Figure 4. Graphical Comparison of Fabricated Positive Samples.
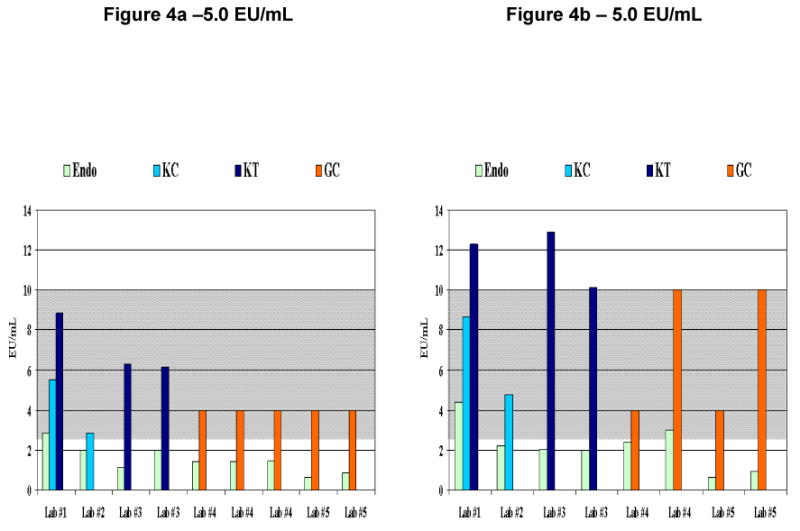
Figures 4a represent runs from one fabricated positive sample and figure 4b represent runs from a second fabricated positive sample using the Endosafe® test system and 0.05 –5.0 EU/mL sensitivity cartridges and the in-house method at the participating laboratories. The expected value for each of the positive samples (Figures 4a and 4b) is 5.0 with an expected endotoxin range (EER) of 2.5 EU/mL 10 EU/mL representing the 2-fold margin of error shown in gray shading. These samples were chosen from the pool of fabricated positive samples since they were representative of how a true positive sample might appear in laboratory analysis. Legend for 4a: Endo - Endosafe®, KC - Kinetic-Chromogenic, KT - Kinetic-Turbidimetric, GC - Gel Clot, EER - expected endotoxin range = 2.5 EU/mL-10 EU/mL, Lab #1- Endosafe® run at 1:10 dilution, Lab #1- KC run at 1:10 dilution, Lab #1- KT run at 1:10 dilution, Lab #2 - Endosafe®, and KC runs at 1:40 dilution, Lab #3 - Endosafe® run at 1:10 and 1:40 dilutions, Lab #3 - KT run at 1:20 and 1:40 dilutions, Lab #4 - Endosafe® runs at 1:1(undiluted) and 1:20 dilutions, Lab #4 - GC run using serial dilutions, Lab #5 - Endosafe® run at 1:1(undiluted) and 1:10 dilutions, Lab #5 - GC run using serial dilutions, Legend for 4b: Endo - Endosafe®, KC - Kinetic-Chromogenic, KT - Kinetic-Turbidimetric, GC - Gel Clot, EER - expected endotoxin range = 2.5 EU/mL-10 EU/mL, Lab #1- Endosafe® run at 1:10 dilution, Lab #1- KC run at 1:1(undiluted), Lab #1 - KT run at 1:10 dilution, Lab #2 - Endosafe® and KC runs at 1:40 dilution, Lab #3 - Endosafe® run at 1:10 and 1:40 dilutions, Lab #3 - KT run at and 1:20 and 1:40 dilutions, Lab #4 - Endosafe® run at 1:10 dilution, Lab #4 - GC run using serial dilutions, Lab #5 - Endosafe® run at 1:10 dilution, Lab #5 - GC run using serial dilutions
