Abstract
Cyclodextrins and antibodies have been used as affinity agents to improve relative recovery during microdialysis sampling. Two neuropeptides, methionine-enkephalin (ME) and leucine-enkephalin (LE), were chosen to compare the use of cyclodextrins and antibodies as possible affinity agents for improving their relative recovery across polycarbonate and polyethersulfone membranes during in vitro sampling. Cyclodextrins (CD) including β-CD, 2-hydroxypropyl-β-cyclodextrin (2HPβ-CD), and γ-CD gave improvements of relative recovery for both peptides of less than 2-fold as compared to controls. Comparisons of relative recovery between tyrosine-glycine-glycine, tyrosine, and phenylalanine using different cyclodextrins in the perfusion fluid were also obtained. Inclusion of an antibody against met-enkephalin in the microdialysis perfusion fluid resulted in relative recovery increases of up to 2.5-fold. These results show that using antibodies as affinity agents during microdialysis sampling may be more effective agents to improve the relative recovery of these opioid neuropeptides.
Keywords: microdialysis sampling, neuropeptides, affinity agents, cyclodextrins, antibodies
1.0 Introduction
Neuropeptides are a class of neurotransmitters that are the most structurally diverse group of neuromodulators [1]. Enkephalins are endogenous opioid neuropeptides that have morphine-like action or pain relief in the body and are readily associated with the neurochemistry of addiction [2]. Enkephalins also play significant roles in mediating body temperature control, food intake, reward mechanisms, and stress [3].
Microdialysis sampling has been used for over thirty years for chemical sampling applications in neuroscience, pharmacokinetics, and drug metabolism [4–6]. It is by far the most commonly used method for the collection of low molecular weight hydrophilic neurotransmitters, such as acetylcholine, dopamine, and glutamate from mammalian brain with high relative recovery [7–9]. Despite the successful applications of microdialysis sampling, the main limitations for sampling large neuropeptides are a combination of their low relative recoveries and low basal concentrations. A neuropeptide with a high molecular weight will exhibit a smaller aqueous diffusion coefficient, which directly affects its relative recovery. An additional difficulty with quantitation of neuropeptides is their picomolar to nanomolar concentrations [10]. Therefore, there is a great need for improved sampling methods to increase analyte concentration for neuropeptides. Such sampling methods that could ultimately be applied to in vivo studies would be complemented by the ongoing research towards significant improvements in neuropeptide detection and identification in low microliter volume samples [11–13].
Microdialysis sampling is a diffusion-based process where a perfusion fluid is passed through an inlet tube and then passed through a semi-permeable membrane with a defined molecular weight cutoff. Analytes being sampled can freely diffuse into the probe. The analytes are then collected and carried by the perfusion fluid to an outlet tube where the sample is collected and then analyzed using an appropriate detection method. Bungay et al. described a microdialysis calibration model shown below [14]. The extraction efficiency (EE), also called RR for steady-state conditions is shown in Eq 1, where Cinlet is the analyte inlet concentration, Coutlet is the analyte outlet concentration, and Csample,∞ is the sample concentration far away from
| (Eq 1) |
the probe.
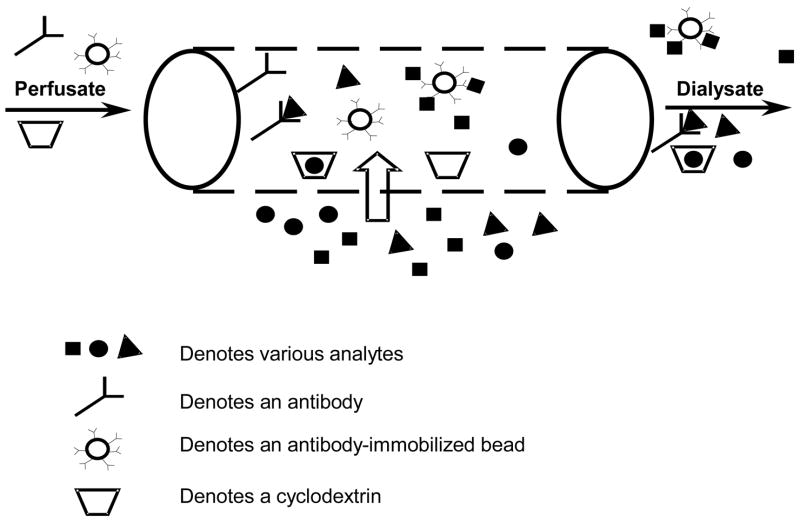
Increasing the RR of larger molecular weight analytes can be obtained by using lower perfusion volumetric flow rates, larger molecular weight cut-off membranes, adding osmotic agents to the perfusion fluid, and/or including affinity agents to the perfusion fluid. Several agents including antibodies and antibody-immobilized beads [15,16], chelating agents [17], colloids [18], cyclodextrins [19], and resins [20] have been used to increase the RR of various analytes. During microdialysis sampling, the affinity agent binds to the targeted analyte causing an increase in diffusive flux into the probe as compared to conventional microdialysis sampling (Figure 1) [21].
Figure 1.
A schematic of a microdialysis probe with various affinity agents included in the perfusion fluid. Analytes can freely diffuse into the semi-permeable probe and can be trapped by the affinity agent.
Cyclodextrins (CD) are cyclic oligosaccharides that are widely used as complexing agents [22], have varied internal diameter 4.7 (α-CD) to 8.3 Å (γ-CD) [23], and have been previously used in microdialysis to improve the RR of drugs. Binding occurs primarily through hydrogen bonding, van der Waal’s forces and hydrophobic interactions with the CD cavity [22]. Cyclodextrins, were chosen as an affinity agent because they are inexpensive, widely available, used in pharmaceutical [24] and food preparations [25], have significant published thermodynamic binding data for numerous compounds [26], and have been reported to bind to opioid peptides [27–29].
Antibodies are obvious affinity agents for peptides and proteins. The use of free antibodies added to the perfusion fluid to enhance the RR of an analyte has been previously done. Pich et al. added corticotropin-releasing factor (CRF) antibodies into the perfusion fluid to increase the RR of CRF and found that a 2-fold increase was observed [15]. Antibody-immobilized beads have previously been used to increase the flux of cytokine proteins into the microdialysis probe [16].
This work chose to compare different cyclodextrins for their ability to increase the RR of leu- and met-enkephalin during in vitro microdialysis sampling. A comparison among different individual N- and C- terminal ends of the enkephalin peptides including phenylalanine, tyrosine and the tripeptide (Try-Gly-Gly) was also included in this work. Enhanced RR using cyclodextrins was compared against an antibody for FITC-labeled met-enkephalin.
2.0 Experimental
2.1 Chemicals
Methionine-enkephalin (ME), leucine-enkephalin (LE), β-cyclodextrin (β-CD), and 2-hydroxylpropyl-β-cyclodextrin (2-HPβ-CD), γ-cyclodextrin (γ-CD), tyrosine-glycine-glycine (YGG), L-tyrosine, L-phenylalanine, and FluoroTag Fluorescein Isothiocyanate (FITC) conjugation kit were purchased from Sigma (St. Louis, MO). Polyclonal anti-met-enkephalin and anti-leu-enkephalin antibodies raised in rabbits were purchased from Chemicon International (Temecula, CA). Artificial cerebral spinal fluid (aCSF) that contains 128 mM NaCl, 3.0 mM KCl, 1.3 mMCaCl2, 1.0 mM MgCl2, 21 mM Na2HPO4 and 1.3 mM NaH2PO4 and a phosphate buffer (PBS) containing 10 mM sodium phosphate buffer, 27 mM KCl, 138 mM NaCl, pH 7.4 were the buffers used to collect the control microdialysis samples. This PBS buffer was also used in the FITC-labeling conjugation kit All other chemicals were reagent grade or better.
2.2 Preparation and Purification of Fluorescein Isothiocyanate (FITC) labeled ME
ME was labeled with FITC using the Fluoro Tag FITC conjugation kit. FITC (1 mg mL−1) was dissolved in acetone and 250 μL of it was added drop-wise to a 1 mL solution of 5 mg mL−1 ME dissolved in 100 mM sodium carbonate-bicarbonate buffer, pH 9.0. The reaction mixture was allowed to react for 2 hours in the dark at room temperature on a plate shaker. The PBS buffer was used to pre-equilibrate a Sephadex G-25M column. The reaction mixture was separated to remove low molecular mass components and free FITC. The fractions were monitored by absorbance at 280 nm using a spectrophotometer and the appropriate fractions determined by mass spectral analysis were combined and stored at 4°C until further purification. The appropriate FITC–ME fractions were then purified by reversed-phase HPLC using a Vydac Protein/Peptide column, 250 mm × 2.1 mm C18 packed with 5 μm particles as previously described [30]. The following gradient was used for purification at 0.2 mL min−1 flow rate: 0–15 min. (15–35%B), 15–25 min (35–65% B), 25–32 min. (65% B), and 32–33 min. (65–15% B), and 33–40 min. (15% B), where A was 0.1% TFA in water and B was 0.1% trifluoroacetic acid (TFA) in acetonitrile. The peaks were monitored by UV absorbance at 220 nm and fluorescence wavelengths at excitation 490 nm and emission 525 nm. Purified FITC–ME fractions were collected, evaporated under nitrogen gas, and reconstituted with PBS buffer, pH 7.4. The purified FITC-ME was divided into aliquots and kept frozen at −20°C until used.
2.3 Microdialysis sampling
A BAS Bee microdialysis syringe pump and controller (Bioanalytical Systems Inc., West Lafayette, IN, USA) was used with a 1000-series gastight glass syringe (Hamilton, Reno, NV). CMA/12 polycarbonate (PC) and polyethersulfone (PES) microdialysis probes with 4 mm length were purchased from CMA Microdialysis (North Chelmsford, MA) and were prepared for first use per manufacturer’s instructions. The control probes were perfused with aCSF.
2.3.1. Cyclodextrin samples
Two PC (20 kDa) or PES (100 kDa) probes were used for the collection of ME and LE. Probes were placed in either a stirred or non-stirred solution in a microcentrifuge tube of 800 μL containing 25 μM ME and LE. A 25 μM solution of YGG and a 200 μM solution of tyrosine and phenylalanine were used and sampled with a PES probe. CD probes were perfused with different concentrations of β-CD, 2HP-β-CD or γ-CD (w/v%). Peptides were collected either at room temperature or 37°C at flow rates ranging between 0.5 to 2 μL min−1. Samples were analyzed using the HPLC-UV assay described below.
2.3.2. Antibody samples
Two PC CMA/12 (4 mm) probes were used for the collection of FITC-ME. Probes were placed in a quiescent solution of 800 μL of 62.5 μM of FITC-ME. Samples were collected in duplicate at room temperature at flow rates ranging between 0.5 to 2 μL min−1 and were protected from light. Samples (40 μL) were added to a 96-well half-area fluorescent plate and analyzed using a plate reader at excitation 492 nm and emission 535 nm. Standards were also made in antibody dilution solutions to test background interferences and no significant differences were observed when the standards were prepared in aCSF buffer. MALDI-MS was performed to determine the molecular weight of the antibody.
2.4 Analysis of Peptides using HPLC-UV
A Shimadzu LC system, which included a SIL-10ADvp autoinjector, an LC-10ADvp pump, a DGU-14A degasser, a CTO-10ASvp column oven, and an SCL-10Avp system controller, was used for separation. The ME and LE dialysates collected from the CD experiments were quantified from a standard curve of ME and LE in water from 500 μM to 2.1 μM. A Vydac Protein/Peptide column (218TP52), 250 mm × 2.1 mm C18 packed with 5 μm particle was used at a 0.2 mL min−1 flow rate. Solvent A consisted of 0.1% TFA in water and solvent B consisted of 0.1% TFA in acetonitrile. Five microliters of each sample was injected into the system and the gradient 15% B to 40% B in 0–12.5 minutes was used to separate the sample. Wavelengths of 220 nm and 280 nm were monitored.
3.0 Results and Discussion
3.1 Cyclodextrins
The primary goal of the studies performed with cyclodextrins was to determine their effectiveness towards improving the microdialysis RR of opioid peptides. Table 1 shows the RR values for ME and LE across a PC membrane using different cyclodextrins in the perfusion fluid. A statistically significant increase in RR was observed for all w/v% solutions of CDs used, xcept for 0.1% 2HPβ-CD. However, the increases in RR were modest. The mean increase in RR for ME using 0.5% 2HPβ-CD, 1% 2HPβ-CD, 1% β-CD and 1% γ-CD was 1.4, 1.8, 1.4, and 1.4 times, respectively and for LE was 1.4, 1.9, 1.7 and 1.5, respectively. Higher relative recoveries were obtained for 1% 2HPβ-CD compared to 1% β-CD. Relative recovery values were similar between 1% β-CD and 1% γ-CD despite their differences in rim diameters of 0.78 nm (β-CD) vs. 0.95 nm (γ-CD).
Table 1.
Comparison of the RR% for different concentrations of 1% 2HP-β-CD, 1% βCD and 1% γ-CD as affinity agents for the RR of 25 μM of ME and LE using a 20 kDa (PC) probe at 0.5 μL min−1 at room temperature (n = 3). *Indicates there is no significant difference in CD recoveries compared to the control (p = 0.05).
| [Cyclodextrin] | Met-Enkephalin | Leu-Enkephalin |
|---|---|---|
| aCSF (control) | 17.6 ± 1.1 | 16.2 ± 1.2 |
| 0.1% 2HP-β-CD | 20.7 ± 2.1 * | 19.2 ± 2.3* |
| 0.5% 2HP-β-CD | 24.7 ± 1.2 | 23.1 ± 0.9 |
| 1% 2HP-β-CD | 31.2 ± 3.5 | 31.6 ± 3.5 |
| 1% β-CD | 25.5 ± 1.2 | 27.1 ± 2.5 |
| 1% γ-CD | 24.4 ± 1.6 | 23.7 ± 1.8 |
The RR of ME and LE using a PES probe (100 kDa) with 1% β-CD and 1% γ-CD was compared. Table 2 shows that in an unstirred solution of ME and LE, 1% β-CD actually causes the RR to significantly decrease across different flow rates while 1% γ-CD shows statistically significantly higher relative recovery at each flow rate compared to the control. It has been reported that LE has a binding constant to β-CD of approximately 120 M−1 [27]. Compared to the results obtained with a PC probe (Table 1), the level of improved RR was less for both enkephalins using the PES probe when the affinity agents β-CD and γ-CD were perfused at 0.5 μL min−1. This observation may be explained due to the larger pores in the PES membrane allowing more CD to diffuse out of the probe into the sample medium. Once CD is outside the probe, it can bind to ME and LE. This results in a decrease of free ME and LE available to be sampled and thus influences the flux of free analyte toward the probe. Additionally, any bound ME or LE to cyclodextrin would be expected to have a lower aqueous diffusion coefficient which would also affect the amount of analyte collected.
Table 2.
Comparison of the RR% for 1% βCD and 1% γCD as affinity agents using a 100 kDa (PES) probe for the collection of 25 μM of ME and LE at 37 °C. Mean ± S.D. (n = 3) *Indicates there is no significant difference in CD recoveries compared to the control (p=0.05).
| Flow Rate | Met-Enkephalin | Leu-Enkephalin | ||||
|---|---|---|---|---|---|---|
| μL min−1 | aCSF | 1% βCD | 1% γCD | aCSF | 1% βCD | 1% γCD |
| 0.5 | 51.9 ± 0.3 | 54.6 ± 0.5* | 64.9 ± 1.1 | 45.2 ± 1.1 | 45.3 ± 0.8* | 57.4 ± 1.3 |
| 1.0 | 32.5 ± 1.7 | 17.4 ± 0.8 | 46.9 ± 0.1 | 24.7 ± 2.8 | 15.6 ± 0.5 | 40.8 ± 0.3 |
| 1.5 | 25.4 ± 0.1 | 11.2 ± 1.4 | 33.9 ± 0.9 | 17.9 ± 1.9 | 9.7 ±1.8 | 28.9 ± 2.8 |
To further investigate the results obtained in Table 2, the PES probes were placed in a well-stirred sample medium while samples were collected. Sampling from a well-stirred solution serves to provide the maximum possible RR [31]. In an unstirred solution, CD that has diffused out the probe membrane can bind to some of the ME and/or LE and form an inclusion complex. This complex will now have a smaller diffusion coefficient because of its larger size and will result in a lower RR back into the probe. In a stirred solution, cyclodextrin will be removed from the region nearest the probe that has diffused out of the probe. This removal process will allow free ME and/or LE to diffuse directly through the probe without being extensively bound by cyclodextrin and result in a larger enhancement than the unstirred solution. ME and LE when using 1% β-CD as significantly higher recoveries were observed (Table 3). Larger enhancements for the stirred solutions of ME and LE were generally observed using β-CD and γ-CD when compared to the unstirred solution.
Table 3.
Summary of stirred and unstirred relative recoveries for microdialysis sampling of 25 μM ME and LE using 1%βCD and 1% γ CD as affinity agents at 37 °C. Samples were collected using a 100 kDa probe. Mean ± S.D. (n = 3) *Indicates there is no significant difference in CD recoveries compared to the control (p = 0.05).
| Conditions | Met-Enkephalin | Leu-Enkephalin | ||||
|---|---|---|---|---|---|---|
| aCSF | 1%βCD | 1% γ CD | aCSF | 1%βCD | 1% γ CD | |
| 0.5 μL min−1 | ||||||
| Unstirred | 51.9 ± 0.3 | 54.6 ± 0.5* | 64.9 ± 1.1 | 45.2 ± 1.1 | 45.3 ± 0.8* | 57.4 ± 1.3 |
| Stirred | 52.6 ± 1.4 | 71.5 ± 3.8 | 79.7 ± 7.1 | 50.5 ± 3.1 | 73.6 ± 4.0 | 77.9 ± 7.7 |
| 1.5 μL min−1 | ||||||
| Unstirred | 25.4 ± 0.1 | 11.2 ± 1.4 | 33.9 ± 0.9 | 17.9 ± 1.9 | 9.7 ± 1.8 | 28.9 ± 2.8 |
| Stirred | 29.9 ± 3.5 | 31.2 ± 4.4 | 39.2 ± 5.7 | 29.1 ± 3.0 | 26.8 ± 3.1 | 38.4 ± 4.4 |
Table 4 compares several analytes sampled using PC and PES probes with 1% β-CD as an affinity agent for stirred conditions [32]. The known binding constants of each analyte are also listed. It can be seen that LE has the smallest log K value for cyclodextrin and is the only analyte that does not show enhanced microdialysis RR across both the PC and PES membranes at 1.0 μL min−1 flow. These two types of probes have nearly identical geometry with respect to internal and (PC 400 μm and PES 420 μm) external diameter (500 μm). Theoretically, the mass transport enhancement is related to the probe dimensions [19]. While there are no significant enhancements observed for LE with either probe, enhancement values of 1.81 (PC - data not shown) and 1.52 (PES) times were observed at 0.5 μL min−1.
Table 4.
Comparison of enhanced microdialysis RR of 1% β-CD for different analytes across PC and PES probes at a flow rate of 1.0 μL min−1 at stirred conditions. Table used is from reference [32].
| Analyte | log K (β-CD) | PC (4 mm) | PES (4 mm) |
|---|---|---|---|
| Leu-enkephalin (LE) | |||
| Control | 2.09a | 14.4 ± 1.2 | 35.9 ± 4.7 |
| 1% β-CD | 13.7 ± 2.1* | 32.8 ± 3.1* | |
| 4-nitrophenol | |||
| Control | 2.28 | 49.1 ± 13.7 | 43.2 ± 4.6 |
| 1% β-CD | 118.3 ± 6.1 | 93.3 ± 7.6 | |
| Carbamazepine | |||
| Control | 2.61 | 64.1 ± 6.0 | 58.8 ± 3.3 |
| 1% β-CD | 134.1 ± 7.5 | 74.1 ± 2.7 | |
| Amitriptyline | |||
| Control | 4.38 | 52.0 ± 4.0 | 37.5 ± 2.0 |
| 1% β-CD | 137.8 ± 2.2 | 146.3 ± 7.2 | |
| Ibuprofen | |||
| Control | 3.33 | 37.2 ± 1.5 | 22.3 ± 1.9 |
| 1% β-CD | 75.5 ± 3.6 | 54.1 ± 8.4 | |
Binding constant value is from ref [27].
Indicates there is no significant difference in CD recoveries compared to the control (p = 0.05).
The amino acids, tyrosine and phenylalanine, and the tripeptide, tyrosine-glycine-glycine (YGG), were sampled to determine if known binding interactions between the CDs and these more hydrophilic analytes would improve the RR through the probe compared to those of ME and LE (Table 5). There was a significant difference in RR enhancement at 0.5 μL min−1 for 1% α-CD for both tyrosine and phenylalanine, while no increases in RR were observed when they were collected at 1.0 and 1.5 μL min−1 with 1% β-CD and 1% γ-CD. YGG showed significant differences in recoveries for all flow rates collected using 1% γ-CD. In addition, YGG showed significantly higher enhanced RR using 1% γ-CD than both ME and LE (Table 2). These results may suggest that the hydrophobic residues, methionine, and leucine, play an important factor influencing the RR of enkephalins. Table 6 summarizes the reported binding constants for L-tyrosine and L-phenylalanine to α- and β-CDs. It is difficult to determine whether the binding of tyrosine is stronger to β-CD than for α-CD due to the wide variation in the reported values, however, tyrosine recoveries for α-CD were higher than β-CD. Phenylalanine has a larger binding constant for α-CD than tyrosine, which may explain why it has higher enhanced recoveries at 1.0 and 1.5 μL min−1 flow rates.
Table 5.
Comparison of the RR% for 200 μM of Tyrosine and Phenylalanine using 1% αCD, 1% βCD and 1% γCD and 25 μM Tyr-Gly-Gly (YGG) using 1% γCD as affinity agents using a 100 kDa (PES) probe at room temperature. Mean ± S.D. (n = 3) *Indicates there is a significant difference in CD recoveries compared to the control (p = 0.05).
| Flow Rate | Tyrosine | Phenylalanine | Tyr-Gly-Gly | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| μL min−1 | aCSF | 1% αCD | 1% βCD | 1% γCD | aCSF | 1% αCD | 1% βCD | 1% γCD | aCSF | 1% γCD |
| 0.5 | 67.7 ± 2.8 | 76.8 ± 4.9* | 68.2 ± 11.5 | 67.2 ± 5.7 | 69.9 ± 4.0 | 76.7 ± 7.9* | 66.5 ± 0.5 | 66.1 ± 14.2 | 63.0 ± 7.2 | 84.0 ± 4.1* |
| 1.0 | 33.8 ± 4.6 | 37.4 ± 6.8 | 23.6 ± 6.9 | 27.5 ± 2.9 | 35.3 ± 9.1 | 46.7 ± 3.6 | 29.1 ± 3.2 | 29.3 ± 6.8 | 16.2 ± 7.4 | 47.7 ± 1.2* |
| 1.5 | 19.9 ± 3.6 | 22.5 ± 4.5 | 17.7 ± 4.7 | 14.2 ± 2.4 | 22.6 ± 7.3 | 28.9 ± 1.6 | 17.7 ± 2.7 | 16.3 ± 3.8 | 13.3 ± 4.5 | 33.9 ± 3.9* |
Table 6.
Summary of published binding constants (log K) for L-Tyrosine, L-Phenylalanine and L-Leucine to α- and β-cyclodextrins.
3.2 Antibodies
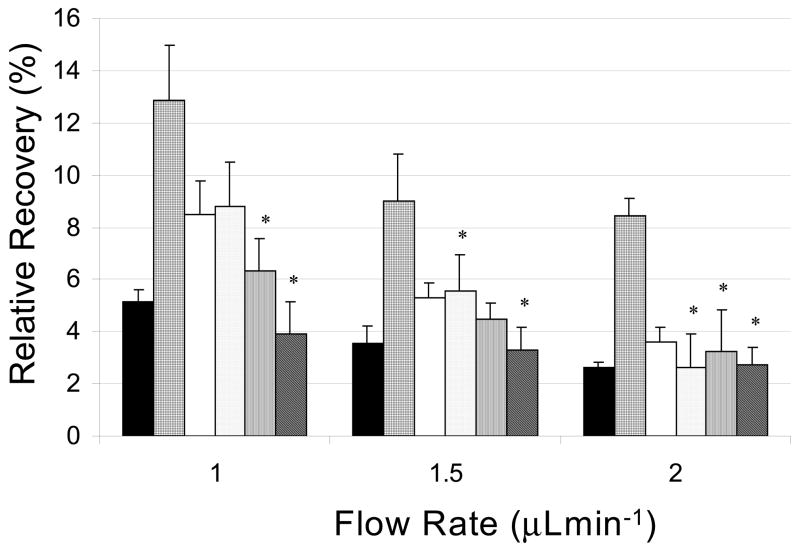
The second affinity agent studied for enhancing the collection of enkephalins was an antibody for ME. To simply test whether or not this antibody will enhance the RR for met-enkephalin, ME was fluorescently-labeled. Then, by measuring the fluorescence signal from the sample, the peptide content can be quantified. Different antibody concentrations from 7.5 to 750 nM were used. The RR of the control dialysate of FITC-ME was 5% or 3.1 μM of FITC-ME collected. The 0.75 μM antibody concentration gave an enhanced RR of 13%. At a control RR of 3.1 μM FITC-ME being collected, the antibody concentration of 0.75 μM is 4 times less than the amount of FITC-ME being collected and should allow for the favored 1:2 complex of one antibody with two FITC-ME. This 1:2 binding will occur due to a limiting amount of antibody present [33], thus resulting in an increase in RR. The 0.15 μM, 75 nM and 15 nM antibody concentrations show a statistically significant enhanced RR compared to controls with decreasing effectiveness. However, the 7.5 nM antibody concentration showed no enhancement compared to the control most likely because the concentration is too low. The 2.5-fold increase in RR at 0.5 μL min−1 flow rate is comparable to the 2-fold increase in RR observed for corticotropin-releasing factor when it was collected with antibodies and detected by RIA [15]. A calculated Kd binding constant of 25 nM was determined for LE binding to its antibody by equilibrium dialysis (data not shown) and is comparable to the Kd value of 21 nM for CRF [34]. The much stronger binding affinity between the enkephalin and its antibody, Kd in the nM range compared to the CDs, which have Kd values in the mM range is likely the driving force behind the higher RR values with lower affinity agent concentration.
4.0 Conclusions
The addition of affinity agents to the perfusion fluid during microdialysis has been shown to increase the RR of leu- and met-enkephalin. However, some unanswered questions remain regarding why certain affinity agents enhance the RR for some analytes and not for others. Cyclodextrins have shown promise for enhanced RR for small molecules; however, the results shown here confirm that CDs are not suitable affinity agents to enhance RR for met-enkephalin and leu-enkephalin. An antibody has shown promising results for enhancing met-enkephalin RR.
Figure 2.
In vitro ME antibody RR enhancement of 62.5 μM FITC-ME quiescent solution at room temperature using various antibody dilutions as an affinity agent through a PC probe: 0.75 μM (grid), 0.15 μM (white), 75 nM (dots), 15 nM (lines), and 7.5 nM (diagonal lines), aCSF (black) at flow rates 1.0, 1.5 and 2.0 μL min−1 (n = 3). * Indicates there is no significant difference in recoveries compared to the control (p = 0.05).
Acknowledgments
We gratefully acknowledge NIH DA-020577 for funding of this research. We thank Amy Munson for collecting some microdialysate samples that were used for data presented in Table 5.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Snyder S, lnnis R. Annu Rev Biochem. 1979:755. doi: 10.1146/annurev.bi.48.070179.003543. [DOI] [PubMed] [Google Scholar]
- 2.Nutt DJ. Human Psychopharmacology. 1997;12:S53. doi: 10.1002/hup.388. [DOI] [PubMed] [Google Scholar]
- 3.Vaccarino AL, Kastin AJ. Peptides. New York: 1975. Endogenous opiates: 1999. 2000 21. [DOI] [PubMed] [Google Scholar]
- 4.Bourne JA. Clin Exp Pharmacol Physiol. 2003;30:16. [Google Scholar]
- 5.Hansen DK, Davies MI, Lunte SM, Lunte CE. J Pharm Sci. 1999;88:14. doi: 10.1021/js9801485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lunte CE, Scott DO, Kissinger PT. Anal Chem. 1991;63:773A. [Google Scholar]
- 7.Maidment NT, Brumbaugh DR, Rudolph VD, Erdelyi E, Evans CJ. J Neurosci. 1989;33:549. doi: 10.1016/0306-4522(89)90407-7. [DOI] [PubMed] [Google Scholar]
- 8.Hows MEP, Organ AJ, Murray S, Dawson LA, Foxton R, Heidbreder C, Hughes ZA, Lacroix L, Shah AJ. J Neurosci Methods. 2002;121:33. doi: 10.1016/s0165-0270(02)00228-5. [DOI] [PubMed] [Google Scholar]
- 9.Kendrick KM. J Neurosci Methods. 1990;34:35. doi: 10.1016/0165-0270(90)90040-m. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy RT, Watson CJ, Haskins WE. Curr Opin Chem Biol. 2002;6:659. doi: 10.1016/s1367-5931(02)00373-3. [DOI] [PubMed] [Google Scholar]
- 11.Myasein KTM, Pulido JS, Hatfield RM, McCannel CA, Dundervill RF, III, Shippy SA. Analyst. 2007;132:1046. doi: 10.1039/b707783a. [DOI] [PubMed] [Google Scholar]
- 12.Monroe EB, Koszczuk BA, Losh JL, Jurchen JC, Sweedler JV. Int J Mass Spectrom. 2007;260:237. [Google Scholar]
- 13.Wei H, Nolkrantz K, Parkin MC, Chisolm CN, O’Callaghan JP, Kennedy RT. Anal Chem. 2006;78:4342. doi: 10.1021/ac052196x. [DOI] [PubMed] [Google Scholar]
- 14.Bungay PM, Morrison PF, Dedrick RL. Life Sci. 1990;46:105. doi: 10.1016/0024-3205(90)90043-q. [DOI] [PubMed] [Google Scholar]
- 15.Pich EM, Koob GF, Heilig M, Menzaghi F, Vale W, Weiss F. J Neurosci. 1993;55:695. doi: 10.1016/0306-4522(93)90435-i. [DOI] [PubMed] [Google Scholar]
- 16.Ao X, Sellati TJ, Stenken JA. Anal Chem. 2004;76:3777. doi: 10.1021/ac035536s. [DOI] [PubMed] [Google Scholar]
- 17.Mogopodi D, Torto N. Anal Chim Acta. 2005;534:239. [Google Scholar]
- 18.Hamrin K, Rosdahl H, Ungerstedt U, Henriksson J. J Appl Physiol. 2002;92:385. doi: 10.1152/jappl.2002.92.1.385. [DOI] [PubMed] [Google Scholar]
- 19.Khramov AN, Stenken JA. Anal Chem. 1999;71:1257. doi: 10.1021/ac9811930. [DOI] [PubMed] [Google Scholar]
- 20.Pettersson A, Amirkhani A, Arvidsson B, Markides K, Bergquist J. Anal Chem. 2004;76:1678. doi: 10.1021/ac035305l. [DOI] [PubMed] [Google Scholar]
- 21.Duo J, Fletcher H, Stenken JA. Biosens Bioelectron. 2006;22:449. doi: 10.1016/j.bios.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 22.Schneiderman E, Stalcup AM. J Chromatogr B. 2000;745:83. doi: 10.1016/s0378-4347(00)00057-8. [DOI] [PubMed] [Google Scholar]
- 23.Saenger W. Angew Chem Int Ed Engl. 1980;19:344. [Google Scholar]
- 24.Carrier RL, Miller LA, Ahmed IJ. Control Release. 2007;123:78. doi: 10.1016/j.jconrel.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 25.Szejtli J, Szente L. Eur J Pharm Biopharm. 2005;61:115. doi: 10.1016/j.ejpb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 26.Rekharsky MV, Inoue Y. Chemical Reviews. 1998;98:1875. doi: 10.1021/cr970015o. [DOI] [PubMed] [Google Scholar]
- 27.Bekos E, Gardella J, Jr, Bright F. J Inclusion Phenom Mol Recognit Chem. 1996;26:185. [Google Scholar]
- 28.Chun IK, Chien YW. Int J Pharm. 1995;121:217. [Google Scholar]
- 29.Yaksh TL, Jang J, Nishiuchi Y, Braun KP, Ro S, Goodman M. Life Sciences. 1991;48:623. doi: 10.1016/0024-3205(91)90537-l. [DOI] [PubMed] [Google Scholar]
- 30.Babu CVS, Chung BC, Lho DS, Yoo YS. J Chromatogr A. 2006;1111:133. doi: 10.1016/j.chroma.2005.06.031. [DOI] [PubMed] [Google Scholar]
- 31.Stenken JA, Topp EM, Southard MZ, Lunte CE. Anal Chem. 1993;65:2324. doi: 10.1021/ac00065a026. [DOI] [PubMed] [Google Scholar]
- 32.Ao X, Stenken JA. Analyst. 2003;128:1143. doi: 10.1039/b308057a. [DOI] [PubMed] [Google Scholar]
- 33.Wang H, Lu M, Weinfeld M, Le XC. Anal Chem. 2003;75:247. doi: 10.1021/ac026204a. [DOI] [PubMed] [Google Scholar]
- 34.van Oers JW, Tilders JH, Berkenbosch F. Endocrinology. 1989;124:1239. doi: 10.1210/endo-124-3-1239. [DOI] [PubMed] [Google Scholar]
- 35.Kahle C, Holzgrabe U. Chirality. 2004;16:509. doi: 10.1002/chir.20068. [DOI] [PubMed] [Google Scholar]