Abstract
Acrolein, a widely distributed environmental pollutant, reacts with dGuo in DNA to form two pairs of 1,N2-propano-dGuo adducts: (6R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-6-hydroxypyrimido[1,2-a]purine-10(3H)one (α-OH-Acr-dGuo) and (8R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)one (γ-OH-Acr-dGuo). α-OH-Acr-dGuo is the more mutagenic and induces mainly G→T transversions. A recent study demonstrated that acrolein DNA adducts are preferentially formed in p53 mutational hotspots in human lung cancer, but there are no reports on the presence of these adducts in human lung. To directly investigate this question, we have developed a sensitive and specific liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method for quantitative analysis of Acr-dGuo adducts in DNA. Our method is based on the enzymatic hydrolysis of DNA isolated from human lung in the presence of [13C10,15N5]Acr-dGuo as internal standards. Acr-dGuo adducts are enriched from the hydrolysates by solid phase extraction and analyzed by LC-ESI-MS/MS using selected reaction monitoring. The method is accurate and precise, and the identity of the adducts was confirmed by monitoring different transitions from the same parent ion, and by carrying out reactions with NaOH and NaBH4, which produced N2-(3-hydroxypropyl)dGuo or 1,N2-(1,3-propano)dGuo from γ-OH-Acr-dGuo and α-OH-Acr-dGuo, respectively. Thirty DNA samples from lung tissue were analyzed and Acr-dGuo adducts were detected in all samples. Both α-OH- and γ-OH-Acr-dGuo were observed in most of the samples; total adduct concentrations ranged from 16 – 209 adducts/109 nucleotides. These results demonstrate for the first time that both types of Acr-dGuo adducts are present in human lung DNA. There was no difference in adduct levels between current and ex-smokers. Collectively, the results support a plausible role for acrolein as one cause of p53 mutations in human lung.
Introduction
Acrolein, a highly reactive α,β-unsaturated aldehyde, is a widely distributed environmental pollutant (1) and is also formed endogenously through lipid peroxidation (2). Its concentration in cigarette smoke is relatively high, about 18 - 98 μg per cigarette (3). It is mutagenic in bacteria (4–6) and in cultured human cells (7,8). However, it is generally considered non-carcinogenic, except that one study reported the induction of bladder tumors in rats treated with acrolein (9,10). The weak carcinogenicity of acrolein may be due to efficient detoxification by glutathione or other sulfhydryls. Nonetheless, acrolein is strongly suspected to be responsible for the induction of secondary bladder tumors in cyclophosphamide-treated patients (11).
Acrolein reacts readily with dGuo in DNA to form cyclic 1,N2-propanodeoxyguanosine adducts (Acr-dGuo, Scheme 1). Depending on the direction of the initial Michael addition, two pairs of stereoisomers of (6R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-6-hydroxypyrimido[1,2-a]purine-10(3H)one (α-OH-Acr-dGuo) and (8R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)one (γ-OH-Acr-dGuo) are formed (12). α-OH-Acr-dGuo in particular is mutagenic in human cells and induces predominantly G→T transversions (8). Acr-dGuo adducts have been detected in various human tissues as well as tissues from untreated animals (13–16). Early studies used 32P-postlabeling coupled with HPLC and detected γ-OH-Acr-dGuo as the major adduct in vivo. Its level was 3-fold higher in oral tissues of cigarette smokers compared with non-smokers (15). Although extremely sensitive, 32P-postlabeling lacks internal standards for quantitation. A capillary liquid chromatography nanoelectrospray isotope dilution tandem MS method was recently developed for analysis of γ-OH-Acr-dGuo in DNA hydrolysates (16). Its levels were significantly higher in brain tissues from Alzheimer’s disease subjects compared with age-matched controls.
Scheme 1.
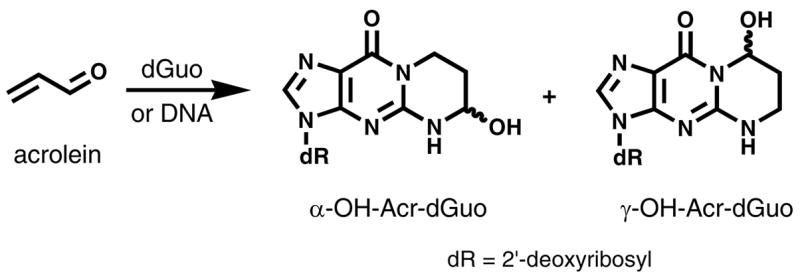
Structures of Acr-dGuo adducts.
A recent study demonstrated that acrolein DNA adducts are preferentially formed at p53 mutational hotspots in human lung cancer and inhibit DNA repair (17). This study challenged the hypothesis that the p53 mutations are due to reactions with polycyclic aromatic hydrocarbon diol epoxides. These results raise the possibility that acrolein, which occurs in quantities up to 10,000 times as great as benzo[a]pyrene in cigarette smoke, may be a major etiological agent for cigarette smoking-related lung cancer. However, there are no reports in the literature on the presence of acrolein-DNA adducts in human lung tissue. In a previous study, we developed a liquid chromatography-electrospray ionization-tandem mass spectrometry (LC-ESI-MS/MS) method to analyze the crotonaldehyde- and acetaldehyde-derived 1,N2-propanodeoxyguanosine (Cro-dGuo), a structurally related adduct (18).
In the present study, we investigated Acr-dGuo adducts in human lung DNA using a similar method. The results demonstrate their presence in relatively high quantities.
Experimental Section
HPLC-UV analysis
This was carried out using Waters Associates (Milford, MA) instruments equipped with a UV detector (Shimadzu Scientific Instruments, Columbia, MD) operated at 254 nm or a model 996 photodiode array detector. System 1 used a 4.6 mm × 25 cm 5 μm Luna C18 column (Phenomenex, Torrance, CA) with isocratic elution by 5% acetonitrile in H2O at a flow rate of 0.7 mL/min. This system was used for the purification of Acr-dGuo and [13C10,15N5]Acr-dGuo. System 2 used the same column as system 1 with a gradient from 5 to 40% CH3OH in H2O over the course of 35 min at a flow rate of 0.7 mL/min. This system was used for the analysis of dGuo.
Chemicals and Enzymes
[13C10,15N5]dGuo was obtained from Spectra Stable Isotopes (Columbia, MD). Ethanol was obtained from AAPER Alcohol and Chemical Co. (Shelbyville, KY). 2-Propanol was purchased from Acros Organics (Morris Plains, NJ). Puregene DNA purification solutions were procured from Gentra Systems (Minneapolis, MN). Calf thymus DNA, micrococcal nuclease, and phosphodiesterase II were obtained from Worthington Biochemical Co. (Lakewood, NJ). Alkaline phosphatase was obtained from Roche Diagnostics Corporation (Indianapolis, IN). All other chemicals were purchased from Sigma-Aldrich.
Acr-dGuo and [13C10,15N5]Acr-dGuo
Acr-dGuo standards were prepared as described (12) from the reaction of dGuo and acrolein. In brief, acrolein (56 mg, 1 mmol) was allowed to react with dGuo (25 mg, 0.09 mmol) in 10 mL of 0.1 M phosphate buffer (pH 7) at 37 ºC overnight. The products were purified by HPLC system 1 (15). α-OH-Acr-dGuo eluted earlier as two interchanging peaks of equal height, corresponding to the two diastereomers, while γ-OH-Acr-dGuo eluted as a single peak. The two peaks of α-OH-Acr-dGuo were collected together, and both α-OH- and γ-OH-Acr-dGuo were characterized and quantified by 1H NMR, using toluene as an internal standard. The NMR spectra were consistent with published results (11, see Supporting Information). α-OH-Acr-dGuo: UV λmax (ε) 259 nm (16800); positive ESI-MS m/z 324 [M + H]+; MS/MS of m/z 324 (collision energy 30 eV): m/z (relative intensity) 208 [BH]+ (100), 190 [BH - H2O]+ (53), 152 [Gua + H]+. γ-OH-Acr-dGuo: UV λmax (ε) 259 nm (18000); positive ESI-MS m/z 324[M + H]+; MS/MS of m/z 324 (collision energy 30 eV): m/z (relative intensity) 208 [BH]+ (48), 190 [BH - H2O]+ (29), 164 [BH - CH3CHO]+ (100), 152[Gua + H]+(18), 135 [Gua- NH3 + H]+ (13). The approximate yields were 5% for each isomer. [13C10,15N5]Acr-dGuo was prepared the same way from [13C10,15N5]dGuo and quantified by UV at 254 nm. The amount of Acr-dGuo in [13C10,15N5]Acr-dGuo, as determined by LC-MS/MS, was less than 0.5%.
Human tissue samples
This study was approved by the University of Minnesota Research Subjects’ Protection Programs Institutional Review Board Human Subjects Committee. Thirty lung samples were obtained from The Cancer Center Tissue Procurement Facility. The samples were histologically confirmed as normal tissue. They were obtained at surgery, immediately frozen in liquid N2, and stored at -80 ºC until DNA isolation. Urine samples were also obtained from some subjects just prior to surgery. They were analyzed for nicotine and cotinine as described previously (19).
DNA Isolation
This was performed as previously described (20), following the “DNA Purification from 1 g Animal Tissue” protocol (Gentra Systems) with several modifications. Isolated DNA was stored at −20 ºC until sample preparation. For the artifact study, lung tissue samples were split into 2 portions. One was isolated as usual, and the other was homogenized in cell lysis solution containing 100 mM NaBH3CN. For this portion of the sample, isopropanol, ethanol, and 70% ethanol all contained 100 mM NaBH3CN.
Analysis of DNA for Acr-dGuo
For enzymatic hydrolysis, DNA (0.1 – 1.0 mg) was dissolved in 900 μL of 10 mM sodium succinate/5 mM CaCl2 buffer (pH 7.0) to which 25 fmol of each isomer of [13C10,15N5]Acr-dGuo was added as internal standard. The mixture was heated at 100 ºC for 30 min and cooled to room temperature. Enzymatic hydrolysis was performed by incubation with 75 units of micrococcal nuclease (from Staphylococcus aureus) and 0.45 unit of phosphodiesterase II (from bovine spleen) at 37 ºC for 6 h. Then, 150 units of alkaline phosphatase (from calf intestine) were added, and the mixture was incubated at 37 ºC overnight. A 10 μL aliquot was removed for dGuo quantitation, and the remaining hydrolysate was purified using a solid phase extraction (SPE) cartridge [Strata-X, 33 μm, 30 mg/1 mL (Phenomenex)]. After the sample was applied, the cartridge was washed with 1 mL H2O and 1 mL 5% CH3OH/H2O, and the analyte was eluted with 1 mL 70% CH3OH/H2O. The eluants were evaporated to dryness, and dissolved in 20 μL of H2O for LC-ESI-MS/MS analysis. A buffer control which lacked DNA was prepared each time and processed in the same way to exclude any contamination; while a calf thymus DNA (0.5 mg) sample was prepared and served as a positive control.
LC-ESI-MS/MS analysis was carried out with an Agilent 1100 capillary flow HPLC (Agilent Technologies, Palo Alto, CA) equipped with a 100 mm × 0.5 mm 1.8 μm particle size C18 column (Agilent Zorbax SB-C18) and coupled to either a Finnigan Quantum Ultra AM or Discovery Max (ThermoElectron, San Jose, CA) triple quadrupole mass spectrometer. The solvent elution program was a gradient from 5 to 25% CH3OH in 15 mM ammonium acetate buffer in 20 min at a flow rate of 10 μL/min at 50 ºC. The ESI source was set in the positive ion mode as follows: voltage, 3.7 kV; current, 3 μA; and heated ion transfer tube, 275 ºC. The adducts were analyzed by MS/MS using selected reaction monitoring (SRM). Ion transitions of m/z 324 → m/z 208 (Acr-dGuo) and m/z 339 → m/z 218 ([13C10,15N5]Acr-dGuo) with collision energy of 12 eV were used for quantitation and those of m/z 324 → m/z 164 and m/z 324 → m/z 190 (Acr-dGuo) and m/z 339 → m/z 174 and m/z 339 → m/z 200 ([13C10,15N5]Acr-dGuo) with collision energy of 32 eV were used for structural confirmation. Other MS parameters were optimized to achieve maximum signal intensity.
Calibration curves were constructed before each analysis using standard solutions of Acr-dGuo and [13C10,15N5]Acr-dGuo. A constant amount of [13C10,15N5]Acr-dGuo (10 fmol) was mixed with differing amounts of Acr-dGuo (0.5 – 100 fmol) and analyzed by LC-ESI-MS/MS-SRM. dGuo content was determined by HPLC system 2, and total nucleotides calculated from the amount of dGuo, considering that dGuo accounts for 19.9% of the nucleotides in human DNA (21). The adduct levels were expressed per 109 nucleotides.
Reaction of Acr-dGuo with NaOH and NaBH4
The eluant from SPE containing the adducts was dissolved in 0.5 mL of 0.5 N NaOH containing an excess of NaBH4. The resulting mixture was heated at 100 °C for 1 h, cooled to room temperature, and neutralized to pH 7 with 1 N HCl. The mixture was loaded on another Strata-X SPE cartridge and washed with 2 mL of H2O to remove salts. The corresponding products were eluted by 1 mL 70% CH3OH/H2O and analyzed by LC-ESI-MS/MS, with the following ion transitions: m/z 326 → m/z 210 [N2-(3-hydroxypropyl)-dGuo], m/z 341 → m/z 220 {[13C10,15N5]N2-(3-hydroxypropyl)-dGuo}, m/z 308 → m/z 192 [1,N2-(1,3-propano)-dGuo], and m/z 323 → m/z 202 {[13C10,15N5]1,N2-(1,3-propano)-dGuo}.
Results
Standard characterization and calibration curves
Acr-dGuo standards were prepared as described (12), and characterized by NMR (see Supporting Information). They exist as two regioisomers, α-OH- and γ-OH-Acr-dGuo. The two diastereomers of α-OH-Acr-dGuo eluted earlier on HPLC as two interchanging peaks, while γ-OH-Acr-dGuo eluted as a single peak. The internal standard for our analysis was [13C10,15N5]Acr-dGuo, prepared by reacting acrolein with [13C10,15N5]dGuo, and characterized by UV and LC-ESI-MS, and comparison to Acr-dGuo. For LC-ESI-MS/MS-SRM analysis, the transitions monitored were m/z 324 → m/z 208 for Acr-dGuo and m/z 339 → m/z 218 for [13C10,15N5]Acr-dGuo. Calibration curves were plotted for the concentration ratios versus the integrated peak area ratios of analyte and internal standards. The two peaks corresponding to α-OH-Acr-dGuo were both integrated to reflect the total amount of this isomer, and linear responses were observed for both α-OH- and γ-OH-Acr-dGuo.
Method Development and Validation
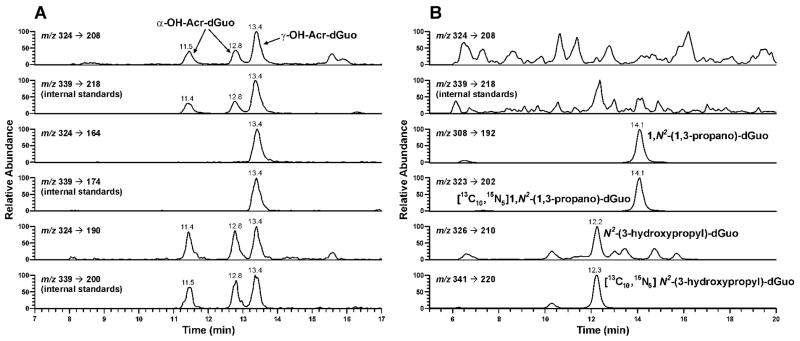
The method was developed based on our previous study on the analysis of Cro-dGuo adducts (18), with some modifications. DNA was enzymatically hydrolyzed in the presence of [13C10,15N5]Acr-dGuo as internal standard, and the analytes were enriched from the hydrolysate by SPE. The enzymatic hydrolysis procedures were optimized to reduce background noise and signal suppression in the MS analysis. Our previous method used DNase I, phophodiesterase I, and alkaline phosphatase with incubation at 37 °C for 1 h. When calf thymus DNA was hydrolyzed in this way, significant signal suppression was observed for Acr-dGuo, which affected quantitation. This was probably due to the polarity of Acr-dGuo, which eluted close to unmodified dAdo. Our current method employed heating DNA to 100 °C for 30 min, followed by the addition of micrococcal nuclease, phosphodiesterase II, and alkaline phosphatase, and overnight incubation (22). Heat-denatured DNA was hydrolyzed much more rapidly by micrococcal nuclease (23,24). In addition, alkaline phosphatase contained a small amount of adenosine deaminase, which converted dAdo to dIno during the longer incubation. When heating was omitted, the measured level of Acr-dGuo was much lower than when heating was included (data not shown). Overall, this method gave more complete hydrolysis of DNA and removed dAdo which interfered with the Acr-dGuo analysis. This resulted in lower background and less signal suppression. Also, various HPLC conditions were investigated for better separation of the three isomeric peaks of Acr-dGuo. LC-ESI-MS/MS-SRM chromatograms obtained upon analysis of untreated calf thymus DNA are shown in Figure 1 (Panel A). All three product peaks were observed in the transition m/z 324 → m/z 208 and they coeluted with the internal standards peaks in the transition m/z 339 → m/z 218. No peaks were observed at this retention time in a buffer control which lacked DNA (data not shown). When the collision energy was increased to 32 eV, a peak at the retention time of γ-OH-Acr-dGuo was observed in the transition m/z 324 → m/z 164 [BH - CH3CHO]+, while all three peaks were observed in the transition of m/z 324 → m/z 190 [BH - H2O]+. Identical peaks were observed for the internal standards at the corresponding transitions. These results were fully consistent with the MS/MS analysis of standards. To further investigate peak identity, eluants from SPE were treated with NaOH and NaBH4. Under these conditions, α-OH- and γ-OH-Acr-dGuo react differently (Scheme 2). γ-OH-Acr-dGuo undergoes base-catalyzed ring-opening followed by reduction of the intermediate aldehyde, producing N2-(3-hydroxypropyl)-dGuo, while treatment of α-OH-Acr-dGuo with NaOH and NaBH4 results in the elimination of H2O followed by reduction, giving unsubstituted 1,N2-(1,3-propano)-dGuo (12). The results of analysis of calf thymus DNA after the reaction with NaOH and NaBH4 are shown in Figure 1 (Panel B). The peaks in the transition m/z 324 → m/z 208 disappeared. By comparing with standards that underwent the same procedures, the peak eluting at 14.1 min in the transition m/z 308 → m/z 192 was assigned as 1,N2-(1,3-propano)-dGuo, which came from reduction of α-OH-Acr-dGuo, while the peak eluting at 12.3 min in the transition m/z 326 → m/z 210 was N2-(3-hydroxypropyl)-dGuo, which came from reduction of γ-OH-Acr-dGuo. Both peaks coeluted with peaks in the transitions m/z 323 → m/z 202 and m/z 341 → m/z 220, from the internal standards. These results unambiguously demonstrate the identity of the adducts as those shown in Scheme 1.
Figure 1.
Chromatograms obtained upon LC-ESI-MS/MS analysis of calf thymus DNA. Calf thymus DNA was enzymatically hydrolyzed, purified by SPE, and analyzed (panel A); or the eluants from SPE were treated with NaOH and NaBH4 and analyzed (panel B).
Scheme 2.
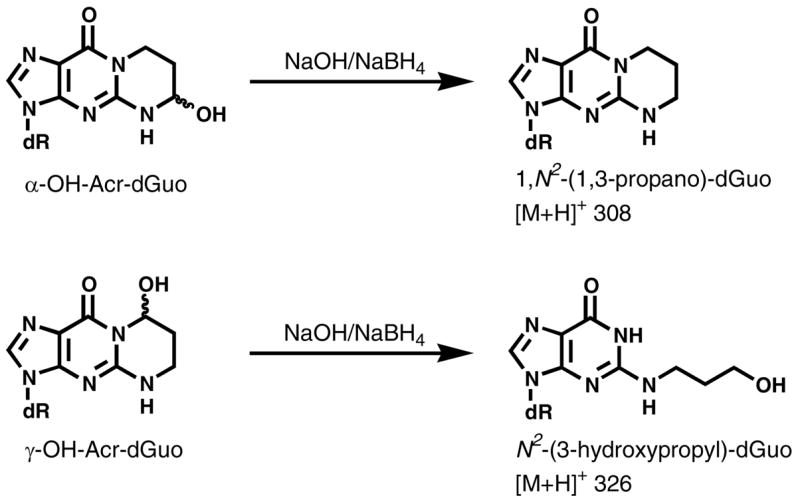
Reaction of α-OH- and γ-OH-Acr-dGuo with NaOH and NaBH4.
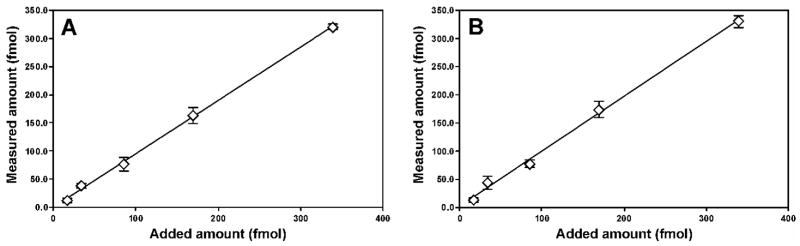
Accuracy was determined by analyzing calf thymus DNA spiked with differing levels of Acr-dGuo standards. Each sample was analyzed in triplicate. The results are summarized in Figure 2, which shows good agreement between expected and measured values. Precision of the method was investigated by analyzing calf thymus DNA in six replicates on three separate days. The interday CVs were 5% for α-OH-Acr-dGuo and 7% for γ-OH-Acr-dGuo, as summarized in Table 1. The limit of quantitation (LOQ) for pure standard was 0.5 fmol injected on column (S/N = 10) as well as a linear MS response. LOQ in DNA samples was estimated as 4 adducts/109 nucleotides, starting from 0.5 mg of DNA. The recovery of 25 fmol of internal standard during sample processing was 88% and 84% for α-OH-Acr-dGuo and →-OH-Acr-dGuo, respectively. The stability of the analytes was investigated by analyzing 25 fmol of standards that were processed through the same enzymatic hydrolysis procedure, and no decomposition was observed.
Figure 2.
Relationship of added to detected Acr-dGuo. Various amounts of α-OH- and γ-OH-Acr-dGuo standards (17, 34, 85, 170, and 340 fmol) were added to calf thymus DNA (0.5 mg) and analyzed by the method described in the text. Background levels in calf thymus DNA (40 adducts/109 nucleotides for α-OH-Acr-dGuo, and 30 adducts/109 nucleotides for γ-OH-Acr-dGuo) were subtracted from each amount detected. Each point represents a triplicate measurement. A, α-OH-Acr-dGuo, R2 = 1.0; B, γ-OH-Acr-dGuo, R2 = 1.0.
Table 1.
Precision of the LC-ESI-MS/MS method for analysis of Acr-dGuoa.
| Acr-dGuo level (adducts/109 nucleotides)
|
|||||
|---|---|---|---|---|---|
| Day 1 Mean ± SD | Day 2 Mean ± SD | Day 3 Mean ± SD | Average | %RSD | |
| α-OH | 40 ± 7 | 37 ± 6 | 43 ± 5 | 40 ± 3 | 7 |
| γ-OH | 30 ± 7 | 27 ± 3 | 29 ± 6 | 29 ± 1 | 5 |
Six aliquots of calf thymus DNA were analyzed on 3 separate days.
It was possible that Acr-dGuo adducts could have been formed as artifacts from the reaction of intracellular acrolein with DNA during DNA isolation. In this case, acrolein could be pre-existing in the cell or produced from lipid peroxidation during DNA isolation. We tested this possibility by adding NaBH3CN to the DNA isolation solutions. NaBH3CN would reduce any acrolein released during DNA isolation. Three lung tissue samples were each divided into two portions and DNA was isolated using solutions containing or not containing NaBH3CN. In other respects, the enzymatic hydrolysis, SPE, and LC-MS/MS analysis procedures were identical. The results indicated that levels of both α-OH- and γ-OH-Acr-dGuo were similar under these two conditions, which eliminated the possibility of artifactual formation of Acr-dGuo adducts.
Analysis of Human Lung DNA for Acr-dGuo
Lung tissue samples were obtained at surgery from 30 subjects (Table 2). Ten were male, 19 were female, and gender information was not available for one subject. Ages ranged from 37 – 81 years (mean ± S.D. 62.2 ± 12), and the age of one subject was unknown. All subjects were current or ex-smokers, based on self report. Urinary nicotine and cotinine levels were available for 14 subjects. These analyses established that 5 of the subjects – numbers 1, 2, 8, 9, 10 – were current smokers, and disagreed with self-report for 2 subjects – numbers 8 and 13 (although we cannot exclude the possibility that the subjects with positive urinary cotinine and nicotine may have been using nicotine replacement therapy). Acr-dGuo adducts were found in all samples. Figure 3 shows a representative chromatogram. Five DNA samples were also analyzed after reaction with NaOH and NaBH4, which gave similar chromatograms to those shown in Figure 1, Panel B. Both α-OH- and γ-OH-Acr-dGuo were detected in all samples except one in which only γ-OH-Acr-dGuo was observed. Adduct levels ranged from ND – 154 adducts/109 nucleotides for α-OH-Acr-dGuo and 6.4 – 159 adducts/109 nucleotides for γ-OH-cr-dGuo, which were much higher than the Cro-dGuo levels we analyzed before (18). There was no difference in adduct levels between confirmed current smokers (N = 5) and non-smokers (N = 9), nor was there any relationship of adduct levels to self-reported time since cessation of smoking, gender, or age.
Table 2.
Levels of Acr-dGuo detected in human lung DNA.
| urinary biomarkers
|
Acr-dGuo levelc (adducts/109 nucleotides)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| subject | gender | race | age at surgery | self-reported smoking historya | cotinine (ng/ml) | nicotine (ng/ml) | amount of DNA analyzed (mg)b | α-OH | γ-OH | total |
| 1 | F | W | 60 | current | 652 | 115 | 0.14 | 91 | 47 | 138 |
| 2 | F | W | 47 | current | 5084 | 1462 | 0.51 | 45 | 21 | 66 |
| 3 | F | W | 52 | 2 yr | 6.8 | 1.3 | 1.25 | 18 | 9 | 27 |
| 4 | M | W | 69 | 2 yr | 25.2 | 4.5 | 1.09 | 18 | 10 | 27 |
| 5 | F | W | 72 | 10 yr | 3.7 | 0.7 | 0.20 | 25 | 49 | 74 |
| 6 | F | W | 79 | 10 yr | 9.2 | 1.3 | 0.10 | 78 | 49 | 127 |
| 7 | M | W | 66 | 26 yr | 4.3 | 1.5 | 0.03 | 154 | 55 | 209 |
| 8 | F | W | 51 | 3 months | 2397 | 534 | 0.70 | 10 | 6 | 16 |
| 9 | M | W | 53 | current | 2346 | 410 | 0.81 | 15 | 10 | 25 |
| 10 | M | W | 53 | current | 692 | 117 | 0.54 | ND | 159 | 159 |
| 11 | F | W | 61 | 9 yr | 26.7 | 4.1 | 0.29 | 75 | 47 | 121 |
| 12 | F | B | 52 | 6 yr | 34.1 | 16.4 | 0.55 | 25 | 12 | 36 |
| 13 | F | W | 37 | current | 3.3 | 1.7 | 0.61 | 16 | 9 | 25 |
| 14 | M | W | 78 | 24 yr | 7.2 | 0.6 | 0.09 | 85 | 53 | 138 |
| 15 | F | W | 68 | 1 month | NA | NA | 0.65 | 33 | 23 | 56 |
| 16 | F | W | 72 | 2 months | NA | NA | 0.49 | 20 | 13 | 33 |
| 17 | M | W | 78 | NA | NA | NA | 0.77 | 12 | 18 | 29 |
| 18 | F | W | 60 | 2 yr | NA | NA | 0.10 | 44 | 24 | 68 |
| 19 | M | W | 72 | 7 yr | NA | NA | 0.86 | 10 | 6 | 16 |
| 20 | F | W | 46 | 1 week | NA | NA | 0.72 | 10 | 9 | 19 |
| 21 | F | W | 81 | 23 yr | NA | NA | 0.48 | 17 | 17 | 34 |
| 22 | F | W | 58 | 18 yr | NA | NA | 1.08 | 13 | 13 | 26 |
| 23 | F | W | 76 | 10 yr | NA | NA | 0.04 | 110 | 82 | 192 |
| 24 | M | W | 66 | 17 yr | NA | NA | 0.64 | 16 | 14 | 29 |
| 25 | F | W | 51 | 1 yr | NA | NA | 0.53 | 10 | 13 | 24 |
| 26 | F | W | 56 | 1 yr | NA | NA | 0.33 | 35 | 21 | 56 |
| 27 | M | W | 74 | 16 yr | NA | NA | 0.79 | 15 | 11 | 26 |
| 28 | M | W | 69 | 9 yr | NA | NA | 0.84 | 20 | 22 | 42 |
| 29 | F | W | 49 | 2 months | NA | NA | 0.98 | 17 | 12 | 29 |
| 30 | NAe | NA | NA | NA | NA | NA | 0.04 | 111 | 47 | 158 |
|
| ||||||||||
| Mean ± S.D. | 62.2 ± 12 | 0.54 ± 0.35 | 40 ± 38 | 29 ± 31 | 68 ± 58 | |||||
Reported time since quitting
Calculated based on dGuo
Each value represents a single measurement. dGuo was determined by HPLC-UV.
ND, not detected
NA not available
Figure 3.
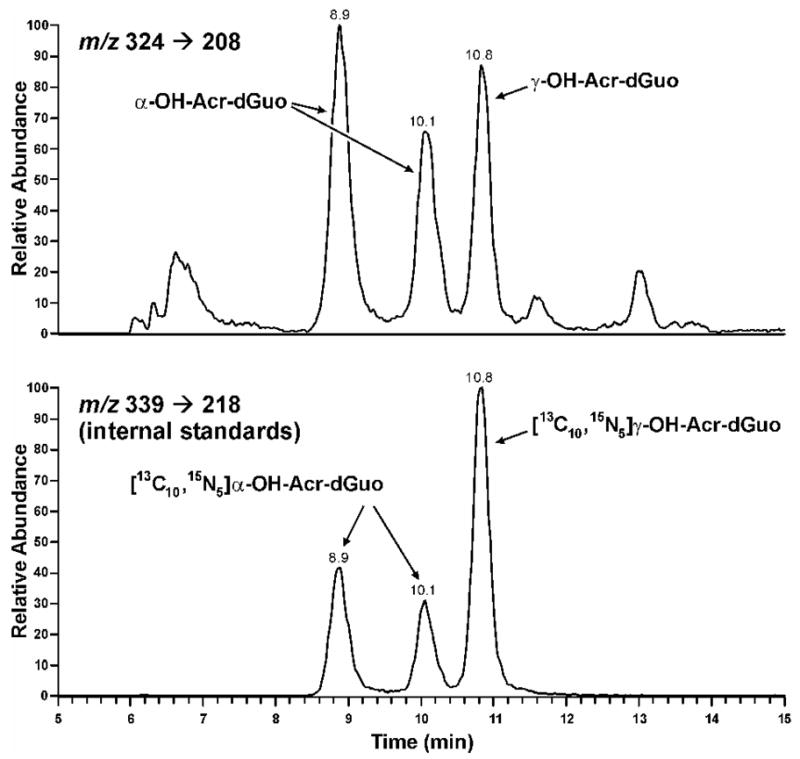
Chromatograms obtained upon LC-ESI-MS/MS-SRM analysis of DNA from human lung.
Discussion
The results of this study demonstrate for the first time that acrolein-DNA adducts, including the mutagenic adduct α-OH-Acr-dGuo, are present in human lung. This is significant in view of a recent study which showed that acrolein produces a spectrum of DNA damage in the p53 gene that is remarkably similar to the spectrum of mutations found in this gene in lung tumors from smokers (17). The total levels of acrolein-DNA adducts quantified here, about 1 per 107 nucleotides, are higher than “PAH-DNA adducts” reported in lung tissue by ELISA, typically about 0.3 adducts per 107 nucleotides, and benzo[a]pyrene diol epoxide – DNA adducts, which are frequently undetectable and typically amount to 0.1 – 0.5 per 107 nucleotides when they are detected (25–27). Collectively, these results indicate that acrolein could contribute significantly to the mutations seen in the p53 gene in lung cancer and challenge the widely held view that these mutations are due to polycyclic aromatic hydrocarbon diol epoxides (28). The weak or non-existent carcinogenicity of acrolein and the similarity in adduct levels between a limited number of confirmed current and ex-smokers on the other hand argue against a significant direct role for this compound in tobacco smoke - induced lung cancer, and further work is clearly needed to resolve these issues (29).
The structure of acrolein DNA adducts was first characterized in 1984 (12). Using 32P-postlabeling/HPLC, Chung and co-workers have detected Acr-dGuo adducts in various human and untreated animal tissues (13–15), suggesting the existence of an endogenous source. γ-OH-Acr-dGuo was the major adduct detected, and the levels of α-OH-Acr-dGuo were too low to be quantified or detected in most tissues. 32P-Postlabeling has certain limitations, such as the inability to provide unambiguous structural identification and the lack of internal standards for reliable quantitation. In the present study, we have developed a sensitive and specific LC-ESI-MS/MS method for the quantitative analysis of Acr-dGuo adducts. Compared with the method described by Liu et al (16), ours does not involve the setup of a nanospray source for the mass spectrometer, and uses commercially available capillary columns. Although the sensitivity is slightly lower than the previously published method, it is still sufficient to detect these adducts in human tissues.
The levels of Acr-dGuo in human lung were higher than the structurally related Cro-dGuo adducts which we have analyzed before (18). This is consistent with previous studies by Chung and co-workers, who also detected higher levels of Acr-dGuo than Cro-dGuo (13,14). Cigarette smoking is not the only source of these adducts. Acrolein from the environment and from endogenous formation through lipid peroxidation should also contribute. As reported by Pan and Chung (30), the rate of Acr-dGuo formation from polyunsaturated fatty acids under oxidative conditions was much higher than that of Cro-dGuo, consistent with our results.
We failed to observe a relationship between levels of Acr-dGuo adducts and urinary nicotine and cotinine, or time since cessation of smoking based on self-report. However, nicotine and cotinine data were available only for a small number of subjects, and self-report can be unreliable. Nothing is known about the kinetics of Acr-dGuo adduct removal after smoking cessation. Exposures to acrolein other than cigarette smoking as well as endogenous formation of acrolein from lipid peroxidation could contribute to adduct levels and will vary among people. Furthermore, individuals will have different abilities to detoxify acrolein and to repair Acr-dGuo adducts. Further research is needed to establish the relationship, if any, of Acr-dGuo adducts to cigarette smoking.
In summary, we have developed a sensitive and specific MS method for the quantitative analysis of Acr-dGuo adducts in human tissue DNA. Our results clearly demonstrate the presence of Acr-dGuo adducts in human lung DNA. Additional research is required to assess the contribution of acrolein to lung cancer caused by cigarette smoking.
Acknowledgments
This study was supported by Grant ES-11297 from the National Institute of Environmental Health Sciences. S.S.H. is an American Cancer Society Research Professor, supported by Grant RP-00-138. Mass spectrometry was carried out in the Analytical Biochemistry Core Facility of The Cancer Center, supported in part by Cancer Center Support Grant CA-77598. We thank Menglan Chen and Steven Carmella for analysis of nicotine and cotinine in urine, Sarah Bowell and Diane Rauch of the Tissue Procurement Facility for obtaining lung tissue samples, and Bob Carlson for assistance in manuscript preparation.
Footnotes
Abbreviations: α-OH-Acr-dGuo, (6R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-6-hydroxypyrimido[1,2-a]purine-10(3H)one; γ-OH-Acr-dGuo, (8R/S)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-8-hydroxypyrimido[1,2-a]purine-10(3H)one; Acr-dGuo, both α-OH-Acr-dGuo and γ-OH-Acr-dGuo; Cro-dGuo, (6S, 8S)- and (6R, 8R)-3-(2′-deoxyribos-1′-yl)-5,6,7,8-tetrahydro-8-hydroxy-6-methylpyrimido[1,2-a]purine-10(3H)one; LC-ESI-MS/MS, liquid chromatography-electrospray ionization-tandem mass spectrometry; LOQ, limit of quantitation; SPE, solid phase extraction; SRM, selected reaction monitoring.
References
- 1.Izard C, Libermann C. Acrolein. Mutat Res. 1978;47:115–138. doi: 10.1016/0165-1110(78)90016-7. [DOI] [PubMed] [Google Scholar]
- 2.Chung FL, Chen HJC, Nath RG. Lipid peroxidation as a potential source for the formation of exocyclic DNA adducts. Carcinogenesis. 1996;17:2105–2111. doi: 10.1093/carcin/17.10.2105. [DOI] [PubMed] [Google Scholar]
- 3.Roemer E, Stabbert R, Rustemeier K, Veltel DJ, Meisgen TJ, Reininghaus W, Carchman RA, Gaworski CL, Podraza KF. Chemical composition, cytotoxicity and mutagenicity of smoke from US commercial and reference cigarettes smoked under two sets of machine smoking conditions. Toxicology. 2004;195:31–52. doi: 10.1016/j.tox.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Lutz D, Eder E, Neudecker T, Henschler D. Structure-mutagenicity relationship in α,β-unsaturated carbonylic compounds and their corresponding allylic alcohols. Mutat Res. 1982;93:305–315. [Google Scholar]
- 5.Eder E, Henschler D, Neudecker T. Mutagenic properties of allylic and α,β-unsaturated compounds: consideration of alkylating mechanisms. Xenobiotica. 1982;12:831–848. doi: 10.3109/00498258209038955. [DOI] [PubMed] [Google Scholar]
- 6.Marnett LJ, Hurd HK, Hollstein MC, Levin DE, Esterbauer H, Ames BN. Naturally occurring carbonyl compounds are mutagens in salmonella tester strain TA104. Mutat Res. 1985;148:25–34. doi: 10.1016/0027-5107(85)90204-0. [DOI] [PubMed] [Google Scholar]
- 7.Curren RD, Yang LL, Conklin PM, Grafstrom RC, Harris CC. Mutagenesis of xeroderma pigmentosum fibroblasts by acrolein. Mutat Res. 1988;209:17–22. doi: 10.1016/0165-7992(88)90104-2. [DOI] [PubMed] [Google Scholar]
- 8.Yang IY, Chan G, Miller H, Huang Y, Torres MC, Johnson F, Moriya M. Mutagenesis by acrolein-derived propanodeoxyguanosine adducts in human cells. Biochemistry. 2002;41:13826–13832. doi: 10.1021/bi0264723. [DOI] [PubMed] [Google Scholar]
- 9.The Carcinogenic Potency Database (CPDB) 2007 http://potency.berkeley.edu.
- 10.Cohen SM, Garland EM, John M, Okamura T, Smith RA. Acrolein intiates rat urinary bladder carcinogenesis. Cancer Res. 1992;52:3577–3581. [PubMed] [Google Scholar]
- 11.Gomes R, Meek ME, Eggleton M. Concise International Chemical Assessment Document No. 43; World Health Organization, Geneva. 2002. [Google Scholar]
- 12.Chung FL, Young R, Hecht SS. Formation of cyclic 1, N2-propanodeoxyguanosine adducts in DNA upon reaction with acrolein or crotonaldehyde. Cancer Res. 1984;44:990–995. [PubMed] [Google Scholar]
- 13.Nath RG, Chung FL. Detection of exocyclic 1, N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc Natl Acad Sci, USA. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nath RG, Ocando JE, Chung FL. Detection of 1, N2-propanodeoxyguanosine adducts as potential endogenous DNA lesions in rodent and human tissues. Cancer Res. 1996;56:452–456. [PubMed] [Google Scholar]
- 15.Nath RG, Ocando JE, Guttenplan JB, Chung FL. 1, N2-Propanodeoxyguanosine adducts: Potential new biomarkers of smoking-induced DNA damage in human oral tissue. Cancer Res. 1998;58:581–584. [PubMed] [Google Scholar]
- 16.Liu X, Lovell MA, Lynn BC. Development of a method for quantification of acrolein-deoxyguanosine adducts in DNA using isotope dilution-capillary LC/MS/MS and its application to human brain tissue. Anal Chem. 2005;77:5982–5989. doi: 10.1021/ac050624t. [DOI] [PubMed] [Google Scholar]
- 17.Feng Z, Hu W, Hu Y, Tang MS. Acrolein is a major cigarette-related lung cancer agent: Preferential binding at p53 mutational hotspots and inhibition of DNA repair. Proc Natl Acad Sci U S A. 2006;103:15404–15409. doi: 10.1073/pnas.0607031103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang S, Villalta PW, Wang M, Hecht SS. Analysis of crotonaldehyde-and acetaldehyde-derived 1, N2-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography-electrsopray ionization-tandem mass spectrometry. Chem Res Toxicol. 2006;19:1386–1392. doi: 10.1021/tx060154d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht SS, Carmella SG, Chen M, Dor Koch JF, Miller AT, Murphy SE, Jensen JA, Zimmerman CL, Hatsukami DK. Quantitation of urinary metabolites of a tobacco-specific lung carcinogen after smoking cessation. Cancer Res. 1999;59:590–596. [PubMed] [Google Scholar]
- 20.Wang M, Yu N, Chen L, Villalta PW, Hochalter JB, Hecht SS. Identification of an acetaldehyde adduct in human liver DNA and quantitation as N2-ethyldeoxyguanosine. Chem Res Toxicol. 2006;19:319–324. doi: 10.1021/tx0502948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Snustad DP, Simmons MJ, Jenkins JB. Principles of Genetics. John Wiley and Sons; New York: 1997. p. 189. [Google Scholar]
- 22.Thomson NM, Mijal RS, Ziegel R, Fleischer NL, Pegg AE, Tretyakova NY, Peterson LA. Development of a quantitative liquid chromatography/electrospray mass spectrometric assay for a mutagenic tobacco specific nitrosamine-derived DNA adduct, O6-[4-Oxo-4-(3-pyridyl)butyl]-2′-deoxyguanosine. Chem Res Toxicol. 2004;17:1600–1606. doi: 10.1021/tx0498298. [DOI] [PubMed] [Google Scholar]
- 23.von Hippel PH, Felsenfeld G. Micrococcal nuclease as a probe of DNA conformation. Biochemistry. 1964;3:27–39. doi: 10.1021/bi00889a006. [DOI] [PubMed] [Google Scholar]
- 24.Cuatrecasas P, Fuchs S, Anfinsen CB. Catalytic properties and specificity of the extracellular nuclease of Staphylococcus aureus. J Biol Chem. 1967;242:1541–1547. [PubMed] [Google Scholar]
- 25.Boysen G, Hecht SS. Analysis of DNA and protein adducts of benzo[a]pyrene in human tissues using structure-specific methods. Mutation Res. 2003;543:17–30. doi: 10.1016/s1383-5742(02)00068-6. [DOI] [PubMed] [Google Scholar]
- 26.Kriek E, Rojas M, Alexandrov K, Bartsch H. Polycyclic aromatic hydrocarbon-DNA adducts in humans: relevance as biomarkers for exposure and cancer risk. Mutat Res. 1998;400:215–231. doi: 10.1016/s0027-5107(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 27.Beland FA, Churchwell MI, Von Tungeln LS, Chen S, Fu PP, Culp SJ, Schoket B, Gyorffy E, Minarovits J, Poirier MC, Bowman ED, Weston A, Doerge DR. High-performance liquid chromatography electrospray ionization tandem mass spectrometry for the detection and quantitation of benzo[a]pyrene-DNA adducts. Chem Res Toxicol. 2005;18:1306–1315. doi: 10.1021/tx050068y. [DOI] [PubMed] [Google Scholar]
- 28.Pfeifer GP, Denissenko MF, Olivier M, Tretyakova N, Hecht SS, Hainaut P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene. 2002;21:7435–7451. doi: 10.1038/sj.onc.1205803. [DOI] [PubMed] [Google Scholar]
- 29.International Agency for Research on Cancer. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Vol. 63. IARC; Lyon, FR: 1995. Acrolein; pp. 337–372. [PMC free article] [PubMed] [Google Scholar]
- 30.Pan J, Chung FL. Formation of cyclic deoxyguanosine adducts from omega-3 and omega-6 polyunsaturated fatty acids under oxidative conditions. Chem Res Toxicol. 2002;15:367–372. doi: 10.1021/tx010136q. [DOI] [PubMed] [Google Scholar]