Abstract
Of 994 patients admitted to the Bangkok Hospital for Tropical Diseases for P. vivax malaria, 104 (10.5%) experienced appearance of Plasmodium falciparum following drug treatment for P. vivax . In all patients, P. falciparum parasites were not found by microscopic examination upon admission. The mean time for P. falciparum appearance was 12.6 days after the commencement of chloroquine treatment. Patients experiencing appearance of P. falciparum. had significantly lower hematocrit, and greater initial P. vivax parasite counts. We use a mathematical model to explore the consequences of chloroquine treatment of such mixed infections. Both clinical results and features of the model suggest that such “hidden infections” may be quite common, and that the appearance of P. falciparum may be stimulated by treatment of P. vivax.
INTRODUCTION
Plasmodium vivax and P. falciparum are the most prevalent of the four species that cause human malaria, and mixed infections of the two are common and frequently recorded in field surveys (for reviews, see Cohen, 1973; Richie, 1988; McKenzie and Bossert, 1997, 1999). Recent studies using acridine orange, nested PCR, and microtiter-plate hybridization methods have indicated that mixed infections are far more prevalent than has been suspected based on conventional microscopy (Brown, et al, 1992; Snounou et al, 1993; Kawamoto et al, 1996; Postigo, 1998; Zhou et al, 1998; May et al, 1999). It is not surprising, therefore, that numerous hospital studies following patients longitudinally have indicated substantial underreporting of mixed infections in cases thought to be P. falciparum alone (Meek et al, 1986; Looareesuwan et al, 1987; 1994a; 199b; 1997). More recently, we found that 10.5% of patients diagnosed with P. vivax alone actually harbored P. falciparum as well (Krudsood et al, 1999). While the appearance of P. vivax following treatment for P. falciparum might be attributed to vivax relapse from hypnozootic forms, the appearance of P. falciparum following admission and treatment for P. vivax is less easily accounted for, as it is not clear why: (1) such mixed infections are missed during blood examination, and (2) P. falciparum appears following the commencement of chloroquine treatment of P. vivax.
The dynamics of mixed infections have been explored using models of mixed infection for P. falciparum and P. malariae (Mason et al, 1999) and for P. vivax and P. falciparum (Mason and McKenzie, 1999). Both studies suggested that one parasite can greatly affect the dynamic of the second through non-specific and cross-specific immune response, thus leading to significant differences in clinical status. The model of P. vivax- P. falciparum explored the effects of drug treatment of a mixed infection, and found that mistaken treatment of a mixed infection as a single P. vivax infection could lead to a reappearance of P. falciparum. Here we discuss the clinical features of P. falciparum reappearance after treatment for P. vivax with chloroquine and apply our model to suggest a mechanism for this reappearance.
PATIENTS AND METHODS
The study was conducted in concert with a study on chloroquine resistance in Thailand (Looareesuwn et al 1999). The study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University. Patients older than 12 years who were admitted to the Bangkok Hospital for Tropical Diseases between April 1992 and December 1997 were enrolled if they had acute vivax malaria upon admission, had not received antimalarial treatment before admission, had no patent P. falciparum upon admission and signed a consent form. Reasons for exclusion were pregnancy or unwillingness to remain hospitalized for at least 28 days. All patients were treated with a standard regimen of 1,500 mg CQ (chloroquine phosphate from the Government Pharmaceutical Organization of Thailand, in tablets containing 150 mg each) over 3 days: 600 mg base initially (0 hours), followed by 300 mg base at 6, 24, and 48 hours, giving a total dose of approximately 25 mg/kg. P. falciparum appearance was defined as the microscopic diagnosis of P. falciparum following the commencement of therapy. Standard descriptive and statistical analysis were conducted using version 6.04 of the Epi-Info software (Centers for Disease Control, Atlanta, GA). Comparisons were made using chi-square and Student’s t-test.
Computer modeling was conducted using the model for P. vivax-P. falciparum mixed infections developed by Mason and McKenzie (1999) and is described there in detail. For chloroquine we assumed a parasite reduction rate of 100, based on the published range of 10–1,000 (White, 1997). The pharmacodynamics of chloroquine are notoriously complex, due to a wide range of reported blood half-lives (Desjardins et al, 1988), conversion to the active metabolite desethyl chloroquine, and use of multiple doses. Thus, we included a variable representing the time chloroquine remained active (i.e, the time before serum concentrations fell below the minimum inhibitory concentration (MIC)), rather than the value of the MIC itself. The parameter space of immune coefficients, super-inoculation timing, and parasite growth rates was searched for conditions which would result in low p falciparum parasitemia relative to P. vivax density. In addition to the parameters described in Mason and McKenzie (1999) we tested the model for P. vivax erythrocytic schizogony rates of 13 and 18 merozoites/schizont/2-day interval. These parameter sets were then used for simulated chloroquine administration at different P. falciparum levels of sensitivity.
RESULTS
Clinical study
Overall, 992 patients were enrolled in the study. Of these 10.5% (104/992) experienced an appearance of P. falciparum during their 28 days in the hospital. P. falciparum appearance varied from 1 to 28 days following commencement of CQ treatment for P. vivax. A summary of days of P. falciparum appearance is shown in Fig 1. Due to patients dropping out of the study, charts were available for only 87 of the 104 who experiened an appearance of P. falciparum. As can be seen from the graph, the day of appearance was distributed over the entire study time, with the exception of days 24–27. The mean time of appearance was 12.6 days (SD = 6.82). An example of a mixed infection in a patient is given in Fig 2. Briefly, measurements found to be significantly associated with patients experiencing an appearance of P. falciparum were: higher P. vivax density on admission, depressed hematocrit, elevated albumin, elevated globulin, and elevated alkaline phosphatase (Krudsood et al, 1999).
Fig 1.
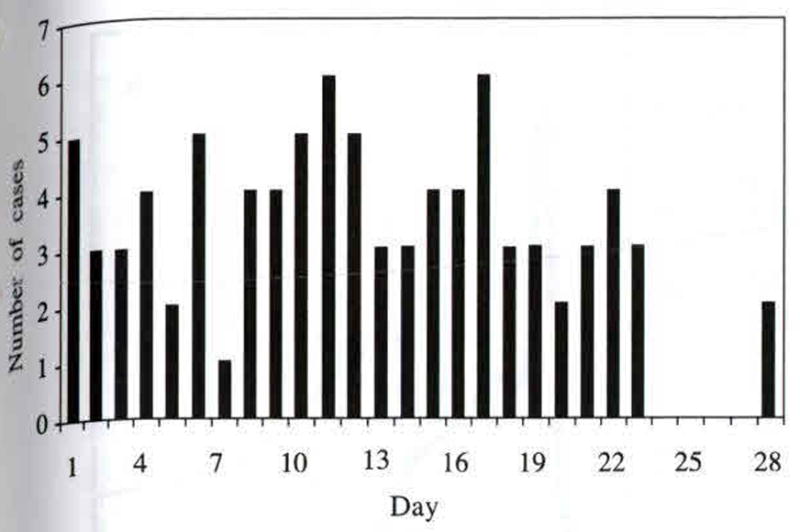
Number of cases of P.falciparum appearance by day, following the commencement of chloroquine treatment for P. vivax infection.
Fig 2.
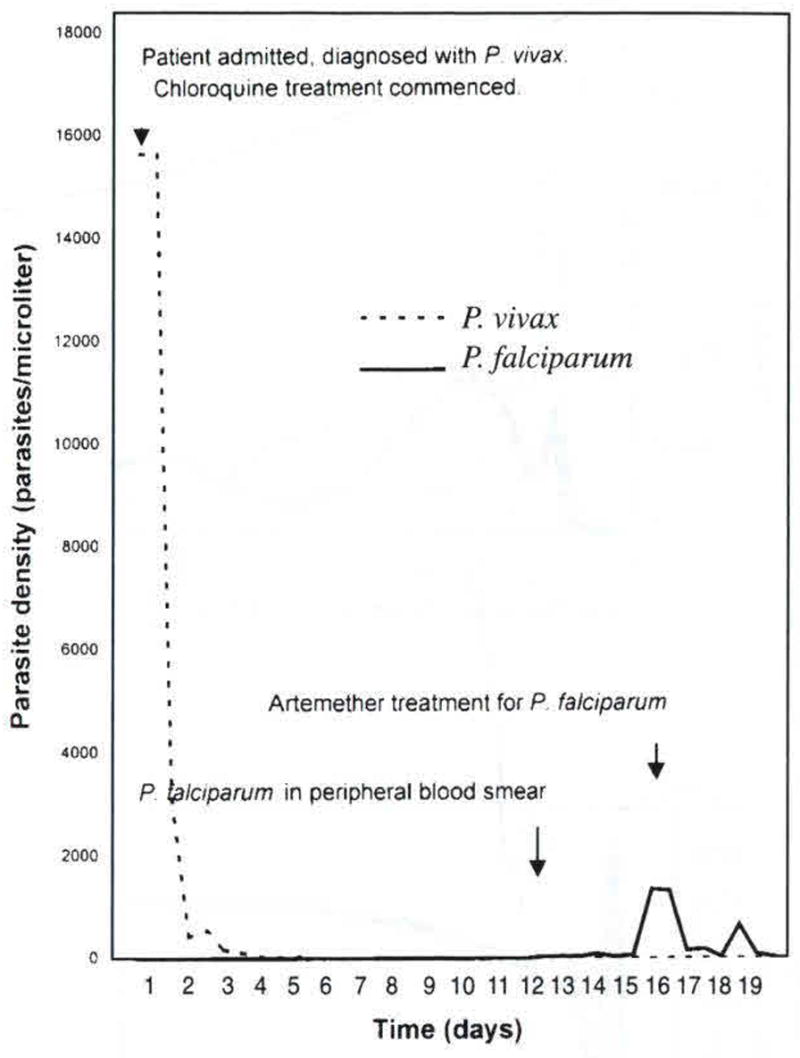
Sample parasite density chart of patient 58, a 20-year old man admitted with P. vivax malaria on day 1, and treated with chloroquine on day 1. P. falciparum appeared in the peripheral blood smear on day 13, and was treated with artemether on day 18.
Application of the model
The structure of the model is described in Mason and McKenzie (1999) which also contains a detailed exploration of parasite equilibria and timing. With regard to the specific questions raised by this study, three conditions were found in which P. vivax parasites greatly outnumbered those of P. falciparum: (1) P. falciparum troughs during out-of-phase P. vivax-P. falciparum parasitemia oscillation, (2) early phases in a P. falciparum superinfection of P. vivax, and (3) mixed infections in which P. vivax erythrocytic schizogony produced more merozoites than did P. falciparum schizogony. An example of each is illustrated in Fig 3. It is important to note the distinction between these figures and the sample patient chart shown in Fig 2: clinically, we could only record parasite density once the patient was admitted; the model gives us insight into the dynamics which preceded and produced the high P. vivax/ low P. falciparum levels observed in the patient.
Fig 3. Conditions resulting in low P. falciparum para-sitemia, relative to P. vivax density. P. vivax density is shown as a havy line, P. falciparum as a thin line.
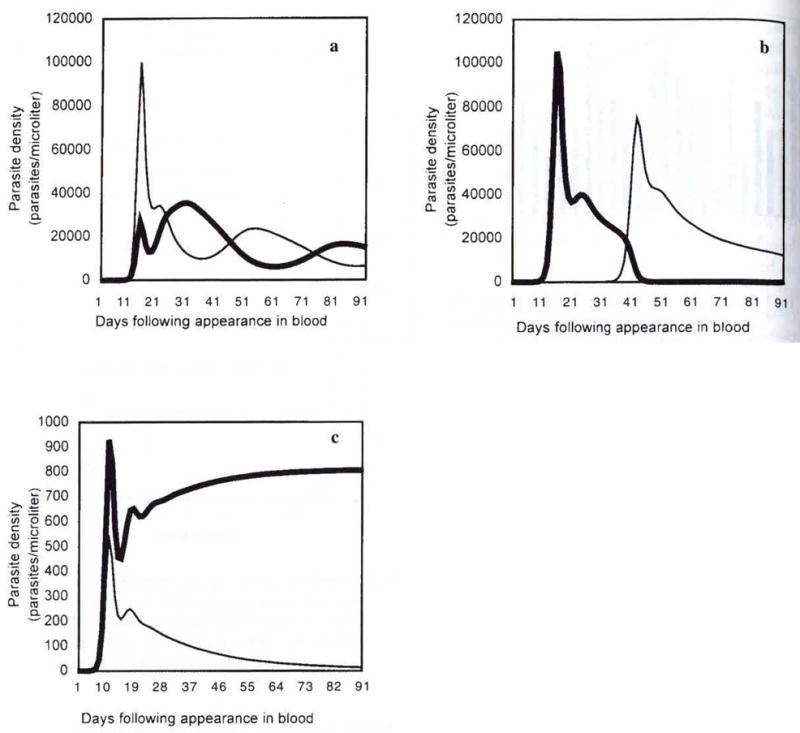
3a. P. falciparum and P. vivax oscillate out of phase with each other. Troughs in P. falciparum correspond to peaks in P. vivax parasitemia. (parameters set at c=0.001; cn=0.01; s=0.001; sn=0.001; x=y=0.0).
3b. P. falciparum superinfection occurs 15 days after that of P. vivax. P. falciparum does not reach patency until day 30, and is outnumbered by P. vivax until day 38. (c=0.001; cn=0.01; s=0.001; sn=0.001; x=y=0.1).
3c. P. vivax strain with greater than average multiplication rate (= 18 merozoites per schizonl). P. falciparum approaches equilibrium at subdetectable levels. (c=0.001; cn=0.01; s=0.001; sn=0.l; x=y=0.1) (Note different y-axis scales, due to variations in immune coefficients).
Assuming varying degrees of P.falciparum chloroquine resistance, treatment in all three scenarios led to rise in P. falciparum parasitemia (Fig 4). The length of the period before parasites became patent varied according to P. falciparum density at time of treatment (higher density lead to faster recrudescence), immune parameters (stronger immune response, slower recrudescence), and MIC. Parasites with greater MIC reached patency faster, but peak parasitemia was lower due to the persistence of P. vivax-raised non-specific immunity.
Fig 4.
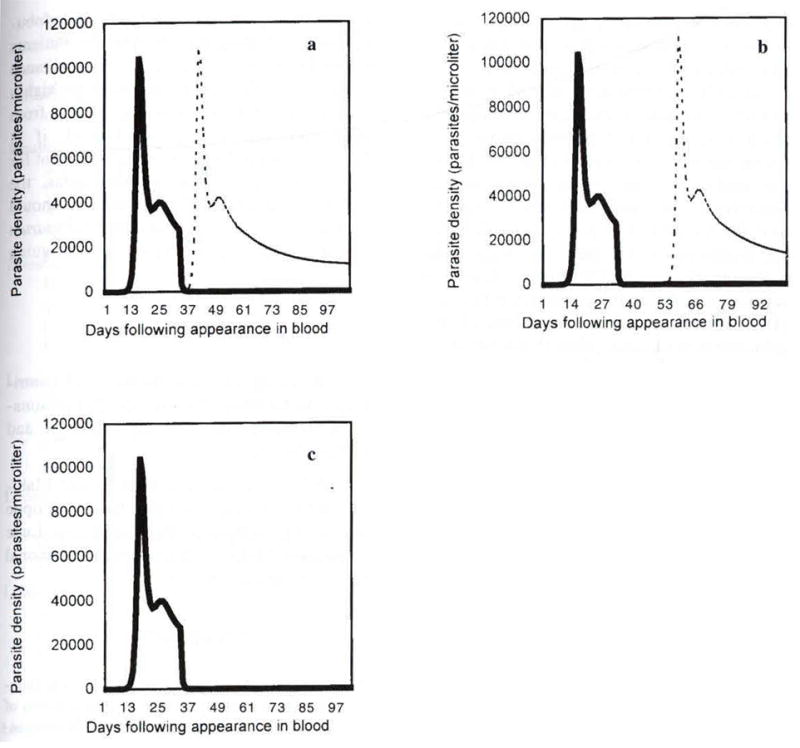
Parasite dynamics of a mixed P. vivax- P. falciparum mixed infection misdiagnosed as P. vivax only. In this example, the patient presented within 30 days following the appearance of P. vivax in the blood, and was treated immediately with chloroquine. P. falciparum infection occurred 15 days after P. vivax infection, and was not yet patent at the time of microscope analysis. P. vivax density is shown as solid line, P. falciparum a dashed line. Graphs indicate differences in P. falciparum density for varying periods of chloroquine activity against P. falciparum. (4a) P. falciparum is completely chloroquine resistant (RIII) (MIC> initial dose). (4b) The MIC is reached in 10 days. P. falciparum is RI resistant, decreases slightly and recrudesces to patent levels after 18 days. (4c) P. falciparum is sensitive to chloroquine. The MIC is reached in 20 days, by this point the sensitive P. falciparum has been eliminated from the blood. (cs=0.001, cn=0.01, ss=0.001, sn=0.001, x=y=0.1, PRR=100).
It is important to note that it is impossible to know which of the three possible conditions illustrated in Fig 3 and discussed in the text produced the low P. falciparum density. Rather, clinically, we can only observe the dynamics following treatment, but must rely on the model to suggest dynamic prior to treatment,
DISCUSSION
Although the phenomenon of “hidden mixed infections” has become increasingly familiar, the cause for both the misdiagnosis of mixed-species as single-species infections, as well the rise of P. falciparum following chloroquine treatment is less certain. Conducting this study in a hospital setting where malaria cannot be transmitted allowed us to eliminate the possibility that the P. falciparum appearance is due to a new infection. And unlike P. vivax appearance following treatment which we have documented above, P. falciparum does not form hypnozoites; thus such an appearance cannot be due to relapse from the liver stage.
The first possible explanation for a missed diagnosis is that the patient was bitten by a P. falciparum-infected mosquito less than 9–10 days (the P. falciparum pre-patency period) prior to admission to the hospital for P. vivax malaria. However, this could not account for the 59% (51/87) of cases which appeared more than 10 days following chloroquine treatment. Although it is possible that later appearances may be due to either heterogeneity in either the host immune response or the parasites themselves, we think it is unlikely that over half the parasites examined have such widely variant pre-patent periods.
If P. falciparum is present in the blood, it may be missed due to low parasitemia. P. falciparum is noted for its ability to sequester in organs; thus observed parasite density is may be considerably less than total parasite load (White, 1997). Low parasitemia is especially likely to be missed in the presence of another parasite at greater density. Indeed, the danger of misdiagnosing mixed infections as single infections has been noted since Knowles and White (1930) who described the “flexible stopping rule”, the tendency of workers to stop examining a blood smear once parasites have been found.
Using the mathematical model, we found that P. vivax parasitemia will be high relative to P. falciparum under three conditions. First, parasite density oscillates due to interspecific suppression, with P. falciparum trough densities corresponding to P. vivax peaks. Second, in a mixed infection, P. falciparum takes a longer time to reach detectable parasitemias (especially if P. falciparum is super-inoculated over a standing P. vivax infection). Finally, although the original model considered the average P. vivax multiplication rate to be 13 merozoites/schizont (Garnham, 1988) in reality this figure is quite variant (Garnham, 1966). According to the model, if P. vivax multiplication is greater than 16, P. falciparum density levels are pushed even lower.
Even if low P. falciparum parasitemia is a product of coinfecting along with P. vivax it does not necessarily follow that appearance of P. falciparum after treatment for P. vivax, is caused by the treatment. Indeed, in two of the three conditions producing low P. falciparum levels (oscillation and super-inoculation), P. falciparum parasitemia eventually rises to surpass that of P. vivax without drug treatment. Nevertheless, the model indicates that the rise of P. falciparum can be precipitated following the treatment for P. vivax. We suggest the following mechanism: Chloroquine resistance is widespread and well documented in Thailand (Trig and Kondrachine, 1998). Thus chloroquine removes the susceptible P. vivax, causing a concomitant fall in non-and cross-specific immune effectors raised by P. vivax. Assuming chloroquine levels drop below the MIC before P. falciparum is removed, P. falciparum will recrudesce.
The time period for P. falciparum appearance varied according to P. falciparum density at the time of treatment (treatment at periods of higher density led to faster recrudescence), strength of the immune response (a stronger immune response, especially persistent specific immunity, slowed recrudescence), and degree of Plasmodium resistance to chloroquine (greater MIC produced faster recrudescence; Fig 3). It is important to note that although it varied in time and peak parasitemia, the surge in P. falciparum parasitemia occurred regardless of the complexities of previous parasite dynamics.
P. falciparum liberation from a non- and cross-specific immune response raised by P. vivax remains a hypothesis, indeed without being able to compare our cohort with untreated P. vivax patients, we cannot be certain whether or not treatment precipitated the rise in P. falciparum. Nevertheless, given the severity of P. falciparum malaria, the potential for P. falciparum appearing following elimination of P. vivax must be considered. There is too little data to currently recommend changes in treatment procedure. Physicians should be aware of the risk factors cited above: namely, higher P. vivax density on admission, depressed hematocrit, depressed albumin, elevated globulin, and elevated alkaline phosphatase. Patients should be warned to report any reappearance of fever, and follow-up blood checks are highly indicated. Overall, we await other studies from other geographical regions. Indeed, if p falciparum appearance following treatment for P. vivax is as common in other areas, the question of whether all vivax malaria should be treated as a mixed infection with P. falciparum appears a highly controversial yet intriguing proposal.
Acknowledgments
We are grateful for the help and contributions of NJ White, WH Bossert, M Phanumaphorn, F Kawamoto, C Wongsrichanalai, and K Nontabutra.
This study was supported by the Mahidol University Research Fund. Daniel Philippe Mason was supported by the Henry Luce Foundation, F Ellis McKenzie by a NIH National Research Service Award.
References
- Brown AE, Kain KC, Pipithkul J, Webster HK. Demonstration by the polymerase chain reaction of mixed Plasmodium falciparum and P. vivax infections undetected by conventional microscopy. Trans R Soc Trap Med Hyg. 1992;86:609–12. doi: 10.1016/0035-9203(92)90147-5. [DOI] [PubMed] [Google Scholar]
- Cohen JE. Heterologous immunity in human malaria. Quart Rev Biol. 1973;48:467–89. doi: 10.1086/407705. [DOI] [PubMed] [Google Scholar]
- Desjardins RE, Doberstyn EB, Wernsdorfer WH. The treatment and prophylaxis of malaria. In: Wernsdorfer WH, McGregor I, editors. Malaria. Edinburgh: Churchill Livingstone; 1988. pp. 827–64. [Google Scholar]
- Garnham PCC. Malaria parasites and other haemosporidia. Oxford: Blackwell Scientific Publications; 1966. [Google Scholar]
- Garnham PCC. Malaria parasites of man: life-cycles and morphology (excluding ultrastructure) In: Wernsdorfer WH, McGregor I, editors. Malaria. Edinburgh: Churchill Livingstone; 1988. pp. 61–96. [Google Scholar]
- Kawamoto F, Miyake H, Kaneko O, Kimura M, Dung NT. Sequence variation in the 18S rRNAgene, a target for PCR-based malaria diagnosis, in Plasmodium ovale from southern Vietnam. J Clin Microbiol. 1996;34:2287–9. doi: 10.1128/jcm.34.9.2287-2289.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles R, White RS. Studies in the parasitology of malaria. Indian Med Res Mem. 1930:18. [Google Scholar]
- Krudsood S, Wilairatana P, Mason DP, Treeprasertsuk S, Singhasivanon P, Looareesuwan S. Hidden Plasmodium falciparum infections. Southeast Asian J Trap Med Public Health. 1999;30:623–4. [PubMed] [Google Scholar]
- Looareesuwan S, Vanijanonta S, Viravan C, et al. Randomised trial of mefloquine-tetracycline and quinine-tetracycline for acute uncomplicated falciparum malaria. Acta Tropicar. 1994a;57:47–53. doi: 10.1016/0001-706x(94)90092-2. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Viravan C, Vanijanonta S, et al. Randomized trial of mefloquine-doxycycline, and artesunate-doxycycline for treatment of acute uncomplicated falciparum malaria. Am J Trop Med Hyg. 1994b;50:784–9. doi: 10.4269/ajtmh.1994.50.784. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, White NJ, Chittamas S, Bunnag D, Harinasuta T. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet. 1987;2:1052–5. doi: 10.1016/s0140-6736(87)91479-6. [DOI] [PubMed] [Google Scholar]
- Looareesuwan S, Wilairatana P, Viravan C, Vanijanonta S, Pitisuttitum P, Kyle DE. Open randomized trial of oral artemether alone and a sequential combination with mefloquine for acute uncomplicated falciparum malaria. Am J Trop Med Hyg. 1997;56:613–17. doi: 10.4269/ajtmh.1997.56.613. [DOI] [PubMed] [Google Scholar]
- Looareesuwan SL, Wilairatana P, Krudsood S. Chloroquine sensitivity of Plasmodium vivax in Thailand. Ann Trop Med Parasitol. 1999;93:225–300. [PubMed] [Google Scholar]
- Mason D, McKenzie FE, Bossert WH. The blood-stage dynamics of mixed Plasmodium malariae-P. falciparum infections. J Theor Biol. 1999;198:549–66. doi: 10.1006/jtbi.1999.0932. [DOI] [PubMed] [Google Scholar]
- Mason D, Mckenzie F. Blood stage dynamics and clinical implications of mixed Plasmodium vivax-Plasmodium falciparum infections. Am J Top Med Hyg. 1999;61:367–74. doi: 10.4269/ajtmh.1999.61.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May J, Mockenhaupt F, Ademowo O, et al. High rate of mixed and subpatent malarial infections in southwest Nigeria. Am J Trop Med. 1999;61:339–43. doi: 10.4269/ajtmh.1999.61.339. [DOI] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. Mixed-species Plasmodium infection of humans. J Parasitol. 1997;83:593–600. [PMC free article] [PubMed] [Google Scholar]
- McKenzie FE, Bossert WH. Multispecies Plasmodium infection of humans. J Parasitol. 1999;85:12–8. [PMC free article] [PubMed] [Google Scholar]
- Meek SR, Doberstyn EB, Gauzere BA, Thanapanich C, Nordlander E, Phuphaisan S. Treatment of falciparum malaria with quinine and tetracycline or combined mefloquine, sulfadoxine, pyrimethamine on the Thai-Kampuchean border. Am J Trop Med Hyg. 1986;35:246–50. doi: 10.4269/ajtmh.1986.35.246. [DOI] [PubMed] [Google Scholar]
- Postigo M, Mendoza-Leon A, Perez HA. Malaria diagnosis by the polymerase chain reaction: a field study in south-eastern Venezuela. Trans R Soc Trop Med Hyg. 1998;92:509–11. doi: 10.1016/s0035-9203(98)90893-8. [DOI] [PubMed] [Google Scholar]
- Richie TL. Interactions between malaria parasites infecting the same vertebrate host. Parasitology. 1988;96:607–39. doi: 10.1017/s0031182000080227. [DOI] [PubMed] [Google Scholar]
- Snounou G, Pinheiro L, Goncalves A, et al. The importance of sensitive detection of malaria parasites in the human and insect hosts in epidemiological studies, as shown by the analysis of field samples from Guinea Bissau. Trans R Soc Trop Med Hyg. 1993;87:649–53. doi: 10.1016/0035-9203(93)90274-t. [DOI] [PubMed] [Google Scholar]
- Trigg PI, Kondrachine AV. The current global malaria situation. In: Sherman IA, editor. Malaria: Parasite Biology, Pathogenesis, and Protection. Washington DC: ASM Press; 1998. pp. 11–22. [Google Scholar]
- White NJ. Assessment of the pharmacodynamic properties of antimalarial drugs in vivo. Antimicrob Agents Chemother. 1997;41:1413–22. doi: 10.1128/aac.41.7.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M, Liu Q, Wongsrichanalai C, Suwonkerd W, et al. High prevalence of Plasmodium malariae and Plasmodium ovale in malaria patients along the Thai-Myanmar border, as revealed by acridine orange staining and PCR-based diagnoses. Trop Med Int Health. 1998;3:304–12. doi: 10.1046/j.1365-3156.1998.00223.x. [DOI] [PubMed] [Google Scholar]


