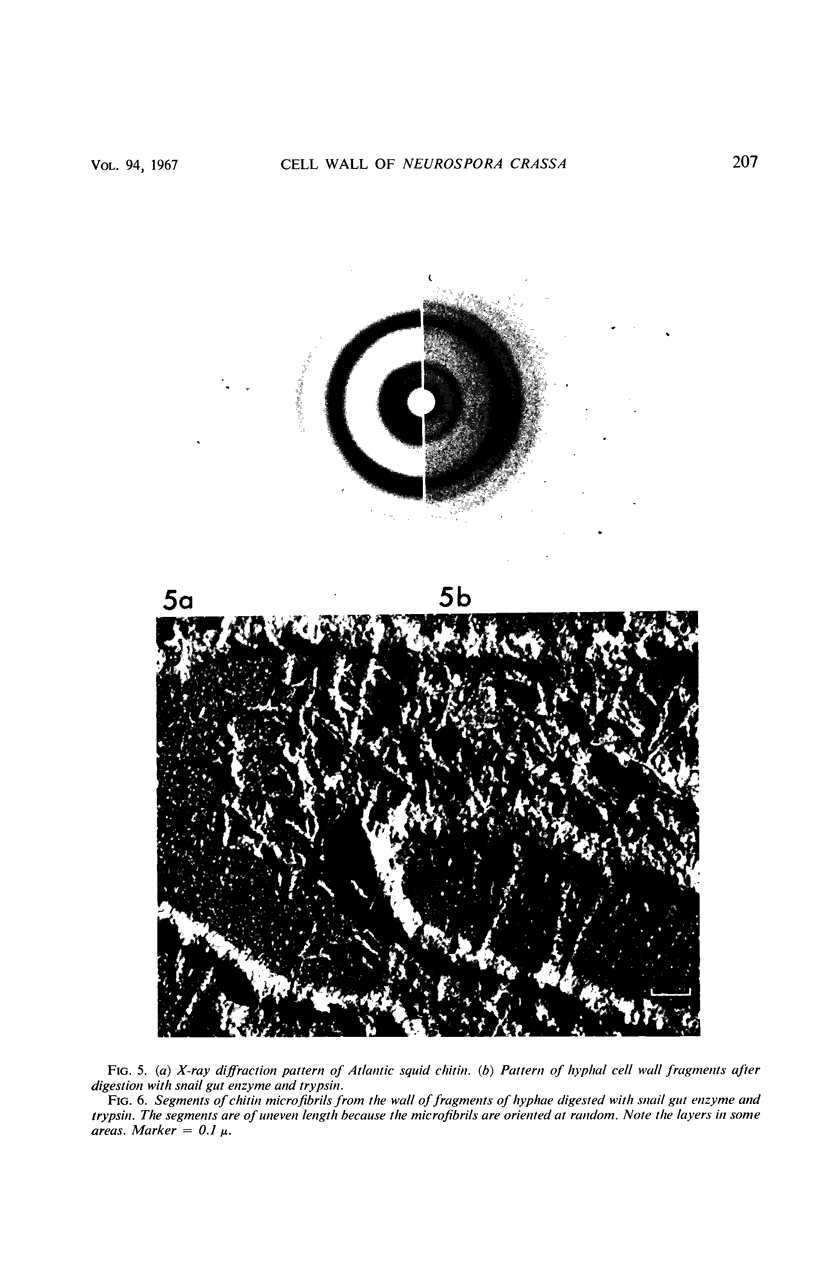
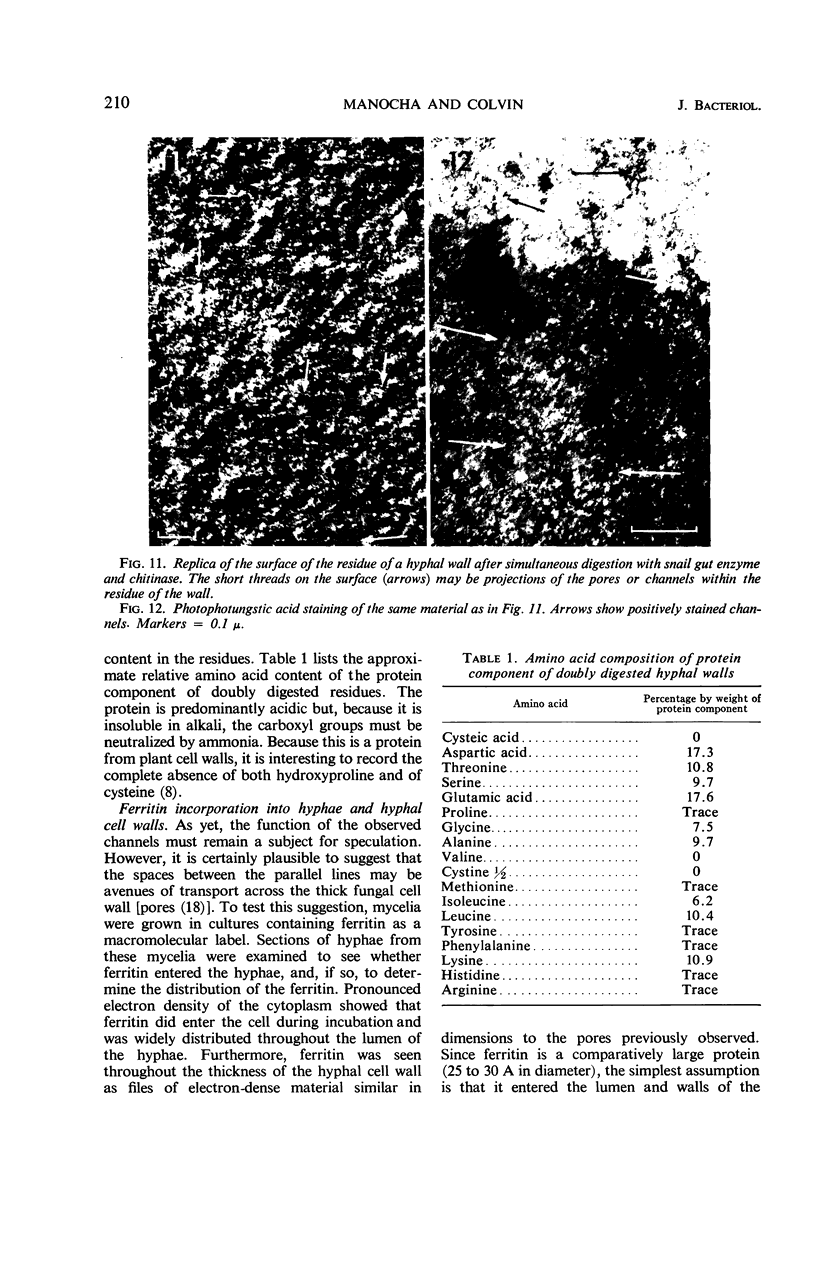
The structure and composition of the cell walls of hyphae of Neurospora crassa were investigated by electron microscopy, chemical analysis, and X-ray diffraction both before and after progressive enzymatic degradation by snail gut enzymes, chitinase, and trypsin. The wall consists of two phases: randomly disposed skeletal microfibrils of chitin only and an amorphous matrix which contains both β-glucans and protein. The protein contains a high percentage of the amides of aspartic and glutamic acid but no hydroxy-proline or cysteine. A portion of this protein is a component of or is associated with a system of pores which is embedded in the matrix of the wall. These pores, 40 to 70 A in outside diameter, sometimes branch and seem to provide a three-dimensional network from one side of the wall to the other. They may be a general system of transport across the walls.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARONSON J. M., PRESTON R. D. An electron microscopic and x-ray analysis of the walls of selected lower Phycomycetes. Proc R Soc Lond B Biol Sci. 1960 Jun 14;152:346–352. doi: 10.1098/rspb.1960.0043. [DOI] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Isolation, composition, and structure of cell walls of filamentous and yeast-like forms of Mucor rouxii. Biochim Biophys Acta. 1962 Mar 26;58:102–119. doi: 10.1016/0006-3002(62)90822-3. [DOI] [PubMed] [Google Scholar]
- BAYLEY S. T., COLVIN J. R., COOPER F. P., MARTIN-SMITH C. A. The structure of the primary epidermal cell wall of Avena coleoptiles. J Biophys Biochem Cytol. 1957 Mar 25;3(2):171–182. doi: 10.1083/jcb.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLONIG G. A modified procedure for lead staining of thin sections. J Biophys Biochem Cytol. 1961 Dec;11:736–739. doi: 10.1083/jcb.11.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POTGIETER H. J., ALEXANDER M. POLYSACCHARIDE COMPONENTS OF NEUROSPORA CRASSA HYPHAL WALLS. Can J Microbiol. 1965 Feb;11:122–125. doi: 10.1139/m65-017. [DOI] [PubMed] [Google Scholar]
- SHATKIN A. J., TATUM E. L. Electron microscopy of Neurospora crassa mycelia. J Biophys Biochem Cytol. 1959 Dec;6:423–426. doi: 10.1083/jcb.6.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trevithick J. R., Metzenberg R. L. Molecular sieving by Neurospora cell walls during secretion of invertase isozymes. J Bacteriol. 1966 Oct;92(4):1010–1015. doi: 10.1128/jb.92.4.1010-1015.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara T., Yamada M. Cytological structure of Aspergillus niger by electron microscopy. Jpn J Microbiol. 1965 Mar;9(1):35–48. doi: 10.1111/j.1348-0421.1965.tb00273.x. [DOI] [PubMed] [Google Scholar]
- Whitaker D. R., Roy C., Tsai C. S., Jurásek L. Lytic enzymes of Sorangium sp. A comparison of the proteolytic properties of the alpha- and beta-lytic proteases. Can J Biochem. 1965 Dec;43(12):1961–1970. doi: 10.1139/o65-219. [DOI] [PubMed] [Google Scholar]