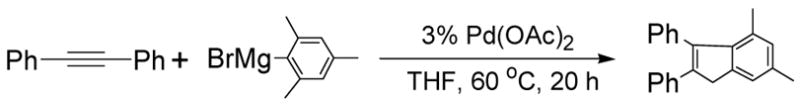
Abstract
Two types of domino reactions from the same internal alkynes and hindered Grignard reagents based on carbopalladation, Pd-catalyzed cross-coupling reaction and C-H activation strategy are described. The realization of these domino reactions relied on the control of the use of the ligand and the reaction temperature. Our study provides an efficient access to useful polysubstituted indenes and cis-substituted stilbenes, and may offer new means to the development of tandem/domino reactions in a more efficient way.
The development of transition metal-catalyzed tandem or “domino” reactions, which combine two or more bond-forming reactions into one synthetic operation, represents one of the most attractive subjects in synthetic organic chemistry.1,2 Such tandem/domino reactions allow the concomitant formation of two or more bonds with rapid increase in molecular complexity with minimized separation/purification efforts. Since arranging two or more bond-forming reactions to occur in a tandem or domino fashion is always challening, it is not surprising to observe that almost all tandem/domino reactions were developed on a basis of one type of tandem/domino reaction per one set of substrates/reagents.1–3 Developing two or more types of tandem/domino reactions from the same substrates and reagents, which represents a strategy that could further heighten the efficiency of conducting reactions in a tandem/domino fashion, is apparently very attractive, but remains to be largely unexplored.4
We have recently documented the palladium-catalyzed tandem reaction of 1,2-dhalobenzenes and 2-haloaryl tosylates with hindered Grignard reagents to form substituted fluorenes,5 in which palladium-associated arynes were believed to be the intermediates when the reaction was carried out in the absence of phosphines or N-heterocyclic carbenes ligands.5b The triple bond nature of arynes led us to consider that alkynes might also function similarly as in situ generated arynes. We thus envisioned that carbopalladation of alkynes could generate vinylpalladium(II)X complexes I (Scheme 1).6 I could then (a) undergo cyclization via C-H activation7,8 to afford substituted indenes, which are structural constituents of metallocene-based catalysts for olefin polymerizations, of biologically active compounds and of functional materials;9,10 and (b) undergo transmetalation followed by reductive elimination (cross-coupling process) to yield cis-stilbenoid hydrocarbons, which are potentially useful in the fields of molecular sensors and molecular electronics.11,12 Therefore, two types of domino reactions, namely domino carbopalladation-cyclization to form polysubstituted indenes and domino carbopalladation–cross-coupling to form cis-stilbenoid hydrocarbons containing highly substituted phenyl groups, might be developed from the same alkynes and hindered Grignard reagents if the two competing pathways could be controlled (Scheme 1). Herein, we report our successful realization of these two types of reactions by controlling the use of ligand and the reaction temperature.
Scheme 1.
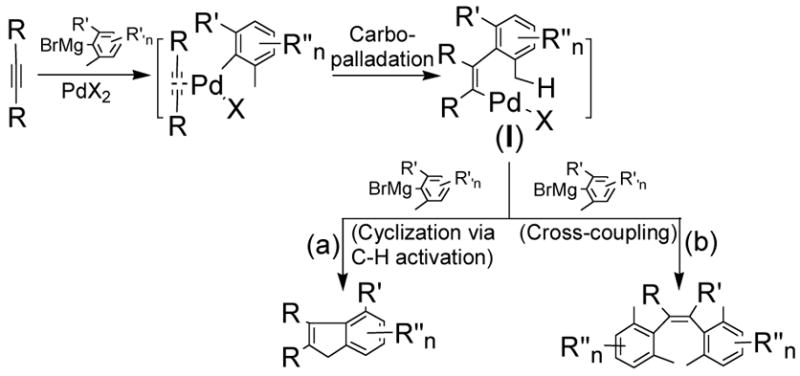
Domino Carbopalladation-Cyclization via sp3 C-H Activation vs. Domino Carbopalladation-Cross-Coupling Reaction
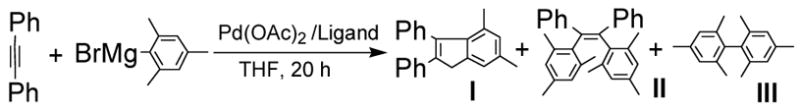
Based on the consideration that the activation of C-H bond would involve the interaction of C-H bond with Pd(II) center and such interaction would be disfavored at higher reaction temperature and/or in the presence of ligands, we surmised the cyclization via sp3 C-H activation process would be favored in the absence of ligands and at lower reaction temperature. We thus began our study by examining the reaction of diphenylacetylene with 2-mesitylPd(II)(OAc), in situ generated from 2-mesitylmagnesium bromide with Pd(OAc)2. We found the domino carbopalladation-cyclization product 4,6-dimethyl-2,3-diphenylindene was the major product with only Pd(OAc)2 as the promoter, either at room temperature, 60 C or refluxing (Table 1, entries 1, 2, 5). The use of PPh3 as a ligand decreases the formation of the cyclization product as well as slowed down the reaction (Table 1, entries 2–4). By using 4 equiv. of PPh3 and in refluxing THF, the domino carbopalladation–cross-coupling product became the major product, along with the self-coupling of Grignard reagent as the main side reaction (Table 1, entry 7). Our results suggested that by controlling the use of ligand and reaction temperature, it is possible to control the domino reaction pathways.
Table 1.
Pd(OAc)2-Promoted Domino Reaction of Diphenylacetylene with 2-Mesitylmagnesium Bromide a
 | ||||||
|---|---|---|---|---|---|---|
| ratiob |
||||||
| entry | ligand | temperature | conversion | I | II | III |
| 1 | None | R. T. | 85% | 97 : | 2 : | 1 |
| 2 | None | 60 | 99% | 90 : | 2 : | 8 |
| 3 | 2 equiv PPh3 | 60 | 75% | 81 : | 9 : | 10 |
| 4 | 4 equiv PPh3 | 60 | 60% | 20 : | 24 : | 56 |
| 5 | None | Reflux | 99% | 93 : | 3.5 : | 3.5 |
| 6 | 2 equiv PPh3 | Reflux | 99% | 69 : | 12 : | 19 |
| 7 | 4 equiv PPh3 | Reflux | 81% | 2 : | 67 : | 31 |
Reaction conditions: diphenylacetylene (1.0 equiv), Grignard reagent (2.5 equiv), Pd(OAc)2 (1 equiv), THF (2 mL), 20 h.
Based on 1H NMR.
As Pd(II)X2 would be reduced to Pd(0) species after every reaction cycle, after establishing factors that influence the reaction competing pathways, we next turned our attention to develop the catalytic version of these two types of domino reactions by identifying oxidants that could oxidize Pd(0) species to Pd(II) species. We have tested several commonly available oxidants and found 1,2-dibromoethane can be served as an excellent oxidizer (Table 2). By using a stoichiometric amount of 1,2-dibromoethane and 3% Pd(OAc)2, the domino carbopalladation-cyclization process proceeded smoothly to give 4,6-dimethyl-2,3-diphenylindene in excellent yield (Table 2, entry 6).
Table 2.
Pd(II)-Catalyzed Domino Reaction of Diphenylacetylene with Mesitylmagnesium Bromide a
Reaction conditions: diphenylacetylene (1.0 equiv), Grignard reagent (2.5 equiv), THF (2 mL).
Conversion based on 1H NMR
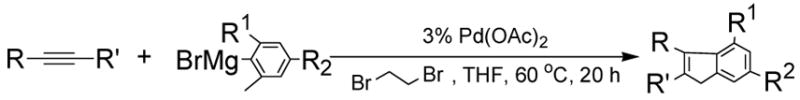
With 1,2-dibromoethane as the oxidant, a number of alkynes were examined for the Pd(OAc)2-catalyzed domino carbopalladation-cyclization reaction and our results are listed in Table 3. We found that diaryl-, dialkyl- and alkylarylacetylenes were all suitable substrates. When unsymmetrical alkylarylacetylenes were employed as the substrates, we found the domino reaction occurred mainly from the alkyl sides of alkyarylacetylenes,13 as evidenced by the ratio of two isomeric products (Table 3, entries 9–13). To determine whether other types of hydrogens (nonbenzylic 1° hydrogens, benzylic 2°, and 3° hydrogens) could also be activated under our condition, we have tested 2-ethyl-6-methylphenylmagnesium bromide and 2-isopropyl-6-methylphenylmagnesium bromide for the domino reaction. We found that the sp3 C-H activation exclusively occurred at the benzylic methyl group, suggesting that nonbenzylic 1° hydrogens (nonbenzylic methyl group), 2° (ethyl group) and 3° (isopropyl group) benzylic hydrogens could not be activated (Table 3, entries 14, 15). This was further confirmed by the fact that no reaction was observed for 2,6-diethylphenylmagnesium bromide with diphenyl-acetylene (Table 3, entry 16).
Table 3.
Pd(OAc)2-Catalyzed Cyclizations of Internal Alkynes with Hindered Grignard Reagentsa
 | ||||
|---|---|---|---|---|
| entry | alkyne | ArMgBr | product | yield(%)b |
| 1 |
|
|
|
87 |
| 2 |
|
|
|
85 |
| 3 |
|
|
|
81 |
| 4 |
|
|
|
87 |
| 5 |
|
|
|
97 |
| 6 |
|
|
|
94 |
| 7 |
|
|
|
71 |
| 8 |
|
|
|
77 |
| 9 |
|
|
|
78 |
| 10 |
|
|
|
72d,e |
| 11 |
|
|
|
64d,f |
| 12 |
|
|
|
85 |
| 13 |
|
|
|
67 |
| 14 |
|
|
|
69 |
| 15 |
|
|
|
78 |
| 16 |
|
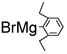
|
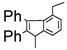
|
<2%g |
Reaction conditions: alkyne (1.0 equiv), Grignard reagent (2.5 equiv), Pd(OAc)2 (3%), 1,2-dibromoethane (1.0 equiv.), THF (2 ml), 60 °C.
Isolated yields.
Ratio based on 1H NMR.
Reaction condition: room temperature, 30 h.
15% Crossc-oupling product was observed.
21% Cross-coupling product was observed.
Reaction time: 45 h.
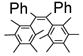
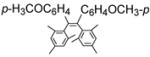
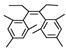
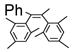
By using 1,2-dibromoethane as the oxidant, 4 equivalent of PPh3 relative to Pd(OAc)2 and in refluxing THF, we were also able to realize the second type of domino reaction, the carbopalladation followed by cross-coupling, to form cis-stilbenes,11,12 in a catalytic fashion. cis-Substituted stilbenes containing highly substituted phenyl groups were obtained in good yields from the same alkynes and hindered Grignard reagents that form polysubstituted indenes (Table 4). Our results also suggested that Pd(PPh3)2Cl2-catalyzed reactions of trans-1,2-dibromoalkenes with Grignard reagents in refluxing THF to give cis-substituted stilbenes12a most likely also proceeded with alkynes and I as the reaction intermediates.14
Table 4.
Pd(OAc)2-Catalyzed Domino Carbopalladation-Cross-coupling of Internal Alkynes and Hindered Grignard Reagents a
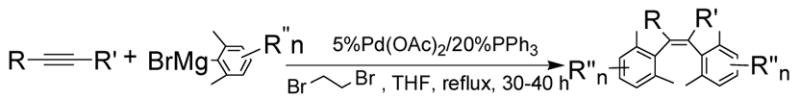 | ||||
|---|---|---|---|---|
| entry | yield(%)b | |||
| 1 |
|
|
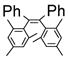
|
71 |
| 2 |
|
|
|
65 |
| 3 |
|
|
|
72 |
| 4 |
|
|
|
69 |
| 5 |
|
|
|
60 |
| 6 |
|
|
|
81 |
| 7 |
|
|
|
78 |
| 8 |
|
|
|
74 |
| 9 |
|
|
|
81 |
Reaction conditions: alkyne (1.0 equiv), Grignard reagent (4.0 equiv), 1,2-dibromoethane (1.5 equiv), Pd(OAc)2 (5%), PPh3 (20%), THF (2 mL), refluxing, 30–40 h.
Isolated yields.
In summary, we developed two types of Pd-catalyzed domino reactions from the same alkynes and hindered Grignard reagents by controlling the use of ligand and the reaction temperature. We also showed that only benzylic methyl hydrogens might be activated by Pd(II) species. Our study provided an efficient access to useful polysubstituted indenes and cis-substituted stilbenes from simple, commercially available starting materials/reagents. The ligand and temperature factors for controlling the domino reaction pathways identified in this study may also be applicable for other cross-coupling and C-H activation-based tandem/domino reactions. Work toward this direction is underway.
Supplementary Material
General procedures and product characterizations for palladium-catalyzed domino reactions.
Acknowledgments
We gratefully thank the NIH (GM69704) for funding. Partial support from the Petroleum Research Fund-administered by the American Chemical Society (44428-AC1) and PSC-CUNY Research Award Programs is also gratefully acknowledged. We also thank Dr. Hsin Wang at the College of Staten Island for his help on the NMR NOE experiments and Frontier Scientific, Inc. for its generous gifts of palladium acetate.
References
- 1.(a) Wasilke JC, Obrey SJ, Baker RT, Bazan GC. Chem Rev. 2005;105:1001–1020. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]; (b) Tietze LF, Rackelmann N. Pure Appl Chem. 2004;76:1967–1983. [Google Scholar]; (c) Nicolaou KC, Montagnon T, Snyder SA. Chem Commun. 2003:551–564. doi: 10.1039/b209440c. [DOI] [PubMed] [Google Scholar]; (d) Parsons PJ, Penkett CS, Shell AJ. Chem Rev. 1996;96:195–206. doi: 10.1021/cr950023+. [DOI] [PubMed] [Google Scholar]; (e) Tietze LF. Chem Rev. 1996;96:115–136. doi: 10.1021/cr950027e. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 2.(a) de Meijere A, von Zezschwitz P, Brase S. Acc Chem Res. 2005;38:413–422. doi: 10.1021/ar980025r. [DOI] [PubMed] [Google Scholar]; (b) Ritleng V, Sirlin C, Pfeffer M. Chem Rev. 2002;102:1731–1770. doi: 10.1021/cr0104330. [DOI] [PubMed] [Google Scholar]; (c) Ikeda S-i. Acc Chem Res. 2000;33:511–519. doi: 10.1021/ar9901016. [DOI] [PubMed] [Google Scholar]; (d) Montgomery J. Acc Chem Res. 2000;33:467–473. doi: 10.1021/ar990095d. [DOI] [PubMed] [Google Scholar]; (e) Heumann A, Reglier M. Tetrahedron. 1996;52:9289–9346. [Google Scholar]; (f) Malacria M. Chem Rev. 1996;96:289–306. doi: 10.1021/cr9500186. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 3.Selected recent examples of Pd-catalyzed tandem/domino reactions: Mariampillai B, Alberico D, Bidau V, Lautens M. J Am Chem Soc. 2006;128:14436–14437. doi: 10.1021/ja064742p.Bertrand MB, Wolfe JP. Org Lett. 2006;8:2353–2356. doi: 10.1021/ol0606435.Leclerc JP, Andre M, Fagnou K. J Org Chem. 2006;71:1711–1714. doi: 10.1021/jo0523619.Henderson JL, Edwards AS, Greaney MF. J Am Chem Soc. 2006;128:7426–7427. doi: 10.1021/ja0615526.Pinto A, Neuville L, Retailleau P, Zhu J. Org Lett. 2006;8:4927–4930. doi: 10.1021/ol062022h.Martins A, Marquardt U, Kasravi N, Alberico D, Lautens M. J Org Chem. 2006;71:4937–4942. doi: 10.1021/jo060552l.Hay MB, Wolfe JP. J Am Chem Soc. 2005;127:16468–16476. doi: 10.1021/ja054754v.Tsang WCP, Zheng N, Buchwald SL. J Am Chem Soc. 2005;127:14560–14561. doi: 10.1021/ja055353i.Bressy C, Alberico D, Lautens M. J Am Chem Soc. 2005;127:13148–13149. doi: 10.1021/ja054472v.Zhao J, Larock RC. Org Lett. 2005;7:701–704. doi: 10.1021/ol0474655.Ohno H, Yamamoto M, Iuchi M, Tanaka T. Angew Chem, Int Ed. 2005;44:5103–5106. doi: 10.1002/anie.200500159.Yang Q, Ney JE, Wolfe JP. Org Lett. 2005;7:2575–2578. doi: 10.1021/ol050647u.Cheung WS, Patch RJ, Player MR. J Org Chem. 2005;70:3741–3744. doi: 10.1021/jo050016d.Zhao J, Larock RC. Org Lett. 2005;7:701–704. doi: 10.1021/ol0474655.Ganton MD, Kerr MA. Org Lett. 2005;7:4777–4779. doi: 10.1021/ol052086c.Abbiati G, Arcadi A, Canevari V, Capezzuto L, Rossi E. J Org Chem. 2005;70:6454–6460. doi: 10.1021/jo050882q.Huang Q, Fazio A, Dai G, Campo MA, Larock RC. J Am Chem Soc. 2004;126:7460–7461. doi: 10.1021/ja047980y.Wolfe JP, Rossi MA. J Am Chem Soc. 2004;126:1620–1621. doi: 10.1021/ja0394838.Ney JE, Wolfe JP. Angew Chem, Int Ed. 2004;43:3605–3608. doi: 10.1002/anie.200460060.
- 4.For examples: Nakhla JS, Kampf JW, Wolfe JP. J Am Chem Soc. 2006;128:2893–2901. doi: 10.1021/ja057489m.Ney JE, Wolfe JP. J Am Chem Soc. 2005;127:8644–8651. doi: 10.1021/ja0430346. Also Ref. 1 and 2.
- 5.(a) Dong CG, Hu Q-S. Org Lett. 2006;8:5057–5060. doi: 10.1021/ol061989i. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dong CG, Hu Q-S. Angew Chem, Int Ed. 2006;45:2289–2292. doi: 10.1002/anie.200504310. [DOI] [PubMed] [Google Scholar]
- 6.Recent reviews for carbopalladation of alkynes: Zeni G, Larock RC. Chem Rev. 2004;104:2285–2310. doi: 10.1021/cr020085h.de Meijere A, von Zezschwitz P, Brase S. Acc Chem Res. 2005;38:413–422. doi: 10.1021/ar980025r.
- 7.Recent reviews for C-H activation: Dyker G, editor. Handbook of C-H Transformations: Application in Organic Synthesis. Vol. 2. Wiley-VCH; Weinheim: 2005. Kakiuchi F, Chatani N. Advan Synth Catal. 2003;345:1077–1101.Ritleng V, Sirlin C, Pfeffer M. Chem Rev. 2002;102:1731–1770. doi: 10.1021/cr0104330.Jia C, Kitamura T, Fujiwara Y. Acc Chem Res. 2001;34:633–639. doi: 10.1021/ar000209h.
- 8.For examples of Pd-catalyzed cyclizations via sp3 C-H activation: Baudoin O, Herrbach A, Gueritte F. Angew Chem Int Ed. 2003;42:5736–5740. doi: 10.1002/anie.200352461.Suau R, Lopez-Romero JM, Rico RD. Tetrahedron Lett. 1996;37:9357–9360.Dyker G. J Org Chem. 1993;58:6426–6428.Dyker G. Angew Chem Int Ed. 1994;33:103–105.Dyker G. Angew Chem Int Ed. 1992;31:1023–1025.
- 9.(a) Alt HG, Köppl A. Chem Rev. 2000;100:1205–1222. doi: 10.1021/cr9804700. [DOI] [PubMed] [Google Scholar]; (b) Korte A, Legros J, Bolm C. Synlett. 2004;13:2397–2399. and references therein. [Google Scholar]; (c) Barbera J, Rakitin OA, Ros MB, Torroba T. Angew Chem, Int Ed. 1998;37:296–299. doi: 10.1002/(SICI)1521-3773(19980216)37:3<296::AID-ANIE296>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 10.Substituted indenes were typically prepared in more than one step. For recent examples of one-pot access to substituted indenes with different substitution patterns: From 2-(2-(1-alkynyl)phenyl)malonates or 2-(2-halophenyl)malonate: Guo LN, Duan XH, Bi HP, Liu XY, Liang YM. J Org Chem. 2006;71:3325–3327. doi: 10.1021/jo0601361.Zhang D, Yum EK, Liu Z, Larock RC. Org Lett. 2005;7:4963–4966. doi: 10.1021/ol051907a. Also see: Kuninobu Y, Nishina Y, Takai K. Org Lett. 2006;8:2891–2893. doi: 10.1021/ol0611292.Nakamura I, Bajracharya GB, Wu H, Oishi K, Mizushima Y, Gridnev ID, Yamamoto Y. J Am Chem Soc. 2004;126:15423–15430. doi: 10.1021/ja044603c.
- 11.(a) Rathore R, Lindeman SV, Kochi JK. Angew Chem. 1998;110:1665–1667. [Google Scholar]; Angew Chem, Int Ed. 1998;37:1585–1587. doi: 10.1002/(SICI)1521-3773(19980619)37:11<1585::AID-ANIE1585>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]; (b) Irie M. Chem Rev. 2000;100:1685–1716. doi: 10.1021/cr980069d. [DOI] [PubMed] [Google Scholar]
- 12.The most efficient preparation methods reported so far involve the use of 1,2-dibromoalkenes with hindered Grignard reagents: Rathore R, Deselnicu MI, Burns CL. J Am Chem Soc. 2002;124:14832–14833. doi: 10.1021/ja027421w. For other methods: Maeda K, Okamoto Y, Morlender N, Haddad N, Eventova I, Biali SE, Rappoport Z. J Am Chem Soc. 1995;117:9686–9689.Bottino FA, Finocchiaro P, Libertini E, Reale A, Recca A. J Chem Soc, Perkin Trans 2. 1982:77–81.
- 13.A similar regioselectivity trend was observed in Pd-catalyzed three-component reactions of aryl iodides, internal alkynes and arylboronic acids: Zhou C, Larock RC. J Org Chem. 2005;70:3765–3777. doi: 10.1021/jo048265+.
- 14.Pd(PPh3)2Cl2-catalyzed reactions of trans-1,2-dibromoalkenes with Grignard reagents in refluxing THF were reported to give cis-substituted stilbenes in excellent yields (Ref. 12a). In our hands, we found Pd(PPh3)2Cl2-catalyzed reaction of trans-1,2-dibromo-1,2-diphenyl-ethene with pentamethlphenylmagnesium bromide indeed gave cis-substituted stilbene in 86% yield. However, we also found Pd(PPh3)2Cl2-catalyzed reactions of trans-1,2-dibromo-1,2-diphenylethene or trans-3,4-dibromo-3-hexene with 2-mesitylmagnesium bromide or 2,6-dimethylhenylmagnesium bromide in refluxing THF gave significant amounts of substituted indenes (>18%); When the reaction temperature was 60°C, substituted indenes were obtained in 70–98% yields
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General procedures and product characterizations for palladium-catalyzed domino reactions.