Abstract
There has been much recent investigation into the role of parietal cortex in memory retrieval. Proposed hypotheses include attention to internal memorial representations, an episodic working memory-type buffer, and an accumulator of retrieved memorial information. The current investigation used event-related potentials (ERPs) to test the episodic buffer hypothesis, and to assess the memorial contribution of parietal cortex in younger and older adults, and in patients with circumscribed lateral parietal lesions. In a standard recognition memory paradigm, subjects studied color pictures of common objects. One-third of the test items were presented in the same viewpoint as the study phase, one-third were presented in a 90-degree rotated viewpoint, and one-third were presented in a noncanonical viewpoint. Conflicting with the episodic buffer hypothesis, results revealed that the duration of the parietal old/new effect was longest for the canonical condition and shortest for the noncanonical condition. Results also revealed that older adults demonstrated a diminished parietal old/new effect relative to younger adults. Consistent with previous data reported by Simons et al., patients with lateral parietal lesions showed no behavioral impairment compared to controls. Behavioral and ERP data from parietal lesion patients are presented and discussed. From these results, the authors speculate that the parietal old/new effect may be the neural correlate of an individual’s subjective recollective experience.
Keywords: parietal cortex, recollection, event-related potentials, parietal effect, aging
Introduction
Over the last 50 years, neuropsychology research has established that medial temporal and frontal regions of the brain are critical areas for memory (Aggleton & Brown, 1999; Janowsky, Shimamura, & Squire, 1989; Simons & Spiers, 2003). For nearly half of this time, event-related potential (ERP) researchers have associated parietal activity in healthy individuals with successful recollection on tests of recognition memory (see Rugg & Curran, 2007, for review). Due to the lack of spatial resolution associated with ERPs, these researchers could only speculate as to the neural generators of this parietal activity. More recently, the advent of functional neuroimaging techniques such as PET and fMRI have allowed us to examine the functional workings of the brain with high spatial resolution while subjects engage in memory retrieval. Converging with ERP findings, a number of PET and fMRI studies have reported parietal activation in both medial and lateral parietal cortices associated with successful memory retrieval (Rugg, Otten, & Henson, 2002; Wagner, Shannon, Kahn, & Buckner, 2005).
There are strong anatomic connections from the parietal lobe to medial temporal and prefrontal regions, which make it plausible that the parietal lobe might be important for mnemonic processing (Suzuki & Amaral, 1994; Rockland & Van Hoesen, 1999). This suggestion is supported by the ubiquitous medial and lateral parietal activation seen in fMRI studies during recognition memory tasks (see meta-analysis in Simons et al., in press, Figure 1). Although the exact role of the parietal lobe in episodic memory remains unclear, recent fMRI and ERP studies have attempted to answer this intriguing question. Several hypotheses have been proposed as to the mnemonic functions supported by the parietal lobes, with two highly discussed suppositions. One hypothesis is that parietal activity may index attentional orienting to recollected information (Rugg & Henson, 2002; Wagner et al., 2005). Another hypothesis posed is that the parietal cortex supports the representation of recollected information, and acts as an episodic working memory-type buffer holding contents of retrieval “on-line” for a decision to be made by a central executive (Baddeley, 2000; Ravizza, et al., 2004; Vilberg, Moosavi, & Rugg, 2006).
Figure 1.
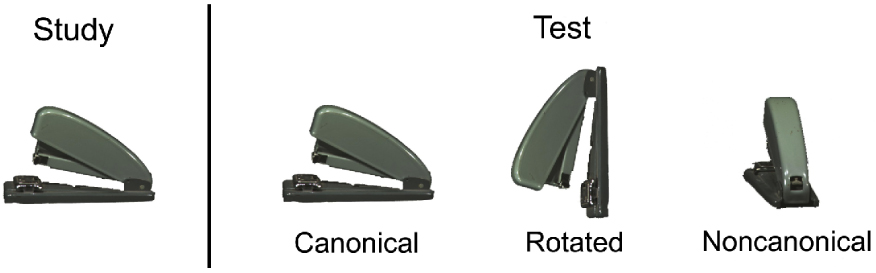
Example of study and test stimuli
Neuroimaging researchers have consistently reported greater activity in parietal regions for correctly recognized studied items (hits) than for correctly identified unstudied items (correct rejections) (Henson et al., 1999; Konishi et al., 2000; Shannon & Buckner, 2004). This difference between hits and correct rejections is commonly referred to as the parietal old/new effect. Several researchers using fMRI designs have reported greater activity in parietal regions for items that subjects reported as vividly recollected compared to when subjects reported having a general sense of knowing that an item was studied (Henson et al., 1999; Eldridge et al., 2000; Wheeler & Buckner, 2004). Consistent with these findings, Yonelinas et al. (2005) suggested that the lateral region of the parietal cortex could perhaps differentiate whether the recognition of an item was accompanied by recollection. In a more recent fMRI study, Vilberg and Rugg (2007) reported that activity in the left lateral parietal cortex was sensitive to the amount of information retrieved. This finding, in combination with previous studies reporting parietal activation across a number of study materials and modalities (i.e., visual, auditory, pictures, words), have advanced the hypothesis that the parietal lobe may play a role in the cortical reinstatement of encoded information (Vaidya et al., 2002; Wagner et al., 2005; Wheeler & Buckner, 2004; Woodruff et al., 2005). These studies have suggested that parietal cortex may work in a similar manner to the multimodal episodic buffer proposed by Baddeley (2000), acting as an interface between episodic memory and the prefrontal central executive (Wagner et al., 2005).
ERP studies have advanced similar hypotheses regarding retrieval-related parietal activity. ERPs can provide precise temporal resolution of cognitive processes on the order of tens of milliseconds. The parietal ERP old/new effect begins around 500 ms after a subject encounters a studied test item, is predominantly left-sided, and lasts approximately 400 to 600 ms in duration (Friedman & Johnson, 2000). The parietal effect is invariant to changes in familiarity strength, and has been associated with recollection (Ally & Budson, 2007; Friedman & Johnson, 2000; Trott et al., 1999; Woodruff, Hayama, & Rugg, 2006). Although there have been no investigations to our knowledge that have directly investigated the duration of the parietal old/new effect, analysis of the magnitude of the effect has produced similar results to fMRI studies. Research has shown that the parietal ERP old/new effect is greater for items that are vividly recollected compared to when one has a more general sense of simply knowing that an item was studied (Duzel et al., 1997; Wolk et al., 2006; Woodruff, Hayama, & Rugg, 2006). An ERP study that served as a precursor to the fMRI study above (Vilberg and Rugg, 2007) showed that the magnitude of the parietal old/new effect was greater when subjects retrieved both words in a word pair compared to only remembering one of the words (Vilberg, Moosavi, & Rugg, 2006). Because the old/new effect was evident in a graded fashion varying with the amount of information retrieved, Vilberg et al. (2006) suggested that the parietal ERP effect may reflect the amount of information recollected at test.
Taken together, the anatomical connectivity in conjunction with the fMRI and ERP studies provide strong evidence that parietal cortex is involved in memory retrieval success, and more specifically that it may be involved when retrieval is based on recollection of the context in which stimuli were previously encountered. If parietal cortex were involved in memory retrieval in general and in recollection in particular, we would expect that individuals with parietal cortex dysfunction would show deficits in recollection-based memory. The parietal lobe is one of the areas to suffer neural degeneration as a result of the aging process (Good et al., 2001; Sowell et al., 2003), and reduced parietal activity has been observed in older adults during recognition (Daselaar et al., 2006). However, aging also results in degeneration of frontal and numerous other cortical areas, so the specificity of this parietal effect is difficult to determine from comparison solely based between younger and older adults. Until recently, there were no studies investigating performance on a recollection task in patients with circumscribed lesions affecting parietal cortex. However, in a recent study using a source memory task involving words and famous faces that elicited significant parietal activation in healthy subjects using fMRI, Simons et al. (in press) found that patients with left or right unilateral parietal lesions did not show memory impairment compared to controls. These authors concluded that although the processes supported by the parietal lobe likely contribute to memory function, these processes may not be critical for accurate source recollection to occur.
While each of the scientific methods reviewed above has its limitations, interpretations of the fMRI, ERP, and lesion data have led to intriguing hypotheses as to the role of the parietal lobes in memory retrieval (Ally & Budson, 2007; Simons et al., in press; Wagner et al., 2005). The current study was designed to better understand the role of the parietal lobes in memory by seeking convergence across multiple complementary domains: ERPs, effects of aging, and effects of circumscribed parietal lesions. We used a novel recognition memory paradigm specifically designed to target processes thought to be dependent on parietal lobe function. Among the experimental manipulations that have been shown to elicit particularly robust parietal activation is the recognition of common objects from unusual or noncanonical viewpoints (Kosslyn et al., 1994; Faillenot et al., 1997; Sugio et al., 1999). In the present experiment, subjects studied 210 color pictures of objects in a standard or canonical view, and were then tested on 420 pictures, half old and half new. One-third of both the old and new pictures were presented in the same canonical view, one-third were presented in the canonical view but rotated 90 degrees to either left or right, and one-third were presented in a noncanonical view (see Figure 1 for an example of the stimuli). Subjects were instructed to respond “old” if the object was studied, even if it was now rotated or shown from a different vantage point. We used this paradigm to examine the role of the parietal lobes in memory in several different ways.
First, we elected to use the excellent temporal resolution of ERPs to investigate the episodic buffer hypothesis. This hypothesis posits that activity in parietal cortex reflects retrieved information that is held for the central executive to execute decision-making processes. In the context of a task involving recognition of objects from different viewpoints, one would expect that the demands on a system that holds information “on-line” for further processing would depend on the amount of mental imagery transformation required for a perceived object to be matched against those stored in long-term memory (Ally & Budson, 2007; Shepard & Metzler, 1988; Warrington & Taylor, 1973). Thus, we predicted that the duration of the ERP parietal old/new effect would be shortest for the canonical condition, longest for the noncanonical condition, and in-between for the rotated condition. It should be noted that due to cognitive processes involved in mental rotation and transformation associated with the rotated and noncanonical conditions, it is possible that the episodic buffer will be loaded at differing onset points. To account for this, our analyses of the scalp topographic data examined not only duration of the parietal old/new effect, but onset differences as well.
Second, we examined the ERP activity of healthy older adults on this paradigm relative to the younger adults. Numerous studies have reported differential effects of aging on parietal activity during the performance of recognition memory tasks (Ally et al., 2008; Grady et al., 2002; Cabeza et al., 2002; Daselaar et al., 2006). It follows that as the need for parietal lobe activity increases in a memory task, older adults should show increasing amplitude differences in the parietal old/new effect when compared to the younger adults. Therefore, we predicted that older adults would show the least difference in parietal old/new amplitude when compared to younger adults on the canonical condition, the greatest difference in parietal old/new amplitude compared to the younger adults for the noncanonical condition, and the difference in parietal old/new amplitude for the rotated items would fall somewhere between the canonical and noncanonical conditions. Further, given the extensive evidence suggesting decreased parietal activity during retrieval in healthy older adults (Ally et al., 2008; Daselaar et al., 2006; Fjell et al., 2005; Morcom & Rugg, 2004; Nielsen-Bohlman & Knight, 1995; Rugg et al., 1997; Senkfor & Van Patten, 1998; Swick & Knight, 1997), we also predicted that when collapsed over all three conditions, the healthy older adults would show a diminished parietal old/new ERP effect relative to younger adults.
Lastly, we had the opportunity to examine the performance of several patients with circumscribed unilateral parietal lobe lesions. If the assertion made by Simons et al. (in press) that parietal cortex plays a supportive role but is not central to recollection were correct, we would expect an interaction of group and condition such that the patients would show the least impairment for the canonical condition, the most impairment for the noncanonical condition, and in-between performance for the rotated items. We therefore predicted that as the need for parietal lobe activity increases, the patients should perform increasingly worse relative to an age and gender matched lesion control group. However, if parietal cortex were central to recollection, we would expect performance to be significantly impaired for the patients compared to controls on all three conditions, with no interaction of group and condition.
Methods
Design Overview
Each subject viewed 210 color pictures in a standard canonical viewpoint at study. After a 10-minute delay, subjects viewed 420 color pictures (50% old) at test. The 420 test items were presented in three different viewpoints. One-third of the test items were presented in the same viewpoint as the study phase (canonical), one-third were presented in a 90-degree rotated viewpoint, and one-third were presented in a noncanonical viewpoint. High-density ERPs were recorded at test.
Subjects
Twenty-four younger adults, 24 older adults, 4 patients with parietal lobe lesions, and 4 age and gender-matched lesion control subjects participated in the experiment. Older adult subjects were excluded if they had a first-degree relative with a history of Alzheimer's disease or other memory disorder, if they had a significant history of cerebrovascular disease, a neurodegenerative disorder, or if they were currently being treating for a psychiatric disorder. All subjects were right-handed, English was their native language, and were required to have corrected 20/30 or better color vision. Demographic information for the lesion patients can be seen in Figure 2. The study was approved by the human studies committees of the Edith Nourse Rogers Memorial Veterans Hospital, Bedford, Massachusetts, USA, and the psychology research ethics committee of the University of Cambridge, Cambridge, UK. All subjects gave IRB-approved informed consent before participating in the study, and were compensated at the rate of $25 per hour. The data from one younger adult and two older adults were excluded from all analyses due to either poor performance or excessive artifact in the electrophysiological data. Data are reported from the remaining 23 younger adults (16 female) with a mean education of 15.1 years, and an age range of 18 to 25 (mean 21.38) and 22 older adults (16 female) with a mean education of 16.4 years, and an age range of 62 to 83 (mean 73.86). Of note, ERP data was obtained from only one of the lesion patients, and was not obtained from the 4 lesion control subjects. The ages of the four parietal lesion patients (1 female) ranged from 49 to 74 (mean 59.5), and the ages of the four lesion control group subjects (1 female) ranged from 49 to 77 (mean 60.6).
Figure 2.
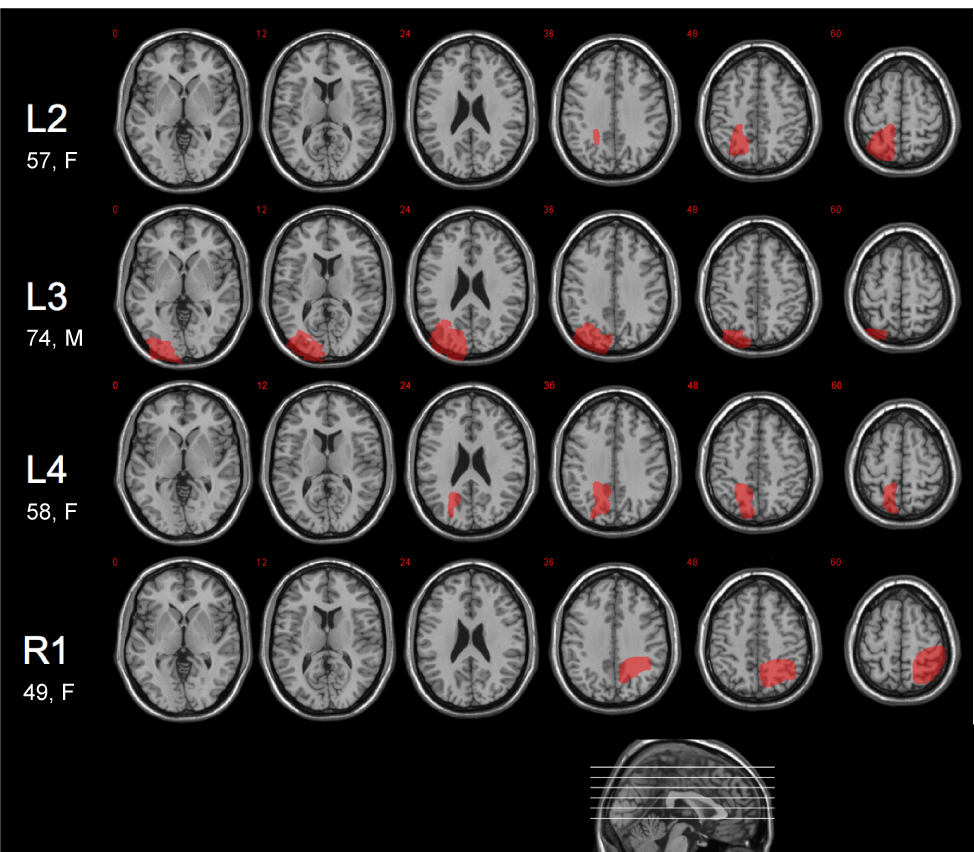
Lesion diagrams for the patients with parietal lobe lesions. Each patient's lesion was manually traced on a structural MRI of their brain, normalized to MNI space, and displayed on axial slices of a canonical structural image (slice positions indicated on sagittal section at foot of figure). Patient L2's lesion affected BA 5 and 7, with some extension into BA 40. Patient L3's lesion involved BA 7 and 39, as well as BA 17–19. Patient L4’s lesion is predominantly in BA 5, with some extension into BA 18 and 19. Patient R1's lesion involved BA 7 and 39, in addition to BA 1–3.
The older adults completed a brief neuropsychological battery to confirm cognitive functioning in the average range. These tests were administered in a separate 45-minute session. Subjects were first administered the Mini Mental State Examination (Folstein, Folstein,& McHugh, 1975), on which a minimum score of 27 was required for participation. Subjects were then administered the CERAD word list memory test (Morris et al., 1989), Trail Making Test Part B (Adjutant General’s Office, 1944), Verbal Fluency (Monsch, et al., 1992), and the 15-item Boston Naming Test (Mack, et al., 1992). Mean scores (and standard deviations) for the older adult group on our neuropsychological battery were the following: MMSE = 29.23 (1.2); CERAD word list immediate recall over 3 trials /30 = 20.6 (3.8), delayed recall /10 = 6.91 (1.6), recognition /10 = 9.77 (0.4); Trails B = 84.31 (26.9) seconds; Word fluency to letters (F, A, S) = 42.04 (10.6); Word fluency to categories (animals, fruits, vegetables) = 46.99 (6.8); 15 item Boston Naming Test = 14.14 (1.2). No older adult scored greater than 1 standard deviation from the group mean on any neuropsychological test. Neuropsychological data was also obtained from parietal lesion patients, and can be seen in Supplemental Table 1.
Experimental Material and Methods
The color pictures used in the current study were of common objects, and were obtained from several online databases including the Princeton 3D Model Search Engine, http://shape.cs.princeton.edu/search.html, the Amsterdam Library of Object Images, http://staff.science.uva.nl/~aloi; and The Tarr Lab Object Databank, http//titan.cog.brown.edu:8080/TarrLab/stimuli/objects/objectdatabank.zip/view. The studied and unstudied items, and the canonical, rotated, and noncanonical views at test, were counterbalanced across subjects. Half of the rotated items were rotated 90 degrees to the left, and the other half were rotated 90 degrees to the right. Pictures were presented in central vision on a white background, with an average height of 12.7 cm and an average width of 15 cm. Subjects were presented with three example study-test trials (one in each view; canonical, rotated, noncanonical) immediately prior to the experiment. Subjects were instructed that a test item is “old” if the object was studied from either the same viewpoint or from a different viewpoint. Examples of study and test stimuli can be seen in Figure 1.
During the study phase, subjects were asked to make like/dislike judgments of the stimuli, and asked to remember the stimuli for a subsequent memory test. Each trial began with a 1500 ms fixation character (“+”) prior to the presentation of study stimuli. Study stimuli were then presented for 2000 ms followed by the question, “Do you like this item?” Subjects were then prompted to button press to signify their like/dislike judgment.
The test phase began with another example study-test trial to assure that the subject understood the task. Each trial began with a 1000 ms fixation character (“+”) prior to the presentation of the stimuli. Test stimuli were presented in either a canonical view, a 90 degree rotated view, or a noncanonical view with the question, “Is this item old or new?” The test item remained on the screen until subjects button pressed to signify their response.
ERP Procedure
Subjects were seated in a hardback chair and fitted with an Active Two electrode cap (Behavioral Brain Sciences Center, Birmingham, UK). A full array of 128 Ag-AgCl BioSemi (Amsterdam, Netherlands) “active” electrodes were connected to the cap in a pre-configured montage which places each electrode in equidistant concentric circles from 10–20 position Cz (Supplemental Figure 1). Active electrodes are amplified through the electrode at the source and do not require abrading of the skin or measuring skin-electrode impedance levels. In addition to the 128 scalp electrodes, two mini-biopotential electrodes were placed on each mastoid process. Finally, vertical and horizontal EOG activity was recorded from bipolar electrodes placed below the left eye and on the outer canthus of the left and right eye. EEG and EOG activity was amplified with a bandwidth of 0.03–35 Hz (3 dB points) and digitized at a sampling rate of 256 Hz. Recordings were referenced to a vertex reference point, but were later re-referenced to a common average reference to minimize the effects of reference-site activity and accurately estimate the scalp topography of the measured electrical fields (Curran, et al., 2006; Dien, 1998).
The sampling epoch for each test trial lasted for a total of 1700 ms, which included a 200 ms pre-stimulus baseline period. This pre-stimulus period was used to baseline correct averaged ERP epochs lasting 1500 ms. ERPs were averaged and corrected using the EMSE Software Suite (Source Signal Imaging, San Diego, CA). Trials were corrected for excessive EOG activity using the empirical EMSE Ocular Artifact Correction Tool, in which artifact data are manually distinguished from the clean data by the investigator. The Ocular Artifact Tool then produces a logarithmic ratio of artifact data versus clean data and subtracts artifact data from all channels where it is detected. Trials were discarded from the analyses if they contained baseline drift or movement greater than 90 µV. Individual bad channels were corrected with the EMSE spatial interpolation filter.
Results
Recognition accuracy, response bias (C), reaction time (RT), and electrophysiological data were analyzed for the younger and older adults using ANOVAs. Nonparametric analyses were used to examine scalp topographic differences over time between the three conditions. Analysis of memorial accuracy for the three conditions was also completed using ANOVA for the four lesion patients and age and gender-matched controls. Although the lesion data is from a small number of patients, we believe that such data are essential to our understanding of the role of the parietal lobes in memory retrieval. Because of the small number of patients we are careful not to overreach our conclusions.
Behavioral Performance
Recognition accuracy was calculated using both the discrimination index Pr (%Hits - %False Alarms) and d’ (Snodgrass & Corwin, 1988); because these results were nearly identical only the Pr analyses are presented (all data are presented in Table 1). To examine differences in accuracy, a repeated measures ANOVA was performed with the factors of Group (Young, Old) and Condition (Canonical, Rotated, Noncanonical). Results revealed an effect of Group [F(1, 43) = 53.13, p < .001], indicating the overall accuracy was better for the younger adults compared to the older adults. An effect of Condition [F(2, 86) = 488.34, p > .001] showed that accuracy was better for the canonical condition compared to the rotated condition [F(1, 46) = 20.95, p < .001] and the noncanonical condition [F(1, 46) = 535.59, p < .001], and also for the rotated condition compared to the noncanonical condition [F(1, 46) = 526.52, p < .001]. There was also an interaction of Group and Condition [F(2, 86) = 7.96, p = .002]. This interaction was likely due to performance becoming increasingly worse for the older adults compared to the younger adults as the task became more difficult. As can be seen in Table 1, the difference in Pr between the two groups for the canonical condition was relatively small (.12), but became greater for the rotated condition (.17), and was at its greatest for the noncanonical condition (.24). Follow-up t-tests for the younger adult group revealed that accuracy was better in the canonical [t(22) = 16.39, p < .001] and rotated [t(22) = 15.17, p < .001] conditions compared to the noncanonical condition. However, the difference in performance between the canonical and rotated conditions was not significant [t(22) = 1.80, p = .095].
Table 1.
Discrimination and response bias values for each of the three conditions
| Group | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Condition | Younger Adults | Older Adults | Lesion Patients | |||||||||
| Hits | FA | d’ | C | Hits | FA | d’ | C | Hits | FA | d’ | C | |
| Canonical | .93 | .06 | 3.12 | −.03 | .88 | .13 | 2.35 | .00 | .93 | .15 | 2.98 | −.03 |
| Rotated | .92 | .08 | 2.93 | −.03 | .84 | .16 | 2.08 | −.03 | .89 | .16 | 2.36 | −.11 |
| Noncanonical | .66 | .17 | 1.46 | .32 | .45 | .19 | 0.78 | .53 | .58 | .17 | 1.19 | .31 |
Response bias was calculated using the measure C (Snodgrass & Corwin, 1988), and can be seen in Table 1. Positive values of C indicate a conservative response bias, and negative values indicate a liberal response bias. A repeated measures ANOVA with the factors of Groups (Young, Old) and Condition (Canonical, Rotated, Noncanonical) revealed an effect of Condition [F(2, 86) = 88.28, p < .001] and an interaction of Group and Condition [F(2, 86) = 4.20, p = .018]. Follow-up analyses showed that response bias was more conservative for both groups on the noncanonical condition compared to the canonical [F(1, 44) = 109.63, p < .001] and rotated [F(1, 44) = 104.26, p < .001] conditions. Further analysis showed that the interaction was present because the older adults demonstrated a more conservative response bias than the younger adults only for the noncanonical condition [t(43) = 2.49, p = .017].
The RT data are shown in Table 2. To analyze differences in median RTs, a repeated measures ANOVA was performed with the factors of Group (Young, Old), Condition (Canonical, Rotated, Noncanonical), and Item Type (Hits, Correct Rejections). The results revealed an effect of Group [F(1, 43) = 31.80, p < .001], indicating that overall reaction times were faster for younger adults than for older adults. There was also an effect of Item Type [F(1, 43) = 49.92, p < .001] showing that reaction times were faster for hits than correct rejections, an effect of Condition [F(2, 86) = 115.53, p < .001] indicated that reaction times were faster for the canonical condition compared to the rotated condition [F(1, 44) = 75.19, p < .001] and the noncanonical condition [F(1, 44) = 121.05, p < .001], and also for the rotated condition compared to the noncanonical condition [F(1, 44) = 96.77, p < .001]. The ANOVA also revealed a marginal 3-way interaction of Group, Condition, and Item Type [F(2, 86) 3.58. p = .060]. This marginal interaction was likely due to the fact that the reaction time difference between hits and correct rejection remained relatively constant for the older adults, whereas the reaction time difference between hits and correct rejections increased significantly as the condition became more difficult for the younger adults (Table 2).
Table 2.
Median reaction times (ms) for each of the three conditions
| Group | ||||||
|---|---|---|---|---|---|---|
| Condition | Younger Adults | Older Adults | Lesion Patients | |||
| Hits | CR | Hits | CR | Hits | CR | |
| Canonical | 0.92 | 1.11 | 1.24 | 1.57 | 0.97 | 1.66 |
| Rotated | 0.97 | 1.21 | 1.18 | 1.75 | 1.17 | 1.68 |
| Noncanonical | 1.33 | 1.77 | 2.02 | 2.32 | 1.96 | 4.44 |
ERP Results
The ERP analysis was guided by previous research and began with an ANOVA performed on the 500 to 800 ms time interval associated with the parietal old/new effect. Mean amplitudes were calculated for this time interval, which were then averaged across groups of electrodes that formed four separate regions of interest [Left Parietal (LP), Right Parietal (RP), Left Occipital (LO), Right Occipital (RO)]. Each region of interest (ROI) consisted of a seven-electrode cluster. See Supplemental Figure 1 for the scalp topography of the four ROIs. The initial analysis consisted of a repeated measures ANOVA with the factors of Group (Young, Old), Condition (Canonical, Rotated, Noncanonical), Item Type (Hits, Correct Rejections), and ROI (LP, RP, LO, RO). Follow-up analyses were performed as necessary. Group grand average ERP waveforms for the three conditions can be seen in Supplemental Figure 2. In addition, nonparametric permutation tests were used to examine topographic differences in 50 ms intervals throughout the recording epoch. Typically used in imaging studies to compare voxels between two different conditions, nonparametric permutation tests can be useful in understanding temporal differences in high-density ERP data (Ally & Budson, 2007; Galan et al., 1997; Greenblatt & Pflieger, 2004; Karniski et al., 1994). Old/new scalp topographies for the younger and older adult groups can be seen in Figure 3 and Figure 5. The nonparametric comparison p-value maps can be seen in Figure 4 and Figure 6. Please note that all 50 ms topographic maps represent an average of 50 ms going forward from the labeled time, such that 0 ms is the average from 0 to 49 ms, etc. Only significant statistics will be discussed.
Figure 3.
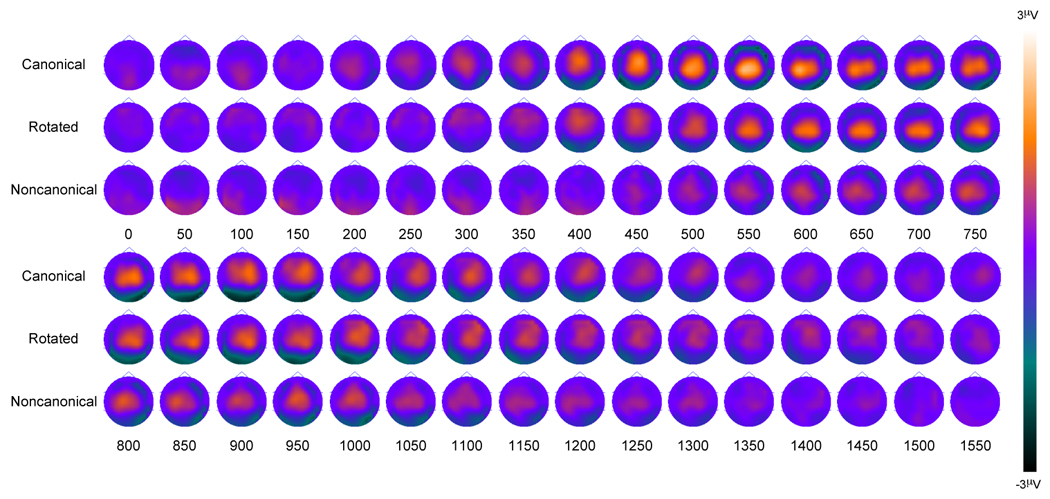
Younger Adult old/new scalp topography maps for the three conditions. Topographies are presented in 50 ms averages going forward.
Figure 5.
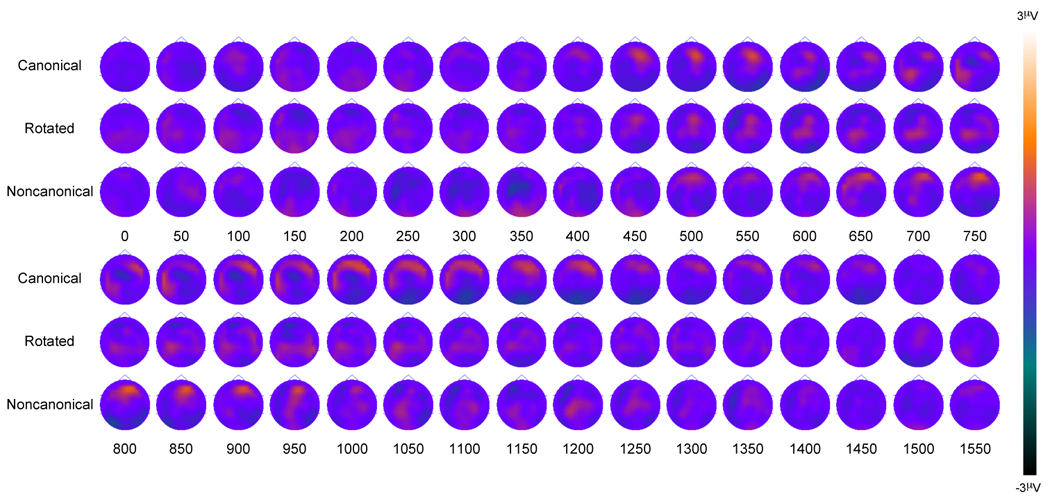
Older Adult old/new scalp topography maps for the three conditions. Topographies are presented in 50 ms averages going forward.
Figure 4.
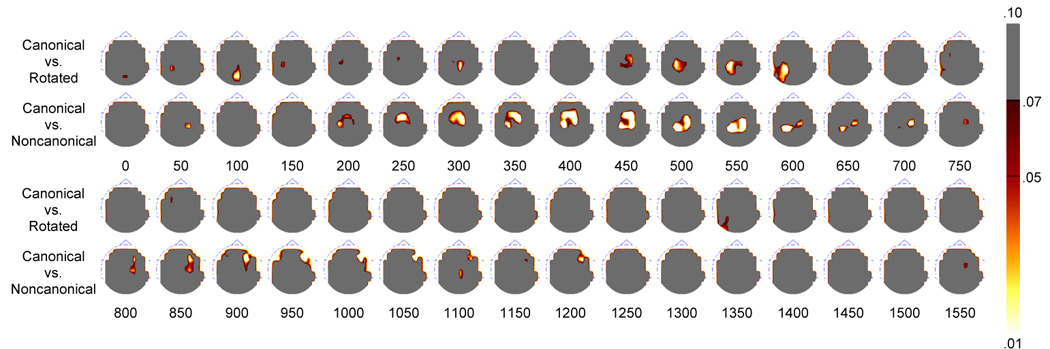
Younger Adult topographic p-value maps created by the nonparametric permutation test. Topographies are presented in 50 ms averages going forward.
Figure 6.
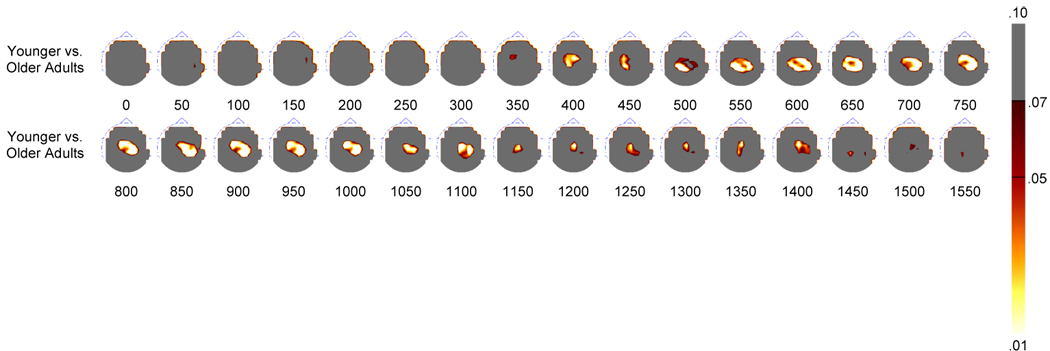
Topographic p-value maps created by the nonparametric permutation test between the younger and older adults. Topographies are presented in 50 ms averages going forward.
The between-subjects ANOVA revealed an interaction of Condition and Group [F(2, 86) = 7.02, p = .002], due to the fact that there was an effect of Condition on the activity at the parietal regions for the younger adults [F(2, 44) = 10.87, p < .001], but not for the older adults [F(2, 42) = 2.42, p = .119] (see Figure 7). An interaction of Item Type, Condition, and ROI [F(6, 258) = 4.57, p = .002] indicated that when collapsed across groups, hits were more positive than correct rejections at parietal regions for the canonical condition compared to the noncanonical condition [F(1, 44) = 5.76, p = .021] but not for the canonical condition compared to the rotated condition [F(1, 44) = 1.46, p = .233). Hits were also more positive than correct rejections at parietal regions for the rotated condition compared to the noncanonical condition [F(1, 44) = 4.61, p = .037]. An interaction of Item Type, ROI, and Group [F(3, 129) = 16.73, p < .001] indicated that hits were more positive than correct rejections at parietal regions for the younger adults [F(1, 22) = 74.02, p < .001], but not for the older adults [F(1, 21) = 1.90, p = .182]. Lastly, there was an interaction between Item Type and ROI [F(3, 129) = 53.00, p < .001]. Follow-up t-tests revealed that the magnitude of the old/new effect was significantly greater for the younger adults for all three conditions compared to the older adults: canonical [t(43) = 5.09, p < .001], rotated condition [t(43) = 2.44, p = .014], and noncanonical [t(43) = 2.56, p = .018].
Figure 7.
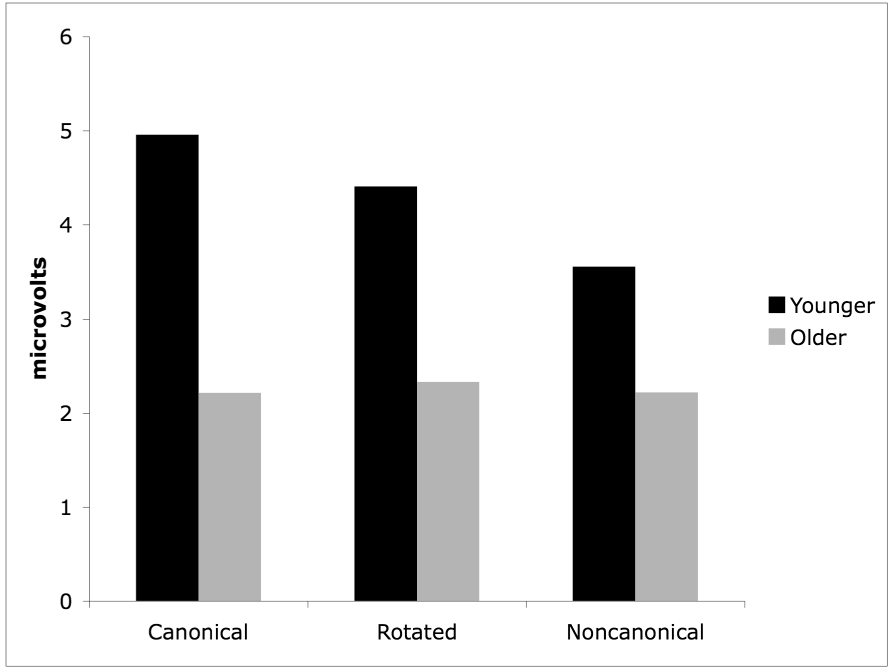
Mean amplitudes of the parietal old/new effect for the older and younger adults on all three conditions.
To follow-up on the interactions of Item Type X ROI X Group and Item Type X ROI, a follow-up ANOVA of Item Type, Condition, and ROI was performed for the younger adult group alone. This revealed an effect of Item Type [F(1, 22) = 4.74, p = .040], and an interaction of Condition, Item Type and ROI [F(6, 132) = 3.48, p = .016]. Follow-up t-tests revealed that the magnitude of the parietal old/new effect was greater for the canonical condition than for the rotated condition at the left parietal region [t(22) = 2.96, p = .007], but not at the right parietal region [t(22) <1]. The magnitude of the old/new effect was greater at both the left [t(22) = 3.90, p = .001] and right [t(22) = 3.32, p = .003] parietal regions for the canonical condition compared to the noncanonical condition. Finally, the effect was greater at the right parietal region for the rotated condition compared to the noncanonical condition [t(22) = 2.94, p = .008].
The scalp topographies and nonparametric analyses are also helpful in determining spatial and temporal differences in the parietal old/new effect between conditions for the younger adults. The topographies and p-value maps can be seen in Figure 3 and Figure 4. As can be seen in Figure 4, parietal activity is statistically more robust in the canonical condition compared to the rotated condition at three distinct periods in time. Early parieto-occipital differences are first seen at around 100 ms, due to a parietal positivity observed in the canonical but not rotated condition. Parietal differences are next seen at around 300 ms, where it appears that the parietal effect begins earlier for the canonical condition than for the rotated condition. Finally, more robust differences are seen from approximately 450 ms to 600 ms, due to the magnitude of the parietal old/new effect here being greater in the canonical condition compared to the rotated condition (consistent with the above ANOVAs). Nonparametric differences between the canonical and noncanonical conditions show robust fronto-parietal differences from approximately 200 ms to around 750 ms. Here the parietal effect also began earlier and was more robust in the canonical condition compared to the noncanonical condition. These within-subjects differences can be seen dynamically by viewing a video clip in the Supplemental Data Appendix. The video clip (Supplemental Figure 3) shows the electrical activity of the brain during successful retrieval of the canonical, rotated, and noncanonical test items.
Following-up the above ANOVA for the older adult group revealed no main effect of Item Type [F(1, 21) < 1]. There were significant interactions of Condition and ROI [F(6, 126) = 8.34, p < .001] and Item Type and ROI [F(3, 63) = 7.62, p = .001]. However, further post hoc analyses demonstrated that there were no significant old/new magnitude differences at parietal or occipital ROIs for the three conditions. Nonparametric comparisons between older and younger adults showed similar differences for each of the three comparisons. For this reason, the three conditions were averaged to create a composite condition for both groups. As can be seen in Figure 6, when the composite topographies were analyzed nonparametrically, significant parietal differences were seen throughout the recording epoch beginning at approximately 400 ms, consistent with and expanding upon the results obtained with the ANOVA.
Lesion Data
Table 1 and Table 2 provide mean discrimination, response bias, and median reaction time values for the lesion patients and the age-matched lesion control group. A repeated measures ANOVA was performed on the accuracy data (Pr) with the factors of Group (lesion patient, lesion control) and Condition (canonical, rotated, noncanonical). To examine effect size in order to assure that any negative results were not attributable to insufficient power, eta squared (η2) was also calculated. As expected the results revealed a robust effect of Condition [F(2, 12) = 81.59, p < .001, η2 = .931]. However, there was no effect of Group [F(1, 6) = .09, p = .773, η2 = .015] or interaction of Group and Condition [F(2, 12) = .67, p = .470, η2 = .101]. Consistent with the previous lesion data (Simons, et al., in press), these patients performed within the normal range of healthy age-matched control subjects. Figure 8 shows the behavioral data for the parietal lesion patients compared to the lesion controls as well as the younger and older adults for each of the conditions.
Figure 8.
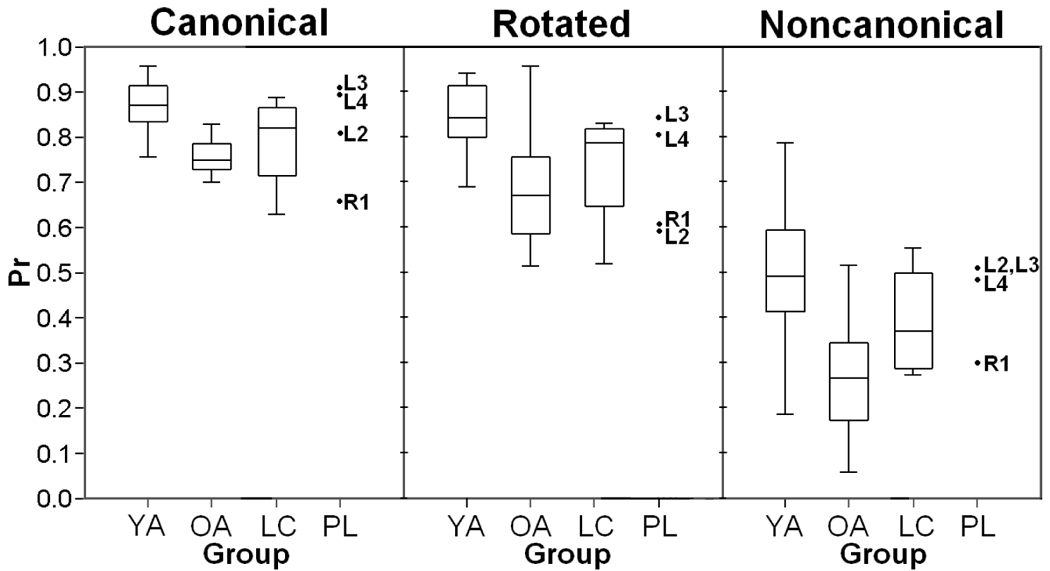
Boxplots showing the accuracy data for the parietal lesion patients and the younger and older adults for the three conditions. Lesion patient data are plotted individually. (YA – Younger Adults; OA – Older Adults; LC – Lesion Controls; PL – Lesion Patients).
We also had the opportunity to acquire ERP data from one patient with a right lateral parietal lesion (patient R1). The topographic data from patient R1 are displayed in Figure 9, where it can be seen that there was a distinct absence of right parietal activity. This was confirmed using Crawford and Howell's (1998) modified t-test for comparing an individual score with a small control group (20 subjects from the younger adult group and 20 subjects from the older adult group, mean age 48.4 years, for comparison with patient R1, aged 49). The t-test revealed that parietal activity during the 500 to 800 ms time interval was significantly diminished in patient R1 compared to the controls [t(39) = 2.044, p = .047]. Of particular interest, patient R1 demonstrated significantly enhanced frontal activity during the 500 to 800 ms time interval associated with recollection compared to the control group [t(39) = 2.335, p = .024]. These frontal differences will be discussed in detail below.
Figure 9.
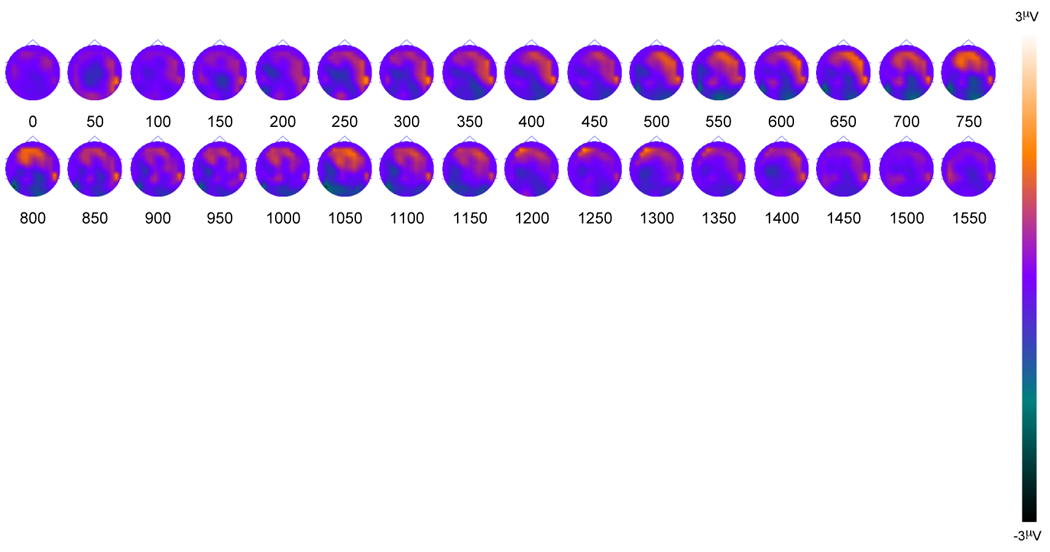
Old/new scalp topography maps for parietal lesion patient R1, collapsed across the three conditions. Topographies are presented in 50 ms averages going forward Supplementary Table 1. Neuropsychological test data for the parietal lesion patients and controls.
Discussion
The present study provided several interesting findings regarding the contribution of the parietal lobes during memory retrieval. First, to assess the validity of the episodic buffer hypothesis, we analyzed the duration of the parietal old/new effect. The episodic buffer hypothesis posits that parietal cortex may dynamically represent or hold retrieved information in a form accessible to executive decision-related processing (Baddeley, 2000; Wagner et al., 2005). Therefore, we predicted that the parietal effect would be longest in duration for the noncanonical condition, as information would likely need to be held for a longer period of time for a memorial decision to be made, and shortest in duration for the canonical condition, which likely requires a shorter period of time for a memorial decision to be made. The analyses of our data, however, yielded the opposite result: the parietal old/new effect was longer in duration for the canonical condition compared to the rotated and noncanonical conditions. Thus, these data are in apparent conflict with the episodic buffer hypothesis of parietal function in recollection.
Second, we predicted that as parietal lobe activity increased on a memory retrieval task, older adults would show increasing differences in the parietal old/new effect compared to the younger adults. As can be seen in Figure 7, this prediction was borne out, although in a slightly different way than expected. Specifically, we predicted that older adults would show the least difference in parietal old/new amplitude when compared to younger adults on the canonical condition, the greatest difference in parietal old/new amplitude compared to the younger adults for the noncanonical condition, and the difference in parietal old/new amplitude for the rotated items would fall somewhere between the canonical and noncanonical conditions. However, because the younger adults showed the greatest activity in the canonical condition and the least activity in the noncanonical condition, the results showed the opposite pattern than originally predicted. The greatest difference in the parietal old/new effect between the groups was in the canonical condition, the least difference was in the noncanonical, and the rotated condition fell in between the canonical and noncanonical conditions. And, consistent with our original predictions, the older adults showed significantly diminished parietal activity compared to the younger adults when collapsed over all three conditions.
Third, the lesion patients in the current investigation performed similar to healthy age and gender-matched control subjects on all three conditions. Consistent with Simons et al. (in press), these findings suggest that parietal cortex is not necessary for accurate remembering, although our ERP data suggests that it plays some important supportive role. In their initial investigation of memory retrieval in patients with lateral parietal lesions, Simons et al. showed that performance was not impaired compared to controls on a source recollection test that elicited parietal activation on fMRI. In addition to the behavioral data in the present study, we also had the opportunity to acquire ERP data from a right hemisphere lesion patient (subject R1). Although substantial conclusions cannot be drawn from a single subject, the old/new scalp topographies provide some preliminary data for speculation. As expected, there was diminished parietal activity, particularly on the right side. However, there was significant bilateral frontal activity, perhaps reflecting compensatory mechanisms or strategies. This bifrontal activity is particularly interesting in light of anecdotal post-experiment interview data acquired from the lesion patients. Patient R1 stated that she has developed numerous strategies to compensate for her memory since tumor resection. These strategies included looking for distinctive colors in a picture and determining whether study items are animate or inanimate objects. Perhaps the utilization of these strategies is responsible for the frontal activity seen in the scalp topographies. Additional anecdotal evidence from parietal lesion patients in the current study and others may also be informative. Two other subjects with parietal lobe lesions in the current study, as well as several of those from Simons et al. (in press), reported low confidence in their overall memory abilities, and/or stated that their memories lacked richness. Similarly, a parietal lesion patient (S.M.) in a recent study by Davidson et al. (in press) reported low confidence in her memories and that they lacked episodic richness or vividness.
Combining (1) these anecdotal data, (2) the patients’ normal memorial accuracy, and (3) our ERP data in young subjects showing the greatest parietal activity in the canonical condition leads us to speculate as to what role parietal cortex may play in memory retrieval. We propose that parietal activity may index the magnitude of the subjective experience occurring at recognition. Hassabis et al. (2007) suggested that recollection entails a number of component processes, including both objective and subjective processes. The fact that parietal lesion patients are relatively unimpaired on tests of recognition, suggests that parietal activity may support a subjective component of recollection rather than an objective one. Consistent with this view, Chua et al. (2006) showed that activity in medial and lateral parietal regions was greater during memory assessment compared to recognition accuracy.
Impaired subjective recollection with parietal damage has also found support from studies of autobiographical memory. Davidson et al. (in press) recently showed that patients with unilateral parietal lesions were able to freely recall memories from their past, but that their memories were significantly impoverished of details compared to control subjects. Findings from Berryhill et al. (2007) also support this idea by showing that two patients with bilateral parietal lesions could recall memories from their past, but only with severely diminished content. In the same study, Berryhill and colleagues (2007) demonstrated that the patients were not impaired on tasks of global mental imagery, suggesting that mental imagery is not the same as the subjective episodic experience associated with recollection.
It has been argued that perhaps parietal cortex provides a critical link between consciousness and cognition (Naghavi & Nyberg, 2005). Recent research has reported that activity in the posterior precuneus appears to correlate with self-reflection in episodic and autobiographical retrieval (Cavanna, 2007; Cavanna & Trimble, 2006). Further, Simons et al. (2005) observed precuneus activity that was greater during recollection of self-generated context details than those derived from the external world, and Hassabis et al. (2007) showed that activation in parietal regions could distinguish real memories from contrived imaginary ones. The idea of self-reflective awareness being important in memorial recollection is of course not new. Ebbinghaus (1885) and James (1890) both stated that for proper memory to occur, one must have knowledge of an event with additional recognition or consciousness that the event has been personally experienced. More modern connections can be made to Tulving’s autonoetic consciousness (self-knowing) and mental time travel in episodic memory (Tulving, 1984, 1985, 2005). The data provided by the current study and Simons et al. (in press) are consistent with the idea that this sense of personal experience or self-knowing memory discussed by Ebbinghaus, James, and Tulving becomes disrupted with parietal damage. If this idea is correct, we could further speculate that the parietal old/new effect may be the neural correlate of one’s subjective episodic experience.
This hypothesis is further supported by the current ERP data. The magnitude of the parietal old/new effect in our younger adults was greatest in the canonical condition, where the test items were an exact match to the study items, compared to the rotated and noncanonical conditions, and also greater for the rotated condition compared to the noncanonical condition. We speculate that due to the exact match of study and test items during recognition in the canonical condition, the subjective experience of recollection of the study episode would be the most rich relative to the rotated and noncanonical conditions. This speculation is consistent with prior studies showing greater magnitude of the parietal effect when recognition is accompanied by the vivid recollection of study details (Duzel et al., 1997; Wolk et al., 2006; Woodruff, Hayama, & Rugg, 2006), and when study and test stimuli are an exact perceptual match (Ally & Budson, 2007; Schloerscheidt & Rugg, 2004). This idea is further supported by ERP (Goldmann et al., 2003) and fMRI (Wheeler & Buckner, 2003) studies showing that false recognition elicits similar parietal activity to correct recognition. These studies suggest that parietal cortex is involved in the subjective experience of a memory despite whether or not the item was previously encountered.
The ERP data from older adults also lends support to the hypothesis that the parietal old/new indexes the amount of subjective episodic experience during recollection. Similar to numerous previous studies of aging (Ally et al., 2008; Daselaar et al., 2006; Fjell, et al., 2005; Joyce, et al., 1998; Morcom & Rugg, 2004; Nielson-Bohlman & Knight, 1995; Rugg, et al., 1997; Senkfor & Van Petten, 1998; Swick & Knight, 1997), the older adults in the current study demonstrated significantly diminished parietal activity compared to young adults. If the hypothesis of subjective episodic experience were true, older adults likely experience diminished subjective recollection. This hypothesis is supported by previous studies showing that older adults lack self-related episodic details when recollecting a past event compared to younger adults (Addis, Wong, & Schacter, in press), and that they exhibit decreased estimates of recollection compared to younger adults (Howard, et al., 2006; Jennings & Jacoby, 1993, 1997; Prull et al., 2006). For these reasons, the subjective aspects of recollection may be similar between older adults and patients with parietal lesions. Indeed, research investigating healthy older adults has reported neural degeneration, (Good et al., 2001; Sowell et al., 2003) cortical volume loss (Kochunov, Mangin, Coyle, Lancaster, Thompson, Riviere, et al., 2005; Rettmann, Kraut, Prince, & Resnick, 2006; Resnick, Goldszal, Davatzikos, Golski, Kraut, Metter, et al., 2000; Resnick. Pham, Kraut, Zonderman, & Davatzikos, 2003), and Alzheimer pathology in parietal regions (McKee, Au, Cabral, Kowall, Seshadri, Kubilus, Drake, Wolf, 2006), as well as poor functional connectivity between medial temporal regions and parietal cortex during memory tasks (Damoiseaux, Beckmann, Arigita, Barkhof, Scheltens, Stam, Smith, Rombouts, in press; Daselaar, Fleck, Dobbins, Madden, Cabeza, 2006) in healthy older adults. Future studies can help to determine what role subjective recollection may play in both confidence and accuracy (see Chua et al., 2006).
An alternative explanation that should be considered as to the role of parietal cortex in recollection is awareness or attention to internal memorial representations (Wagner et al., 2005). Rugg and Henson (2002) and others have suggested that posterior parietal cortex may contribute to shifting attention to, or maintaining attention on, internally generated memory representation. Providing some support for this argument, the patients with bilateral parietal lesions in Berryhill et al. (2007) demonstrated simultanagnosia; a neurological disorder where patients can perceive objects in their visual field, but can only attend to one at a time. Berryhill and colleagues suggested that parietal damage might make it impossible for patients to attend to or report on an entire memory, focusing on only a single aspect. Analogous to the way patients with visual neglect following parietal damage can attend to items when pointed out by an examiner, the patients in Berryhill et al. could remember or report specific memorial details when given directed probes by the examiner. Based on these findings, Berryhill and colleagues proposed a variation of Wagner et al.’s (2005) attention to internal memorial representations hypothesis, drawing on distinctions between top-down and bottom-up attention (Corbetta & Shulman, 2002). They suggested that their patient data could be attributed to a deficit in the capture of bottom-up attention by internal memorial representations. It should be noted, however, that a deficit that can be improved by the provision of external probes could equally be considered to reflect impaired top-down control because the patients are unable spontaneously to implement top-down strategies to guide performance in the absence of the external probes (Desimone & Duncan, 1995; Duncan, Emslie, Williams, Johnson, & Freer, 1996).
It will be interesting to see how the top-down vs. bottom-up debate unfolds, but either way, we would argue that the attention to memorial representation hypothesis does not fit the present data as well as our proposed episodic experience hypothesis. If successful recollection were to cause attention to be disengaged from the environment to focus on the contents of retrieval, we would expect that the noncanonical and rotated conditions would require greater attentional demands than the canonical condition, producing greater parietal ERP activity. Yet, the current findings yielded the opposite result. Parietal activity was greatest in magnitude and longest in duration for the canonical condition. Further, our analyses showed that increasing cognitive demands required to mentally rotate or transform an object at test resulted in delayed onset of the parietal effect, possibly reflecting load effects. However, the parietal effect also ended earlier in these conditions. If the parietal old/new effect represented attentional processes, we would once again expect activity to be longest in duration in the noncanonical condition, as in the episodic buffer hypothesis. In addition, based on the reaction time data, we would expect the parietal effect to end later in the epoch for the noncanonical condition if the parietal effect was attributable to attentional processes. While we agree that retrieved items likely demand attentional resources for memorial decisions to be made, it is of course unlikely that more easily recognized items would capture the greatest amount of attention.
In conclusion, results from the present investigation allowed us to attain several insights into the possible contributions of parietal cortex in memory retrieval. First, our younger adult ERP data showed that the duration of the parietal old/new effect is not consistent with the episodic buffer hypothesis. In contrast to recent hypotheses focusing on the amount of information retrieved at test, our data suggest that the parietal old/new effect may reflect subjective aspects of recollection. In support of this hypothesis, debriefing and anecdotal reports from the patients with parietal lesions from the current study and others suggest that parietal damage may lead to a diminished sense that an event has been personally experienced. Consistent with previous behavioral research demonstrating that healthy older adults experience decreased recollection, the older adults in the current study showed significantly diminished parietal activity compared to the younger adults. Lastly, we speculate that the subjective episodic experience during memory retrieval discussed by Ebbinghaus (1885) and James (1890), and the experience of mental time travel described by Tulving (1985), may be actualized within the parietal cortex.
Supplementary Material
Video clip showing the electrical activity of the brain during successful retrieval of the canonical, rotated, and noncanonical test items. The view is of the back of the head.
Neuropsychological test data for the parietal lesion patients and controls.
Positions of the 128 electrodes on the Bio-Semi ActiveTwo headcap with the four posterior regions of interest shown.
Grand average hit and correct rejection ERP waveforms for both groups for the three conditions. Waveforms on the left are from the left parietal region of interest, and waveforms on the right are from the right parietal region of interest.
Acknowledgments
This research was supported by National Institute on Aging grants F32 AG027632 (BAA), R01 AG025815 (AEB), and P30 AG13846 (AEB). This material is also the result of work supported with resources and the use of facilities at the Edith Nourse Rogers Memorial Veterans Hospital in Bedford, MA. The authors would like to thank Dr. Neil Kowall for his helpful insights into the workings of the parietal cortex, and the three anonymous reviewers for their thoughtful comments and suggestions for the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Addis DR, Wong AT, Schacter DL. Age-related changes in the episodic simulation of future events. Psychological Science. doi: 10.1111/j.1467-9280.2008.02043.x. (In press). [DOI] [PubMed] [Google Scholar]
- Adjutant General’s Office. Army Individual Test Battery: Manual of Directions and Scoring. Washington, D.C.: War Department; 1944. [Google Scholar]
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampalanterioir thalamic axis. Behavioral and Brain Sciences. 1999;22:425–489. [PubMed] [Google Scholar]
- Ally BA, Budson AE. The worth of pictures: Using high density event-related potentials to understand the memorial power of pictures and the dynamics of recognition memory. NeuroImage. 2007;35:378–395. doi: 10.1016/j.neuroimage.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Waring JD, Beth EH, McKeever JD, Milberg WP, Budson AE. Aging memory for pictures: Using high-density event-related potentials to understand the effect of age on the picture superiority effect. Neuropsychologia. 2008;46:287–297. doi: 10.1016/j.neuropsychologia.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddeley AD. The episodic buffer: a new component of working memory? Trends Cognitive Science. 2000;4:417–423. doi: 10.1016/s1364-6613(00)01538-2. [DOI] [PubMed] [Google Scholar]
- Berryhill ME, Phuong L, Picasso L, Cabeza R, Olson IR. Parietal lobe and episodic memory: Bilateral damage causes impaired free recall of autobiographical memory. Journal of Neuroscience. 2007;27:14415–14423. doi: 10.1523/JNEUROSCI.4163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budson AE, Wolk DA, Chong H, Waring JD. Episodic memory in Alzheimer's disease: separating response bias from discrimination. Neuropsychologia. 2006;44:2222–2232. doi: 10.1016/j.neuropsychologia.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage. 2002;17:1394–1402. doi: 10.1006/nimg.2002.1280. [DOI] [PubMed] [Google Scholar]
- Cavanna AE. The precuneuos and consciousness. CNS Sprect. 2007;12:545–552. doi: 10.1017/s1092852900021295. [DOI] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Chua EF, Schacter DL, Rand-Giovannetti E, Sperling RA. Understanding metamemory: Neural correlates of the cognitive process and subjective level of confidence in recognition memory. Neuroimage. 2006;29:1150–1160. doi: 10.1016/j.neuroimage.2005.09.058. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crawford JR, Howell DC. Regression equations in clinical neuropsychology: An evaluation of statistical methods for comparing predicted and obtained scores. Journal of Clinical and Experimental Neuropsychology. 1998;20:755–762. doi: 10.1076/jcen.20.5.755.1132. [DOI] [PubMed] [Google Scholar]
- Curran T, DeBuse C, Woroch B, Hirshman E. Combined pharmacological and electrophysiological dissociation of familiarity and recollection. J Neurosci. 2006;26:1979–1985. doi: 10.1523/JNEUROSCI.5370-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: An event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Beckmann CF, Arigita EJ, Barkhof F, Scheltens P, Stam CJ, Smith SM, Rombouts SA. Reduced resting-state brain activity in the “default network” in normal aging. Cerebral Cortex. doi: 10.1093/cercor/bhm207. (in press). [DOI] [PubMed] [Google Scholar]
- Davidson PSR, Anaki D, Ciaramelli E, Cohn M, Kim A, Levine B, Murphy KJ, Troyer AK, Moscovitch M. Does parietal cortex support episodic memory? A review of patient data. Neuropsychologia. doi: 10.1016/j.neuropsychologia.2008.01.011. (in press, current issue). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Dien J. Addressing misallocation of variance in principal components analysis of event-related potentials. Brain Topogr. 1998;11:43–55. doi: 10.1023/a:1022218503558. [DOI] [PubMed] [Google Scholar]
- Duncan J, Emslie H, Williams P, Johnson R, Freer C. Intelligence and the frontal lobe: The organization of goal-directed behavior. Cognitive Psychology. 1996;30:257–303. doi: 10.1006/cogp.1996.0008. [DOI] [PubMed] [Google Scholar]
- Duzel E, Yonelinas AP, Mangun GR, Heinze HJ, Tulving E. Event-related brain potential correlates of two states of conscious awareness in memory. Proc Natl Acad Sci. 1997;94:5973–5978. doi: 10.1073/pnas.94.11.5973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbinghaus H. On Memory. New York: Dover Edition; 18851964. (original work published in 1885). [Google Scholar]
- Eldridge LL, Knowlton BT, Furmanski CS, Bookheimer SY, Engel SA. Remembering episodes: A selective role for the hippocampus during retrieval. Nature Neuroscience. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- Faillenot I, Toni I, Decety J, Gregoire MC, Jeannerod M. Visual pathways for object-oriented action and object recognition: functional anatomy with PET. Cereb Cortex. 1997;7:77–85. doi: 10.1093/cercor/7.1.77. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Reinvang I. Age-differences in verbal recognition memory revealed by ERP. Clin EEG Neurosci. 2005;36:176–187. doi: 10.1177/155005940503600308. [DOI] [PubMed] [Google Scholar]
- Fletcher PC, Frith CD, Baker SC, Shallice T, Frackowiak R, S J, Dolan RJ. The mind’s eye – Precuneus activation in memory-related imagery. NeuroImage. 1995;2:195–200. doi: 10.1006/nimg.1995.1025. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State". A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Friedman D, Johnson R., Jr Event-related potential (erp) studies of memory encoding and retrieval: A selective review. Microsc Res Tech. 2000;51:6–28. doi: 10.1002/1097-0029(20001001)51:1<6::AID-JEMT2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Galan L, Biscay R, Rodriguez JL, Perez-Abalo MC, Rodriguez R. Testing topographical differences between event-related brain potentials by using non-parametric combinations of permutation tests. Electroencephalography and Clinical Neurophysiology. 1997;102:240–247. doi: 10.1016/s0013-4694(96)95155-3. [DOI] [PubMed] [Google Scholar]
- Goldmann RE, Sullivan AL, Droller DB, Rugg MD, Curran T, Holcomb PJ, Schacter DL, Daffner KR, Budson AE. Late frontal brain potentials distinguish true and false recognition. Neuroreport. 2003;14:1717–1720. doi: 10.1097/00001756-200309150-00012. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grady CL, Bernstein LJ, Beig S, Siegenthaler AL. The effects of encoding task on age-related differences in the functional neuroanatomy of face memory. Psychol Aging. 2002;17:7–23. doi: 10.1037//0882-7974.17.1.7. [DOI] [PubMed] [Google Scholar]
- Greenblatt RE, Pflieger ME. Randomization-based hypothesis testing from event-related data. Brain Topogr. 2004;16:225–232. doi: 10.1023/b:brat.0000032856.48286.18. [DOI] [PubMed] [Google Scholar]
- Hassabis D, Kumaran D, Maguire EA. Using imagination to understand the neural basis of episodic memory. Journal of Neuroscience. 2007;27:14365–14374. doi: 10.1523/JNEUROSCI.4549-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henson RN, Rugg MD, Shallice T, Josephs O, Dolan RJ. Recollection and familiarity in recognition memory: An event-related functional magnetic resonance imaging study. J Neurosci. 1999;19:3962–3972. doi: 10.1523/JNEUROSCI.19-10-03962.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: Evidence from modeling and receiver operating characteristic curves. Psychol Aging. 2006;21:96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. New York: Dover Publications; 1890. [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. Automatic versus intentional uses of memory: Aging, attention, and control. Psychol Aging. 1993;8:283–293. doi: 10.1037//0882-7974.8.2.283. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: Telling effects of repetition. Psychol Aging. 1997;12:352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Joyce CA, Paller KA, McIsaac HK, Kutas M. Memory changes with normal aging: Behavioral and electrophysiological measures. Psychophysiology. 1998;35:669–678. [PubMed] [Google Scholar]
- Kahn I, Davachi L, Wagner AD. Functional-neuroanatomic correlates of recollection: implications for models of recognition memory. J Neurosci. 2004;24:4172–4180. doi: 10.1523/JNEUROSCI.0624-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniski W, Blair RC, Snider AD. An exact statistical method for comparing topographic maps, with any number of subjects and electrodes. Brain Topogr. 1994;6:203–210. doi: 10.1007/BF01187710. [DOI] [PubMed] [Google Scholar]
- Kochunov P, Mangin JF, Coyle T, Lancaster J, Thompson P, Riviere D, Cointepas Y, Regis J, Schlosser A, Royall DR, Zilles K, Mazziotta J, Toga A, Fox PT. Age-related morphology trends of cortical sulci. Human Brain Mapping. 2005;26:210–220. doi: 10.1002/hbm.20198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi S, Wheeler ME, Donaldson DI, Buckner RL. Neural correlates of episodic retrieval success. Neuroimage. 2000;12:276–286. doi: 10.1006/nimg.2000.0614. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Alpert NM, Thompson WL, Chabris CF, Rauch SL, Anderson AK. Identifying objects seen from different viewpoints: A P FT investigation. Brain. 1994;117:1055–1071. doi: 10.1093/brain/117.5.1055. [DOI] [PubMed] [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: Shortened versions for use in Alzheimer's disease. J Gerontol. 1992;47:P154–P158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- McKee AC, Au R, Cabral HJ, Kowall NW, Seshadri S, Kubilus CA, Drake J, Wolf PA. Visual association pathology in preclinical Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 2006;65:621–630. doi: 10.1097/00005072-200606000-00010. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol. 1992;49:1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morcom AM, Rugg MD. Effects of age on retrieval cue processing as revealed by ERPs. Neuropsychologia. 2004;42:1525–1542. doi: 10.1016/j.neuropsychologia.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part i. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Naghavi HR, Nyberg L. Common fronto-parietal activity in attention, memory, and consciousness: shared demands on integration? Conscious Cogn. 2005;14:390–425. doi: 10.1016/j.concog.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Nielsen-Bohlman L, Knight RT. Prefrontal alterations during memory processing in aging. Cereb Cortex. 1995;5:541–549. doi: 10.1093/cercor/5.6.541. [DOI] [PubMed] [Google Scholar]
- Prull MW, Dawes LL, Martin AM, 3rd, Rosenberg HF, Light LL. Recollection and familiarity in recognition memory: Adult age differences and neuropsychological test correlates. Psychol Aging. 2006;21:107–118. doi: 10.1037/0882-7974.21.1.107. [DOI] [PubMed] [Google Scholar]
- Ravizza SM, Delgado MR, Chein JM, Becker JT, Fiez JA. Functional dissociations within the inferior parietal cortex in verbal working memory. Neuroimage. 2004;22:562–573. doi: 10.1016/j.neuroimage.2004.01.039. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, Bryan RN, Zonderman AB. One-year changes in MRI brain volumes in older adults. Cerebral Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Resnick SM, Pham DL, Kraut MA, Zonderman AB, Davatzikos C. Longitudinal magnetic resonance imaging studies of older adults: A shrinking brain. Journal of Neuroscience. 2003;23:3295–3301. doi: 10.1523/JNEUROSCI.23-08-03295.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettmann ME, Kraut MA, Prince JL, Resnick SM. Cross-sectional and longitudinal analyses of anatomical sulcal changes associated with aging. Cerebral Cortex. 2006;16:1584–1594. doi: 10.1093/cercor/bhj095. [DOI] [PubMed] [Google Scholar]
- Rockland KS, Van Hoesen GW. Some temporal and parietal cortical connections converge in CA1 of the primate hippocampus. Cerebral Cortex. 1999;9:232–237. doi: 10.1093/cercor/9.3.232. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. Trends in Cognitive Neuroscience. 2007;11:251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Henson RNA. Episodic memory retrieval: an (event-related) functional neuroimaging perspective. In: Parker AE, Wilding EL, Bussey T, editors. The Cognitive Neuroscience of Memory Encoding and Retrieval. New York: Psychology Press; 2002. pp. 3–37. [Google Scholar]
- Rugg MD, Mark RE, Gilchrist J, Roberts RC. ERP repetition effects in indirect and direct tasks: effects of age and interim lag. Psychophysiology. 1997;34:572–586. doi: 10.1111/j.1469-8986.1997.tb01744.x. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Otten LJ, Henson RNA. The neural basis of episodic memory: Evidence from functional neuroimaging. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 2002;357:1097–1110. doi: 10.1098/rstb.2002.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloerscheidt AM, Rugg MD. The impact of change in stimulus format on the electrophysiological indices of recognition. Neuropsychologia. 2004;42:451–466. doi: 10.1016/j.neuropsychologia.2003.08.010. [DOI] [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what? An event-related potential investigation of source and item memory. J Exp Psychol Learn Mem Cogn. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24:10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepard S, Metzler D. Mental rotation: effects of dimensionality of objects and type of task. J Exp Psychol Hum Percept Perform. 1988;14:3–11. [PubMed] [Google Scholar]
- Simons JS, Gilbert SJ, Owen AM, Fletcher PC, Burgess PW. Distinct roles for lateral and medial anterior prefrontal cortex in contextual recollection. J Neurophysiol. 2005;94:813–820. doi: 10.1152/jn.01200.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Peers PV, Hwang DW, Ally BA, Fletcher PC, Budson AE. Is the parietal lobe necessary for recollection in humans? Neuropsychologia. doi: 10.1016/j.neuropsychologia.2007.07.024. (in press). [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal interactions in long-term memory. Nature Reviews Neuroscience. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. J Exp Psychol Gen. 1988;117:34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Thompson PM, Welcome SE, Henkenius AL, Toga AW. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- Sugio T, Inui T, Matsuo K, Matsuzawa M, Glover GH, Nakai T. The role of the posterior parietal cortex in human object recognition: a functional magnetic resonance imaging study. Neurosci Lett. 1999;276:45–48. doi: 10.1016/s0304-3940(99)00788-0. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. The Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Swick D, Knight RT. Event-related potentials differentiate the effects of aging on word and nonword repetition in explicit and implicit memory tasks. J Exp Psychol Learn Mem Cogn. 1997;23(1):123–142. doi: 10.1037//0278-7393.23.1.123. [DOI] [PubMed] [Google Scholar]
- Trott CT, Friedman D, Ritter W, Fabiani M, Snodgrass JG. Episodic priming and memory for temporal source: event related potentials reveal age-related differences in prefrontal functioning. Psychol Aging. 1999;14:390–413. doi: 10.1037//0882-7974.14.3.390. [DOI] [PubMed] [Google Scholar]
- Tulving E. Précis of elements of episodic memory. The Behavioral and Brain Sciences. 1984;7:223–268. [Google Scholar]
- Tulving E. Memory and consciousness. Canadian Psychology. 1985;26:1–12. [Google Scholar]
- Tulving E. In: Episodic memory and autonoesis: Uniquely human? Terrace HS, Metcalfe J, editors. NewYork, NY: Oxford University Press; 2005. pp. 4–56. The Missing Link in Cognition. [Google Scholar]
- Vaidya CJ, Zhao M, Desmond JE, Gabrieli JD. Evidence for cortical encoding specificity in episodic memory: memory-induced re-activation of picture processing areas. Neuropsychologia. 2002;40:2136–2143. doi: 10.1016/s0028-3932(02)00053-2. [DOI] [PubMed] [Google Scholar]
- Vilberg KL, Moosavi RF, Rugg MD. The relationship between electrophysiological correlates of recollection and amount of information retrieved. Brain Res. 2006;1122:161–170. doi: 10.1016/j.brainres.2006.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilberg KL, Rugg MD. Dissociation of the neural correlates of recollection and familiarity: Further evidence from fMRI. Neuropsychologia. 2007;45:2216–2225. doi: 10.1016/j.neuropsychologia.2007.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AD, Shannon BJ, Kahn I, Buckner RL. Parietal lobe contributions to episodic memory retrieval. Trends Cogn Sci. 2005;9:445–453. doi: 10.1016/j.tics.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Warrington EK, Taylor AM. The contribution of the right parietal lobe to object recognition. Cortex. 1973;9:152–164. doi: 10.1016/s0010-9452(73)80024-3. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional dissociation among components of remembering: Control, perceived oldness, and content. J Neurosci. 2003;23:3869–3880. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. NeuroImage. 2004;21:1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wilding EL, Rugg MD. An event-related potential study of recognition memory with and without retrieval of source. Brain. 1996;119:889–905. doi: 10.1093/brain/119.3.889. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Hayama H, Rugg MD. Electrophysiological dissociation of the neural correlates of recollection and familiarity. Cognitive Brain Research. 2006;1100:125–135. doi: 10.1016/j.brainres.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Woodruff CC, Johnson J, Uncapher M, Rugg M. Content-specificity of the neural correlates of recollection. Neuropsychologia. 2005;43:1022–1032. doi: 10.1016/j.neuropsychologia.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Lygizos M, Mandu-Sen N, Holcomb PJ, Daffner KR, Budson AE. ERP correlates of recognition memory: Effects of retention interval and false alarms. Brain Research. 2006;1096:148–162. doi: 10.1016/j.brainres.2006.04.050. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Otten LJ, Shaw KN, Rugg MD. Separating the brain regions involved in recollection and familiarity in recognition memory. J Neurosci. 2005;25:3002–3008. doi: 10.1523/JNEUROSCI.5295-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video clip showing the electrical activity of the brain during successful retrieval of the canonical, rotated, and noncanonical test items. The view is of the back of the head.
Neuropsychological test data for the parietal lesion patients and controls.
Positions of the 128 electrodes on the Bio-Semi ActiveTwo headcap with the four posterior regions of interest shown.
Grand average hit and correct rejection ERP waveforms for both groups for the three conditions. Waveforms on the left are from the left parietal region of interest, and waveforms on the right are from the right parietal region of interest.


