Abstract
Background
Despite evidence that optimal care for diabetes can result in reduced complications and improved economic outcomes, such care is often not achieved. The Vermont Diabetes Information System (VDIS) is a registry-based decision support and reminder system based on the Chronic Care Model and targeted to primary care physicians and their patients with diabetes.
Purpose
To develop and evaluate a regional decision support system for patients with diabetes.
Methods
Randomized trial of an information system with clustering at the practice level. Ten percent random sub sample of patients selected for a home interview. Subject and setting includes 10 hospitals, 121 primary care providers, and 7,348 patients in 55 Vermont and New York primary care practices.
Results
We report on the study design and baseline characteristics of the population. Patients have a mean age of 63 years and a mean glycosolated hemoglobin A1C of 7.1%. Sixty percent of the population has excellent glycemic control (A1C<7%); 45% have excellent lipid control (serum LDL-cholesterol < 100mg/dl and serum triglycerides < 400mg/dl). Twenty-five percent have excellent blood pressure control (<130/80 mm Hg). These results compare favorably to recent national reports. However, only 8% are in optimal control for all three of hyperglycemia, lipids and blood pressure.
Conclusions
Our experience to date indicates that a low cost decision support and information system based on the chronic care model is feasible in primary care practices that lack sophisticated electronic information systems. VDIS is well accepted by patients, providers, and laboratory staff. If proven beneficial in a rigorous, randomized, controlled evaluation, the intervention could be widely disseminated to practices across America and the world with a substantial impact on the outcomes and costs of diabetes. It could also be adapted to other chronic conditions. We anticipate the results of the study will be available in 2006.
Background and Significance
Diabetes mellitus is one of the most common chronic diseases treated in the United States, affecting almost 8% of the adult population. [1, 2] Because diabetes leads to a variety of debilitating complications, it also accounts for a disproportionately high amount of health care spending. [3-5] Treatment of hyperglycemia has been shown to lower the risk of microvascular complications. [6-10] There is increasing evidence that management of associated cardiac risk factors, in particular hypertension and dyslipidemia, results in reduced macrovascular complications. [11, 12] One study, using an approach targeted at treatment of hyperglycemia, hypertension, dyslipidemia, microalbuminuria, and cardiac risk reduction with aspirin, achieved a 50% reduction in cardiovascular and microvascular complications. [13]
Despite evidence that optimal care can result in reduced complications and improved economic outcomes, such care is often not achieved. [14-17] A recent study of outcomes in diabetic patients from the National Health and Nutrition Examination Survey found that 37% had poor glycemic control (A1C>8%), 40% had blood pressure values >140/90 mm Hg, and over half had cholesterol levels greater than 200 mg/dl. In total, only 7.3% of patients were on target for all three indicators. [17]
Although it is generally accepted that expert, best-practice, clinical guidelines will lead to improvement in clinical care processes and outcomes [18], these effects may not persist without a comprehensive and ongoing system for quality improvement. [19-22] Decision support information is a key element in future efforts to improve clinical performance. Decision support is difficult to provide without an information system that can provide reminders, alerts, and feedback to the provider (and patient) at the point of care.
Several studies have reported improvement in outcomes for diabetic patients by using population-based, decision support approaches. These studies have been conducted largely in staff-model managed care organizations with robust information systems. [23-25] The majority of health care in the United States is, however, delivered in settings where a wide variety of insurance plans are accepted and a central information system is not used.
We have developed and implemented a regional evidence-based disease management system for primary care providers and their patients called VDIS (Vermont Diabetes Information System). The system supports primary care providers in meeting treatment targets for management of diabetes and associated vascular risk factors. Our primary goal in this randomized controlled trial is to study the effect of the information system (including patient and physician reminders, feedback, and decision support) on disease control as measured by glycosolated hemoglobin A1C (A1C). Secondary questions address the effect of the system on hyperlipidemia, renal monitoring and function, adherence to guideline recommendations, blood pressure, patient satisfaction, medication use, and functional status. We hypothesize that the information system will result in improvements in the process and outcomes of clinical care. In this paper, we describe the design and implementation of the system and the baseline characteristics of the study population.
Methods
VDIS is a decision support and reminder system for primary care practices and their patients with diabetes. It is based on the principles of quality improvement of Donabedian [26] and the Chronic Care Model of illness management. [27, 28] The Chronic Care Model emphasizes the importance of bringing together for an ideal clinical encounter a prepared, proactive health care team and an informed, activated patient. Chronic disease registries are a central aspect of this model. While other implementations of the Chronic Care Model require substantial investment by the practice and major changes in the providers' usual activities, we designed VDIS to require a minimum of effort and no new financial resources on the part of the providers.
VDIS is a joint effort of the study investigators, the Vermont Program for Quality in Health Care (VPQHC), and The North East Community Laboratory Alliance (NECLA). VPQHC is a non-profit peer-review organization founded in 1988 with a mission to develop and implement a system of quality design and measurement in Vermont. The board of directors includes representatives of consumers, hospitals, insurers, employers, physicians, and state government. NECLA is a regional network of community-based clinical laboratories formed in 1996 to provide patients and physicians with coordinated access to high quality, cost-effective laboratory services that improve the health status of local communities. [29] The network currently includes 13 of the 14 acute care hospitals in Vermont. One of the network's missions is to develop and share disease management strategies. The development and evaluation of VDIS is funded by the U.S. National Institute of Diabetes and Digestive and Kidney Diseases (R01 DK61167).
Technical Description of VDIS
There are five defining components that characterize VDIS: 1) Use of the Chronic Care Model as an organizing framework, 2) Daily data feeds from otherwise independent laboratories, 3) Automatic test interpretation using algorithms based on consensus guidelines, 4) Use of fax and mail to report to providers and patients not easily reached by electronic networks, and 5) Report formats that are accessible and useful to patients and providers. The primary function of the system is to collect pertinent clinical information and to provide accurate and timely flow sheets, reminders, and alerts to physicians and their patients with diabetes. Secondly, the system generates summary population reports for physicians regarding their roster of diabetic patients. The intended effects of the interventions are outlined in Table 1.
Table 1. Anticipated effects of VDIS interventions.
| Intervention | Anticipated effect |
|---|---|
| Directed to the Practice and Primary Care Provider | |
|
Provide decision support and stimulate appropriate action by provider. |
| Stimulate follow-up of patients who are lost to follow up or otherwise overdue. | |
| Provide the provider a population-based view of his or her entire diabetes patient roster for targeted case management. Allow provider to keep roster of patients up to date. Peer comparison may motivate a practice to modify office processes for chronic illness management. | |
|
| |
| Directed to the Patient | |
|
Engage and activate patients to know and understand the goals of therapy and to be prepared for interaction with the provider. |
| Remind patient to schedule follow up testing or an office visit. | |
Data Loading
For each participating practice, an initial list of patients is developed by the lab, based on all patients who have had an A1C test performed in the previous two years. This list is verified by the Primary Care Provider (PCP) to determine the eligibility of each patient. Once the PCP has verified the list, the patient demographic data are loaded into a custom Oracle data repository. Subsequently, the lab prepares a two-year historical report of lab results for those patients and this information is loaded into the database for seeding of flow sheets, reminders, and alerts. The lab results that are pertinent to management of most patients with diabetes, and that are the subject of guideline recommendations, are the A1C, serum lipid tests, urinary microalbumin to creatinine ratio (MCR), and the serum creatinine.
Nightly Data Collection and Processing
The collection of the laboratory data in a timely manner is essential to the creation and distribution of the flow sheets. A nightly program automatically reports that day's A1C, lipid, microalbumin, and creatinine results on the population of identified subjects. This file is transferred using File Transfer Protocol (FTP) and a variety of secure connection methods. Most of the connections are done via branch-to-branch Virtual Private Network (VPN) connections over the Internet or private leased data lines. These daily report files are then processed into the registry database. The system also allows manual data input via a secure Internet forms software function. The software accepts the Medical Record Number and test results and processes them into the registry. This function allows practices performing point of care testing in the office to directly enter test results.
Report Triggering
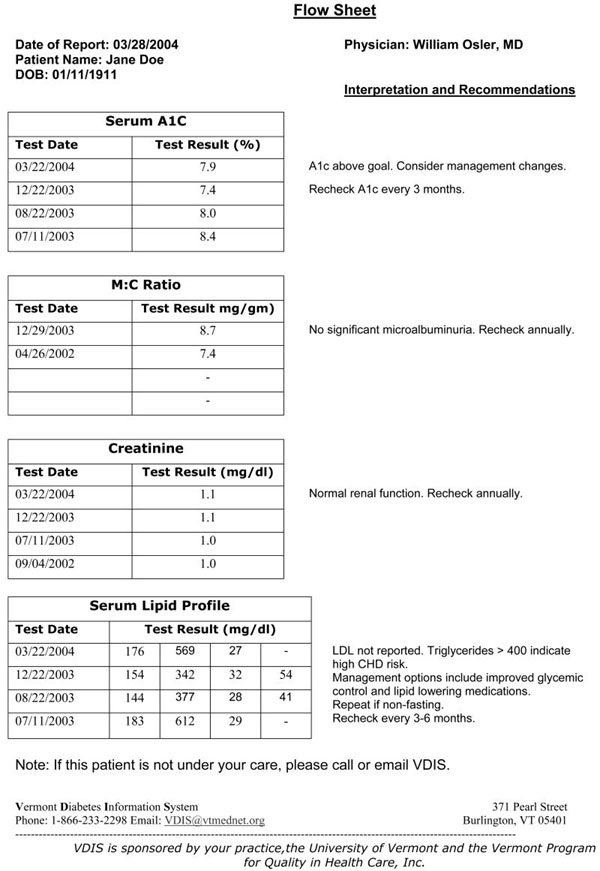
The report generator function runs automatically each night after results are received. Any lab result for A1C, LDL, creatinine, or MCR triggers the creation and faxing to the PCP of a flow sheet displaying the current results, the previous four results in the database (to display trends), and decision support recommendations based on published guidelines [30,31]. If a result is above a threshold level, an alert letter is electronically sent to a mail and production service for mailing to the patient. If a patient is overdue for a lab test, an alert fax is sent to the provider, and a letter is mailed to the patient to remind them both of the recommended testing. See the appendix for examples of these outputs. None of the VDIS output is part of the permanent medical record and does not require filing in the chart. The labs continue to send their routine reports to the practices.
The thresholds for designating a result to be high were taken from a Vermont guideline [30] based on the American Diabetes Association Clinical Practice Recommendations [31] for a change in therapy (A1C>8%; LDL >130mg/dl; MCR>300 μg/gm). An A1C is overdue if the previous A1C is more than six months old, or if the previous A1C is 7.0% or greater and more than three months old. A one month grace period is allowed, so a patient reminder letter is not generated until seven or four months have elapsed. A six to twelve month overdue period (plus the one month grace period) is applied to LDL and MCR depending on the result range. Since microalbumin testing is often stopped after the development of proteinuria (and appropriate therapy with medications directed at the renin-angiotensin system), we suppress MCR reminders once the patient has microalbuminuria.
Quarterly population reports are intended to provide the PCP with a population-based view of his or her roster of diabetic patients. PCPs are encouraged to use the roster for identification of patients who are off guideline or lost to follow-up. The population report also contains comparisons of individual PCP performance with the performance of the entire study population for both on-target and on-time with guideline-based goals. We also include a top ten percent performance measure, the Achievable Benchmark of Care. [32, 33]
Practices and Study Subjects
Labs were recruited for VDIS through the Northeast Community Laboratory Alliance and personal communication with lab directors and hospital administrators. Eight of the fourteen hospital-based labs in Vermont as well as four in nearby New York and another in nearby New Hampshire have joined the study. Ten are currently reporting data in the project. Technical personnel from each lab work with the investigators to create a secure connection for the daily transmission of lab results.
To be eligible, an Internal Medicine or Family Medicine practice must: 1) use one of the participating labs, 2) care for patients with diabetes, 3) be able to receive faxes, and 4) provide consent. Practices using point of care testing devices for a small proportion of their testing were invited to participate if we were able to arrange for an efficient method of data acquisition. This was accomplished by daily fax of point of care test results to the VDIS office and web-based data entry into the system by VDIS staff. Some of the largest practices in the state, most notably the faculty practices of the University of Vermont, were not eligible to participate because they were involved in pilot work for this study.
We identified and contacted 141 practices that were potentially eligible for participation in the study from the customer lists of the participating labs and by personal communication with providers around the state. Practices were invited to participate in the project by mail, phone and presentations at Grand Rounds or Medical Staff meetings at four of the hospitals. Twenty-two practices were ineligible for the following reasons: Practice uses point of care testing device for A1C or lipids making data acquisition impractical (9), practice participating in a conflicting practice improvement study (6), practice changes such as a new practice, retiring or ill provider (4), scope of practice does not include diabetes care (2), practice uses an EMR that includes reminder and decision support functions (1). Of the 119 eligible practices 74 (62%) agreed to participate in the study; 45 practices (38%) declined participation or did not respond to multiple attempts at contact. Introductory meetings were held with interested practices to explain the project and solicit participation. If a practice agreed to participate, all PCPs from the practice gave written informed consent. An overview of the principles of the Chronic Care Model and the current Vermont Department of Health diabetes treatment guidelines were provided.
Once a practice was enrolled, a list of all patients with a test for A1C in the previous two years was generated by the lab. These lists were reviewed by each PCP to identify those patients who met the following eligibility criteria: 1) diabetes type 1 or type 2, 2) age 18 or older, 3) under the care of that PCP for diabetes, and 4) not suffering from cognitive impairment that would prevent understanding reminders, per the judgment of the PCP. Any conflicts were resolved by discussion with the PCP offices. If a patient was receiving the majority of diabetes care from an endocrinologist or other provider, they were not included on the final PCP roster. We did not distinguish between Type 1 and Type 2 DM because the ADA guidelines do not differ substantially regarding testing frequency or therapeutic goals, and because it is often unclear clinically which type of diabetes is present. If a new patient with diabetes is encountered in the course of the study, they may be added to the system for clinical purposes, but are not part of the study population.
A practice is affiliated with one and only one laboratory. We desired to ensure that no laboratory had a gross preponderance of active or control practices. Each laboratory represented a stratum in a stratified and blocked randomization scheme. A series of numbered, sealed, opaque envelopes were created for each stratum (each laboratory). The envelopes contained a card indicating either CONTROL or ACTIVE condition. Blocks of four or six envelopes were filled with balanced numbers of ACTIVE and CONTROL cards, sealed, and shuffled thoroughly within blocks. In that way, each stratum was likely to have an approximately equal number of active and control practices. After each practice was recruited and consented, the next envelope in their laboratory stratum's series was opened to determine the assignment for that practice. The practice was chosen as the unit of randomization because of the sharing of patients and systems of care among PCPs in the same office. Intervention practices receive the VDIS intervention while the control practices have patient data collected behind the scenes, and otherwise continue with usual care. The initial practice start date was June 5, 2003 and the most recent practice start date is September 18, 2004. New practices continue to be enrolled and randomized as new labs are brought into the study.
Consent Process and Privacy Issues
Decision support services (such as the information systems, registry functions, reminders, and reports of VDIS) are clinical quality improvement activities that require personal health information as defined and protected under the Health Insurance Portability and Accountability Act (HIPAA). Providers may generally conduct such activities without a specific consent from the patient, although certain restrictions apply such as protection of patient confidentiality. To ensure that the registry data could not be accessed by others, VDIS is structured as a regional quality improvement initiative under the direction and supervision of the Vermont Program for Quality in Health Care (VPQHC), a state chartered peer-review organization. Vermont law protects findings, reports and deliberations of peer review activities from legal discovery, thereby relieving any potential concern on the part of the practices or laboratories that the data could be used to embarrass or legally disadvantage them. (Vermont 26 V.S.A. Sec 144, et seq.)
Research activities face stricter regulations under HIPAA and the federal “Common Rule” governing human investigations and generally require patients to provide informed consent. With identical language, however, both sets of regulations allow an Institutional Review Board (IRB) or Privacy Board to waive active consent if the study meets four criteria set out in Federal guidelines [45CFR46 §46.116(d)]:
“An IRB may approve a consent procedure which does not include, or which alters, some or all of the elements of informed consent set forth in this section, or waive the requirements to obtain informed consent provided the IRB finds and documents that:
the research involves no more than minimal risk to the subjects;
the waiver or alteration will not adversely affect the rights and welfare of the subjects;
the research could not practicably be carried out without the waiver or alteration; and
whenever appropriate, the subjects will be provided with additional pertinent information after participation.”
Because it meets all four criteria, the study protocol was approved by the University's Committee for the Protection of Human Subjects, by legal review at VPQHC, and by further legal and IRB review at the participating hospital labs. An additional special review was held at the National Institutes of Health.
Although not required by law, we employ a passive (“opt-out”) consent process for inviting patients into the study. After the patient is identified, but before any services are initiated, we mail a letter to the patient on behalf of the PCP. The letter describes the study and invites the patient to participate. It requests that the patient call the provider or a toll-free number at the University, if they prefer not to participate. All laboratory data for these patients are removed from the database.
We considered using an active (“opt-in”) consent process for VDIS that requires each potential subject to sign and return a traditional consent form. We saw this as a potentially fatal flaw in that it would have likely resulted in a greatly reduced sample size and would have introduced a response bias that would limit generalizabilty. In an evaluation of community-based interventions that are designed to be easily reproducible in common clinical settings, it is especially important to have the study population closely resemble the target population.
The PCPs are also considered subjects of this research. Therefore, each participating provider signs an informed consent agreement.
VDIS Survey
One advantage of the design of VDIS is that, once the connection to the lab is made, the cost of acquisition of lab data is negligible. One disadvantage is that these data are limited to laboratory results, sex, and date of birth. In order to obtain a deeper understanding of the study population and the impact of the intervention, we designed a survey targeted at a randomly selected 10% sub-sample of patient subjects. Practice rosters are randomly sorted and patients invited by phone to participate in an in-home interview consisting of a questionnaire, measurement of height using a portable stadiometer (SECA, Inc.), weight (LB Dial Scale HAP200KD-41, Healthometer, Inc.), blood pressure (Omron automated sphygmomanometer, Model HEM-711), and administration of a test of health literacy. Blood pressure is obtained in the seated position in the left arm (unless contraindicated), using the cuff size recommended by the manufacturer. Three readings are obtained at 5-minute intervals and are averaged for the final result. The research assistant reviews questionnaires for completeness at the time of the interview. Patients are reimbursed $20 for their time. Patients who are enrolled in the sub-study provide full written informed consent before they are interviewed. Table 2 lists the variables included in the VDIS study, including those in the survey.
Table 2. Study Variables in the VDIS Trial.
| Dimension | Variables |
|---|---|
| Laboratory Data | |
| Glycemic control | A1C |
| Lipid control | Total Cholesterol, Triglyceride, High Density Lipoprotein, Low Density Lipoprotein |
| Renal function | Creatinine, microalbumin:creatinine ratio |
| Demography | Date of birth, sex |
|
| |
| Physical examination and direct observation | |
| Obesity | Height, Weight, Body Mass Index |
| Hypertension | Blood pressure |
| Heart Rate | Pulse |
| Functional Health Literacy | Short Test of Functional Health Literacy in Adults [40] |
| Medications | Medication list with name, dose, frequency of all prescription, over-the-counter, herbal or supplement preparations used in the last month |
|
| |
| Self Report | |
| Demography | Income, education, marital status, race/ethnicity, health insurance |
| Health Habits | Smoking, drinking, exercise habits |
| Functional Status | Medical Outcomes Trust SF-12 [34] |
| Diabetes-related quality of life | The Audit of Diabetes-Dependant Quality of Life [36] |
| Diabetes Self Care | Summary of Diabetes Self Care Activities Measure [61] |
| Health Care Utilization | Self-report of visits to primary care, Emergency Room, Endocrinology, Ophthalmology, Diabetes Educator, Dietician |
| Complication Status | Self-report of diabetes complications |
| Comorbidity | Self Administered Comorbidity Questionnaire [39] |
| Patient Satisfaction | Primary Care Assessment Survey [42] |
| Diabetes Utility | Paper Standard Gamble [37] |
| Depression | Patient Health Questionnaire-9 [62] |
The Medical Outcomes Trust SF-12 is a widely used, validated instrument for assessment of general (rather than disease-specific) functional status [34]. Summary scales covering Mental and Physical Functioning are calculated: the Physical Component Summary and the Mental Component Summary.
The Audit of Diabetes-Dependant Quality of Life is an 18-item questionnaire regarding the impact of diabetes on specific aspects of a person's life with patient weighting of the impact of each domain. [35, 36]
Another approach to health related quality of life is to measure the subject's quantitative preference for their current health. This measure, called “utility,” is widely used in cost-effectiveness analyses and other economic studies. The Paper Standard Gamble is a one page assessment of patient utility that has been validated for use in postal surveys. [37]
The Self-Administered Comorbidity Questionnaire is a modification of the widely used Charlson Index. It uses patient interview or questionnaire rather than chart abstraction for assessment of comorbidity and has excellent agreement with the chart-based Charlson Index. [38, 39]
The Short Test of Functional Health Literacy in Adults is a 7-minute timed instrument that measures the ability to read health-related material. [40, 41]
The Primary Care Assessment Survey is a validated, 51-item patient-completed questionnaire designed to measure the essential elements of primary care. It measures seven characteristics of primary care through eleven summary scales: accessibility, continuity, comprehensiveness, integration of care, clinical interaction, interpersonal treatment, and trust. [42]
The Patient Health Questionnaire-9 is a brief self-report instrument that quantifies the presence and degree of mental depression [43]
Statistical approach
This is a two-arm randomized trial with clustering by practice. Our primary null hypothesis is that there will be no difference between the intervention and control groups in mean A1C level at study's end. Secondary analyses will focus on group differences in lipids, creatinine, proportion on guideline, and proportion adhering to specific guideline components (overdue for specific tests or out of range for specific tests). We will use a general linear mixed model for outcomes with normally distributed residual errors, or a generalized linear mixed model for outcomes with binomial distribution for residual errors. [44] The primary analysis will include all participants and use final hemoglobin A1C as the dependent variable. Independent variables will be dichotomous variables representing randomization status (1=active; 0=control) and patient sex, and continuous variables representing hemoglobin A1C at baseline and patient age. Since the unit of randomization is the practice, we will adjust all standard errors for clustering on practice.
Clustering reduces statistical power in proportion to the degree that subjects within each cluster are similar. To account for this, we modeled sample size using the methods of Donner and others [45-47], which require an estimate of the intraclass (or within practice) correlation coefficient to use in a variance inflation factor. Initial data from VDIS indicate a standard deviation of A1C of 1.4% and an intra-class correlation of 0.02. There are, on average, 125 eligible subjects per practice. Using alpha=0.05 and a power of 80%, we require 20 randomized practices (10 per arm) to detect a difference between control and active groups of 0.3%. To detect a difference of only 0.2% requires 44 randomized practices per arm. Currently, 55 practices have been activated and another 17 are in the process of coming into the system.
Results
We report on the 10 hospitals, 55 practices, 121 primary care providers, and 7,348 patients who are currently active in the VIDS system. The baseline characteristics of the patient population are shown in Table 3. The demographic characteristics match the population of Vermont. [47]
Table 3. Baseline Characteristics of the VDIS Patient Population.
| Characteristic | Result |
|---|---|
| Registry Data (N=7,348) | |
| Age in years, mean (range) | 62.9 (18-99) |
| Female | 51% |
| A1C, mean (SD) | 7.1 (1.4) |
| A1C in excellent control (<7%) | 60% |
| A1C on time (within 3 months if A1C<7%; 6 months if A1C ≥7%) | 49% |
| Lipids in control (LDL <100mg/dl; triglyceride <400 mg/dl) | 45% |
| Lipids on time (within 12 months) | 67% |
| Microalbuminuria absent (<30 mg/gm) | 69% |
| Microalbumin test on time (within 12 months) | 23% |
|
| |
| Survey data (n=746) | |
| Race (% white) | 97% |
| Education (% some college) | 41% |
| Smoking (% current smokers) | 15% |
| Income (<$30,000/y) | 56% |
| Body Mass Index (SD) | 33.7 (7.8) |
| Excellent blood pressure control (<=130/80 mm Hg) | 25% |
| Poor blood pressure control (>140/90 mm Hg) | 49% |
| SF-12 Physical Component Summary, mean (SD) | 41.8 (12.3) |
| SF-12 Mental Component Summary, mean (SD) | 50.2 (10.5) |
| Duration of diabetes in years, mean (range) | 10.9 (0.3-63) |
| Number of comorbid conditions, mean (range) | 1.8 (0-13) |
SD = standard deviation; LDL = Low density lipoprotein cholesterol
Two-hundred-seven invited patients have declined participation. The refusal rate is 207/7555 or 2.7%. Patients cite a variety of reasons including “feeling too ill,” “too old,” concerns regarding privacy and sharing of lab data, and not identifying oneself as a diabetic.
The number of primary care providers per practice averages 2.1 with a range of 1-6. Of the PCPs, 93 are physicians, 13 are nurse practitioners and 15 are physician assistants. The mean PCP panel size is 59 patients with a range of 1-201. The mean practice panel size is 125 patients with a range of 12-353.
Discussion
Despite evidence that optimal care of diabetes can result in reduced complications and improved economic outcomes, such care is often not achieved. [14-17] We have developed and are testing a registry-based system that delivers decision support, reminders, and population reporting to the primary care provider, and aims to provide patient activation and knowledge through reminders and alerts to patients regarding their own lab results. Our primary analytic goal is to determine the impact of the intervention on glycemic control.
Our intervention was designed to incorporate many aspects of the Chronic Care Model. This model has been promoted as a paradigm for overcoming barriers to optimal care of patients with chronic conditions by the Robert Wood Johnson Foundation in their Improving Chronic Illness Care program. [27, 28] [49] The Health Disparities Collaborative, supported by the US Health Resources and Services Administration is using the model to support chronic illness care in federally qualified Community Health Centers. [50] The model includes aspects of traditional case management which been found to be effective in improving outcomes in diabetes. [51]
Our design raises important issues regarding the role of patient consent for studies based on disease registries. A recent report on the impracticability of active consent for a Canadian stroke network highlighted the biases introduced by the consent process.[52] Ingelfinger, in an accompanying editorial, highlights several examples of important problems experienced by registries related to privacy and consent issues. We have been successful in recruiting almost 8,000 patients into a registry that is overseen by an independent peer review and quality improvement organization. Our refusal rate has been very low at 2.7%. HIPAA and IRB regulations have provisions that allow for the use of registries in conducting clinical research which were vital to the implementation of this project. We have learned that the passive consent process is acceptable to patients and providers in this trial. Of the 207 patients who declined to participate in the study, only 3 raised significant concerns about potential violations of privacy. These cases have resulted in interesting discussions with the IRB and the Data Safety Monitoring Board as this type of trial represents new territory for these boards. As an extension of this trial, we are planning further study of the patient and provider experience with the consent process.
Several studies have reported improvement in outcomes for diabetic patients by using population-based, decision support approaches in staff-model managed care organizations with robust information systems.[23-25] The majority of health care in the United States though, is delivered in settings where a wide variety of insurance plans are accepted and a central information system is not used. There have been reports describing improvements in diabetes clinical process measures in these settings using population-based approaches, but many of these studies suffer from limitations such as a single-institution setting, lack of physiologic outcomes, short duration, or observational design. [53-58]
Like many of these studies, our quarterly population reports provide a current roster of patients for each PCP, sorted by level of control of A1C, lipids and urinary albumin excretion. This allows the provider to identify patients who may benefit from a more intensive intervention. We have deliberately not required a specific case management approach because we are working with a diverse group of practices with widely varying office processes. A mandated case management approach would add significantly to the burden on the practice and on the cost of the intervention itself. Practice participation in VDIS does not require additional personnel, or require a major change in the office processes or referral patterns of the participating practices. In designing the system, we focused on avoiding data entry and minimizing roster maintenance by the primary care practice staff or providers. We feel that this approach would be reproducible in any environment where a single clinical lab provides the bulk of services for each patient. It is potentially adaptable to other clinical conditions where lab testing plays a central role in the management of a chronic illness, such as hyperlipidemia, thyroid disorders, etc.
Peer comparison can be a potent stimulus for providers to examine their office processes and may stimulate a practice to implement systematic improvements. For this reason, our quarterly population report provides two benchmarks: 1) the top 10% performers in the VDIS registry, using the Achievable Benchmark of patient consent for studies based on disease registries.
Several studies have reported improvement in outcomes for diabetic patients by using population-based, decision support approaches in staff-model managed care organizations with robust information systems.[23-25] The majority of health care in the United States though, is delivered in settings where a wide variety of insurance plans are accepted and a central information system is not used. There have been reports describing improvements in diabetes clinical process measures in these settings using population-based approaches, but many of these studies suffer from limitations such as a single-institution setting, lack of physiologic outcomes, short duration, or observational design. [52-57]
Like many of these studies, our quarterly population reports provide a current roster of patients for each PCP, sorted by level of control of A1C, lipids and urinary albumin excretion. This allows the provider to identify patients who may benefit from a more intensive intervention. We have deliberately not required a specific case management approach because we are working with a diverse group of practices with widely varying office processes. A mandated case management approach would add significantly to the burden on the practice and to the cost of the intervention itself. Practice participation in VDIS does not require capital expense, additional personnel, or major changes in office processes or referral patterns. In designing the system, we focused on avoiding data entry and minimizing roster maintenance by the primary care practice staff or providers. We feel that this approach is reproducible in any environment where a single clinical lab provides the bulk of services for each patient. It is potentially adaptable to other clinical conditions where lab testing plays a central role in the management of a chronic illness, such as hyperlipidemia, thyroid disorders, etc.
Peer comparison can be a potent stimulus for providers to examine their office processes and may stimulate a practice to implement systematic improvements. For this reason, our quarterly population report provides two benchmarks: 1) the top performing 10% of providers in the VDIS registry, using the achievable benchmark of control approach [32-33], and 2) the American Diabetes Association Provider Recognition Program benchmark. There are significant limitations to the use of physician report cards, particularly in settings where the average patient panel size is small, which we point out to our participating PCPs. [59, 60]
Limitations
One of the limitations of our study design is that if we find improved outcomes in the intervention group, the relative effect of the various components of the intervention will not be known. The effect may, in fact, vary by provider or by patient. Some providers may actively use the population report to track down patients who are lost to follow up, while others may respond to the flow sheets. Likewise, patients are likely to be at varying levels of engagement in the care process and may respond differently to notification about specific results versus overdue reminders. However, given the low marginal cost per patient of providing the entire collection of services, it may not matter exactly which component is most effective.
In some ways, the study population may not generalize to other groups around the country and the world. Our subjects are less racially diverse than the rest of the country, more rural, and less likely to have access to specialty care. However, they are similar to other American diabetes patients in regard to their age, income, sex, comorbid conditions, tobacco use, functional status, and other social and demographic characteristics. Importantly, they get their diabetes care from PCPs without high-tech electronic medical records.
Point-of-care testing devices are becoming more widely used for the office measurement of A1C, MCR, and lipids. If data from these devices are not captured in an electronic format, then they are not available for formatting and reporting to the practices or patients by VDIS. We have eliminated some practices that rely heavily on such machines. In several cases, we developed special hand data entry systems for practices with relatively low use of on-site testing. Unfortunately, this is not feasible on a large scale. The importance of developing interfaces between point-of-care testing devices and central lab computers or electronic health records has been recognized. [61]
Conclusions
Our experience to date indicates that a low cost decision support and information system based on the chronic care model is feasible in primary care practices that lack sophisticated electronic information systems. VDIS is well accepted by patients, providers, and laboratory staff. If proven beneficial in a rigorous, randomized, controlled evaluation, the intervention could be widely disseminated to practices across America and the world with a substantial impact on the outcomes and costs of diabetes. It could also be adapted to other chronic conditions. We anticipate the results of the study will be available in 2006.
Acknowledgments
The authors would like to acknowledge the contributions made by the patients, physicians, Nurse Practitioners, Physician Assistants, the office staffs of the participating practices, and the staffs at the participating hospitals, without whom this project would not be feasible.
Funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK61167)
Appendix: Vermont Diabetes Information System
References
- 1.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289(1):76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 2.Narayan KM, Boyle JP, Thompson TJ, Sorensen SW, Williamson DF. Lifetime risk for diabetes mellitus in the United States. JAMA. 2003;290(14):1884–90. doi: 10.1001/jama.290.14.1884. [DOI] [PubMed] [Google Scholar]
- 3.Saydah SH, Eberhardt MS, Loria CM, Brancati FL. Age and the burden of death attributable to diabetes in the United States. Am J Epidemiol. 2002;156(8):714–9. doi: 10.1093/aje/kwf111. [DOI] [PubMed] [Google Scholar]
- 4.Gu K, Cowie CC, Harris MI. Mortality in adults with and without diabetes in a national cohort of the U.S. population, 1971-1993. Diabetes Care. 1998;21(7):1138–45. doi: 10.2337/diacare.21.7.1138. [DOI] [PubMed] [Google Scholar]
- 5.Economic Costs of Diabetes in the U.S. in 2002. Diabetes Care. 2003;26(3):917–932. doi: 10.2337/diacare.26.3.917. [DOI] [PubMed] [Google Scholar]
- 6.The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329(14):977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 7.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34) Lancet. 1998;352(9131):854–65. [PubMed] [Google Scholar]
- 8.UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–53. [PubMed] [Google Scholar]
- 9.UK Prospective Diabetes Study Group. Cost effectiveness analysis of improved blood pressure control in hypertensive patients with type 2 diabetes: UKPDS 40. BMJ. 1998;317(7160):720–6. [PMC free article] [PubMed] [Google Scholar]
- 10.The Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of Intensive Therapy on the Microvascular Complications of Type 1 Diabetes Mellitus. JAMA. 2002;287(19):2563–2569. doi: 10.1001/jama.287.19.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK Prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ. 1998;317(7160):713–20. [PMC free article] [PubMed] [Google Scholar]
- 12.UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–13. [PMC free article] [PubMed] [Google Scholar]
- 13.Gaede P, Vedel P, Larsen N, Jensen GV, Parving HH, Pedersen O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N Engl J Med. 2003;348(5):383–93. doi: 10.1056/NEJMoa021778. [DOI] [PubMed] [Google Scholar]
- 14.Saaddine JB, Engelgau MM, Beckles GL, Gregg EW, Thompson TJ, Narayan KM. A diabetes report card for the United States: quality of care in the 1990s. Ann Intern Med. 2002;136(8):565–74. doi: 10.7326/0003-4819-136-8-200204160-00005. [DOI] [PubMed] [Google Scholar]
- 15.Harris MI. Health care and health status and outcomes for patients with type 2 diabetes. Diabetes Care. 2000;23(6):754–8. doi: 10.2337/diacare.23.6.754. [DOI] [PubMed] [Google Scholar]
- 16.Beckles GL, Engelgau MM, Narayan KM, Herman WH, Aubert RE, Williamson DF. Population-based assessment of the level of care among adults with diabetes in the U.S. Diabetes Care. 1998;21(9):1432–8. doi: 10.2337/diacare.21.9.1432. [DOI] [PubMed] [Google Scholar]
- 17.Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA. 2004;291(3):335–42. doi: 10.1001/jama.291.3.335. [DOI] [PubMed] [Google Scholar]
- 18.Grimshaw JM, Russell IT. Effect of clinical guidelines on medical practice: a systematic review of rigorous evaluations. Lancet. 1993;342(8883):1317–22. doi: 10.1016/0140-6736(93)92244-n. [DOI] [PubMed] [Google Scholar]
- 19.Goldfarb S. The utility of decision support, clinical guidelines, and financial incentives as tools to achieve improved clinical performance. Jt Comm J Qual Improv. 1999;25(3):137–44. doi: 10.1016/s1070-3241(16)30433-3. [DOI] [PubMed] [Google Scholar]
- 20.Kirkman MS, Williams SR, Caffrey HH, Marrero DG. Impact of a program to improve adherence to diabetes guidelines by primary care physicians. Diabetes Care. 2002;25(11):1946–51. doi: 10.2337/diacare.25.11.1946. [DOI] [PubMed] [Google Scholar]
- 21.Lomas J, Anderson GM, Domnick-Pierre K, Vayda E, Enkin MW, Hannah WJ. Do practice guidelines guide practice? The effect of a consensus statement on the practice of physicians. N Engl J Med. 1989;321(19):1306–11. doi: 10.1056/NEJM198911093211906. [DOI] [PubMed] [Google Scholar]
- 22.Renders CM, Valk GD, Griffin SJ, Wagner EH, Eijk Van JT, Assendelft WJ. Interventions to improve the management of diabetes in primary care, outpatient, and community settings: a systematic review. Diabetes Care. 2001;24(10):1821–33. doi: 10.2337/diacare.24.10.1821. [DOI] [PubMed] [Google Scholar]
- 23.Brown JB, Nichols GA, Glauber HS. Case-control study of 10 years of comprehensive diabetes care. West J Med. 2000;172(2):85–90. doi: 10.1136/ewjm.172.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McCulloch DK, Price MJ, Hindmarsh M, Wagner EH. A population-based approach to diabetes management in a primary care setting: early results and lessons learned. Effective Clin Practice. 1998;1:12–22. [PubMed] [Google Scholar]
- 25.Peters AL, Davidson MB. Application of a diabetes managed care program. The feasibility of using nurses and a computer system to provide effective care. Diabetes Care. 1998;21(7):1037–43. doi: 10.2337/diacare.21.7.1037. [DOI] [PubMed] [Google Scholar]
- 26.Donabedian A. The Definition of Quality and Approaches to its Assessment. Vol. 1. Ann Arbor: Health Administration Press; 1980. [Google Scholar]
- 27.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288(14):1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 28.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness: the chronic care model, Part 2. JAMA. 2002;288(15):1909–14. doi: 10.1001/jama.288.15.1909. [DOI] [PubMed] [Google Scholar]
- 29.NECLA Northeast Commnity Laboratory Alliance. [February 17 2004]; http://www.necla.org/
- 30.Vermont Program for Quality in Health Care. Recommendations for management of Diabetes in Vermont. Montpelier: Vermont Program for Quality in Health Care; 2004. [Google Scholar]
- 31.ADA. American Diabetes Association. Clinical Practice Recommendations. Diabetes Care. 2004;27 1 [PubMed] [Google Scholar]
- 32.Kiefe CI, Allison JJ, Williams OD, Person SD, Weaver MT, Weissman NW. Improving quality improvement using achievable benchmarks for physician feedback: a randomized controlled trial. JAMA. 2001;285(22):2871–9. doi: 10.1001/jama.285.22.2871. [DOI] [PubMed] [Google Scholar]
- 33.Weissman NW, Allison JJ, Kiefe CI, et al. Achievable benchmarks of care: the ABCs of benchmarking. J Eval Clin Pract. 1999;5(3):269–81. doi: 10.1046/j.1365-2753.1999.00203.x. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Kosinski M, Turner-Bowker DM, Gandek B. How to Score Version 2 of the SF-12 Health Survey. Lincoln, RI: Quality Metric Inc.; 2002. [Google Scholar]
- 35.Bradley C, Todd C, Gorton T, Symonds E, Martin A, Plowright R. The development of an individualized questionnaire measure of perceived impact of diabetes on quality of life: the ADDQoL. Qual Life Res. 1999;8(12):79–91. doi: 10.1023/a:1026485130100. [DOI] [PubMed] [Google Scholar]
- 36.Bradley C, Speight J. Patient perceptions of diabetes and diabetes therapy: assessing quality of life. Diabetes Metab Res Rev. 2002;18 3:S64–9. doi: 10.1002/dmrr.279. [DOI] [PubMed] [Google Scholar]
- 37.Littenberg B, Partilo S, Licata A, Kattan MW. Paper Standard Gamble: the reliability of a paper questionnaire to assess utility. Med Decis Making. 2003;23(6):480–8. doi: 10.1177/0272989X03259817. [DOI] [PubMed] [Google Scholar]
- 38.Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84. doi: 10.1097/00005650-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 39.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–63. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 40.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns. 1999;38(1):33–42. doi: 10.1016/s0738-3991(98)00116-5. [DOI] [PubMed] [Google Scholar]
- 41.Parker RM, Baker DW, Williams MV, Nurss JR. The test of functional health literacy in adults: a new instrument for measuring patients' literacy skills. J Gen Intern Med. 1995;10(10):537–41. doi: 10.1007/BF02640361. [DOI] [PubMed] [Google Scholar]
- 42.Safran DG, Kosinski M, Tarlov AR, et al. The Primary Care Assessment Survey: tests of data quality and measurement performance. Med Care. 1998;36(5):728–39. doi: 10.1097/00005650-199805000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Littell RC, Milliken GA, Stroup WW, Wolfinger RD. SAS System for Mixed Models. Cary, NC: SAS Institute, Inc.; 1996. [Google Scholar]
- 45.Koepsell TH, Wagner EH, Cheadle AC, Patrick DL. Selected methodological issues in evaluating community-based health promotion and disease prevention programs. Ann Rev Publ Health. 1992;13:31–57. doi: 10.1146/annurev.pu.13.050192.000335. [DOI] [PubMed] [Google Scholar]
- 46.Donner A, Birkett N, Buck C. Randomization by cluster: Sample size requirements and analysis. Am J Epidemiol. 1981;114:906–914. doi: 10.1093/oxfordjournals.aje.a113261. [DOI] [PubMed] [Google Scholar]
- 47.Donner A, Klar N. Pitfalls of and Controversies in Cluster Randomization Trials. Am J Public Health. 2004;94(3):416–422. doi: 10.2105/ajph.94.3.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. [March 16, 2004];US Census 2000. www.census.gov/
- 49. [March 16, 2004];Improving Chronic Illness Care. www.improvingchroniccare.org.
- 50. [March 16, 2004];Health Disparities Collaborative. www.healthdisparities.net.
- 51.Norris SL, Nichols PJ, Caspersen CJ, et al. The effectiveness of disease and case management for people with diabetes. A systematic review. Am J Prev Med. 2002;22 4:15–38. doi: 10.1016/s0749-3797(02)00423-3. [DOI] [PubMed] [Google Scholar]
- 52.Tu JV, Willison DJ, Silver FL, et al. Impracticability of informed consent in the Registry of the Canadian Stroke Network. N Engl J Med. 2004;350(14):1414–21. doi: 10.1056/NEJMsa031697. [DOI] [PubMed] [Google Scholar]
- 53.Grant RW, Hamrick HE, Sullivan CM, et al. Impact of population management with direct physician feedback on care of patients with type 2 diabetes. Diabetes Care. 2003;26(8):2275–80. doi: 10.2337/diacare.26.8.2275. [DOI] [PubMed] [Google Scholar]
- 54.Stroebel RJ, Scheitel SM, Fitz JS, et al. A randomized trial of three diabetes registry implementation strategies in a community internal medicine practice. Jt Comm J Qual Improv. 2002;28(8):441–50. doi: 10.1016/s1070-3241(02)28044-x. [DOI] [PubMed] [Google Scholar]
- 55.Frijling BD, Lobo CM, Hulscher ME, et al. Multifaceted support to improve clinical decision making in diabetes care: a randomized controlled trial in general practice. Diabet Med. 2002;19(10):836–42. doi: 10.1046/j.1464-5491.2002.00810.x. [DOI] [PubMed] [Google Scholar]
- 56.Sperl-Hillen J, O'Connor PJ, Carlson RR, et al. Improving diabetes care in a large health care system: an enhanced primary care approach. Jt Comm J Qual Improv. 2000;26(11):615–22. doi: 10.1016/s1070-3241(00)26052-5. [DOI] [PubMed] [Google Scholar]
- 57.Sidorov J, Gabbay R, Harris R, et al. Disease management for diabetes mellitus: impact on hemoglobin A1c. Am J Manag Care. 2000;6(11):1217–26. [PubMed] [Google Scholar]
- 58.Meigs JB, Cagliero E, Dubey A, et al. A controlled trial of web-based diabetes disease management: the MGH diabetes primary care improvement project. Diabetes Care. 2003;26(3):750–7. doi: 10.2337/diacare.26.3.750. [DOI] [PubMed] [Google Scholar]
- 59.Gandhi TK, Francis EC, Puopolo AL, Burstin HR, Haas JS, Brennan TA. Inconsistent report cards: assessing the comparability of various measures of the quality of ambulatory care. Med Care. 2002;40(2):155–65. doi: 10.1097/00005650-200202000-00010. [DOI] [PubMed] [Google Scholar]
- 60.Hofer TP, Hayward RA, Greenfield S, Wagner EH, Kaplan SH, Manning WG. The unreliability of individual physician “report cards” for assessing the costs and quality of care of a chronic disease. JAMA. 1999;281(22):2098–105. doi: 10.1001/jama.281.22.2098. [see comments] [DOI] [PubMed] [Google Scholar]
- 61.Blick KE. The essential role of information management in point-of-care/critical care testing. Clin Chim Acta. 2001;307(12):159–68. doi: 10.1016/s0009-8981(01)00460-0. [DOI] [PubMed] [Google Scholar]
- 62.Toobert DJ, Hampson SE, Glasgow RE. The summary of diabetes self-care activities measure: results from 7 studies and a revised scale. Diabetes Care. 2000;23(7):943–50. doi: 10.2337/diacare.23.7.943. [DOI] [PubMed] [Google Scholar]