Abstract
The definition of estrogen’s actions has expanded from transcriptional regulation to the rapid, membrane-initiated activation of numerous signal transduction cascades. Multiple biological effects of estrogen have been shown in numerous animal, cellular and molecular studies, which support the favorable effects of estrogen on vascular structure, function, and cell signaling. Work from several laboratories has shown that these effects are mediated by distinct forms of estrogen receptor (ER) α. This includes estrogen-stimulated, rapid activation of endothelial nitric oxide synthase (eNOS), resulting elaboration of the athero-protective, angiogenesis-promoting product nitric oxide (NO). We have described the expression of ER46, an N-terminus truncated isoform of the ERα, in human endothelial cells (EC), and its critical role in membrane-initiated, rapid responses to 17β-estradiol (E2). We have proposed a ER46-centered, eNOS activating molecular complex in human EC caveolar membranes, containing c-Src, phosphatidylinositol 3-Kinase (PI3K), Akt and eNOS. Our previous studies support estrogen-induced rapid eNOS activation via a sequential c-Src/PI3K/Akt cascade in EC. In this review, we describe estrogen-induced, rapid, non-genomic actions in endothelium, driven by c-Src-ER46-caveolin-1 interactions, with consequent activation of eNOS. Amidst ongoing controversies in hormone replacement therapy, these molecular and cellular data, defining favorable estrogenic effects on the endothelium, provide a strong impetus to resolve these clinical questions.
Keywords: Estrogen, ER46, c-Src, eNOS, caveolin-1
1. Effects of Estrogen on the Vascular System
Endothelial homeostasis is critical to vascular health. The constitutive and induced production of NO is a key, positive regulatory factor in this homeostatic state, as NO has potent anti-thrombotic, anti-leukocyte adhesive, and anti-smooth muscle cell proliferative properties [1]. Endothelial dysfunction, largely defined as a lack of vasodilation to NO-inducing stimuli, correlates with an increased risk of adverse cardiovascular events [2]. A cardiovascular protective effect of estrogen has been suspected for many years, largely on the basis of the lower incidence of coronary heart disease in premenopausal women, relative to that in age-matched males, as well as on the basis of several retrospective clinical studies [3,4]. Although this has more recently come into question based on large scale, randomized clinical trials [5,6], the potentially favorable biological effects of estrogen, and of ER engagement, in the cardiovascular system is recognized. Estrogen has numerous nuclear-initiated genomic effects on ECs, including the repression of athero-promoting and the induction of athero-protective genes [7]. Here, we discuss those non-genomic endothelial responses to estrogen that enhance the production of the aforementioned athero-protective molecule, NO, through rapid activation of the constitutively expressed enzyme, eNOS.
There are 2 known ER genes encoding ERα and ERβ. Furthermore, there are a variety of ER splice variants, giving rise to a substantial heterogeneity of ER expression which is, in part, tissue-specific. ERα and ERβ are both expressed in ECs and vascular smooth muscle cells, mediating both the rapid, membrane-initiated and genomic cardiovascular effects of estrogens. We have also demonstrated the abundance of an N-terminus (A/B or AF-1)-deleted ERα splice isoform, ER46, in ECs, which is an efficient transducer of membrane-initiated responses (see below).
Several groups have utilized a murine vascular injury model to assess the beneficial effects of 17β-estradiol (E2) within the vasculature. The initially confounding findings that both ERβ knockout (ERβKO) and ERαKO mice retained protective responses to E2 were eventually explained by the retention of ERα splice forms ER55 and ER46 in the exon 1-targeted, ERαKO mice [8,9,10]. The exon 2-targeted, complete ERα KO (ERαKOStrasbourg) mice lost the E2-induced re-endothelialization observed in wild-type mice [11]. It was then understood that products of the ERα gene conferred this form of vascular protection, and that ERα splice variants are capable of mediating these favorable responses. It was subsequently shown that both full-length ERα and the N-terminus truncated ER46 co-localize with eNOS in plasma membranes, and that E2 induces rapid eNOS activation. ER46 mediates E2-stimulated eNOS activation more efficiently, whereas the opposite efficiency profile (ERα greater than ER46) was observed for genomic responses [12,13].
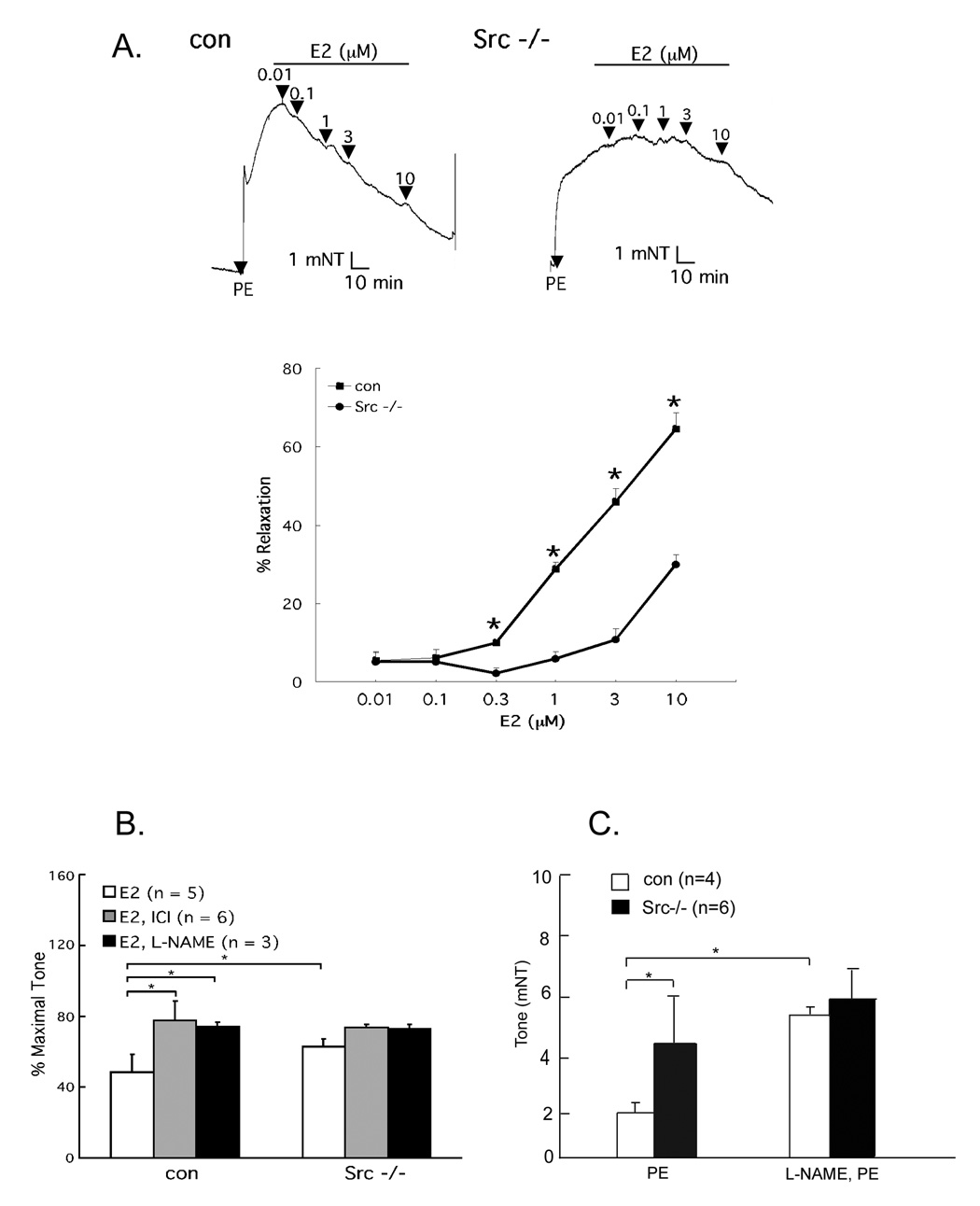
We have recently demonstrated that the non-receptor tyrosine kinase c-Src plays a physiological role in E2-induced vascular responses [14]. Estrogen-stimulated, phenylephrine (PE)-preconstricted aortic rings isolated from c-Src wild-type mice vasodilate in an NO- and ER-dependent fashion (Fig. 1A, B), as determined by suppression of the E2-induced response by the eNOS antagonist L-NAME and the ER antagonist ICI 182,780. When using aortic rings isolated from c-Src−/− mice, E2-stimulated vasodilation was markedly diminished (Fig. 1A). Furthermore, L-NAME greatly augmented PE-induced wild-type aortic ring constriction, but minimally affected c-Src−/− aortic rings (Fig. 1C). These data implicate the tyrosine kinase c-Src as a physiologically relevant modulator of both basal and E2-induced aortic vasorelaxation.
Fig. 1. c-Src-dependent basal and E2-induced vasorelaxation.
(A) Impaired E2 induced vasodilation in PE-preconstricted c-Src−/− aortas. Control and Src−/−thoracic aorta rings were isolated, preconstricted with 10−6 M PE, treated with E2, and the vascular tone was recorded by vessel myograph system. (B) Partially NO and receptor-dependent vasorelaxation (10−8 M E2, 10−5 M ICI 182,780, 10−4 M L-NAME). (C) Suppression of basal NO-dependent vasomotion in c-Src−/− aortas. Rings were pretreated with L-NAME (10−4 M) 20 min before PE preconstriction and remained in the bath throughout the experiment. *, p<0.05 (two-tailed ANOVA). Reprinted with permission [14].
2. ER Membrane Localization and Endothelial ER46 Signal Transduction
Membrane binding sites for estrogen were first described in 1977 [15], when ERs were partially purified and characterized from hepatocyte plasma membranes [16]. Immunohistochemical analyses have led to additional indirect evidence for plasma membrane-associated ERα [17,18]. There have been reports of numerous rapid cellular responses to estrogen, many tissue-specific and which occur through distinct molecular mechanisms, which we have previously summarized [19]. Several ER posttranslational modifications have been described including acetylation, phosphorylation, and S-nitrosylation. We and others have demonstrated that ER palmitoylation is critical for plasma membrane localization and rapid E2-induced signaling responses [13,19]. More specifically, Marino and colleagues described a critical S-palmitoylation of the ERα Cysteine-447 residue, which supports the ERα-caveolin-1 plasma membrane association and E2-induced proliferative signaling via the ERK/MAPK and PI3K/Akt pathways in human cancer cells [21].
We have observed activation of ERK, c-Src and an increase in cGMP within 5 minutes of EC stimulation with E2 [22]. These rapid activation responses occur both with free E2 and with conjugated, membrane-impermeant forms. Most relevant is the E2-induced, rapid phosphorylation of eNOS on the Serine-1177 activation site and the consequent augmentation of EC NO release [22]. In human ECs, we have described the transduction of these membrane-initiated, rapid responses by the aforementioned N-terminus-deleted ERα splice isoform, ER46 [13]. This isoform has also been biochemically identified in human osteoblasts and MCF-7 cells [23,24]. In estrogen-deprived conditions, both primary human umbilical vein EC and immortalized human endothelial EA.hy926 cells predominatly express ER46, which is efficiently targeted to human EC caveolae, specialized plasma membrane lipid rafts [13]. We have proposed that a caveolae-localized, ER46-centered, multi-molecular complex is critical in rapid eNOS activation and endothelial NO release [25].
3. ER46 - c-Src Interaction and Interdependence for eNOS Activation
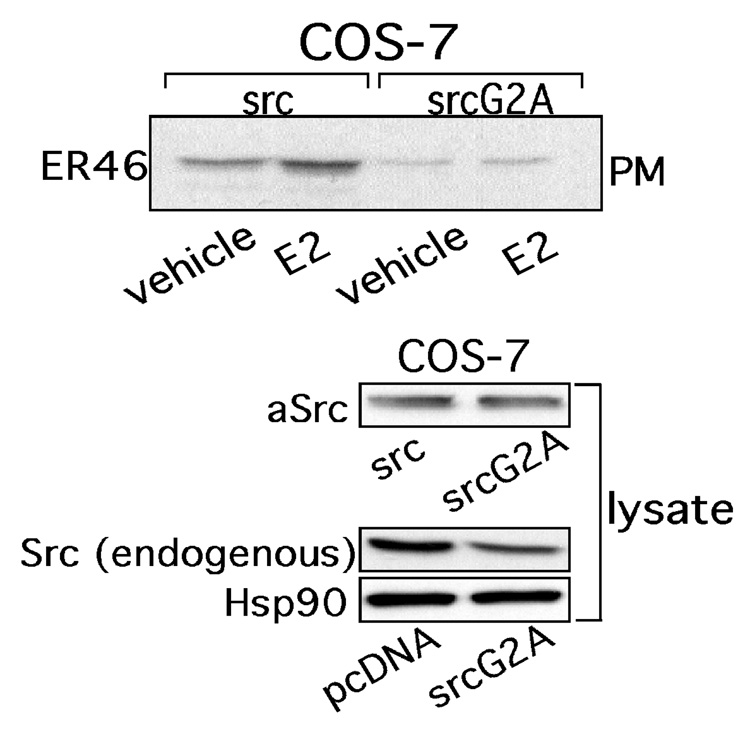
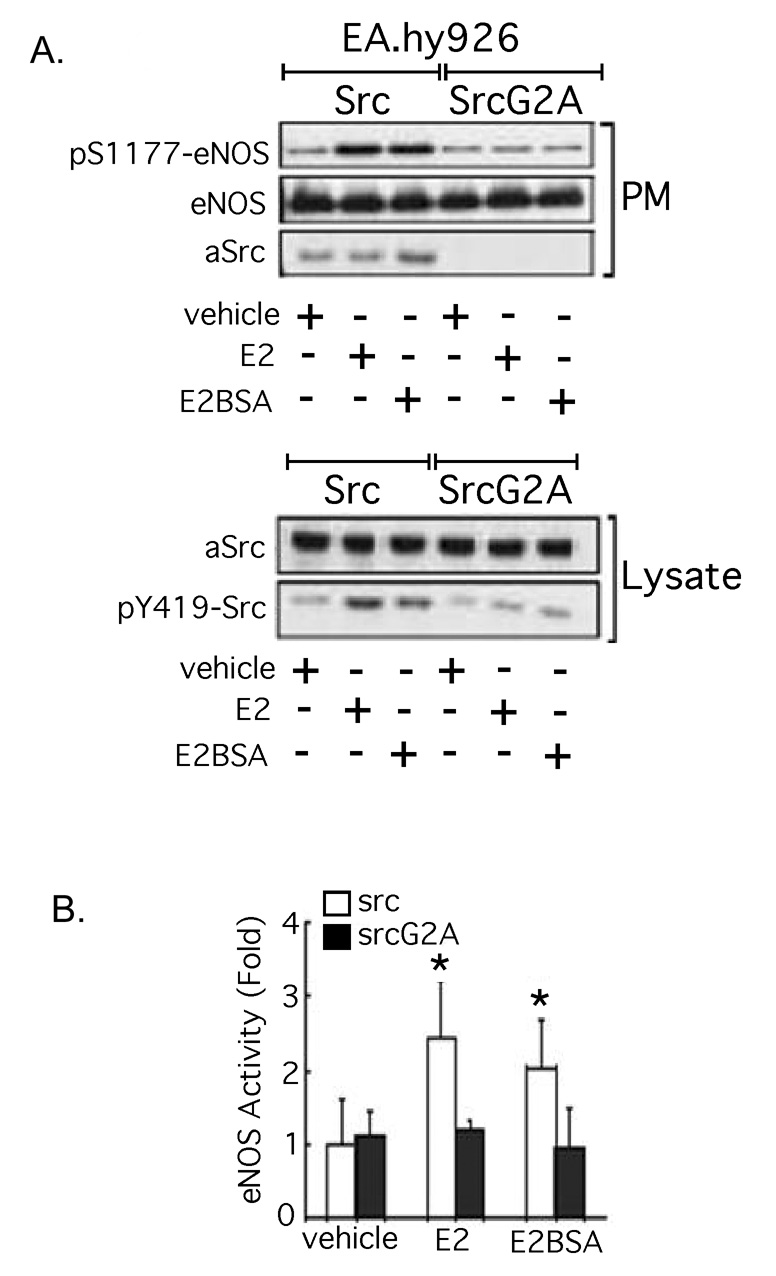
c-Src [26], caveolin-1 [27], Shc [28], the regulatory subunit of PI3K (p85 [29]), multiple receptor tyrosine kinases [30,31], as well as 2 G-protein (Gα) isoforms [32,33] have been reported to bind ERs at the plasma membrane. Several features of c-Src, eNOS and ER membrane targeting, including those described above, have been defined [22,34,35]. We have previously demonstrated that E2-induced activation of EC c-Src directs the generation of a functional signaling complex comprised of ER46, c-Src, and p85, and that the induced ER46-c-Src association is critical for hormone-induced rapid eNOS activation [35]. c-Src membrane targeting is known to require myristoylation on the Glycine-2 residue. Overexpression of a myristoylation-deficient c-Src mutant (SrcG2A) in ER46-transfected COS-7 cells dramatically diminishes ER46 plasma membrane localization (Fig. 2), demonstrating their interdependence for membrane targeting. SrcG2A also does not support membrane-impermeant E2-stimulated phosphorylation of the critical c-Src Tyrosine-419 activation site, nor eNOS activation (Serine-1177 phosphorylation or enzymatic activation) (Fig. 3A, B).
Fig. 2. Interdependent incorporation of c-Src and ER46 in plasma membranes.
COS-7 cells were transfected with ER46, avian Src or avian SrcG2A, treated with 30 nM E2 for 10 min, fractionated and immunoblotted. Reprinted with permission [14].
Fig. 3. Requirement of membrane c-Src kinase activity for eNOS activation in ECs.
EA.hy926 cells electroporated with Src or SrcG2A were treated with 30 nM E2 or E2BSA for 10 min, fractionated, immunoblotted (A), and subjected to eNOS activity assay (n=3) (B). Reprinted with permission [14].
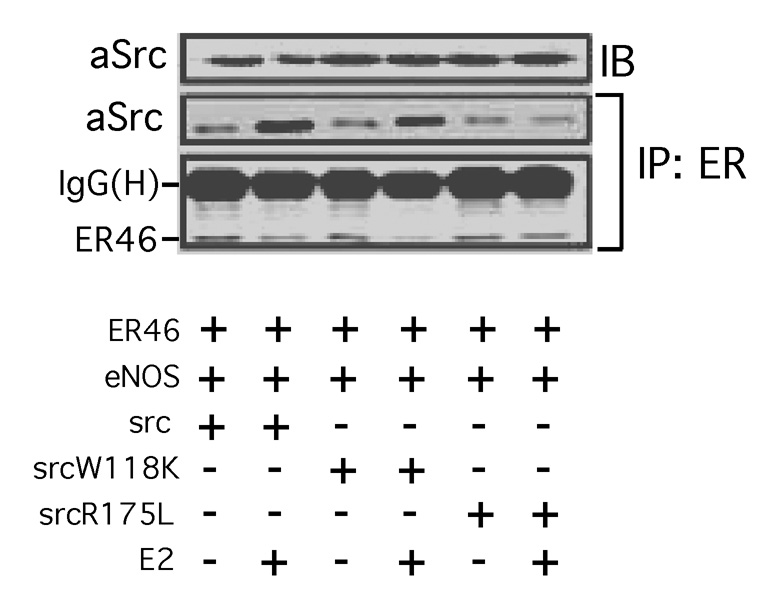
We have attempted to define the most proximal aspects of E2-stimulated, ER-dependent EC signaling responses, as well as the ER46-c-Src interaction domains. In EC ER46-c-Src-eNOS immunocomplexes, E2 stimulates ER46 phosphorylation in vitro, a biochemical event inhibited by both the Src inhibitor PP2 and by the ER antagonist ICI 182,780 [14]. Since Tyrosine-537 is the only known c-Src substrate residue in ERα 36,37], it is likely that this phosphorylation event enhances, or is even responsible for, the endothelial ER46-c-Src interaction. By association, one would predict that the c-Src SH2 domain inducibly interacts with 537-phosphotyrosine. We performed co-immunoprecipitation experiments with cells in which ER46 and key c-Src domain mutants were heterologously expressed. E2 stimulation induced wild-type or SH3 mutant (W118L) association (co-immunoprecipitation) with ER46, whereas the c-Src SH2 mutant (R175L) failed to associate under these conditions (Fig. 4) [14]. This is consistent with a model in which ER phosphotyrosine-537 inducibly interacts with the c-Src SH2 domain, thereby overcoming the intra- and intermolecular restraints conferred by the SH2 domain interacting with its phosphotyrosine-527, and by the SH3 domain-SH2-kinase linker domain interaction [14]. The modulator of non-genomic activity of ER (MNAR) further stabilizes the ER-c-Src interaction [38], although this has not been demonstrated in ECs. Thus, membrane-initiated E2 signaling is directed through c-Src. In ECs, this is achieved through an interdependent plasma membrane targeting of ER46 and c-Src, posttranslationally palmitoylated and myristoylated, respectively. c-Src-dependent E2-induced phosphorylation of ER46, likely Tyrosine-537, results in an amplified c-Src SH2 domain interaction, resulting in eNOS activation and NO release.
Fig. 4. Requirement of c-Src SH2 or SH3 domain in ER46-c-Src interaction.
COS-7 cells were transfected with ER46, eNOS and the indicated avian c-Srcs, E2-deprived for 48 h, serum-starved for 12 h, and stimulated with 33 nM E2 for 10 min. Cell lysates were used for immunoprecipitation with F-10 (anti-ERα C-terminal antibody), and the co-immunoprecipitated avian c-Src was examined by immunoblotting. SrcW118K: c-Src SH3 mutant; SrcR175L: c-Src SH2 mutant. Reprinted with permission [14].
4. ER46 - Caveolin-1 Interaction, Caveolae Localization and eNOS Activation
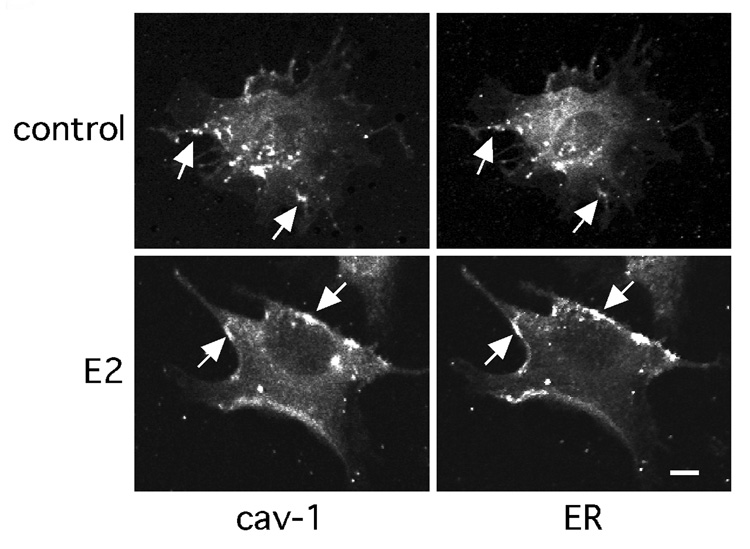
Caveolin-1 is a caveola structural protein that directs caveolae targeting of multiple signaling molecules [39,40,41]. We have demonstrated that ER46 is caveolae-localized in ECs, and that this lipid raft targeting is augmented by E2 stimulation [13]. Confocal images confirm ER46-caveolin-1 colocalization in EA.hy926 cells (Fig 5).
Fig. 5. Caveolin-1-ER46 colocalization in endothelial cells.
Confocal images of cell-surface caveolin-1 and ER46 with anti-cav-1 and anti-ERα (F10) antibodies. Arrows indicate caveolin-1 and ER46 colocalization in digitonin-permeabilized, fixed EA.hy926 cells. Reprinted with permission [13].
The aforementioned S-palmitoylation of ERα at Cysteine-447 has been shown to promote ER-caveolin-1 association [21]. Paradoxically, E2 stimulation in cancer cells reduces the level of ERα palmitoylation within 60 minutes and consequently reduces the ERα-caveolin-1 complex levels [21]. ERα undergoes ligand-dependent allosteric changes. Hegy et al. has proposed that, upon ligand binding, the ERα Cysteine-447 becomes buried in the protein matrix, reducing palmitoylation and caveolin-1 association [42]. Levin and colleagues defined ERα Serine-522 as a critical caveolin-1 interaction residue in the absence of estrogen, and that deletion of the caveolin-1 scaffolding domain (60–100 aa) prevents ERα plasma membrane localization [27]. Together, these studies support that the ERα E-domain Cysteine-447 and Serine-522 residues are involved in caveolin-1 association and membrane localization. In addition, Pedram et al. recently demonstrated that a highly conserved ERα sequence (445–453 aa) is required for plasma membrane localization via palmitoylation [43].
Shaul and colleagues have shown that eNOS activity is augmented by estrogen in caveolae, but not in non-caveolae, subcellular fractions, and that this E2-stimulated event is inhibited by the ER antagonist ICI 182,780 [44]. In this work, ERα levels were much greater in these caveolae fractions. We have found the same preferential localization of ER46 in endothelial caveolae fractions, especially in the presence of estrogen [13]. It is now clear that the ER-mediated activation of eNOS is caveolin-1-dependent, and that the caveola is the signaling organelle in which these activation events occur.
5. Conclusions
Molecular, cellular, and animal studies convincingly demonstrate that estrogen has favorable effects on vascular cells, and that many of these effects are achieved through rapid, membrane-initiated ER-dependent signaling responses. E2 stimulates rapid aortic vasodilation through an ER-, c-Src, and NO-dependent mechanism. The rapid activation of eNOS by estrogen is a key component of both basal and stimulated NO release, affecting vascular homeostasis in many ways. We and others have now defined the molecular components of this critical non-genomic estrogen action. At its center is ERα, or in many endothelial cells, its splice variant ER46. When lipid-modified, c-Src-and caveolin-1-interactive, ERs become plasma membrane caveolae-targeted. E2-induced ER phosphorylation and c-Src SH2 domain interaction are proximal signaling events, leading to PI3K, Akt and, as the key downstream target, eNOS activation. As the clinical paradigms are continuing to develop, it becomes increasingly important to define these signaling events in vascular cells, thereby providing hope that future selective molecular therapeutic targeting will successfully reduce the incidence of cardiovascular disease.
Acknowledgements
This work was supported by the NIH R01 HL61782, T32HL007950 and by the Raymond and Beverly Sackler Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moncada S, Higgs A. The L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- 2.Harrison DG. Cellular and molecular mechanisms of endothelial cell dysfunction. J Clin Invest. 1997;100:2153–2157. doi: 10.1172/JCI119751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stamper MJ, Colditz GA. Estrogen replacement therapy and coronary heart disease: a quantitative assessment of the epidemiologic evidence. Prev Med. 1991;20:47–63. doi: 10.1016/0091-7435(91)90006-p. [DOI] [PubMed] [Google Scholar]
- 4.Grady D, Rubin SM, Petitti DB, Fox CS, Black D, Ettinger B, et al. Hormone therapy to prevent disease and prolong life in postmenopausal women. Ann Intern Med. 1992;117:1016–1037. doi: 10.7326/0003-4819-117-12-1016. [DOI] [PubMed] [Google Scholar]
- 5.Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 6.Heart and Estrogen/progestin Replacement Study (HERS) Research Group. Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, et al. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. JAMA. 1998;280:605–613. doi: 10.1001/jama.280.7.605. [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–1811. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 8.Iafrati MD, Karas RH, Aronovitz M, Kim S, Sulliva TR, Jr, Lubahn DB, et al. Estrogen inhibits the vascular injury response in estrogen receptor-α-deficient mice. Nat Med. 1997;3:545–548. doi: 10.1038/nm0597-545. [DOI] [PubMed] [Google Scholar]
- 9.Pare G, Krust A, Karas RH, Dupont S, Aronovitz M, Chambon P, et al. Estrogen receptor-α mediates the protective effects of estrogen against vascular injury. Cir Res. 2002;90:1087–1092. doi: 10.1161/01.res.0000021114.92282.fa. [DOI] [PubMed] [Google Scholar]
- 10.Karas RH, Hodgin JB, Kwoun K, Krege JH, Aronovitz M, Mackey W, et al. Estrogen inhibits the vascular injury response in estrogen receptor β-deficient female mice. Proc Natl Acad Sci USA. 1999;96:15133–15136. doi: 10.1073/pnas.96.26.15133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brouchet L, Krust A, Dupont S, Chambon P, Bayard F, Arnal JF. Estradiol accelerates reendothelialization in mouse carotid artery through estrogen receptor-α but not estrogen receptor-β. Circulation. 2001;103:423–428. doi: 10.1161/01.cir.103.3.423. [DOI] [PubMed] [Google Scholar]
- 12.Figtree GA, McDonald D, Watkins H, Channon KM. Truncated estrogen receptor α 46-kDa isoform in human endothelial cells. Circulation. 2003;107:120–126. doi: 10.1161/01.cir.0000043805.11780.f5. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor α variant (ER46) in human endothelial cells. Proc Natl Acad Sci USA. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li L, Hisamoto K, Kim KH, Haynes MP, Bauer PM, Sanjay A, et al. Variant estrogen receptor-c-Src molecular interdependence and c-Src structural requirements for endothelial NO synthase activation. Proc Natl Acad Sci USA. 2007;104:16468–16473. doi: 10.1073/pnas.0704315104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pietras RJ, Szego CM. Specific binding sites for oestrogen at the outer surfaces of isolated endometrial cells. Nature. 1977;265:69–72. doi: 10.1038/265069a0. [DOI] [PubMed] [Google Scholar]
- 16.Pietras RJ, Szego CM. Partial purification and characterization of estrogen receptors in subfractions of hepatocyte plasma membranes. Biochem J. 1980;191:743–760. doi: 10.1042/bj1910743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 18.Ropero AB, Soria B, Nadal A. A nonclassical estrogen membrane receptor triggers rapid differential actions in the endocrine pancreas. Mol Endocrinol. 2002;16:497–505. doi: 10.1210/mend.16.3.0794. [DOI] [PubMed] [Google Scholar]
- 19.Moriarty K, Kim KH, Bender JR. Minireview: estrogen receptor-mediated rapid signaling. Endocrinology. 2006;147:5557–5563. doi: 10.1210/en.2006-0729. [DOI] [PubMed] [Google Scholar]
- 20.Acconcia F, Ascenzi P, Fabozzi G, Visca P, Marino M. S-palmitoylation modulates human estrogen receptor-α functions. Biochem Biophys Res Commun. 2004;316:878–883. doi: 10.1016/j.bbrc.2004.02.129. [DOI] [PubMed] [Google Scholar]
- 21.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, et al. Palmitoylation-dependent estrogen receptor α membrane localization: regulation by 17β-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell KS, Haynes MP, Sinha D, Clerisme E, Bender JR. Human vascular endothelial cells contain membrane binding sites for estradiol, which mediate rapid intracellular signaling. Proc Natl Acad Sci USA. 2000;97:5930–5935. doi: 10.1073/pnas.97.11.5930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Flouriot G, Brand H, Denger S, Metivier R, Kos M, Reid G, et al. Identification of a new isoform of the human estrogen receptor-alpha (hER-α) that is encoded by distinct transcripts and that is able to repress hER-α activation function 1. EMBO J. 2000;19:4688–4700. doi: 10.1093/emboj/19.17.4688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denger S, Reid G, Kos M, Flouriot G, Parsch D, Brand H, et al. ERα gene expression in human primary osteoblasts: evidence for the expression of two receptor proteins. Mol Endocrinol. 2001;15:2064–2077. doi: 10.1210/mend.15.12.0741. [DOI] [PubMed] [Google Scholar]
- 25.Kim KH, Bender JR. Rapid, estrogen receptor-mediated signaling: why is the endothelium so special? Sci STKE. 2005;288:pe28. doi: 10.1126/stke.2882005pe28. [DOI] [PubMed] [Google Scholar]
- 26.Castoria G, Migliaccio A, Bilancio A, Di Domenico M, de Falco A, Lombardi M, et al. PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO J. 2001;20:6050–6059. doi: 10.1093/emboj/20.21.6050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor α at the plasma membrane. Mol Cell Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Song RX, McPherson RA, Adam L, Bao Y, Shupnik M, Kumar R, et al. Linkage of rapid estrogen action to MAPK activation by ERα-Shc association and Shc pathway activation. Mol Endocrinol. 2002;16:116–127. doi: 10.1210/mend.16.1.0748. [DOI] [PubMed] [Google Scholar]
- 29.Simoncini T, Hafezi-Moghadam A, Brazil DP, Ley K, Chin WW, Liao JK. Interaction of oestrogen receptor with the regulatory subunit of phosphatidylinositol-3-OH kinase. Nature. 2000;407:538–541. doi: 10.1038/35035131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levin ER. Integration of the extranuclear and nuclear actions of estrogen. Mol Endocrinol. 2005;19:1951–1959. doi: 10.1210/me.2004-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song RX, Barnes CJ, Zhang Z, Bao Y, Kumar R, Santen RJ. The role of Shc and insulin-like growth factor 1 receptor in mediating the translocation of estrogen receptor α to the plasma membrane. Proc Natl Acad Sci USA. 2004;101:2076–2081. doi: 10.1073/pnas.0308334100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERα and ERβ expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 33.Razandi M, Pedram A, Merchenthaler I, Greene GL, Levin ER. Plasma membrane estrogen receptors exist and functions as dimers. Mol Endocrinol. 2004;18:2854–2865. doi: 10.1210/me.2004-0115. [DOI] [PubMed] [Google Scholar]
- 34.Haynes MP, Sinha D, Russell KS, Collinge M, Fulton D, Morales-Ruiz M, et al. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ Res. 2000;87:677–682. doi: 10.1161/01.res.87.8.677. [DOI] [PubMed] [Google Scholar]
- 35.Haynes MP, Li L, Sinha D, Russell KS, Hisamoto K, Baron R, et al. Src kinase mediates phosphatidylinositol 3-kinase/Akt-dependent rapid endothelial nitric-oxide synthase activation by estrogen. J Biol Chem. 2003;278:2118–2123. doi: 10.1074/jbc.M210828200. [DOI] [PubMed] [Google Scholar]
- 36.Migliaccio A, Castoria G, Di Domenico M, de Falco A, Bilancio A, Lombardi M, et al. Steroid-induced androgen receptor-oestradiol receptor β-Src complex triggers prostate cancer cell proliferation. Embo J. 2000;19:5406–5417. doi: 10.1093/emboj/19.20.5406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnold SF, Melamed M, Vorojeikina DP, Notides AC, Sasson S. Estradiol-binding mechanism and binding capacity of the human estrogen receptor is regulated by tyrosine phosphorylation. Mol Endocrinol. 1997;11:48–53. doi: 10.1210/mend.11.1.9876. [DOI] [PubMed] [Google Scholar]
- 38.Wong CW, McNally C, Nickbarg E, Komm BS, Cheskis BJ. Estrogen receptor-interacting protein that modulates its nongenomic activity-crosstalk with Src/Erk phosphorylation cascade. Proc Natl Acad Sci USA. 2002;99:14783–14788. doi: 10.1073/pnas.192569699. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Kim HP, Lee JY, Jeong JK, Bae SW, Lee HK, Jo I. Nongenomic stimulation of nitric oxide release by estrogen is mediated by estrogen receptor α localized in caveolae. Biochem Biophys Res Commun. 1999;263:257–262. doi: 10.1006/bbrc.1999.1348. [DOI] [PubMed] [Google Scholar]
- 40.Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERβ has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- 41.Kelly MJ, Wagner EJ. Estrogen Modulation of G-protein-coupled Receptors. Trends Endocrinol Metab. 1999;10:369–374. doi: 10.1016/s1043-2760(99)00190-3. [DOI] [PubMed] [Google Scholar]
- 42.Hegy GB, Shackleton CH, Carlquist M, Bonn T, Engström O, Sjöholm P, et al. Carboxymethylation of the human estrogen receptor ligand-binding domain-estradiol complex: HPLC/ESMS peptide mapping shows that cysteine 447 does not react with iodoacetic acid. Steroids. 1996;61:367–373. doi: 10.1016/0039-128x(96)00042-6. [DOI] [PubMed] [Google Scholar]
- 43.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 44.Chambliss KL, Yuhanna IS, Mineo C, Liu P, German Z, Sherman TS, et al. Estrogen receptor α and endothelial nitric oxide synthase are organized into a functional signaling module in caveolae. Circ Res. 2000;87:E44–E52. doi: 10.1161/01.res.87.11.e44. [DOI] [PubMed] [Google Scholar]