Abstract
Objectives. Contralateral responses to unilateral stimuli have been well described in animal models. These range from central sensitization to peripheral inflammatory responses. Our aim was to test for contralateral responses following unilateral intradermal capsaicin injection in man.
Methods. Three groups were investigated. A healthy volunteer group (1) was injected with capsaicin into the volar aspect of one forearm. A group of patients with RA (2) was also injected with capsaicin. A control group of healthy volunteers (3) was not injected with capsaicin. All groups were tested for hyperalgesia and allodynia every 10 min for 1 h following the injection using quantitative sensory testing.
Results. A total of 9/14 healthy volunteers (Group 1) and 10/14 patients with RA (Group 2) demonstrated contralateral sensitization that subsided within 1 h following intradermal capsaicin injection. A total of 2/23 control subjects (Group 3) demonstrated positive responses with the monofilaments. The frequency of the contralateral responses in the experimental groups compared with the control group is significant (P < 0.05). The peak hyperalgesia was relatively delayed contralaterally compared with the ipsilateral side (35 min vs 15 min). The area of sensitization, where present, was reduced compared with the ipsilateral side (5–50%).
Conclusions. This is the first demonstration of a contralateral response following a unilateral stimulus in man. Bilateral neural pathways mediating contralateral responses may have a role in the pathophysiology of chronically painful or inflammatory diseases and a confounding influence on using the contralateral limb as a control experimentally. We did not find that a systemic inflammatory disease sensitized for this phenomenon.
Keywords: Human, Capsaicin, Contralateral response, Hyperalgesia, Allodynia, Rheumatoid arthritis, Neurogenic inflammation
Introduction
The nervous system is anatomically symmetrical, both peripherally and centrally at the spinal level. The two sides of the spinal column are thought to function independently from each other. It is clear from decerebrate and spinalized rat models that they do not [1, 2]. Koltzenburg et al. [3] reviewed the literature detailing various unilateral peripheral neuronal insults that result in contralaterally identical, albeit diminished, responses. The topographically precise nature of these contralateral responses can only be due to neural pathways.
We have reviewed the literature concerning contralateral responses following unilateral inflammatory lesions [4]. For example, Bileviciute et al. [5] demonstrated neuropeptide release from the contralateral joint of rats following a monoarthritis with no signs of systemic inflammation. These contralateral responses were temporally delayed and reduced in magnitude compared with the ipsilateral side. Other groups have studied contralateral responses by interrupting the neural supply to abrogate them, but have not been able to do so when interrupting the circulation [6, 7]. This also suggests a neural basis.
Using animal models several groups have described allodynia and hyperalgesia occurring in the contralateral periphery following an inflammatory stimulus. Stimuli that induce such sensitization in animal models include thermal, IL-1β, bee venom, carrageenan, formaldehyde and even repeated saline injections [7–14]. No studies have been performed in man. However, 5% of the patients with complex regional pain syndrome (CRPS), a chronic pain condition with demonstrable hyperalgesia and allodynia, documented bilateral presenting symptoms [15]. Detailed investigations, such as with technetium pertechnetate bone scanning or quantitative sensory testing, demonstrate that a symmetrical involvement might occur even more often than these clinical symptoms suggest [16, 17]. Patients with RA exhibit symmetrical disease in both their clinical symptoms of pain and stiffness as well as radiographic damage. There is evidence that this could be influenced by bilateral neural loops releasing pro-inflammatory cytokines such as substance P and calcitonin gene-related peptide (CGRP).
Our primary aim was to investigate for contralateral sensitization by testing for contralateral hyperalgesia and allodynia following intradermal capsaicin in man. We recruited two groups of subjects, one healthy and the other with RA. We predicted that there might be differences in the primary outcome between the healthy volunteer and RA groups given the above observations. In order to control for the subjective reports of hyperalgesia and allodynia, we recruited a control group of volunteers who did not receive the intradermal capsaicin injection, but were subject to similar experimental conditions.
Capsaicin (8-methyl-N-vanillyl-6-noneamide) opens the non-selective cation channel TRPV1 receptor (vanilloid receptor 1), located on fine sensory afferent nerve fibres. This allows an influx of calcium ions and generates an afferent action potential that is perceived as an intense burning pain [18–20]. Intradermal capsaicin rapidly generates areas of mechanical and thermal hyperalgesia and allodynia through both central and peripheral nervous system sensitization [21, 22]. Sensory testing reliably maps these sensitized areas for quantification [23, 24]. Areas of mechanical hyperalgesia and allodynia produced by intradermal capsaicin are broadly reproducible both within and between individuals [25–27].
Materials and methods
Protocol
Volunteers were seated in a quiet temperature-controlled room (23–24°C) for 30 min. An area on the volar aspect of one forearm was recorded by measurements in two planes: the distance along the line measured from the medial epicondyle to the ulnar styloid, and the plane perpendicular to this. An easily accessible and readily identifiable area was selected about 12 cm distal to the elbow. The exact contralateral site to the capsaicin injection was identified using these measurements. One hundred microlitres of 1% capsaicin (ethanol/Tween-80 vehicle) was then injected intradermally. The capsaicin had been made up on site and stored at 4°C throughout the study. Allodynia and hyperalgesia were then mapped every 10 min over the following hour. Each assessment was colour coded. This was done both ipsi- and contralaterally using standard quantitative techniques (see subsequently) [23, 24]. Due to possible suggestion bias the volunteers were not told of the study objective. It was explained that the capsaicin injection would be very painful and that the study was to assess the response to this pain. Volunteers had been told to avoid smoking, caffeine, strenuous exercise and alcohol on the day of the study.
Sensory detection of hyperalgesia and allodynia
Pinprick hyperalgesia was detected using a stiff Semmes Weinstein monofilament (6.45; 1500 mN) [28]. This was first tested on a control area of skin (anterior chest wall) prior to the capsaicin injection. Most volunteers reported that this felt like a pinprick but was not painful. Volunteers were asked to rate whether the monofilament on their forearm felt different to the sensation felt over the anterior chest wall. Responses were only positive for hyperalgesia if the volunteer reported that the sensation was different to the surrounding skin and was more painful than previously experienced. The central area (capsaicin injection site or contralateral correlate) was linearly approached from eight different directions sequentially about 30 cm from the centre. Each direction was equally spaced around 360°. The monofilament was tested in the same direction until the centre point was reached using intervals of about 3 cm. Should a response be positive, then the skin was marked and the next direction was tested. Most of the surface skin of the arm (about 60 cm in total length) was covered between the shoulder and the wrist. The individual was said to respond positively if three out of the eight approaches elicited a positive response, as a polygon could be then constructed from these three points and the surface area quantified.
Allodynia was detected using a 4.74 (51.9 mN) monofilament, and again the anterior chest wall was used prior to the experiment. This force is not normally perceived as painful, but is well above the sensory threshold [29]. Reponses were only positive for allodynia if the volunteer reported that the sensation was both different to the surrounding skin and painful.
Points referring to positive responses to allodynia and hyperalgesia were then transferred using transparent acetate onto blank paper. Free drawn areas were described by connecting these points and measured using MAVIS, a device used for measuring the surface areas of irregular shapes [30]. Bath Local Research and Ethics Committee approved the protocol and all volunteers gave full informed consent.
Volunteers for intradermal capsaicin
Healthy volunteers were recruited from colleagues and staff at the Royal National Hospital for Rheumatic Diseases and the University of Bath, and had no medical diagnoses. Patients who satisfied the American Rheumatology Association's criteria for RA were also recruited. They were excluded if they had a neurological lesion. Baseline characteristics including disease duration, medication and disease activity were collected.
Fourteen healthy volunteers [mean age 40.5 yrs (range 30–72), 42.8% males] were recruited. Eighteen patients with RA were recruited. As TNF-α is an important mediator for both peripheral and spinal pain, patients receiving anti-TNF-α blockers (n = 4) were excluded from this study. Fourteen patients were therefore analysed with a mean age of 52 yrs (28–66 range) and 35.7% were males. The average duration of disease was 5.9 yrs (range 2–29); twelve were taking anti-rheumatic agents (seven patients taking MTX; one MTX and SSZ; one gold; one AZA; one SSZ; one LEF) and the average disease activity score (DAS) was 5.12, indicating active disease.
Volunteers for controls with no capsaicin
Thresholds to sensory stimuli vary within the same individual depending upon their location due to a variety of factors including the dermal density of nociceptors and environmental cues. Comparing volar forearm responses to anterior chest wall responses might therefore be misleading. To help control for such a factor, and also to control for suggestion bias, a control group was recruited who had not been exposed to the intradermal capsaicin. Areas of hyperalgesia and allodynia were mapped as outlined above on the volar aspect of both forearms. Volunteers in this group were recruited from patients, colleagues and staff at the Royal National Hospital for Rheumatic Diseases and the University of Bath.
Twenty-three volunteers [mean age 35.3 yrs (range 23–56), 39.1% males] were recruited to this control group. Two of these volunteers also had a diagnosis of RA.
Statistical analysis
The primary outcome was the frequency of contralateral responses in the groups exposed to capsaicin compared with the control group not exposed to capsaicin. The secondary outcome was to compare the contralateral responses between the RA and healthy groups. The non-parametric signed rank test was used to analyse both outcomes.
Results
Control group (no capsaicin injection)
A total of 2/23 (8.7%) volunteers demonstrated areas of hyperalgesia and allodynia that both felt different and was more painful than the surrounding tissue on the volar aspect of the forearm. Neither volunteer who responded had RA. One volunteer had areas of hyperalgesia and allodynia of 86.4 and 76.2 cm2, respectively, while the other responder had areas of 4.2 and 3.6 cm2, respectively.
Healthy and RA groups (capsaicin injection)
A total of 9/14 (64.3%) healthy controls demonstrated contralateral hyperalgesia or allodynia within an hour. A total of 10/14 (71.4%) patients with RA demonstrated contralateral sensory changes. Both groups demonstrated statistically significant differences in the frequency of contralateral responses when compared with the control group (P < 0.05). In the healthy group, this achieved significance for hyperalgesia at 5, 15 and 25 min and for allodynia at 15, 25, 35 and 45 min. In the RA group, this achieved significance for hyperalgesia at 15, 25, 35 and 45 min, but did not achieve significance for allodynia at any time point.
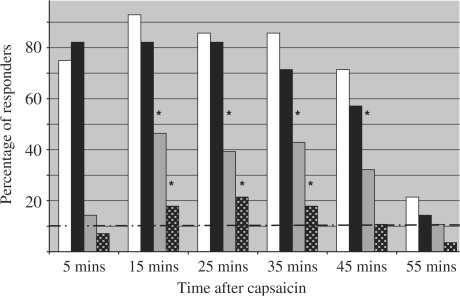
When both groups were combined (Fig. 1), the percentage of responders who demonstrated contralateral hyperalgesia reached significance at time points of 15, 25, 35 and 45 min and those exhibiting contralateral allodynia reached significance at 15, 25 and 35 min. The time point with the highest frequency of contralateral responses was at 15 min for contralateral hyperalgesia and at 25 min for contralateral allodynia. This compares with maximal areas of hyperalgesia and allodynia on the ipsilateral side at 15 min and between 5 and 25 min respectively.
Fig. 1.
The percentage of responders to intradermal capsaicin over time (n = 28). White and black bars are ipsilateral hyperalgesia and allodynia respectively. Grey bars and white-on-black dotted bars are contralateral hyperalgesia and allodynia respectively. Hyperalgesic responses of subjects not exposed to capsaicin are represented by the dashed line. *P < 0.05 for the capsaicin-injected group compared with the non-injected control group. There were no differences between the RA and control groups and both groups (data not shown).
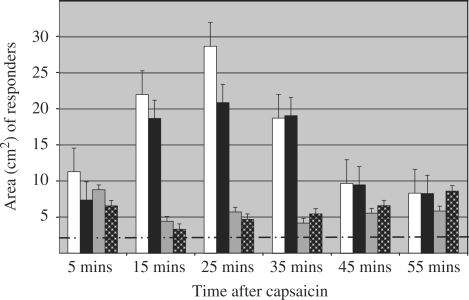
The mean of the maximal ipsilateral hyperalgesic area after capsaicin was injected was 17.0 cm2 (s.e.m. ± 3.1). This was significant (P < 0.05) when compared with the maximal area of contralateral hyperalgesia, 5.9 cm2 (s.e.m. ± 0.7). The mean of the maximal ipsilateral allodynic area following intradermal capsaicin was 14.4 cm2 (s.e.m. ± 2.4), again significant (P < 0.05) compared with the contralateral allodynia, 6.1 cm2 (s.e.m. ± 0.8). In the volunteers who demonstrated responses, the maximal contralateral area varied in magnitude between 5% and 50% of the maximal ipsilateral response to capsaicin (Fig. 2).
Fig. 2.
The area of responses to intradermal capsaicin over time (n = 28). White and black bars are ipsilateral hyperalgesia and allodynia respectively. Grey bars and white-on-black dotted bars are contralateral hyperalgesia and allodynia respectively. Hyperalgesic responses of subjects not exposed to capsaicin are represented by the dashed line. Standard error bars are shown.
There were no significant differences between the proportions of healthy volunteers and patients with RA who described contralateral sensations following capsaicin injection. There was no difference in the temporal pattern of the responses in these two groups. Both groups demonstrated an increase in the frequency of contralateral sensitization that reflected the frequency of the pain responses to the capsaicin injection. There were no differences in the size of the contralateral response between these groups.
There was no correlation between the baseline characteristics of the volunteer groups and the presence of a contralateral response. These were age, sex and, for the RA group, the disease duration, activity and medication as well.
Discussion
We have described novel areas of contralateral hyperalgesia and allodynia occurring in a significant proportion of both healthy volunteers and patients with RA following an intradermal injection of capsaicin using a standardized quantitative sensory testing technique. These changes were not seen in a sample population who had not been exposed to intradermal capsaicin. The contralateral responses mapped a similar time course to the allodynia and hyperalgesia ipsilateral to the capsaicin injection. No differences were seen between healthy controls and patients with RA in the frequency or size of this phenomenon. Although contralateral sensitization following a unilateral inflammatory stimulus has been described in several animal models, this is the first experimental description of topographically precise contralateral sensitization occurring in man.
Supraspinal pathways are bilaterally activated by unilateral stimuli that might be noxious or non-noxious [31]. Primary and secondary sensory cortex is bilaterally activated by vibrotactile non-noxious stimulation. Noxious stimuli, however, activate bilateral regions of the brain associated with descending control pathways including the thalamus and rostral ventral medulla providing a putative mechanism for mediating altered spinal gating contralaterally [32, 33]. This pathway could give a plausible explanation for the contralateral observation but this study gives no information concerning higher CNS responses. The time period between the capsaicin injection and testing the contralateral side was several minutes, which is too long an interval to differentiate whether the contralateral response occurs either directly through the spinal cord or is mediated via long supraspinal pathways.
Contralateral responses studied in animal models demonstrate changes in metabolism and genomic expression in the contralateral dorsal horn following an inflammatory lesion. These are temporally consistent with a functional contribution towards any contralateral peripheral response [34–37]. Furthermore, several histopathologists have anatomically identified the pathways that might mediate such contralateral responses at the spinal level. Fibres have been identified to enter the dorsal horn and cross the mid-line through the posterior commissure terminating in the superficial laminae of the contralateral dorsal horn. Other fibres synapse ipsilaterally before decussating to either the deep or superficial laminae of the contralateral dorsal horn [39, 40]. Koltzenburg et al. [3] suggested the existence of ‘slow’ messengers, possibly nerve growth factors, to explain the contralateral peripheral responses seen following unilateral neural injuries. Communication across the mid-line may be caused by reactions in cell soma and structural changes including sprouting across the mid-line. Alternatively, transmedian commissural interneurons may transmit signals, but rapid synaptic activity is one-way whereas bidirectional flow could be achieved through slower trophic factors. Our results suggest the existence of ‘fast’ messengers because the central sensitization and contralateral peripheral vascular response occurred within 1 h of the capsaicin injection [3].
Pando et al. [41] studied patients with less than a 12-month history of arthritis symptoms by taking specimens from clinically uninflamed joints. Their results confirmed that histological evidence of synovitis can be present, as defined by increased thickness of the synovial lining layer, altered morphology of synovial cells, perivascular and interstitial cellular infiltrates and increased vascularity in these clinically ‘normal’ joints [41]. Further investigation into such observations is needed to study the existence of peripheral changes contralateral to an inflammatory stimulus in man. Using a power calculation corrected for the non-parametric data distribution, 81 patients would need to be recruited for the contralateral allodynic responses to be significantly different between the RA and healthy control groups at 25 min using an α = 0.05 and β = 0.80.
This could be done using more sensitive neurophysiological techniques such as functional imaging. Peripheral induction of a contralateral response completes the loop between homologous body parts and this has been well described in animal models [4]. The role of these pathways in symmetrical diseases such as RA and skin psoriasis is an important question to be investigated as it could lead to new therapeutic strategies. The role of these pathways in chronically painful conditions, such as CRPS, also needs to be explored further.
It is interesting to note that patients with RA did not exhibit different responses when compared with the normal control group. This may be because this study was not powered to detect such differences but may also illustrate the lack of appropriate stimulus and detection mechanisms. This could be studied further by examining the contralateral inflammatory response histologically in patients with RA and normal controls.
Work on animal models has suggested that the tachykinins are important chemical mediators for neurogenically transmitted inflammation. For example, Decaris et al. [6] produced a model of contralateral cartilage degeneration following a monoarthritis induced by complete Freund's adjuvant. They were able to ameliorate this change by injecting neurokinin-1 antagonist intrathecally prior to arthritis induction. Kidd et al. [42] induced a monoarthritis using latex spheres injected into the right knee of male Wistar rats. Contralateral knee cellular infiltrates, mainly monocytes, were significantly elevated when compared with control animals and joints elsewhere in the affected rat. The neonatal administration of capsaicin, which is known to reduce the expression of tachykinins, reduced this contralateral cell count whilst having no effect on the ipsilateral arthritic joint. Donaldson et al. [43] induced a monoarthritis with Freund's complete adjuvant in the left ankle of adult male Han Wistar rats that had previously been shown to spread to the contralateral joint. The application of capsaicin to the sciatic nerve 2 weeks prior to the arthritis induction prevented the full expression of this contralateral arthritis.
There are clear difficulties in evaluating hyperalgesia and allodynia responses in man related to the subjectivity of the sensory experience. It may therefore be argued that the contralateral responses are the result of the experimental design. However, it must be emphasized that none of the volunteers were aware of the objective of the experiment. Furthermore, the sensations in the contralateral area changed over time reflecting the dynamic changes on the ipsilateral side, which suggests an underlying physiological mechanism rather than an artefact. The responses were remarkably consistent between volunteers. Finally, we obtained a control group for the allodynia and hyperalgesia responses to the Semmes Weinstein monofilaments that had not been exposed to capsaicin. A total of 2/23 (8.7%) of this group were seen to demonstrate allodynic and hyperalgesic responses that were different to that felt over the chest wall. Both of these responses are significantly lower in both frequency and magnitude than in either of the experimental groups.
The observed contralateral responses are unlikely to reflect a global change in pain thresholds as only the topographically precise contralateral area was sensitized and there was no sensitization of anatomically mismatched areas, even though these were tested using quantitative sensory testing. The responses are therefore a result of a specific contralateral effect rather than a generalized sensitization of the central nervous system.
Some volunteers did not exhibit contralateral sensory changes. This is most likely to be because the threshold to generate contralateral central sensitization had not been reached. It may also have been that the testing equipment was not sensitive enough to detect contralateral changes, or, albeit less likely, the pathways needed to generate contralateral central sensitization are not present in everyone. This last point implies that a degree of genetic control could exist in these pathways.
We chose to quantitatively assess contralateral sensory changes using mapping techniques rather than that of sensory thresholds using a manual mechanical technique, similar to that which had previously been used following intradermal capsaicin [23, 24]. Using this method, at least three positive answers in the eight mapped directions must be given for an area to be calculated. For the purposes of this study there are advantages of area mapping over thresholds. First the area can be mapped quickly without losing reliability, rather than the more temporally laborious threshold detection. As the contralateral responses were transient in nature, any loss of time may result in a false negative. Second, we were concerned that the repeated pinprick stimulus at the same location might sensitize the skin in a ‘wind-up’ phenomenon when tested over an hour resulting in a false positive.
In conclusion, this is the first description of contralateral sensory changes occurring in man. This has implications on the planning of experiments using contralateral body parts as internal controls. The existence of neural pathways that affect the contralateral limb is of the utmost importance when planning such experiments, especially when assessing responses to noxious stimuli. Internal controls should be used in such experiments. This contralateral observation also has implications for the pathophysiology of chronic symmetrical inflammatory diseases, including RA and skin psoriasis.

Acknowledgements
Funding: N.G.S. and R.C.H. received Arthritis Research Campaign fellowships to undertake this research. Funding to pay the Open Access charges for this article was provided by ARC.
Disclosure statement: D.R.B. holds an endowed Chair in Locomotor Sciences, an Arthritis Research Campaign ICAC award that supports the Royal National Hospital for Rheumatic Diseases, Bath, UK. All other authors have declared no conflicts of interest.
References
- 1.Fitzgerald M. The contralateral input to the dorsal horn of the spinal cord in the decerebrate rat. Brain Res. 1982;236:275–87. doi: 10.1016/0006-8993(82)90714-4. [DOI] [PubMed] [Google Scholar]
- 2.Fitzgerald M. Alterations in the ipsi- and contralateral afferent inputs of dorsal horn cells produced by capsaicin treatment of one sciatic nerve in the rat. Brain Res. 1982;248:97–107. doi: 10.1016/0006-8993(82)91151-9. [DOI] [PubMed] [Google Scholar]
- 3.Koltzenburg M, Wall PD, McMahon SB. Does the right side know what the left side is doing? Trends Neurosci. 1999;22:122–27. doi: 10.1016/s0166-2236(98)01302-2. [DOI] [PubMed] [Google Scholar]
- 4.Shenker N, Haigh R, Roberts E, Mapp P, Harris N, Blake D. A review of contralateral responses to a unilateral inflammatory lesion. Rheumatology. 2003;42:1279–86. doi: 10.1093/rheumatology/keg397. [DOI] [PubMed] [Google Scholar]
- 5.Bileviciute I, Lundeberg T, Ekblom A, Theodorsson E. Bilateral changes of substance P, neurokinin A, calcitonin gene-related peptide- and neuropeptide Y-like immunoreactivity in rat knee joint synovial fluid during acute monoarthritis. Neurosci Lett. 1993;153:37–40. doi: 10.1016/0304-3940(93)90071-r. [DOI] [PubMed] [Google Scholar]
- 6.Decaris E, Guingamp C, Chat M, et al. Evidence for neurogenic transmission inducing degenerative cartilage damage distant from local inflammation. Arthritis Rheum. 1999;42:1951–60. doi: 10.1002/1529-0131(199909)42:9<1951::AID-ANR22>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 7.Levine JD, Dardick SJ, Basbaum AI, Scipio E. Reflex neurogenic inflammation. I. Contribution of the peripheral nervous system to spatially remote inflammatory responses that follow injury. J Neurosci. 1985;5:1380–6. doi: 10.1523/JNEUROSCI.05-05-01380.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–8. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 9.Aloisi AM, Porro CA, Cavazzuti M, Baraldi P, Carli G. ‘Mirror pain’ in the formalin test: behavioural and 2-deoxyglucose studies. Pain. 1993;55:267–73. doi: 10.1016/0304-3959(93)90156-J. [DOI] [PubMed] [Google Scholar]
- 10.Coderre TJ, Melzack R. Increased pain sensitivity following heat injury involves a central mechanism. Behav Brain Res. 1985;15:259–62. doi: 10.1016/0166-4328(85)90181-0. [DOI] [PubMed] [Google Scholar]
- 11.Coderre TJ, Melzack R. Cutaneous hyperalgesia: contributions of the peripheral and central nervous system to increase in the pain sensitivity after injury. Brain Res. 1987;404:95–106. doi: 10.1016/0006-8993(87)91359-x. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Luo C, Li H, Chen H. Primary hyperalgesia to mechanical and heat stimuli following subcutaneous bee venom injection into the plantar surface of hindpaw in the conscious rat: a comparative study with the formalin test. Pain. 1999;83:67–76. doi: 10.1016/s0304-3959(99)00075-5. [DOI] [PubMed] [Google Scholar]
- 13.Kissin I, Lee SS, Bradley EL., Jr Effect of prolonged nerve block on inflammatory hyperalgesia in rats: prevention of late hyperalgesia. Anesthesiology. 1998;88:224–32. doi: 10.1097/00000542-199801000-00031. [DOI] [PubMed] [Google Scholar]
- 14.Ganju P, Davis A, Patel S, Nunez X, Fox A. p38 stress-activated protein kinase inhibitor reverses bradykinin B(1) receptor-mediated component of inflammatory hyperalgesia. Eur J Pharmacol. 2001;421:191–9. doi: 10.1016/s0014-2999(01)01048-2. [DOI] [PubMed] [Google Scholar]
- 15.Veldman PH, Goris RJ. Multiple reflex sympathetic dystrophy. Which patients are at risk for developing a recurrence of reflex sympathetic dystrophy in the same or another limb. Pain. 1996;64:463–6. doi: 10.1016/0304-3959(95)00160-3. [DOI] [PubMed] [Google Scholar]
- 16.Kozin F, McCarty SJ, Sims J, Genant H. The reflex sympathetic dystrophy syndrome. Am J Med. 1976;60:320–37. doi: 10.1016/0002-9343(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 17.Rommel O, Malin JP, Zenz M, Janig W. Quantitative sensory testing, neurophysiological and psychological examination in patients with complex regional pain syndrome and hemisensory deficits. Pain. 2001;93:279–93. doi: 10.1016/S0304-3959(01)00332-3. [DOI] [PubMed] [Google Scholar]
- 18.Bevan SJ, Szolcsanyi J. Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol Sci. 1990;11:330–3. doi: 10.1016/0165-6147(90)90237-3. [DOI] [PubMed] [Google Scholar]
- 19.Caterina MJ, Schumacher MA, Tominaga M, et al. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 20.Oh U, Hwang SW, Kim D. Capsaicin activates a non-selective cation channel in cultured rat dorsal root ganglion neurons. J Neurosci. 1996;16:659–67. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–64. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–80. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Simone DA, Baumann TK, LaMotte RH. Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain. 1989;38:99–107. doi: 10.1016/0304-3959(89)90079-1. [DOI] [PubMed] [Google Scholar]
- 24.Pedersen JL, Kehlet H. Secondary hyperalgesia to heat stimuli after burn injury in man. Pain. 1998;76:377–84. doi: 10.1016/S0304-3959(98)00070-0. [DOI] [PubMed] [Google Scholar]
- 25.Serra J, Campero M, Ochoa J. Flare and hyperalgesia after intradermal capsaicin injection in human skin. J Neurophysiol. 1998;80:2801–10. doi: 10.1152/jn.1998.80.6.2801. [DOI] [PubMed] [Google Scholar]
- 26.Liu M, Max MB, Robinovitz E, Gracely RH, Bennett GJ. The human capsaicin model of allodynia and hyperalgesia: sources of variability and methods for reduction. J Pain Symptom Manag. 1998;16:10–20. doi: 10.1016/s0885-3924(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 27.Hughes A, Macleod A, Growcott J, Thomas I. Assessment of the reproducibility of intradermal administration of capsaicin as a model for inducing human pain. Pain. 2002;99:323–31. doi: 10.1016/s0304-3959(02)00161-6. [DOI] [PubMed] [Google Scholar]
- 28.Finnerup NB, Johannesen IL, Fuglsang-Frederiksen A, Bach FW, Jensen TS. Sensory function in spinal cord injury patients with and without central pain. Brain. 2003;126:57–70. doi: 10.1093/brain/awg007. [DOI] [PubMed] [Google Scholar]
- 29.Brennum J, Kjeldsen M, Jensen K, Jensen TS. Measurements of human pressure-pain thresholds on fingers and toes. Pain. 1989;38:211–7. doi: 10.1016/0304-3959(89)90240-6. [DOI] [PubMed] [Google Scholar]
- 30.Plassman P, Jones TD. MAVIS: a non-invasive instrument to measure area and volume of wounds. Med Eng Phys. 1998;20:332–8. doi: 10.1016/s1350-4533(98)00034-4. [DOI] [PubMed] [Google Scholar]
- 31.McGlone F, Kelly EF, Trulsson M, et al. Functional neuroimaging studies of human somatosensory cortex. Behav Brain Res. 2002;135:147–58. doi: 10.1016/s0166-4328(02)00144-4. [DOI] [PubMed] [Google Scholar]
- 32.Bantick SJ, Wise RG, Ploghaus A, et al. Imaging how attention modulates pain in human using functional MRI. Brain. 2002;125:310–19. doi: 10.1093/brain/awf022. [DOI] [PubMed] [Google Scholar]
- 33.Urban MO, Gebhart GF. Supraspinal contributions to hyperalgesia. Proc Natl Acad Sci USA. 1999;96:7687–92. doi: 10.1073/pnas.96.14.7687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Williams S, Evan GI, Hunt SP. Changing patterns of c-fos induction in spinal neurons following thermal cutaneous stimulation in the rat. Neuroscience. 1990;36:73–81. doi: 10.1016/0306-4522(90)90352-5. [DOI] [PubMed] [Google Scholar]
- 35.Herdegen T, Leah JD, Manisali A, Bravo R, Zimmermann M. c-JUN-like immunoreactivity in the CNS of the adult rat: basal and transsynaptically induced expression of an immediate-early gene. Neuroscience. 1991;41:643–54. doi: 10.1016/0306-4522(91)90356-s. [DOI] [PubMed] [Google Scholar]
- 36.Porro CA, Cavazzuti M, Galetti A, Sassatelli L, Barbier GC. Functional activity of the rat spinal cord during formalin-induced noxious stimulation. Neuroscience. 1991;41:655–65. doi: 10.1016/0306-4522(91)90357-t. [DOI] [PubMed] [Google Scholar]
- 37.Schadrack J, Neto FL, Ableitner A, et al. Metabolic activity changes in the rat spinal cord during adjuvant monoarthritis. Neuroscience. 1999;94:595–605. doi: 10.1016/s0306-4522(99)00186-4. [DOI] [PubMed] [Google Scholar]
- 38.Light AR, Perl ER. Reexamination of the dorsal root projection to the spinal dorsal horn including observation of the differential termination of coarse and fine fibres. J Comp Neurol. 1979;186:117–32. doi: 10.1002/cne.901860202. [DOI] [PubMed] [Google Scholar]
- 39.Szentagothai J. Neuronal and synaptic arrangement in the substantia gelatinosa rolandi. J Comp Neurol. 1964;122:219–39. doi: 10.1002/cne.901220207. [DOI] [PubMed] [Google Scholar]
- 40.Culberson JL, Haines DE, Kimmel DL, Brown PB. Contralateral projection of primary afferent fibres to mammalian spinal cord. Expl Neurol. 1979;64:83–97. doi: 10.1016/0014-4886(79)90007-4. [DOI] [PubMed] [Google Scholar]
- 41.Pando JA, Duray P, Yarboro C, et al. Synovitis occurs in some clinically normal and asymptomatic joints in patients with early arthritis. J Rheumatol. 2000;27:1848–54. [PubMed] [Google Scholar]
- 42.Kidd BL, Cruwys SC, Garrett NE, et al. Neurogenic influences on contralateral responses during experimental rat monoarthritis. Brain Res. 1995;688:72–76. doi: 10.1016/0006-8993(95)00512-o. [DOI] [PubMed] [Google Scholar]
- 43.Donaldson LF, McQueen DS, Seckl JR. Neuropeptide gene expression and capsaicin-sensitive primary afferents: maintenance and spread of adjuvant arthritis in the rat. J Physiol. 1995;486:473–82. doi: 10.1113/jphysiol.1995.sp020826. [DOI] [PMC free article] [PubMed] [Google Scholar]