Summary
Objective
To determine which cell fraction(s) of Streptococcus mitis biovar 1 serve as the best source of antigens recognized by salivary SIgA antibodies in infants.
Design
Whole cells of 38 reference and wild-type isolates of Streptococcus mitis, S. oralis, S. gordonii, Enterococcus casseliflavus, and E. faecalis were fractionated into cell walls CW), protease-treated cell walls (PTCW), cell membranes (CM) and cell protein (CP). Whole cells and these fractions were tested for binding by rabbit anti-S. mitis SK145 and anti-S. oralis SK100 sera, and also by salivary SIgA antibodies from infants and adults.
Results
Anti-SK145 and anti-SK100 sera bound whole cells and fractions of all strains of S. mitis and S. oralis variably. Cluster analysis of antibody binding data placed the strains into S. mitis, S. oralis and ‘Non-S. mitis/non-S. oralis’ clusters. Antigens from CW and CM best discriminated S. mitis from S. oralis. CM bound the most infant salivary SIgA antibody and PTCW bound the least. In contrast, adult salivary SIgA antibody bound all of the cell fractions and at higher levels.
Conclusions
Presumably the relatively short period of immune stimulation and immunological immaturity in infants, in contrast to adults, result in low levels of salivary SIgA antibody that preferentially bind CM of S. mitis but not PTCW. By utilizing isolated cell walls and membranes as sources of antigens for proteomics it may be possible to identify antigens common to oral streptococci and dissect the fine specificity of salivary SIgA antibodies induced by oral colonization by S. mitis.
Keywords: Streptococcus mitis, Streptococcus oralis, Bacterial fractions, Antibody binding, Salivary SIgA antibodies
INTRODUCTION
Viridans streptococci are among the first bacteria to colonize the human mouth after birth and generally the predominant colonizing strains are S. mitis and S. oralis.1,2 Considerable diversity exists among the colonizing strains of S. mitis and, in addition, these strains exhibit clonal turnover and replacement.3-6 As early as a few days after birth SIgA antibodies reactive with these colonizing streptococcus strains can be detected in saliva.7 However, whether the SIgA antibody response contains strain-specific antibodies that might pressure clonal turnover and replacement8 in addition to a general response to colonizing streptococci at the genus and/or species7 level remain unclear. One of the major difficulties in attempting to dissect the SIgA antibody response to S. mitis is that relatively little is known about its antigenic structure9 apart from the likelihood that strains carry antigens similar to those of other streptococci. Our limited knowledge about the nature of the antigens of these oral streptococci and the strains that stimulate antibody production7 is compounded by the similarities between S. mitis and S. oralis. It is sometimes difficult to distinguish between strains of these two species using phenotypic tests, because they have closely similar characteristics. However, using whole cell ELISA and antisera against S. mitis biovar 1 (SK145) Kirchherr et al.8 demonstrated that 79% of forty-eight randomly-selected infant strains of S. mitis biovar 1 bound the same amount of rabbit IgG antibody as the homologous strain SK145, suggesting the presence of significant common antigens. In addition, these strains also bound low levels of rabbit antibody to S. oralis strain SK100, showing antigenic similarities between S. mitis biovar 1 and S. oralis. Consequently, given the antigenic similarities between strains of these species, selecting strains to demonstrate specific binding of infants’ salivary SIgA antibody to S. mitis is difficult. Although one could select a single well-described strain of each species to test infant saliva, this approach may not address the inherent phenotypic and serological diversity known to exist among colonizing strains of S. mitis8 and their cross-reaction with S. oralis. Our goal is to determine the nature of the bacterial antigens recognized by salivary SIgA antibody during colonization of neonates and infants with S. mitis. To this end we sought to identify cell fractions bound by salivary SIgA antibodies to use as a source of antigens to analyze the specificity of infant SIgA antibody to S. mitis. In order to provide necessary basic information on antigenic similarities among strains of S. mitis and S. oralis and any antibody binding antigens that are specific or common to each, we have tested binding of rabbit IgG antibody to specific fractions of thirty-eight oral isolates identified as S. mitis or S. oralis. This has been performed using an ELISA8 employing rabbit antisera against S. mitis biovar 1 SK145 and S. oralis SK100. Binding of antibody by whole cells, isolated cell walls, protease-treated cell walls, a crude cell membrane preparation and soluble cell protein was tested to localize significant antibody binding antigens within infant strains of S. mitis biovar 1 and strains of S. oralis. In order to show antigenic similarities among the strains they were subjected to cluster analysis based on the antibody binding of the different cell fractions. As an initial step in using cell fractions as a source of antigens recognized by infants, saliva from three infants was analyzed to determine the amount of SIgA antibody bound to fractions of selected strains.
MATERIALS AND METHODS
Identity and growth of bacterial strains
The strains used in the study are shown in Table 1. These strains included our own S. mitis isolates from infants and generous gifts of strains from workers in the field that were sent as either S. mitis or S. oralis. The purity of all strains was tested by growth on sheep blood agar. Plates were incubated for 24 h in a candle jar. Single colonies were picked off onto fresh plates for three consecutive subcultures and then their identity was confirmed using the API 20 strep panel (bioMérieux, Durham, NC, USA). Once their identity was confirmed colonies were scraped from three blood agar plates into 1 ml of freezing broth consisting of 30% glycerol in Todd-Hewitt broth without dextrose (Difco Laboratories, Detroit, MI) and stored at -80°C. These frozen stock cultures were used as the source of the strains throughout the study. Collection of S. mitis and whole saliva (see later) from infants and adults was approved by the Institutional Review Board of Georgetown University Medical Center.
Table 1.
SPECIES CODE NUMBERS AND SOURCE OF STRAINS USED IN THE STUDY
| SPECIES | STRAIN | ORIGIN | SOURCE |
|---|---|---|---|
| Streptococcus oralis | |||
| 1 | ATCC 35037T | Human Mouth | American Type Culture Collection (ATCC) |
| 2 | NCTC 114127 | Human Mouth | National Collection of Type Cultures (NCTC) |
| 3 | SK100 | Human Dental Plaque | Dr. Mogens Kilian, Aarhus, Denmark |
| 4 | ABA 68 | Dr. David Beighton, London, U.K. | |
| 5 | DBA 1 | “ | |
| 6 | DBC 20 | “ | |
| 7 | HBA 57 | “ | |
| 8 | S2C 45a | “ | |
| 9 | SRC 9-2b | ||
| 10 | SZA 11-2 | “ | |
| 11 | SZC 44 | “ | |
| 12 | SZC 50 | “ | |
| 13 | MKA 36 | “ | |
| 14 | MKA 61 | “ | |
| 15 | MA 7 | Human Dental Plaque | Dr. G. Svensatter, Malmo, Sweden |
| 16 | QB 5 | Human Dental Plaque | “ |
| 17 | RA 1 | Human Dental Plaque | “ |
| 18 | TA 16 | Human Dental Plaque | “ |
| Streptococcus mitis | |||
| 19 | NCTC 12261T | Human Mouth | National Collection of Type Cultures (NCTC) |
| 20 | SK145 | Human Mouth | Dr. Mogens Kilian, Aarhus, Denmark |
| 21 | SK137 | Human Dental Plaque | Dr. Mogens Kilian, Aarhus, Denmark |
| 22 | SK95 | Human Mouth | Dr. Mogens Kilian, Aarhus, Denmark |
| 23 | CBR 393 | Human Mouth | Own isolate, Georgetown University |
| 24 | CBR 515 | Human Mouth | “ |
| 25 | CBR 1495 | Human Mouth | “ |
| 26 | CBR 1516 | Human Mouth | “ |
| 27 | JA | Human Mouth | “ |
| 28 | JC | Human Mouth | “ |
| 29 | JD | Human Mouth | “ |
| 30 | JF | Human Mouth | “ |
| 31 | JG | Human Mouth | “ |
| 32 | JI | Human Mouth | “ |
| 33 | JJ | Human Mouth | “ |
| 34 | JK | Human Mouth | “ |
| Streptococcus gordonii 35 | SK120 | Human Mouth | American Type Culture Collection (ATCC) |
| Enterococcus casseliflavus 36 | ATCC 12818 | Human Gingiva | American Type Culture Collection (ATCC) |
| Enterococcus faecalis 37 | ATCC 4082 | Human Tooth Root Canal | American Type Culture Collection (ATCC) |
| Enterococcus faecalis 38 | ATCC 19433 | American Type Culture Collection (ATCC) |
Fractionation of whole cells
Cells for fractionation were grown in five one-liter batches of Todd-Hewit broth (Difco) for 24h at 37°C and checked for purity by plating aerobically and anaerobically onto blood agar plates. Cells were removed from the medium by centrifugation (4,000 × g at 4°C) for 20 min and the sedimented cells washed three times in distilled water. The cells were re-suspended in distilled water and disrupted using glass beads in a Mickle Tissue Disintegrator (Mickle Engineering Co., Gomshall, England).10 The glass beads were allowed to sediment and the supernatant was removed. The beads were washed twice with 5 ml of distilled water and the washes were added to the supernatant. The combined supernatant and wash was centrifuged (4,000 × g at 4°C) for 20 min to remove any remaining beads and whole cells. The resulting supernatant was centrifuged at 27,000 × g at 4°C for 30 min to separate cell walls and both deposit and supernatant were retained. The deposited cell wall fragments were washed three times in distilled water and freeze-dried (Modulyo, BOC Edwards, Tonawanda, NY). This material was designated “isolated cell walls”. A second fraction was obtained by treating the isolated cell walls with protease to remove protein and any adherent membranes. This was done by suspending 100 mg of freeze-dried isolated cell walls in 25 ml of 0.1M phosphate buffer, pH 8.0, with 0.25 mg of Protease type XVIII (Sigma-Aldrich, St. Louis, MO). A few drops of toluene were added to the cell wall suspension to prevent growth of contaminating organisms during incubation. The reaction mixture was incubated at 37°C for 16 h with gentle shaking. After incubation the suspension was centrifuged at 27,000 × g for 30 min to deposit the cell walls. The supernatant was discarded and the deposited cell walls were washed three times in distilled water and freeze dried. These walls were designated “protease-treated walls”.
The supernatant remaining after obtaining isolated cell walls was centrifuged at 200,000 × g for 1h in an L8-M80 ultracentrifuge (Beckman Coulter Instruments, Fullerton, CA). The supernatant was removed and dialyzed three times against two liters of distilled water. The dialyzed material was freeze-dried and designated “cell protein”. The deposit was an opalescent gel that was insoluble. It was washed three times with distilled water and re-suspended in 2 ml of the same. This fraction was designated “cell membranes”. One-hundred microliters of this suspension were dried to constant weight in order to calculate the volume that contained 10 μg of membranes for use in ELISA.
Selection of strains to use for analysis of infant salivary SIgA antibody binding
Based on the data generated on antibody binding to fractions of the bacterial strains (Tables 2 and 3) we selected ten strains to use as antigens to examine binding of infants’ salivary SIgA antibodies to S. oralis and S. mitis cell fractions. Strains SK145 and SK100 together with the Type strains of S. oralis (ATCC 35037) and S. mitis (NCTC 12261) represented well described strains of these two species. In addition, we tested six infant isolates of S. mitis, two strains (CBR 515, CBR 1516) that were consistently placed into the N cluster and JA, JC, JD and JK (see Tables 2 and 3).
Table 2.
Relative binding of whole cells and of antigenic fractions S. mitis and S. oralis reacted with anti-SK145 and anti-SK100 antisera, respectively.
| ANTIGENIC FRACTIONS | |||||
|---|---|---|---|---|---|
| Steptococcus oralis strains | Whole cells | Cell walls | Protease-treated cell walls | Cell wall protein | Cell membranes |
| ATCC 35037 | 12.5/58.3 | 37.3/74.7 | 20.5/14.5 | 27.6/49 | 11.6/56.7 |
| NCTC 11427 | 20.3/44.7 | 21.6/70.2 | 6.8/2.9 | 23.7/51.2 | 4.8/62.7 |
| SK 100 | 12.1/100 | 18.3/100 | 23.5/100 | 33.7/100 | 34.2/100 |
| ABA 68 | 5.9/4.3 | 12.2/19.4 | 1/6.1 | 3/21.6 | 2.2/34.6 |
| DBA 1 | 14.3/40.6 | 35/100 | 100/52.4 | 49.7/70.1 | 52.3/80.1 |
| DBC 20 | 17.6/95.4 | 12.7/100 | 1/79.7 | 1.3/81.3 | 23.2/79.9 |
| HBA 57 | 9.1/21.8 | 11.3/70.3 | 24/22.8 | 16.6/72.1 | 9.8/57.6 |
| S2C 45a | 7.7/100 | 8.5/78.2 | 2.9/81 | 12.7/79.3 | 24.9/85.4 |
| SRC 9-2b | 23.8/21 | 17.1/25.6 | 1/4.5 | 15.8/18.7 | 2.2/19.4 |
| SZA 11-2 | 10.1/100 | 4.7/39.5 | 1/52.3 | 8.2/66.5 | 22.3/89.5 |
| SZC 44 | 5/73.5 | 3.1/25.8 | 1/51.1 | 17.6/57.7 | 30.6/87.2 |
| SZC 50 | 6.7/100 | 12.7/56 | 1/72.5 | 14.7/55.8 | 58.2/86.3 |
| MKA 36 | 26.7/39.9 | 31.7/56.7 | 100/90.4 | 28.1/44.3 | 47.6/67.4 |
| MKA 61 | 30.5/16.2 | 21/13.8 | 24.3/1.4 | 21.5/20.6 | 30.3/33.3 |
| MA 7 | 1.1/29.3 | 5.2/54 | 6.9/29.1 | 17/97 | 2.7/74.3 |
| QB5 | 3.3/45.6 | 15.3/59.8 | 24.9/16.4 | 11.4/35.5 | 13/60.6 |
| RA 1 | 4.1/100 | 8.9/100 | 4.2/95.2 | 8.6/78.4 | 24/100 |
| TA 16 | 2.3/17 | 6.8/71.6 | 1/3.1 | 14.4/74.4 | 10.4/83.1 |
| NCTC 12261 | 18.5/68 | 17.8/58.6 | 4.5/5.1 | 2.5/33.4 | 6.6/53.3 |
| SK 145 | 100/9.3 | 100/28.4 | 100/3.9 | 100/39.2 | 100/39.1 |
| SK 137 | 66.4/7.3 | 100/33.3 | 87.5/2.7 | 55.8/38.4 | 100/28.1 |
| SK 95 | 25.3/19.6 | 90.3/40 | 47.2/9.5 | 50.5/46.7 | 90.3/50.8 |
| CBR 393 | 30.8/22.8 | 81.3/37.6 | 54.2/8.9 | 23.6/29.2 | 67.8/34.2 |
| CBR 515 | 30.5/31 | 25.6/27.8 | 18.7/7.1 | 17.1/35.6 | 31.1/30.7 |
| CBR1495 | 55.4/25.3 | 100/11.1 | 70.7/5.6 | 29.8/34.6 | 100/37.5 |
| CBR 1516 | 1.6/0 | 4.3/2.1 | 38.7/21.9 | 10.3/14.7 | 17.6/19.3 |
| JA | 92.3/19.2 | 100/46.4 | 75.5/22.67 | 33.1/47.8 | 100/48.2 |
| JC | 91.2/16.1 | 100/42.6 | 100/23.7 | 35.8/40.8 | 64.2/28.4 |
| JD | 13.3/31.1 | 78.8/87.3 | 87.9/53 | 34.1/48.6 | 72.3/98.4 |
| JF | 100/24 | 100/48.3 | 92.1/20.8 | 37.3/44.4 | 100/48.2 |
| JG | 44.2/28.2 | 81.7/46.9 | 45.5/37.6 | 46.5/38.6 | 48/55.6 |
| JI | 24.8/22.5 | 81.7/46.6 | 70.3/23.2 | 21.3/44.5 | 42.6/44.7 |
| JJ | 79.4/19.8 | 100/42.8 | 100/21.5 | 27.3/25.4 | 100/42.3 |
| JK | 81.8/20 | 100/47.4 | 100/33.9 | 61.1/55.3 | 100/47.1 |
| Streptococccus gordonii SK120 | 8.8/36 | 8.4/50.8 | 6.5/3.9 | 2.6/27.9 | 9.1/45.3 |
| Enterococcus strains | |||||
| E. casseliflavus ATCC 12818 | 3.4/6.2 | 2.6/21.6 | 33.3/19.7 | 8/25 | 0.8/22.1 |
| E. faecalis ATCC 4082 | 5.6/33.4 | 8.3/49.6 | 34.9/51.8 | 2.8/26.6 | 0.43/24.6 |
| E. faecalis ATCC 19433 | 19.8/36.2 | 9.6/51.2 | 33/38.8 | 5.8/41.6 | 0.24/37.6 |
Table 3.
Assignment of strains to clusters defined by reactivity of whole cells and cell fractions with anti-SK145 and anti-SK100 antisera
| WC | CW | PTCW | CP | CM | |
|---|---|---|---|---|---|
| Streptococcus oralis | |||||
| ATCC 35037T | |||||
| NCTC 114127 | |||||
| SK100 | |||||
| ABA 68 | |||||
| DBA 1 | |||||
| DBC 20 | |||||
| HBA 57 | |||||
| S2C 45a | |||||
| SRC 9-2b | |||||
| SZA 11-2 | |||||
| SZC 44 | |||||
| SZC 50 | |||||
| MKA 36 | |||||
| MKA 61 | |||||
| MA 7 | |||||
| QB 5 | |||||
| RA 1 | |||||
| TA 16 | |||||
| Streptococcus mitis | |||||
| NCTC 12261T | |||||
| SK145 | |||||
| SK137 | |||||
| SK95 | |||||
| CBR 393 | |||||
| CBR 515 | |||||
| CBR 1495 | |||||
| CBR 1516 | |||||
| JA | |||||
| JC | |||||
| JD | |||||
| JF | |||||
| JG | |||||
| JI | |||||
| JJ | |||||
| JK | |||||
| SK120 | |||||
| ATCC 12818 | |||||
| ATCC 4082 | |||||
| ATCC 19433 |
| S. oralis cluster | |
| S. mitis cluster | |
| Non-S. oralis/non-S. mitis cluster |
WC = Whole Cells PTCW = Protease-treated Cell Wall CP = Cell Protein CW = Cell Walls CM = Cytoplasmic Membrane
Binding of rabbit anti-SK145 and anti-SK100 to whole cells and cell fractions
Immunoglobulin G antibodies against S. mitis SK145 and S. oralis SK100 were produced in rabbits as described previously.8 Antibody binding to antigens contained within the various cell fractions was determined by an ELISA using a reaction volume of 100 μl. The assay has been described in detail previously.8 The blocking step and all antibody incubation steps were performed for 1 h at room temperature on an orbital shaker (Bellco, Vineland, New Jersey). Between blocking and antibody incubation steps wells were washed three times with 0.1 M phosphate-buffered saline, pH 8.0, containing 0.1% Tween-20 (PBS-T) using a Columbus plate washer (Tecan, Durham, NC). Briefly, wells of duplicate 96-well microtiter plates (Immulon II, Dynatech Laboratories Inc., Chantilly, VA) were coated with 10 μg (dry weight) of whole cells or 10 μg of each of the cell fractions in 0.05 M carbonate-bicarbonate buffer, pH 9.6, for 16 h at 4°C. After blocking with PBS-T containing 0.1% globulin-free albumin duplicate wells were charged with serial two-fold dilutions (1:500–1:32,000) of either the rabbit antiserum against SK145 or the rabbit antiserum against SK100 diluted in blocking solution. Individual plates were used for each antiserum and appropriate controls were included on every plate. The dilutions of antisera were incubated in the wells for 4 h. Bound antibody was detected with swine anti-rabbit immunoglobulins conjugated with horseradish peroxidase (DAKO, Carpinteria, CA) diluted 1:1,000, and reported with 1 mg of o-phenylenediamine per ml of citrate-phosphate buffer, pH 4.5, containing 0.012% hydrogen peroxide. Optical densities of the wells were read at 450 nm using a Spectrum Rainbow microtiter plate reader (Tecan, Durham, NC). The sum of the optical densities at each dilution of rabbit anti-SK145 or rabbit anti-SK100 antiserum reacted with each of the cell fractions from the different isolates tested was expressed as a percentage of the sum of the optical density values for the cell fractions of the homologous strain (set at 100%). These values of‘relative binding’ are termed ‘antibody binding’ throughout this paper.
Quantitation of Secretory immunoglobulin A (SIgA) in whole saliva
Total SIgA in saliva samples was quantified using an ELISA as previously described7,11 with minor modifications. The assay conditions were those described for the rabbit antisera in the previous section. The assay was performed in one-half of 96-well Immulon II microtiter plates (Dynatech Laboratories Inc.). With the single exception of the antibody to human secretory component (SC) obtained from Dako all antibodies employed in the ELISA were affinity purified IgG antibodies obtained from Jackson ImmunoResearch (West Grove, PA, USA). Four dilutions of each saliva sample were prepared in PBS-T. For saliva samples collected between birth and 4 months, the dilutions ranged from 1:50 to 1:800. Dilutions of samples collected from infants between 6 and 12 months of age ranged from 1:600 to 1:2,000. Wells were coated with rabbit anti-goat γ chains at a concentration of 3 μg/ml to serve as a presenting antibody. Following washing, wells were blocked. To the blocked wells was added goat anti-human α chains at a concentration of 2.4 μg/ml as the capture antibody. Saliva dilutions were added to the wells in triplicate and incubated for 1 h at room temperature. After washing HRP-conjugated rabbit anti-human SC at a dilution of 1:5,000 was added as the reporter antibody. Following washing captured SIgA was detected using o-phenylenediamine. Absorbance was measured at 450 nm. Dilutions of authentic colostral SIgA (MP Biomedicals/Cappel, Solon, OH) of known concentration were included on each plate to create a standard curve, from which SIgA concentration in saliva samples was determined by interpolation. Adult human whole saliva of known SIgA concentration from our laboratory was used as a positive control. Appropriate background and negative controls were run on every plate.
Assay of salivary SIgA antibodies reactive with cell extracts of S. mitis and S. oralis isolates
Salivary SIgA antibodies reactive with antigens within the cell fractions were determined by ELISA as described above except that the wells in the remaining half of the microtiter plate were coated overnight with 10 μg of the various cell fractions. Performing both quantitation of salivary SIgA and the measurement of salivary SIgA antibodies reactive with the cell fraction antigens in a single plate using the same reporter antibody allowed us to interpolate the absorbance values obtained from assay of the cell fraction-reactive SIgA antibodies into the SIgA standard curve and output amounts of anti-cell fraction SIgA antibodies in primary units.
Cluster analysis
In order to analyze the binding of anti-SK145 and anti-SK100 antisera to the antigenic fractions of different strains the binding values were subjected to cluster analysis using Pearson’s product-moment coefficient with UPGMA (SAS, Cary, NC, U.S.A.).
RESULTS
Almost all of the whole cells and antigenic fractions tested bound both anti-SK145 and anti-SK100 antisera. Several strains showed high levels of relative binding with either anti-SK100 or anti-SK145 antiserum for most or all antigenic fractions, while others gave low binding levels with both antisera (Table 2). Strains DBC 20 and RA 1 are examples of those that bound high levels of anti-SK100 antibody and JK and SK137 are examples of strains that generally bound high levels of anti-SK145 antibody. The strains that gave low relative binding with both antisera included ABA 68, SRC9-2b, CBR 515, and CBR 1516. The amount of antibody bound by different fractions and whole cells of a strain also varied. For example, whole cells of S. oralis strain SZC 50 bound 100% of SK100 antibody but isolated cell walls bound only 56%. Similarly, S. mitis SK137 whole cells bound only 66.4% of anti-SK145 antibody, while the cell walls bound 100%. Strains were also observed that exhibited the same or closely similar patterns of anti-SK145 and anti-SK100 antibody binding to all of the antigenic fractions. Examples of these are S. oralis SZA 11-2, and SZC 44 and S. mitis strains JA, JF and JJ.
In some instances the type of cell fraction used as antigen affected the degree of cross-reactivity of the antisera, between strains. Protease-treated cell walls of S. oralis strains SZA11-2, SZC 44, and SZC 50 only bound approximately 1% of anti-SK145 antibody compared to membrane fractions of these strains that bound 22% to 58% of the antibody (Table 2). It was interesting to note that there were strong similarities in binding among the series of “J “S. mitis strains that had been isolated from infants. All the fractions from strains JA, JC, JF, JJ and JK bound similar amounts of antibody. Three strains JD, JG and JI were exceptions. Whole cells of these strains only bound low levels of SK145 antibody, although their cell walls showed binding equivalent to the other strains. Also, strain JD and JG were unusual among this group in that the cell membrane fraction placed them in the ‘Oralis’ cluster (see below) and JD, in particular, bound 98.4% of anti-SK00 antibody. Streptococcus gordonii and the Enterococcus strains all bound anti-SK145 and anti-SK100 antibody, although at relatively low levels.
Examples of cluster analysis based on the reactivity of both antisera to whole cells and cell walls are shown in Figures 1 and 2, respectively. Cluster analysis placed the strains into three clusters one, termed the Mitis Cluster (M), includes S. mitis SK145 with strains that have closely similar antibody binding patterns, the second, termed the Oralis Cluster (O) is formed around S. oralis SK100 by strains with binding patterns similar to this organism. The third cluster, termed the Non-Mitis/Non-Oral Cluster (N) includes strains that gave relatively low binding with both antisera. In Figure 1, which shows cluster analysis of whole cells, the M cluster contains strains that bind > 55% of anti-SK145 antibody and bind < 26% of anti-SK100 antibody (83.3% mean binding of anti-SK145 antibody and 17.6% mean binding of anti-SK100 antibody). Strains in the O cluster show the inverse, > 58% binding of anti-SK100 antiserum and < 19% binding of anti-SK145 antiserum (10.5% mean binding of anti-SK145 antibody and, 77.2% mean binding of anti-SK100 antibody). Those strains in the N cluster generally gave low binding (17.7% mean binding of anti-SK145 antibody and 25.8% mean binding of anti-SK100 antibody).
Figure 1.
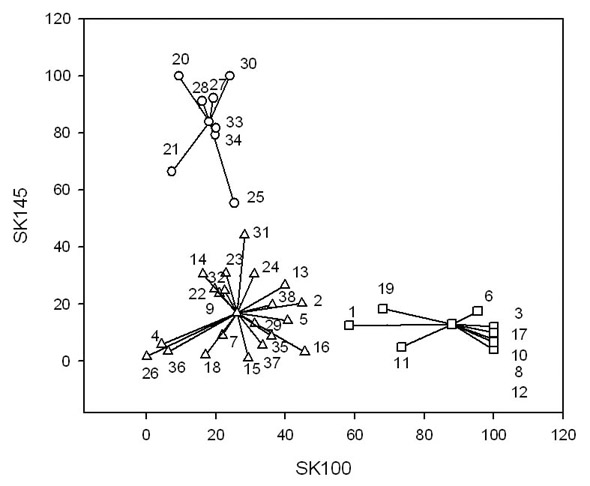
Relative binding of rabbit anti-SK145 and anti-SK100 antisera to whole cells of 38 strains comprising reference strains and wild-type isolates of Streptococcus mitis, S. oralis, S. gordonii, Enterococcus casseliflavus, and E. faecalis as determined by ELISA.
The strains fall into three clusters: Open circles = Mitis (M) cluster; Open squares = Oralis (O) cluster; Open triangles = Non-Mitis/Non-Oralis cluster (N). The numbers identify the strain by reference to the list of strains in Table 1.
Figure 2.
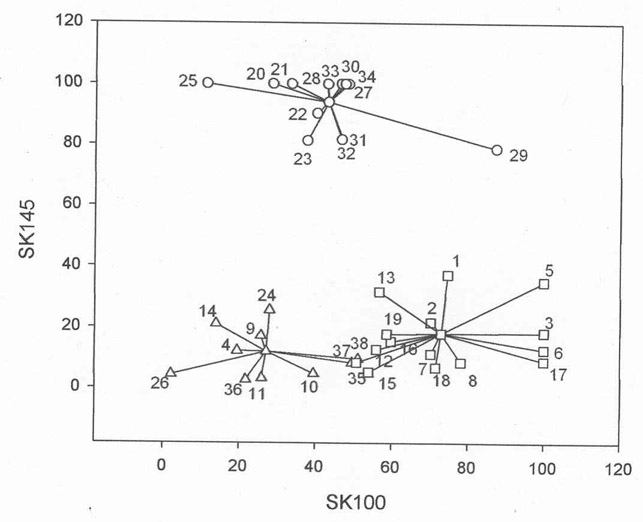
Relative binding of rabbit anti-SK145 and anti-SK100 antisera to isolated cell walls of 38 strains comprising reference strains and wild-type isolates of Streptococcus mitis, S. oralis, S. gordonii, Enterococcus casseliflavus, and E. faecalis as determined by ELISA. The strains fall into three clusters: Open circles = Mitis (M) cluster; Open squares = Oralis (O) cluster; Open triangles = Non-Mitis/Non-Oralis cluster (N). The numbers identify the strain by reference to the list of strains in Table 1.
It should be noted that although the Type strain of S. oralis ATCC 35037T (#1) fell into the O cluster when both whole cells (Fig. 1) and cell walls (Fig. 2) were used as antigen, the same strain (NCTC 11427T) (#2) from another source was included in the N Cluster when its whole cells were used as antigen. However, with the exception of protease-treated cell walls the antibody binding of factions of these strains were similar (Table 2). Also, cell fractions of strain S. mitis NCTC 12261T that fell into the O cluster generally bound more anti-SK100 than anti-SK145 antibody (Table 2).
Figure 2 shows the clusters formed when isolated cell walls were used to bind anti-SK145 and anti-SK100 antibody. The most striking difference observed by use of isolated cell walls was that additional strains were assigned to the M and O clusters. The M cluster based on antibody reactivity with isolated cell walls increased from eight to thirteen strains while the O cluster increased from nine to fifteen strains. Generally, the strains that moved either to the M or O clusters originated in the N cluster and, consequently, this cluster contained only ten strains compared to the twenty-one strains for whole cells (Figure 1). However, strains SZA 11-2 (#10) and SZC 44 (#11) moved from the O to the N cluster. Interestingly, based on antibody reactivity with isolated cell walls (Figure 2), strain NCTC 11427 (#2) was placed together with ATCC 35037 (#1) in the O cluster and SK95 (#22) a well-defined strain of S. mitis joined the M cluster. S. mitis NCTC 12261T (#19) remained in the O cluster.
The cluster assignments of strains based on antibody reactivity with whole cells and the different cell fractions are shown in Table 3. Obviously, strains SK145 and SK100 fell into the M and the O clusters with all fractions. Some strains showed the same cluster assignments as SK145 and SK100. Strains DBC 20, S2C 45a and RA 1 were equivalent to SK100 and strains SK137 and JK were equivalent to SK145 for whole cells and cell fractions. Strains ABA 68, SRC 9-2b, MKA 61, CBR 515, CBR 1516, and E. casseliflavus fell consistently into the N cluster. The other strains in Table 3 show variation in the clusters assigned. Very often the cell wall fraction assigned the strains to the clusters equivalent to their species designation. Thus, using the cell wall fraction placed thirteen strains of S. oralis in the O cluster compared to eight for whole cells, Similarly, thirteen strains of S. mitis fell into the M cluster with cell walls and eleven with whole cells as antigen. Some strains were assigned to the same cluster irrespective of whether whole cells or the various cell fractions served as antigen. Examples of these strains are S. oralis DBC 20, S2C 45a and RA 1 and S. mitis SK137 and JK.
Antibody reactivity with isolated cell walls treated with protease that would leave predominately carbohydrate and mucopeptide10 placed some strains in different clusters compared to those to which they were assigned based on antibody reactivity with untreated isolated cell walls. Interestingly, S. oralis strains DBA 1 and MKA 36 that were placed in the O cluster on the basis of antibody reactivity with untreated cell walls moved to the M cluster when antibody reactivity with protease-treated cell walls was considered. Both of these strains were strongly reactive (100% relative antibody binding) with anti-SK145 antibody (Table 2) and also reacted strongly with anti-SK100 antibody with MKA 36 exhibiting a relative antibody binding value of 90.4%. No strains moved from the M to the O cluster when protease-treated cell walls were used in place of untreated cell walls.
Antibody reactivity with the cell protein fraction assigned a high proportion of the strains to the N cluster. Twenty-five strains were placed in the N cluster on the basis of antibody reactivity with the cell protein fraction compared to 21 with whole cells and ten with cell walls (Figure 3). On the basis of antibody reactivity with cell membranes ten of the eighteen strains of S. oralis were assigned to the O cluster and the remainder of the strains to the N cluster. Similarly, antibody reactivity with cell membranes placed ten of the sixteen S. mitis strains into the M cluster. Strains JD and JG differed from the other S. mitis strains in that they fell into the O cluster. Relative antibody binding of anti-SK145 and anti-SK100 antibody to JD cell membranes was 72.3% and 98.4%, respectively, while binding to JG cell membranes was 48 % and 55.6%, respectively (Table 2).
The concentrations of SIgA antibody from saliva of three infants that bound to fractions of the selected strains are shown in Table 4. The data for two adult saliva samples tested against S. mitis NCTC 12261 and CBR 515 are shown in Table 5. The data in Table 4 show that very little of the SIgA antibody in infant saliva bound the different fractions. The highest concentration of SIgA antibody that bound to S. mitis fractions was observed in infant #3 where 2.7 μg/ml SIgA bound to the membrane fraction of strain S. oralis DBA 1. The lowest concentration (0.1 ug/ml) was observed for infant #1 salivary SIgA binding protease-treated cell walls of S. mitis JC. It was apparent, even at these very low levels, that none of the three saliva samples showed predominant binding of SIgA antibody to any of the antigenic fractions, as was the case with the rabbit antibody. Also, examination of the mean values in Table 4 clearly shows that SIgA binding to the same fraction from different strains was closely similar. However, there were some differences in binding when the individual antigenic fractions were compared. Binding of SIgA to the cell protein fraction ranged from 0.8 to 1.2 μg/ml, cell walls binding ranged from 1.0 to 2.0 μg/ml, protease-treated wall binding ranged from 0.2 to 0.5 μg/ml and cell membrane binding from 1.4 to 2.1 μg/ml. Clearly, the cell membrane fraction bound the most SIgA antibody, while protease-treated cell walls bound the least. Table 5 shows the concentrations of SIgA antibody bound by saliva samples from two adults. Two strains of S. mitis served as positive controls for the assay of infants’ saliva and to show binding of SIgA antibodies to the cell fractions. The concentration of SIgA antibody bound to the cell fractions was much greater than that of the infant saliva. In addition, the differences in SIgA antibody binding seen among the fractions with infant saliva were not so apparent. In particular, adult salivary SIgA antibody bound to protease-treated cell walls at similar levels to the cell protein fraction and the cell membrane fraction bound at lower levels than cell walls.
Table 4.
Concentrations (μg/ml) of salivary SIgA in infant saliva bound to fractions of S. mitis and S. oralis.
| S. mitis and S. oralis Strains | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Fractions | SK 145 | SK 100 | ATCC 35037 | NCTC 12261 | CBR 515 | CBR 1516 | JA | JC | JD | JK | DBA 1 |
| Protein | |||||||||||
| Infant 1 | 1.2 | 0.9 | 1.0 | 1.0 | 1.0 | 0.6 | 0.7 | 1.2 | 1.2 | 0.8 | 0.8 |
| Infant 2 | 1.7 | 0.8 | 0.8 | 0.8 | 0.8 | 1.0 | 1.2 | 1.0 | 0.8 | 1.3 | 0.9 |
| Infant 3 | 0.8 | 1.0 | 0.9 | 0.7 | 0.9 | 1.1 | 1.2 | 0.8 | 0.8 | 1.2 | 0.7 |
| MEAN | 1.2 | 0.9 | 0.9 | 0.8 | 0.9 | 0.9 | 1.1 | 1.0 | 0.9 | 1.1 | 0.8 |
| Cell wall | |||||||||||
| Infant 1 | 1.2 | 1.0 | 0.9 | 1.6 | 1.0 | 1.1 | 1.1 | 1.0 | 1.8 | 1.1 | 1.0 |
| Infant 2 | 1.5 | 1.1 | 1.0 | 1.6 | 1.0 | 1.1 | 1.1 | 1.3 | 2.1 | 1.1 | 2.4 |
| Infant 3 | 1.1 | 1.0 | 1.1 | 1.8 | 1.1 | 1.6 | 1.2 | 1.3 | 2.1 | 1.0 | 2.7 |
| MEAN | 1.3 | 1.1 | 1.0 | 1.7 | 1.0 | 1.3 | 1.1 | 1.2 | 2.0 | 1.1 | 2.0 |
| Protease wall | |||||||||||
| Infant 1 | 0.4 | 0.4 | 0.2 | 0.4 | 0.3 | 0.4 | 0.4 | 0.1 | 0.5 | 0.4 | NS |
| Infant 2 | 0.4 | 0.3 | 0.5 | 0.5 | 0.2 | 0.4 | 0.5 | 0.3 | 0.4 | 0.4 | NS |
| Infant 3 | 0.4 | 0.4 | 0.5 | 0.4 | 0.2 | 0.3 | 0.3 | 0.4 | 0.7 | 0.2 | NS |
| MEAN | 0.4 | 0.4 | 0.4 | 0.4 | 0.2 | 0.4 | 0.4 | 0.3 | 0.5 | 0.3 | - |
| Membrane | |||||||||||
| Infant 1 | 1.4 | 1.3 | 1.6 | 1.3 | 1.5 | 1.5 | 2.1 | 1.2 | 1.8 | 1.3 | 1.6 |
| Infant 2 | 1.5 | 1.7 | 1.7 | 1.5 | 1.4 | 1.8 | 1.5 | 2.1 | 1.8 | 1.8 | 2.8 |
| Infant 3 | 1.6 | 1.1 | 1.6 | 1.4 | 1.5 | 1.3 | 2.1 | 2.0 | 2.1 | 1.8 | 1.9 |
| MEAN | 1.5 | 1.4 | 1.7 | 1.4 | 1.5 | 1.5 | 1.9 | 1.8 | 1.9 | 1.6 | 2.1 |
Table 5.
Concentrations (μg/ml) of adult SIgA in infant saliva bound to fractions of S. mitis and S. oralis.
| Cell Fractions | S. mitis strains | |
|---|---|---|
| NCTC 12261 | CBR 515 | |
| Cell Protein | ||
| Saliva 1 | 107.9 | 108.9 |
| Saliva 2 | 100.6 | 102.1 |
| Mean | 104.3 | 105.5 |
| Cell Wall | ||
| Saliva 1 | 146.3 | 146.7 |
| Saliva 2 | 173.0 | 143.6 |
| Mean | 159.7 | 145.2 |
| Protease-treated Wall | ||
| Saliva 1 | 103.1 | 102.5 |
| Saliva 2 | 100.2 | 98.4 |
| Mean | 101.7 | 100.5 |
| Cell membrane | ||
| Saliva 1 | 138.8 | 136.5 |
| Saliva 2 | 119.3 | 128.1 |
| Mean | 129.1 | 132.3 |
DISCUSSION
Our previous studies8 have shown that strains of S. mitis bound antibody raised against S. mitis SK145 and also against S. oralis SK100. However, thirty-four of forty-eight randomly selected S. mitis strains isolated from infants showed binding of anti-SK145 antibody equivalent to the homologous strain and generally low binding of anti-SK100 antibody, suggesting that these strains represented a group among isolates of S. mitis biovar 1 that was antigenically similar to SK145. Five of the forty-eight strains bound both anti-SK145 and anti-SK100 antisera at between 80% and 100% of the levels of the homologous strains and the remaining nine strains bound lower levels of both antisera. The current study supports these observations, in that it was possible to generate clusters of isolates of S. mitis and S. oralis that included SK145 or SK100. Also, depending on the cell fraction used, of 38 strains examined between ten and twenty-five of the strains tested fell into a third cluster that showed relatively weak binding by these antisera (Tables 2 and 3).
The data in Table 2 clearly show that the type of cell fraction influenced the degree of binding of antibody, although the degree of binding was not consistent for a given fraction from different strains. In general, strains were grouped into their designated species when the cell wall was used as antigen. Protease treatment of the walls reduced this effect (Table 2). However, in some cases protease-treated walls exhibited over 90% binding (S.oralis MKA 36, RA 1, S. mitis JC, JF, JJ and JK) and it is most likely that they share a carbohydrate antigen or antigens with either SK145 or SK100. In particular, strain MKA 36 bound anti-SK145 antibody at 100% and anti-SK100 antibody at 90.4%, reminiscent of those S. mitis strains described previously8 where whole cells bound both antisera at 100%. However, in the present case none of the other MKA 36 cell fractions tested bound the antisera above 67%. It seems possible that MKA 36 has a wall carbohydrate antigen or antigens in common with both SK145 and SK100.
Cluster analysis of the binding data for the cell protein fraction placed twenty-one S. mitis or S. oralis strains into the N cluster (Figure 3) suggesting that, for these strains, cell protein did not contain antigens defining SK145 or SK100. The lower binding shown for both antisera (Table 2) with these strains may indicate the presence of antigens responsible for cross-reactions both within and between S. mitis and S. oralis.
The cell membrane fraction of the isolates very often placed strains in clusters according to their species (Figure 3). Although the cell membrane fractions bound both anti-SK145 and anti-SK100 antibody the binding of the antibody that defined their cluster was generally high (Table 2). This suggests that they contained antigens specific to SK145 or SK100. Along with cell walls, cell membranes would seem to be a good source of potential discriminatory antigens of SK145 or SK100.
In contrast to the finding using the rabbit antisera, there was no obvious difference between the three infant saliva samples tested in terms of binding of SIgA antibody to any of the strains tested. This is probably to be expected because it is most likely that any salivary SIgA antibody response is directed to all colonizing strains. However, there was a trend towards the binding of higher amounts of SIgA antibody to cell wall and cell membrane fractions (Table 4). In particular, protease-treated cell walls bound very little antibody. This observation is consistent with our previous findings that infants appear not to mount an SIgA antibody response to carbohydrate antigens of S. mitis7 unlike adults who clearly do.
The current and our earlier data8 suggest that S. mitis SK145 and S. oralis SK100 are representatives of two groups of oral streptococci that share closely similar antigenic profiles. However, these cannot be said to be the definitive profiles of S. mitis or S. oralis because it is clear from our data that other strains of these species, although cross-reactive, have different antigenic profiles. The simple cell fractionation performed in this study has, we believe, shown that some fractions bind more hyper-immune rabbit antibody than others and probably contain significant antigens of SK145 and SK100. Two of the fractions in particular, the cell wall and the cell membrane fraction, are most likely to be the best from which to isolate such antigens. Also, cell wall carbohydrate from protease-treated cell walls could be a useful source of wall carbohydrate antigens. The results presented here confirm that infant salivary SIgA antibody binds to antigens in the fractions. This finding supports the concept that such antigens can be identified allowing the fine specificity of salivary SIgA antibodies reactive with S. mitis to be mapped during the development of the child. In this manner we may be able to throw further light on the interplay between the secretory immune system and the commensal microbiota.
Acknowledgments
This work was supported by Public Health Service grant DE08171 from the National Institute of Dental and Craniofacial Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pearce C, Bowden GH, Evans M, Fitzsimmons SP, Johnson J, Sheridan MJ, Wientzen R, Cole MF. Identification of pioneer viridans streptococci in the oral cavity of human neonates. J Med Microbiol. 1995;42:67–72. doi: 10.1099/00222615-42-1-67. [DOI] [PubMed] [Google Scholar]
- 2.Kirchherr JL, Bowden GH, Richmond DA, Sheridan MJ, Wirth KA, Cole MF. Distribution of Streptococcus mitis biovar 1 phenotypes on shedding and non-shedding oral surfaces of human infants during the first year of life. Microb Ecol Health Dis. 2005;17:138–145. [Google Scholar]
- 3.Hohwy J, Reinholdt J, Kilian M. Population dynamics of Streptococcus mitis in its natural habitat. Infect Immun. 2001;69:6055–6063. doi: 10.1128/IAI.69.10.6055-6063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hohwy J, Kilian M. Clonal diversity of the Streptococcus mitis biovar 1 population in the human oral cavity and pharynx. Oral Microbiol Immunol. 1995;10:19–25. doi: 10.1111/j.1399-302x.1995.tb00113.x. [DOI] [PubMed] [Google Scholar]
- 5.Fitzsimmons SP, Evans M, Pearce C, Sheridan MJ, Wientzen R, Bowden G, Cole MF. Clonal diversity of Streptococcus mitis biovar 1isolates form the oral cavity of human neonates. Clin Diagn Lab Immunol. 1996;3:517–522. doi: 10.1128/cdli.3.5.517-522.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirchherr JL, Bowden GH, Richmond DA, Sheridan MJ, Wirth KA, Cole MF. Clonal diversity and turnover of Streptococcus mitis bv. 1 on shedding and nonshedding oral surfaces of human infants during the first year of life. Clin Diagn Lab Immunol. 2005;12:1184–1190. doi: 10.1128/CDLI.12.10.1184-1190.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cole MF, Bryan S, Evans MK, Pearce CL, Sheridan MJ, Sura PA, Wientzen R, Bowden GHW. Humoral immunity to commensal oral bacteria in human infants: Salivary secretory immunoglobulin A antibodies reactive with Streptococcus mitis biovar 1, Streptococus oralis, Streptococcus mutans, and Enterococcus faecalis during the first two years of life. Infect Immun. 1999;67:1878–1886. doi: 10.1128/iai.67.4.1878-1886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirchherr JL, Bowden GH, Kawamura Y, Cole MF, Richmond DA, Sheridan MJ, Wirth KA. Physiological and serological variation in Streptococcus mitis biovar 1 from the human oral cavity during the first year of life. Archs Oral Biol. 2007;52:90–99. doi: 10.1016/j.archoralbio.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bergstrom N, Jansson PE, Kilian M, Skov Sorensen UB. Structures of two cell wall-associated polysaccharides of a Streptococcus mitis biovar 1 strain. A unique teichoic acid-like polysaccharide and the group O antigen which is a C-polysaccharide in common with pneumococci. Europ J Biochem. 2000;267:7147–7157. doi: 10.1046/j.1432-1327.2000.01821.x-i2. [DOI] [PubMed] [Google Scholar]
- 10.Slade HD, Slamp EC. Cell-wall composition and the grouping antigens of streptococci. J Bacteriol. 1962;84:345–351. doi: 10.1128/jb.84.2.345-351.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fitzsimmons SP, Evans MK, Sheridan MJ, Wientzen R, Cole MF. Immunoglobulin A subclasses in infant’s saliva and in saliva and milk from their mothers. J Pediatr. 1994;124:566–573. doi: 10.1016/s0022-3476(05)83135-x. [DOI] [PubMed] [Google Scholar]


