Abstract
Background and Purpose
Hypertension is the most important modifiable risk factor for secondary stroke prevention but the immediate management of blood pressure after stroke is uncertain. We evaluated outcomes in the NINDS tPA stroke study in relation to blood pressure declines during the first 24 hours after randomization.
Methods
Declines in blood pressure compared to baseline and preceding time points were analyzed in relationship to favorable outcomes (by a global test), poor outcomes (Rankin scale > 3) and death at 3 months.
Results
551 patients did not receive immediate pre-randomization anti-hypertensive treatment and had available blood pressures. Multivariate analysis showed significantly and progressively reducing likelihoods of a favorable outcome with each 10 mmHg decline in systolic blood pressure (SBP) > 50 mmHg compared to any preceding measurement. Poor outcomes were significantly more likely in patients with > 50 mmHg SBP reduction (or > 30 mmHg compared to any immediately preceding measurement). There was an increased risk of death with blood pressure declines > 60 mmHg. tPA treatment still produced favorable outcomes compared with placebo even with blood pressure declines. The median largest SBP reduction from baseline in patients treated with tPA was 35 mmHg compared to 30 mmHg in placebo-treated patients(p <0.01).
Conclusions
In this post hoc analysis, progressively reducing likelihoods of a favorable outcome were seen with increasing declines in SBP. Despite a greater likelihood of favorable outcomes, tPA treatment was associated with a greater reduction in blood pressure than placebo. Randomized trials of blood pressure management are needed.
Introduction
Elevated blood pressure is considered the single most important modifiable risk factor for preventing ischemic stroke based on its prevalence.1 Randomized trials have demonstrated a reduction in ischemic stroke risk with reduction in blood pressure in patients with a prior history of ischemic stroke.2
The acute management of blood pressure is uncertain. Approximately 75 percent of ischemic stroke patients have elevation of blood pressure within 24 hours of onset.3 Theoretical arguments against lowering blood pressure acutely include irreparably injuring penumbral brain tissue due to a reduction in blood supply.4;5 Theoretical arguments in favor of lowering (or avoiding elevating) blood pressure acutely include reduction in risk of hemorrhagic conversion of tissue due to elevated cerebral perfusion pressures.4The American Heart Association guidelines recommend against lowering blood pressures within the first 24 hours after acute ischemic stroke when systolic blood pressures are less than 220 mmHg without thrombolytic treatment and less than 180 mmHg with thrombolytic treatment.6Recent studies show apparently conflicting results; some suggest an increased risk of worse neurological outcomes following lower blood pressure after acute ischemic stroke7-11 while others find an increased risk of poor outcome with elevated blood pressures,12-14a U-shaped relationship,15-17or no effect.18
In a previous study using the NINDS tPA stroke studies19 data set, the association between anti-hypertensive therapy before and after randomization was explored.20No relationship between pre-randomization treatment of all patients or with post-randomization treatment of placebo patients and outcome was found. Those treated with tPA were less likely to have a good outcome if treated with anti-hypertensive medication post-randomization. In the present study, we used the same data set to look for thresholds of blood pressure declines that were associated with adverse outcomes at 3 months.
Methods
We used the NINDS tPA stroke studies data set which is in the public domain and distributed through the National Technical Information Service (www.ntis.gov). Full details of the study designs have been previously described.19In brief, these were masked, randomized, multi-center studies comparing tPA to placebo for the treatmentof acute ischemic stroke within three hours of symptom onset. All patients were required to have a systolic blood pressure (SBP) of less than 185 mmHg prior to randomization. The primary outcomes were favorable outcomes at 24 hours in Part I and three months in part II. Part I failed to achieve statistical significance although a significant benefit was observed for the tPA group at 3 months. Part II confirmedthe long-term benefits observed in part I.
For the purposes of this analysis, all 624 patients in both trials(N=291 in part I and N=333 in Part II) were included. The two trials had the same recruitment and data collection protocols. Outcomes were analyzed based on 3-month scores collected in the trials. Similar to the analysis in the NINDS tPA studies, a favorable outcome was defined as a global statistic comprising a Barthel index score ≥95, a modified Rankin scale score ≤ 1, NIH stroke scale score (NIHSS) ≤ 1, and Glasgow outcome score = 1. The Barthel index is a 100-point scale which measures performance in activities of daily living including transfers from a bed, walking, stair climbing, bathing, grooming, dressing, eating, continence, and using the toilet.Patients with complete independence are given a score of 100. The modified Rankin scale is a simple 5 point scale which assesses the level of disability. A score of 0 indicates no disability while a score of 4 or 5 indicates inability to walk without assistance and unable to attend to own bodily needs without assistance. The Glasgow outcome scale is a global assessment of function in which a score of 1 indicates a good recovery, a score of 2 to 4 indicates progressing levels of disability or vegetative state, and a score of 5 indicates death. The NIHSS is a 42 point scale that quantifies neurological deficits based on findings in 11 categories. A score of 0 is normal while a score of 1 could represent a mild facial droop or arm drift. In contrast to the definition of a favorable outcome in this study and in the NINDS tPA studies, a poor outcome in this study was defined as a 3-month Rankin score > 3. Therefore, a poor outcome is very different than an “unfavorable” outcome. A single component rather than composite definition for poor outcomes was chosen because the Rankin score is increasingly being used as a 3 month endpoint in stroke clinical trials and because a composite definition of poor outcomes has not been defined previously.
Patients had blood pressure monitoring during the first 24 hours after initiation of treatment with tPA or placebo. Blood pressures were recorded prior to randomization, then every 15 minutes for the next two hours, then every 30 minutes from hours 2 to 8, then hourly from hours 8 to 24. A total of 37 blood pressure measurements were taken during the 24 hours.
The following blood pressure variations were considered (Table 1): 1) the largest decline from the baseline SBP was defined as the largest difference between a blood pressure within 24 hours of randomization compared to the pre-randomization blood pressure (the blood pressure just prior to randomization as opposed to that at emergency room arrival), 2) the largest short-term SBP decline was defined as the largest difference between a SBP compared to an immediately preceding time point, 3) the largest overall SBP decline was defined as the largest difference between a SBP and any preceding time point. Similar analyses were done for diastolic (DBP) and mean (MBP) blood pressures. Blood pressure declines were then dichotomized with cutoff points at 30, 40, 50, 60, 70, and 80 mmHg. The use of this type of modeling rather than consideration of blood pressure as a continuous variable allowed for a more clinically meaningful analysis and a better assessment of the effect of a change in thresholds. Percentage declines were not used in the analysis due to the less straightforward application in clinical practice. Because intervals between blood pressure measurements were not always equal, the analysis was performed using all 37 time points and repeated using only the 24 hourly measurements. The initial analysis excluded patients who had received anti-hypertensive therapy before randomization and included those who had received anti-hypertensive therapy after randomization because that mode of treatment was felt to be consistent with what might be seen in clinical practice. However, considering that pre- or post-randomization anti-hypertensive medication might be associated with adverse outcomes independent of blood pressure lowering effects, additional analyses were performed to: 1) include patients who received pre-randomization anti-hypertensive medication, 2) study the association between blood pressure declines and post-randomization anti-hypertensive medication, and 3) exclude those who received post-randomization anti-hypertensive medication.
Table 1.
Definition of blood pressure declines
| Description | Definition | Example |
|---|---|---|
| Largest decline from baseline blood pressure |
Baseline blood pressure minus the lowest blood pressure in the next 24 hours |
Baseline blood pressure is 180 mmHg and the lowest blood pressure during the next 24 hours is 140 mmHg at 11 hours. The difference is 40 mmHg. |
| Largest short-term blood pressure decline |
Largest difference of blood pressure between a measurement and the one immediately preceding it |
At 5 hours 30 minutes, the blood pressure is 170 mmHg and the next measured blood pressure at 6 hours is 110 mmHg. The difference is 60 mmHg. |
| Largest overall blood pressure decline |
Largest difference of blood pressure between a measurement and any preceding it |
At 2 hours, the blood pressure is 160 mmHg and at 4 hours is 110 mmHg. The difference is 50 mmHg. |
Statistical analysis
Mean blood pressure declines and average heart rate during the 24 hours after randomization were derived. A correlation coefficient for these two variables was then calculated.
We compared the association between blood pressure declines and outcome among tPA and placebo-treated groups using Mantel-Haenszel tests adjusting for randomization stratum. We classified stroke recovery into three categories: 1) a favorable outcome on the global statistic at 3 months as defined by NINDS tPA stroke study group for Part II,19 2)a poor outcome, defined as a 3-month Rankin score > 3 (including death), and 3) death at 3 months.
A global test using the generalized estimating equation (GEE) was performed to study the risk of blood pressure declines on favorable outcome. The initial analysis tested the effect of blood pressure declines after adjusting for treatment with tPA by including treatment/blood pressure decline interactions. Significant effects of blood pressure declines alone (p<0.05) or by treatment interaction (p<0.10) were included in a multivariate model using potentially confounding covariates (age, NIH stroke scale score, history of diabetes,21and early CT changes). Odds ratios and 95% confidence intervals were calculated for each type of blood pressure decline. Odds ratios with confidence intervals less than 1 indicated a reduced likelihood of a favorable outcome.
The logistic regression model was used to study the risk of blood pressure declines on poor outcome and death. A similar analysis for a favorable outcome was used by considering the treatment/blood pressure decline interaction and calculation of odds ratios with 95% confidence intervals. Odds ratios with confidence intervals greater than 1 indicated an increased likelihood of a poor outcome or death. The same analysis approach was use to study association of blood pressure declines on symptomatic intracerebral hemorrhage in tPA-treated patients. The definition of symptomatic intracranial hemorrhage was that used in the NINDS tPA studies i.e. a hemorrhage was considered symptomatic if it was not seen on a previous CT scan and there had subsequently been either a suspicion of hemorrhage or any decline in neurologic status.
Results
Of 624 patients, 18 were excluded because of missing blood pressure readings. In the initial analysis, 56 patients were excluded because of pre-randomization treatment with anti-hypertensive medications (including one patient who also had missing blood pressure readings). Of the 551 remaining patients, 273 were treated with tPA and 278 were treated with placebo. Baseline characteristics of the patients included in this study are shown in table 2.Of the 551 patients included in the initial analysis, 146 (26%) received anti-hypertensive medication after randomization. The results of the analysis excluding those patients are discussed below.
Table 2.
Baseline patient characteristics. SBP = systolic blood pressure, DBP = diastolic blood pressure, MBP = mean blood pressure, HR = heart rate, SD = standard deviation
| Overall | tPA | Placebo | |
|---|---|---|---|
| Prior stroke (%) | 12 | 12 | 12 |
| Prior TIA (%) | 17 | 16 | 17 |
| Aspirin use prior tostroke(%) | 35 | 40 | 29 |
| Diabetes (%) | 21 | 21 | 22 |
| Hypertension (%) | 64 | 65 | 63 |
| Myocardial infarction (%) | 23 | 24 | 22 |
| Atrial fibrillation (%) | 18 | 19 | 18 |
| Angina (%) | 23 | 22 | 24 |
| Congestive heart failure (%) | 17 | 15 | 20 |
| Valvular heart disease (%) | 8 | 10 | 7 |
| Smoking in prior year (%) | 34 | 33 | 35 |
| SBP[mmHg ± SD,(range)] | 151.7±21.3 (90-227) |
152.4±22.0 (102-227) |
150.9±20.6 (90-200) |
| DBP [mmHg ± SD, (range)] | 84.6±13.4 (10-134) |
84.1±12.8 (47-134) |
85.0±13.9 (10-120) |
| MBP [mmHg ± SD, (range)] | 107.0±13.8 (51-161) |
107.0±13.9 (74-161) |
107.0±13.8 (51-153) |
| Mean HR ( ± SD) | 81.8±17.4 (44-155) |
80.9±16.8 (44-136) |
82.6±17.8 (46-155) |
Within 24 hours of randomization, the median largest declines in blood pressure were as follows: 34 mmHg SBP decline from baseline, 33 mmHg short-term SBP decline, 54 mmHg overall SBP decline, 25 mmHg decline from baseline for both DBP and MBP. The median times to a short-term and overall SBP decline were 7 and 13 hours, respectively (range 15 minutes to 24 hours). The average heart rate within 24 hours of randomization was 79 ± 14 (range 50-139). MBP declines from baseline were highly correlated with both SBP and DBP declines with correlation coefficients of 0.76 and 0.88, respectively. The overall SBP declines correlated with short-term SBP declines and SBP declines from baseline with correlation coefficients of 0.75 and 0.66 respectively. The 24-hour average heart rate had low correlation with various types of SBP declines (range of correlation coefficients 0.06 to 0.10). Overall, 57%, 37%, 20% and 11% of patients had SBP declines compared to baseline at cutoffs of 30, 40, 50 and 60 mmHg; 44%, 20%, 11% and 5% of patients had short-term SBP declines at cutoffs of 30, 40, 50 and 60 mmHg; and 51%, 31%, 16% and 9% of patients had overall SBP declines at cutoffs of 50, 60, 70 and 80 mmHg. The tPA treated group, in general, had a significantly higher percentage of blood pressure declines (median SBP decline from baseline = 35 mmHg) compared to the placebo-treated group (median SBP decline from baseline = 30 mmHg) at cut-off points of 50 mmHg for any SBP decline and 30 mmHg SBP decline from baseline (P<0.05) (figure 1).
Figure 1.
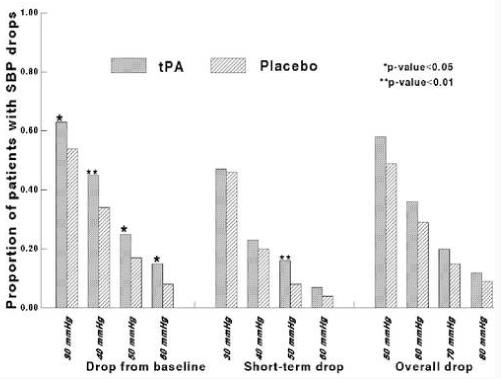
Systolic blood pressure (SBP) declines in tPA and placebo-treated patients at variouspre-defined cutoff points, within 24 hours of randomization.
With respect to a favorable outcome at 3 months, 45% of patients had a Barthel index ≥ 95, 35% had a Rankin score ≤ 1, 38% had a favorable Glasgow outcome score, and 28% had NIHSS ≤ 1. No blood pressure decline by treatment interaction was detected indicating that the beneficial effect of tPA was sustained regardless of blood pressure change (odds ratio [OR] 1.9, 95% confidence intervals [CI] 1.4-2.8, p<0.01 for tPA treatment effect on outcome). In the univariate analysis, patients with short-term SBP declines beyond 30 mmHg and overall SBP declines beyond 50 mmHg were less likely to have a favorable outcome compared to patients who did not (Figure 2). The likelihood of a favorable outcome decreased with each increase in cutoff of 10 mmHg. After adjusting for other covariates, short-term declines in SBP of at least 30 or 40 mmHg were no longer significant predictors of a reduced likelihood of a good outcome while an overall decline in SBP beyond 50 mmHg continued to predict a reduced likelihood of a good outcome (Figure 2). Declines in SBP, DBP, and MBP from baseline were not associated with a reduced likelihood of a favorable outcome.
Figure 2.
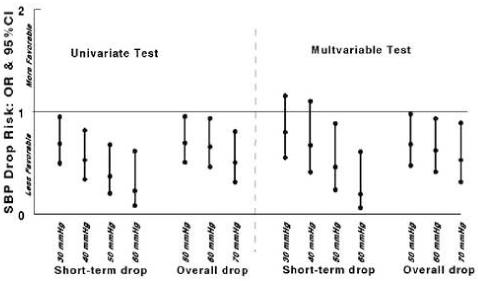
Systolic blood pressure (SBP) declines on 3-month favorable outcomes. The univariate analysis adjusted for treatment with t-PA; the multivariate analysis was adjusted fortreatment with t-PA and other covariates (age, NIH stroke scale score, history of diabetes, and early CT changes). Odds ratios (OR) with 95% confidence intervals (CI) < 1 indicate a significant reduction in the likelihood of a favorable outcome.
At 3 months, 20% of patients died and 23% had a Rankin > 3 (poor outcome). Patients with ≥ 50 mmHg decline in overall SBP or ≥ 30 mmHg short-term SBP were more likely to have poor outcomes at 3 months (figure 3A).Declines in DBP and MBP were not associated with poor outcomes. No SBP by treatment interaction was detected at the 0.10 level indicating that tPA treatment was associated with less poor outcomes regardless of blood pressure decline.
Figure 3.
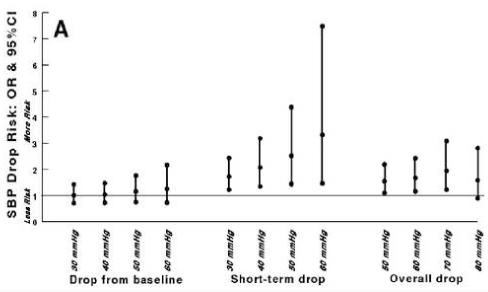
Systolic blood pressure (SBP) declines and 3-month adverse outcomes. A) Rankin score > 3 and B) death. Odds ratios (OR) with 95% confidence intervals (CI) >1 indicate a significantly increased likelihood of poor outcome or death. *Treatment by SBP decline interactions were detected at the 0.10 level.
Patients with ≥ 60 mmHg short-term SBP declines were more likely to be dead at 3 months (OR 2.30, 95% CI 1.03-5.12; p=0.04) (figure 3B).Patients with ≥ 60 mmHg DBP or MBP declines from baseline were also more likely to be dead at 3 months (OR 4.27, 95% CI 1.34-13.60 and 5.33, 95% CI 1.40-20.30, respectively). Treatment by blood pressure decline interactions were observed for SBP declines ≥ 50 mmHg from baseline and ≥ 50 mmHg declines in DBP or MBP from baseline. Compared to placebo, treatment with tPA was associated with a reduced risk of death with SBP declines ≥ 50 mmHg (OR 0.31, 95% CI 0.12-0.78), ≥ 50 mmHg DBP from baseline (OR 0.24, 95% CI 0.07-0.81), and ≥ 50 mmHg MBP decline from baseline (OR 0.15, 95% CI 0.02-0.93). Blood pressure declines were not associated with symptomatic intracerebral hemorrhage.
Figure 4.
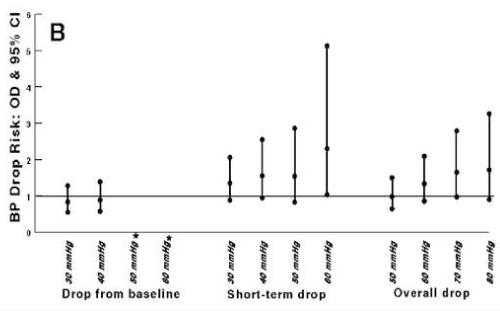
When analyzing by hourly blood pressures rather than consecutively taken blood pressures, results were similar except for the observation that any decline beyond 30 mmHg, rather than a threshold of 50 mmHg, was associated with a reduced likelihood of a favorable outcome at 3 months. Analysis of outcomes after including those who received anti-hypertensive treatment prior to randomization (9% of the total NINDS tPA stroke study population)20 did not affect results.
Analysis of the association between post-randomization anti-hypertensive medication and various SBP declines showed that a larger proportion of patients with various SBP declines had received medication compared to those who did not (p<0.05); however, the percentages of patients with blood pressure declines who had received post-randomization anti-hypertensive medication ranged between 30% and 45%, indicating that over 50% of patients with blood pressure declines did not receive anti-hypertensive medications. The correlation coefficients between post-randomization anti-hypertensive medication and SBP declines, using the polychoric correlation for binary variables, ranged between 0.12 and 0.41. When excluding patients who had received post-randomization anti-hypertensive medication (n=405), the same risk of 3-month adverse outcomes (death and Rankin score > 3) was observed as compared to the risk based on the whole population (n=551).
Discussion
In this post-hoc analysis, regardless of pre- or post- randomization anti-hypertensive medication use, there was significantly reduced likelihood of a favorable outcome (defined by a global outcome test)in patients whose SBP declineped at least 50 mmHg compared to an immediately preceding measurement (termed short-term SBP decline) or by at least 50 mmHg compared to any preceding measurement (termed overall SBP decline). The likelihood of a favorable outcome continually decreased with each 10 mmHg SBP increase. There was an increased likelihood of a poor outcome (defined as Rankin scale > 3) in patients whose overall SBP declineped at least 50 mmHg or patients who had a short-term SBP decline of at least 30 mmHg. Patients with declines in SBP, DBP, or MBP of at least 60 mmHg had an increased risk of death. There was a treatment by blood pressure interaction with respect to death but not with respect to favorable or poor outcomes. The median time to a short-term decline was 13 hours and implies that even later declines in blood pressure may be associated with a less favorable outcome. The results should be interpreted with caution since the study was not designed to study the effect of different blood pressure targets. There is precedence in the stroke literature for a post hoc analysis showing different results from a randomized, controlled trial. A post hoc analysis of the North American Symptomatic Carotid Endarterectomy Trial suggested that high-dose aspirin was beneficial perioperatively;22however, just the opposite was seen in the randomized controlled trial [ASA and Carotid Endarterectomy (ACE) Trial].23
Blood pressure is known to rise within the first 24 hours after acute ischemic stroke and then gradually fall over the following week.24Oliveira-Filho et al found that a reduction in systolic blood pressure ≥ 10% in the first 24 hours (defined as the highest blood pressure minus the lowest blood pressure, regardless of whether the lowest blood pressure preceded or followed the highest) was associated with a nearly two-fold increased risk of a poor outcome (defined as a Rankin score > 2 or Barthel score < 70) at 3 months.7In the ACCESS study, administration of candesartan within the first 24 hours produced no difference in blood pressure and neurological outcome at 3 months compared with placebo but did result in a significant reduction in cardiovascular events at one year.25The authors posited that early neurohumeral inhibition by selective angiotensin type I receptor blockade was the reason for the beneficial effect. If the results of the ACCESS study are confirmed in a separate study, knowledge of the level at which blood pressure reduction might be counterproductive might be useful. The INWEST study, designed to test the neuroprotective effect of nimodipine in acute ischemic stroke, was terminated early because of the finding that a DBP reduction ≥ 20 mmHg induced by nimodipine was associated with a 10-fold increase in the risk of death or dependency.10Previous analysis of the effect of modest blood pressure reduction in the ECASS tPA stroke study found no significant increase in the risk of early neurological deterioration.26
Another finding in this study, not previously described to our knowledge, was the significantly greater median decline in blood pressures in tPA-treated patients compared to placebo-treated patients. All patients in the NINDS tPA studies were required to have a SBP of less than 185 mmHg prior to randomization.19tPA-treated patients were no more likely to receive blood pressure-lowering medications prior to randomization than placebo-treated patients (9% in each group) and were less likely to receive such medications after randomization (24% versus 29%) though this finding did not reach statistical significance.20Possible explanations for this observation include vasodilation, recanalization with decreased neurohumeral activation, and activation of secondary messengers such as nitric oxide (NO). Matte et al found that recanalization with intra-arterial thrombolysis was associated with a lower blood pressure 12 hours later compared to those with inadequate recanalization.27In animal models, tPA has been shown to produce vasodilation by increasing cyclic GMP through a nitric oxide (NO) dependent mechanism.28In spite of lower blood pressures among tPA-treated patients, outcomes were superior to that of placebo-treated patients. Hitherto, the assumption has been that the improved outcomes were entirely due to the effect of recanalization. Because the complete effect of tPA was not be fully realized at 24 hours in the NINDS tPA studies suggests the possibility that additional mechanisms besides recanalization (such as NO-dependent neural regeneration) may play a role in recovery.
There were two potential advantages to using this data set. First, blood pressure measurements were performed a total of 37 times within the first 24 hours after stroke onset. To our knowledge, no other study had as many blood pressure measurements within that period. Because of the frequency of blood pressure evaluation, we were able to assess the effect of consecutive declines in blood pressure over a relatively short period of time. Second, all patients had baseline blood pressure measurements taken within three hours of symptom onset. Therefore, the analysis of blood pressure fluctuations is a true reflection of both early and late changes that occur after acute ischemic stroke. Previous studies used measurements in patients who presented well after three hours limiting the utility of the analysis of change in blood pressure that occurs during the first 24 hours.
Three main weaknesses exist in this data set. First, there was no standard time from symptom onset for the measurement of pre-treatment blood pressures. Entry blood pressures may have been taken as soon as 30 minutes or as late as 150 minutes after stroke onset. Though the differences in the timing of measurements between patients appears to be relatively small, the rapidly dynamic changes in cerebral blood flow and consequent cardiovascular responses may have confounded the defined pre-treatment blood pressures between individuals. Second, there was no standardized blood pressure target prior to treatment. Because this study had the primary purpose of testing tPA as a thrombolytic agent rather than as a blood pressure lowering drug, the true effect of blood pressure reduction may have been masked. Third, patients were not randomized based on blood pressures leading to potential imbalances. Nevertheless, the rate of medication use was the same between placebo and tPA-treated patients prior to randomization (9% in each group)20as were baseline characteristics.
In summary, these findings support the assertion that significant declines in blood pressure are associated with a reduced likelihood of a favorable outcome and an increased risk of a poor outcome following acute ischemic stroke. However, the effect of modest blood pressure reduction remains uncertain. The timing of blood pressure lowering and choice of agent following acute ischemic stroke will only properly be addressed by a randomized clinical trial.
Acknowledgments
Drs. Chopp, Lu, Mitsias, and Silver were supported by an NIH Stroke Center program project grant number NS-23393
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Gorelick PB, Sacco RL, Smith DB, et al. Prevention of a first stroke: a review of guidelines and a multidisciplinary consensus statement from the National Stroke Association. JAMA. 1999;281:1112–1120. doi: 10.1001/jama.281.12.1112. [DOI] [PubMed] [Google Scholar]
- 2.Rashid P, Leonardi-Bee J, Bath P. Blood pressure reduction and secondary prevention of stroke and other vascular events: a systematic review. Stroke. 2003;34:2741–2748. doi: 10.1161/01.STR.0000092488.40085.15. [DOI] [PubMed] [Google Scholar]
- 3.Rodriguez-Garcia JL, Botia E, de La Sierra A, Villanueva MA. Significance of elevated blood pressure and its management on the short-term outcome of patients with acute ischemic stroke. Am J Hypertens. 2005;18:379–384. doi: 10.1016/j.amjhyper.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Wityk RJ, Restrepo L. Hypoperfusion and Its Augmentation in Patients with Brain Ischemia. Curr Treat Options Cardiovasc Med. 2003;5:193–199. doi: 10.1007/s11936-003-0003-2. [DOI] [PubMed] [Google Scholar]
- 5.Lindenauer PK, Mathew MC, Ntuli TS, Pekow PS, Fitzgerald J, Benjamin EM. Use of antihypertensive agents in the management of patients with acute ischemic stroke. Neurology. 2004;63:318–323. doi: 10.1212/01.wnl.0000129831.79811.82. [DOI] [PubMed] [Google Scholar]
- 6.Adams HP, Jr, Adams RJ, Brott T, et al. Guidelines for the early management of patients with ischemic stroke: A scientific statement from the Stroke Council of the American Stroke Association. Stroke. 2003;34:1056–1083. doi: 10.1161/01.STR.0000064841.47697.22. [DOI] [PubMed] [Google Scholar]
- 7.Silva SC, Trabuco CC, Pedreira BB, Sousa EU, Bacellar A. Detrimental effect of blood pressure reduction in the first 24 hours of acute stroke onset. Neurology. 2003;61:1047–1051. doi: 10.1212/01.wnl.0000092498.75010.57. [DOI] [PubMed] [Google Scholar]
- 8.Stead LG, Gilmore RM, Decker WW, Weaver AL, Brown RD., Jr Initial emergency department blood pressure as predictor of survival after acute ischemic stroke. Neurology. 2005;65:1179–1183. doi: 10.1212/01.wnl.0000180939.24845.22. [DOI] [PubMed] [Google Scholar]
- 9.Vlcek M, Schillinger M, Lang W, Lalouschek W, Bur A, Hirschl MM. Association between course of blood pressure within the first 24 hours and functional recovery after acute ischemic stroke. Ann Emerg Med. 2003;42:619–626. doi: 10.1016/s0196-0644(03)00609-7. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed N, Nasman P, Wahlgren NG. Effect of intravenous nimodipine on blood pressure and outcome after acute stroke. Stroke. 2000;31:1250–1255. doi: 10.1161/01.str.31.6.1250. [DOI] [PubMed] [Google Scholar]
- 11.Semplicini A, Maresca A, Boscolo G, et al. Hypertension in acute ischemic stroke: a compensatory mechanism or an additional damaging factor? Arch Intern Med. 2003;163:211–216. doi: 10.1001/archinte.163.2.211. [DOI] [PubMed] [Google Scholar]
- 12.Aslanyan S, Fazekas F, Weir CJ, Horner S, Lees KR. Effect of blood pressure during the acute period of ischemic stroke on stroke outcome: a tertiary analysis of the GAIN International Trial. Stroke. 2003;34:2420–2425. doi: 10.1161/01.STR.0000091233.04524.0C. [DOI] [PubMed] [Google Scholar]
- 13.Aslanyan S, Weir CJ, Lees KR. Elevated pulse pressure during the acute period of ischemic stroke is associated with poor stroke outcome. Stroke. 2004;35:e153–155. doi: 10.1161/01.STR.0000126598.88662.16. [DOI] [PubMed] [Google Scholar]
- 14.Willmot M, Bath PM. High blood pressure in acute stroke and subsequent outcome: a systematic review. Hypertension. 2004;43:18–24. doi: 10.1161/01.HYP.0000105052.65787.35. [DOI] [PubMed] [Google Scholar]
- 15.Castillo J, Leira R, Garcia MM, Serena J, Blanco M, Davalos A. Blood pressure decrease during the acute phase of ischemic stroke is associated with brain injury and poor stroke outcome. Stroke. 2004;35:520–526. doi: 10.1161/01.STR.0000109769.22917.B0. [DOI] [PubMed] [Google Scholar]
- 16.Okumura K, Ohya Y, Maehara A, Wakugami K, Iseki K, Takishita S. Effects of blood pressure levels on case fatality after acute stroke. J Hypertens. 2005;23:1217–1223. doi: 10.1097/01.hjh.0000170385.76826.4a. [DOI] [PubMed] [Google Scholar]
- 17.Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. doi: 10.1161/01.str.0000014509.11540.66. [DOI] [PubMed] [Google Scholar]
- 18.Wong AA, Davis JP, Schluter PJ, Henderson RD, O’Sullivan JD, Read SJ. The effect of admission physiological variables on 30 day outcome after stroke. J Clin Neurosci. 2005;12:905–910. doi: 10.1016/j.jocn.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study. Group N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 20.Brott T, Lu M, Kothari R, et al. Hypertension and its treatment in the NINDS rt-PA Stroke Trial. Stroke. 1998;29:1504–1509. doi: 10.1161/01.str.29.8.1504. [DOI] [PubMed] [Google Scholar]
- 21.Ingall TJ, O’Fallon WM, Asplund K, et al. Findings from the reanalysis of the NINDS tissue plasminogen activator for acute ischemic stroke treatment trial. Stroke. 2004;35:2418–2424. doi: 10.1161/01.STR.0000140891.70547.56. [DOI] [PubMed] [Google Scholar]
- 22.Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. North American Symptomatic Carotid Endarterectomy Trial Collaborators. N Engl J Med. 1991;325:445–453. doi: 10.1056/NEJM199108153250701. [DOI] [PubMed] [Google Scholar]
- 23.Taylor DW, Barnett HJ, Haynes RB, et al. Low-dose and high-dose acetylsalicylic acid for patients undergoing carotid endarterectomy: a randomised controlled trial. ASA and Carotid Endarterectomy (ACE) Trial Collaborators. Lancet. 1999;353:2179–2184. doi: 10.1016/s0140-6736(99)05388-x. [DOI] [PubMed] [Google Scholar]
- 24.Harper G, Castleden CM, Potter JF. Factors affecting changes in blood pressure after acute stroke. Stroke. 1994;25:1726–1729. doi: 10.1161/01.str.25.9.1726. [DOI] [PubMed] [Google Scholar]
- 25.Schrader J, Luders S, Kulschewski A, et al. The ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke. 2003;34:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- 26.Davalos A, Toni D, Iweins F, Lesaffre E, Bastianello S, Castillo J. Neurological deterioration in acute ischemic stroke: potential predictors and associated factors in the European cooperative acute stroke study (ECASS) I. Stroke. 1999;30:2631–2636. doi: 10.1161/01.str.30.12.2631. [DOI] [PubMed] [Google Scholar]
- 27.Mattle HP, Kappeler L, Arnold M, et al. Blood pressure and vessel recanalization in the first hours after ischemic stroke. Stroke. 2005;36:264–268. doi: 10.1161/01.STR.0000153052.59113.89. [DOI] [PubMed] [Google Scholar]
- 28.Armstead WM, Cines DB. Altered NO function contributes to impairment of uPA and tPA cerebrovasodilation after brain injury. J Neurotrauma. 2004;21:1204–1211. doi: 10.1089/neu.2004.21.1204. [DOI] [PubMed] [Google Scholar]


